Abstract
Aims
Previous uncontrolled studies suggested a possible benefit of intravenous immunoglobulin (IVIg) in parvovirus B19 (B19V)‐related dilated cardiomyopathy (DCM). This randomized, double‐blind, placebo‐controlled, single‐centre trial investigated the benefits of IVIg beyond conventional therapy in idiopathic chronic DCM patients with B19V persistence.
Methods and results
Fifty patients (39 men; mean age 54 ± 11 years) with idiopathic chronic (>6 months) DCM on optimal medical therapy, left ventricular ejection fraction (LVEF) <45%, and endomyocardial biopsy (EMB) B19V load of >200 copies/µg DNA were blindly randomized to either IVIg (n = 26, 2 g/kg over 4 days) or placebo (n = 24). The primary outcome was change in LVEF at 6 months after randomization. Secondary outcomes were change in functional capacity assessed by 6‐min walk test (6MWT), quality of life [Minnesota Living with Heart Failure Questionnaire (MLHFQ)], left ventricular end‐diastolic volume (LVEDV), and EMB B19V load at 6 months after randomization. LVEF significantly improved in both IVIg and placebo groups (absolute mean increase 5 ± 9%, P = 0.011 and 6 ± 10%, P = 0.008, respectively), without a significant difference between groups (P = 0.609). Additionally, change in 6MWT [median (interquartile range) IVIg 36 (13;82) vs. placebo 32 (5;80) m; P = 0.573], MLHFQ [IVIg 0 (−7;5) vs. placebo −2 (−6;6), P = 0.904] and LVEDV (IVIg −16 ± 49 mL/m2 vs. placebo −29 ± 40 mL/m2; P = 0.334) did not significantly differ between groups. Moreover, despite increased circulating B19V antibodies upon IVIg administration, reduction in cardiac B19V did not significantly differ between groups.
Conclusion
Intravenous immunoglobulin therapy does not significantly improve cardiac systolic function or functional capacity beyond standard medical therapy in patients with idiopathic chronic DCM and cardiac B19V persistence.
Clinical Trial Registration: ClinicalTrials.gov ID NCT00892112.
Keywords: Dilated cardiomyopathy, Heart failure, Intravenous immunoglobulin, Parvovirus B19, Endomyocardial biopsy
No additional benefit of intravenous immunoglobulin (IVIg) therapy on cardiac systolic function in patients with idiopathic chronic dilated cardiomyopathy (DCM) and cardiac parvovirus B19 (B19V) persistence. EMB, endomyocardial biopsy; LVEF, left ventricular ejection fraction.
Introduction
Virus persistence has been related to the development and progression of dilated cardiomyopathy (DCM). 1 , 2 , 3 , 4 , 5 , 6 In recent decades, parvovirus B19 (B19V) has become the most frequently found cardiotropic virus in endomyocardial biopsies (EMB), with a reported prevalence of up to 80%. 5 , 6 , 7
A possible pathogenic effect of B19V is supported by its activation of pro‐inflammatory cytokines, reduced endothelial regeneration and induction of apoptosis. 5 , 8 , 9 , 10 , 11 If extensive enough, this might result in endothelial damage, which compromises tissue perfusion and causes cardiac dysfunction. 1 , 5 , 8 , 12 However, in recent times B19V genomes are also frequently found in healthy or diseased hearts of individuals without evidence of myocarditis or DCM, making the clinical significance of B19V within the myocardium still unclear. 5 , 13 , 14
While the causal relationship and pathogenic importance of viral persistence and DCM remain controversial, positive effects on viral load and/or cardiac function of intravenous immunoglobulin (IVIg) have been suggested in retrospective and non‐randomized studies. 15 , 16 , 17 , 18 , 19 , 20 Nonetheless, the effect of IVIg therapy in adults with idiopathic chronic DCM and EMB B19V persistence has not yet been prospectively evaluated. We therefore performed a prospective, randomized, double‐blind, placebo‐controlled trial to evaluate the effect of IVIg on systolic cardiac function and EMB B19V load in adult patients with idiopathic chronic DCM and cardiac B19V persistence.
Methods
Study objectives
The objective of the present single‐centre, prospective, randomized, double‐blind, placebo‐controlled trial (NCT00892112) was to evaluate the incremental value of IVIg therapy beyond conventional heart failure therapy vs. conventional heart failure therapy alone in ambulatory patients with idiopathic chronic (>6 months) DCM and an EMB B19V load of >200 copies/µg DNA.
The primary endpoint was the absolute change of echocardiographically assessed left ventricular ejection fraction (LVEF) from baseline to 6 months. The secondary endpoints included changes in EMB B19V load (copies/µg DNA), cardiac CD45+ inflammatory cells, myocardial collagen volume fraction, 6‐min walk test (6MWT) distance, patient quality of life [Minnesota Living with Heart Failure Questionnaire (MLHFQ)], and left ventricular end‐diastolic volume (LVEDV) assessed by echocardiography.
The study was performed according to the Declaration of Helsinki and was approved by the institutional Medical Ethics Committee (METC azM/UM). All patients gave written informed consent.
Patient population
Patients that underwent EMB because of idiopathic DCM were screened for eligibility from November 2009 to January 2018. The primary inclusion criteria were: (i) LVEF <45% with a diagnosis of idiopathic chronic (>6 months) DCM on optimal medical therapy; (ii) EMB B19V load of >200 copies/µg DNA, and (iii) age between 18–75 years.
All patients underwent angiography or non‐invasive screening to exclude coronary artery disease, a transthoracic echocardiogram to rule out significant valvular disease, and right ventricular EMBs before enrolment.
Patients with significant EMB load (>200 copies µg/DNA) of other cardiotropic viruses (enterovirus, adenovirus, human herpesvirus 6, Epstein–Barr virus), systemic autoimmune disease, renal insufficiency (plasma creatinine >115 µmol/L), or non‐idiopathic cardiomyopathy were excluded. The complete list of exclusion criteria is provided in online supplementary Methods S1 .
Randomization and therapeutic protocol
Patients were randomly and blindly assigned using the minimization randomization method to minimize the imbalance between the number of patients in each treatment group over pre‐defined factors (age, gender, LVEF, left ventricular dimensions and EMB B19V load). 21 Patients randomized to the treatment group received a total of 2 g/kg IVIg (Nanogam 50 mg/mL, Sanquin Plasma Products B.V., Amsterdam, The Netherlands) administered as 0.5 g/kg (10 mL/kg) over 6 h on four consecutive days. Placebo consisted of a plasma volume expander (Albuman 40 g/L, Sanquin Plasma Products B.V.) to control for the protein load given by Nanogam, administered as 10 mL/kg over 6 h on four consecutive days. Independent pharmacists prepared the intravenous solutions according to the unique randomization number generated by TEN‐ALEA software. All study personnel and participants were blinded to treatment assignment for the duration of the study.
Clinical evaluations
The baseline measurements and final follow‐up visit at 6 months included physical examination, transthoracic echocardiogram, laboratory tests, assessment of functional capacity by 6MWT and quality of life using the MLHFQ, 22 and right ventricular EMB to evaluate viral persistence and immunohistological markers of inflammation and fibrosis. Additional follow‐up visits with physical examination took place at 2 weeks – including the evaluation of safety and potential side‐effects of the study drug and laboratory tests – and 3 months – including additional transthoracic echocardiogram – after baseline. Circulating B19V antibodies – anti‐NS1 and anti‐VP1/VP2 – were measured at baseline and after the last treatment day, these data were made available after completion of the study to evaluate whether expected differences in circulating B19V antibodies between treatment arms were reached.
More detailed information is provided in online supplementary Methods S1 .
Statistical analysis
The sample size estimation was based on our pilot data – patients with a baseline LVEF <45% in the pilot study were used for this power calculation 15 – as well as potential patient withdrawal. Sample size requirements were determined using the following assumptions: (i) expected absolute therapy effect of 10% LVEF improvement with a standard deviation of 10%; (ii) power of 0.90 and alpha of 0.05; (iii) a drop‐out of n = 4 per group. To ensure enough power for this study, 25 patients per group had to be enrolled.
Normality was assessed visually using histograms and Q‐Q plots. Numerical variables are displayed as mean ± standard deviation or median (interquartile range) where appropriate. Categorical variables are displayed as absolute frequencies and percentage values.
Spearman's correlation (rs) was used to evaluate the correlation between EMB CD45+ cells and EMB B19V load. The changes between 6 months and baseline LVEF (primary outcome), LVEDV, and EMB B19V and the changes between day four and baseline anti‐VP1/VP2 concentrations and anti‐NS1 were calculated for each subject. Subsequently, the differences between groups were calculated by unpaired Student's t‐test or Wilcoxon signed‐rank test for continuous variables, and Chi‐squared or Fisher's exact test for categorical variables as appropriate.
The differences within groups were analysed using paired Student's t‐test, paired Wilcoxon signed‐rank test or McNemar test as appropriate. Additionally, linear mixed‐effects modelling – lme4 package in R 23 – was used to assess the difference in change of LVEF over time (baseline, 3 and 6 months) between the treatment groups (IVIg vs. placebo). Time, group and group*time were included as fixed factors, and a random intercept on subject level was included to adjust for the correlation between repeated measurements. Restricted maximum likelihood was used to obtain unbiased estimates of the treatment effects, while maximum likelihood estimation was used for testing fixed effects.
Missing data at follow‐up were assumed to be missing at random and were not imputed (likelihood‐based approach). In total, three patients (n = 1 IVIg; n = 2 placebo) withdrew – based on their preference – before the first day of treatment and were therefore not included in the analysis. Additionally, two patients did not show up during echocardiography at 3 months (n = 2 IVIg), in three patients LVEDV could not be determined at 6 months (n = 1 IVIg; n = 2 placebo), and two patients refused EMB at 6 months (n = 2 placebo). The former patients have only been excluded from the analysis involving echocardiographic variables at 3 and 6 months, and EMB variables at 6 months, respectively. For the three patients in which LVEDV could not be determined, the Teichholz formula was used to calculate LVEF. The use of the Teichholz formula in all patients did not change the results of this study.
A P‐value ≤0.05 was considered statistically significant. Statistical analysis was performed using RStudio V1.2.5033. 24
Results
A total of 526 patients underwent EMB because of unexplained left ventricular dysfunction (LVEF <45%) at our centre from November 2009 to January 2018. A total of 370 (70%) patients had B19V presence, including 148 (40%) patients with an EMB B19V load of >200 copies/µg DNA. Among them, 95 were excluded according to the exclusion criteria (including four patients with cardiac human herpesvirus 6 >200 copies/µg DNA; no patients were excluded due to significant presence of other cardiotropic viruses). Finally, 53 patients (27 IVIg and 26 placebo) agreed to participate in the study. Three of them, however, withdrew before receiving trial medication (n = 1 IVIg and n = 2 placebo; Figure 1 ) and therefore were not included in the analysis.
Figure 1.
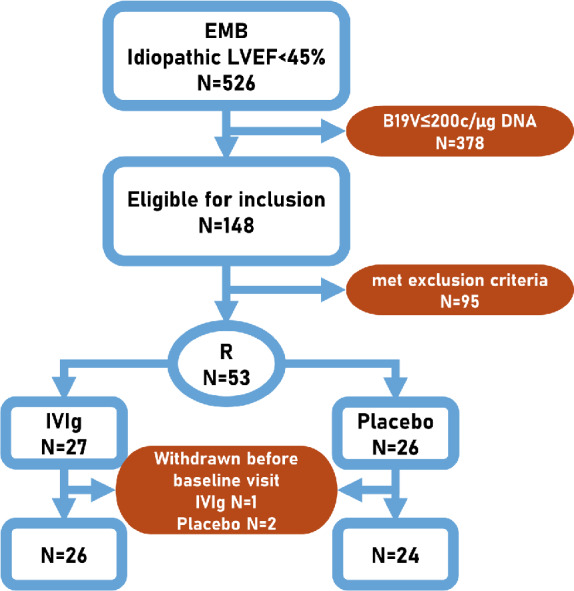
Study flow. B19V, parvovirus B19; c/µg DNA, copies per microgram of DNA; EMB, endomyocardial biopsy; IVIg, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; R, randomized.
Similar clinical characteristics were observed in both treatment groups (Tables 1 and 2 , online supplementary Table S1 ). Male sex predominated (78%), and mean LVEF was 35 ± 6% for the total population at the time of inclusion.
Table 1.
Baseline characteristics: placebo vs. intravenous immunoglobulin
| Demographics/presentation | Placebo (n = 24) | IVIg (n = 26) | P‐value |
|---|---|---|---|
| Age at diagnosis (years) | 53 ± 9 | 54 ± 13 | 0.759 |
| Male sex | 19 (79%) | 20 (77%) | 0.848 |
| Weight (kg) | 86 ± 14 | 87 ± 18 | 0.698 |
| Height (cm) | 177 ± 10 | 178 ± 11 | 0.918 |
| Heart rate (bpm) | 70 ± 8 | 70 ± 9 | 0.817 |
| SBP (mmHg) | 123 ± 13 | 123 ± 18 | 0.857 |
| DBP (mmHg) | 77 ± 9 | 72 ± 10 | 0.045 |
| Diabetes mellitus | 2 (8%) | 3 (12%) | 0.706 |
| Atrial fibrillation | 10 (42%) | 5 (19%) | 0.084 |
| LBBB | 6 (25%) | 5 (19%) | 0.719 |
| Hypercholesterolaemia | 3 (13%) | 2 (8%) | 0.571 |
| Days from first DCM diagnosis | 418 (335;653) | 415 (316;801) | 0.961 |
| Medical history of acute myocarditis | 0 (0%) | 3 (12%) | 0.236 |
| Medical history of HFH | 5 (21%) | 4 (15%) | 0.721 |
| Family history of DCM | 3 (13%) | 1 (4%) | 0.340 |
| NYHA class III or IV | 0 (0%) | 1 (4%) | 1.000 |
| Lab | |||
| Creatinine (µmol/L) | 91 (75;102) | 84 (76;99) | 0.600 |
| NT‐proBNP (pmol/L) | 44 (14;98) | 33 (8;60) | 0.218 |
| CRP | 1 (1;3) | 2 (1;3) | 0.289 |
| Medication a | |||
| Beta‐blocker (yes/no) | 22 (92%) | 24 (92%) | 1.000 |
| ≥50% OMT | 12 (50%) | 16(62%) | 0.592 |
| ACE‐inhibitor/ARB (yes/no) | 23 (96%) | 25 (96%) | 1.000 |
| ≥50% OMT | 21 (88%) | 20 (77%) | 0.467 |
| Aldosterone antagonist (yes/no) | 13 (54%) | 8 (31%) | 0.165 |
| ≥50% OMT | 12 (50%) | 7 (27%) | 0.165 |
| Cardiac devices | |||
| ICD | 1 (4%) | 3 (12%) | 0.611 |
| CRT‐D | 3 (13%) | 1 (4%) | 0.340 |
ACE, angiotensin‐converting‐enzyme; ARB, angiotensin receptor blocker; CRP, C‐reactive protein; CRT‐D, cardiac resynchronization therapy with defibrillator; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; HFH, heart failure hospitalization; ICD, implantable cardioverter‐defibrillator; IVIg, intravenous immunoglobulin; LBBB, left bundle branch block; NYHA, New York Heart Association; OMT, optimal medical therapy; SBP, systolic blood pressure.
OMT was calculated based on the 2016 European Society of Cardiology heart failure guidelines.
Table 2.
Echocardiographic results: placebo vs. intravenous immunoglobulin
| Placebo (n = 24) | IVIg (n = 26) | P‐value | |
|---|---|---|---|
| Baseline | |||
| LVEDV (mL) | 189 ± 52 | 208 ± 69 | 0.283 |
| LVESV (mL) | 122 ± 38 | 135 ± 54 | 0.313 |
| LVEF (%) | 35 ± 6 | 36 ± 7 | 0.552 |
| 3 months a | |||
| LVEDV (mL) | 178 ± 59 | 201 ± 72 | 0.226 |
| LVESV (mL) | 112 ± 44 | 125 ± 53 | 0.380 |
| LVEF (%) | 38 ± 9 | 39 ± 8 | 0.703 |
| LVEF absolute change from baseline (%) | 3 ± 9 | 3 ± 6 | 0.985 |
| 6 months | |||
| LVEDV (mL) | 164 ± 55 | 190 ± 60 | 0.120 |
| LVESV (mL) | 100 ± 41 | 117 ± 46 | 0.187 |
| LVEF (%) | 41 ± 12 | 41 ± 10 | 0.921 |
| LVEF absolute change from baseline (%) | 6 ± 10 | 5 ± 9 | 0.609 |
IVIg, intravenous immunoglobulin; LVEDV, left lentricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume.
IVIg n = 24.
Effect of therapy on left ventricular function
The primary endpoint, i.e. absolute change in LVEF 6 months after therapy, did not differ significantly between the IVIg and placebo group (P = 0.609; Figure 2A ). The mean LVEF improved significantly in the total patient population (5 ± 9%, P < 0.001) from baseline to 6 months. This increase was significant for both the IVIg (5 ± 9%, P = 0.011) and placebo group (6 ± 10%, P = 0.008). Additionally, the linear mixed‐effects model did not show a significant difference in LVEF trajectory over time between groups (P = 0.923 for interaction between group and time), indicating that the change in LVEF from baseline to 3 and 6 months was not significantly different between the treatment groups.
Figure 2.
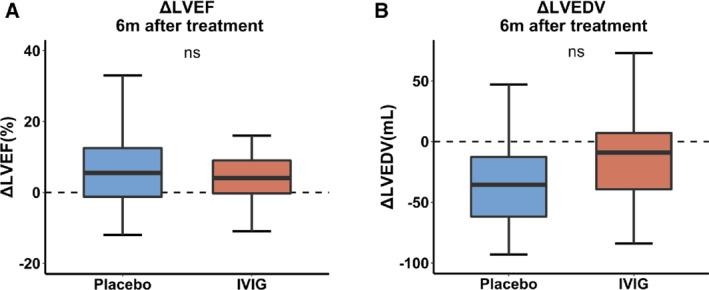
(A) Primary endpoint: comparison of change in left ventricular ejection fraction (LVEF) 6 months after therapy. (B) Comparison of change in left ventricular end‐diastolic volume (LVEDV) 6 months after therapy. IVIG, intravenous immunoglobulin.
The LVEDV decreased significantly in the total patient population from baseline to 6 months (−22 ± 45 mL/m2, P = 0.002). Although this change was only significant in the placebo group (placebo −29 ± 40, P = 0.003; IVIg −16 ± 49 mL/m2; P = 0.116), the difference between groups was not significant (P = 0.334; Figure 2B ).
No significant treatment effect was observed in a subset of patients with baseline EMB B19V load >500 copies/µg DNA (n = 10 placebo; n = 13 IVIg) on change of both LVEF (IVIg 4 ± 7% and placebo 9 ± 8%; P = 0.157) and LVEDV (IVIg −14 ± 57 mL/m2 and placebo −42 ± 22 mL/m2; P = 0.129) 6 months after treatment (online supplementary Tables S3–S6 ).
Histological and biochemical changes
Baseline EMB B19V load did not significantly differ between the IVIg and placebo group [481 (334;907) and 354 (287;883) copies/µg DNA, respectively, P = 0.351; Table 3 ]. There was a significant reduction in B19V load in the total patient population during the trial [−119 (−338;4) copies/µg DNA, P = 0.004],which was comparable between groups (P = 0.718; Figure 3 ).
Table 3.
Endomyocardial biopsy results: placebo vs. intravenous immunoglobulin
| Placebo (n = 24) | IVIg (n = 26) | P‐value | |
|---|---|---|---|
| Baseline | |||
| Cardiac inflammation a | 6 (25%) | 7 (27%) | 0.877 |
| CD3 (cells/mm2) | 5.6 (1.8;7.8) | 5.1 (2.9;7.0) | 0.907 |
| CD45 (cells/mm2) | 6.8 (5.1;12.3) | 7.8 (4.7;11.3) | 0.808 |
| CD68 (cells/mm2) | 1.3 (0.5;4.0) | 2.8 (2.0;4.1) | 0.145 |
| Collagen volume fraction (%) | 7.2 (4.4;11.0) | 7.6 (4.2;13.9) | 0.683 |
| B19V (copies/µg DNA) | 354 (287;883) | 481 (334;907) | 0.351 |
| 6 months b | |||
| Cardiac inflammation a | 3 (12%) | 7 (29%) | 0.286 |
| CD3 (cells/mm2) | 5.4 (2.5;6.1) | 4.5 (3.1;6.5) | 0.645 |
| CD45 (cells/mm2) | 6.4 (5.1;9.1) | 8.9 (7.2;11.2) | 0.060 |
| CD68 (cells/mm2) | 1.2 (0.7;2.8) | 2.1 (1.5;3.6) | 0.090 |
| Collagen volume fraction (%) | 6.5 (4.8;8.7) | 8.4 (4.8;13.9) | 0.177 |
| B19V (copies/µg DNA) | 245 (200;633) | 349 (200;891) | 0.298 |
B19V, parvovirus B19; CD, cluster of differentiation; IVIg, intravenous immunoglobulin.
Defined as ≥14 infiltrating CD45+ cells/mm2 (including up to four infiltrating CD68+ cells/mm2) of total myocardial area.
IVIg n = 24.
Figure 3.
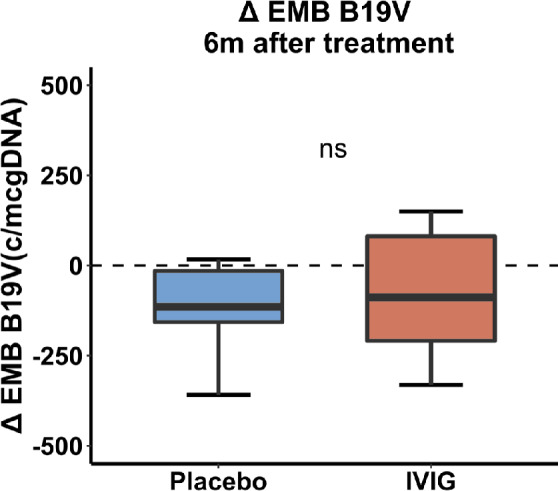
Comparison of change in endomyocardial biopsy (EMB) parvovirus B19 (B19V) load (copies/µg DNA) 6 months after therapy. IVIG, intravenous immunoglobulin.
No significant difference in amount of inflammation or collagen volume fraction in EMB was observed between groups at baseline and 6 months (Table 3 ). Changes of CD45+ cells/mm2 after treatment [placebo −0.9 (−7.2;1.0), P = 0.098; IVIg 2.2 (−1.6;5.6), P = 0.317] did not significantly differ between the treatment arms (P = 0.058). Moreover, B19V and EMB CD45+ cells/mm2 did not significantly correlate at baseline, neither in the total study population (rs = 0.08, P = 0.58), nor in patients (n = 23) with an EMB B19V load of >500 copies/µg DNA at baseline (rs = −0.18, P = 0.42).
Changes in functional capacity and quality of life
The 6MWT distance increased significantly after 6 months in the total patient population [36 (6;81) m, P < 0.001], but did not differ between the IVIg and placebo group [36 (13;82) and 32 (5;80), respectively, P = 0.573; Table 4 ].
Table 4.
Functional capacity and quality of life: placebo vs. intravenous immunoglobulin
| Placebo (n = 24) | IVIg (n = 26) | P‐value | |
|---|---|---|---|
| 6‐min walk test (m) | |||
| Baseline | 487 (466;552) | 468 (421;542) | 0.336 |
| 6 months | 554 (480;581) | 508 (448;613) | 0.778 |
| Change | 32 (5;80) | 36 (13;82) | 0.573 |
| Quality of life (MLHFQ) | |||
| Baseline | 18 (8;34) | 11 (6;36) | 0.514 |
| 6 months | 20 (5;41) | 17 (3;29) | 0.633 |
| Change | −2 (−6;6) | 0 (−7;5) | 0.904 |
IVIg, intravenous immunoglobulin; MLHFQ, Minnesota Living with Heart Failure Questionnaire, with a higher score (range 0–105) indicative of a lower quality of life.
Quality of life did not differ between the assessment at 6 months and baseline in the total patient population [MLHFQ −2 (−6;6), P = 0.517], neither was there a significant difference in change during the trial between the treatment groups [IVIg 0 (−7;5) and placebo −2 (−6;6), P = 0.904; Table 4 ). Two patients (n = 1 in both groups) were classified as New York Heart Association class ≥III after 6 months.
Adverse drug effects and heart failure medication changes
No adverse event led to the interruption or discontinuation of the study treatment, and no serious adverse drug reaction occurred during the study. Headaches were significantly more often reported in the IVIg group compared to placebo (42% and 17%, respectively, P = 0.048). In nine patients, heart failure medication changes occurred during the study period (n = 5 IVIg and n = 4 placebo, respectively; P = 0.99). Medication usage at baseline (Table 1 ) and after 6 months (online supplementary Table S2 ) was not different between the treatment groups. None of the patients underwent cardiac device implantation or upgrade during the study period.
Effect of therapy on circulating parvovirus B19 antibodies
A significant increase in B19V antibodies after 4 days of treatment was observed in patients receiving IVIg as compared to placebo (both P < 0.001), reflected by an increase of positive anti‐NS1 tests (from 4% to 81%, P < 0.001) and increased anti‐VP1/VP2 [+869 (761;1045) U/mL, P < 0.001]. In contrast, anti‐NS1 (from 4% to 9%, P = 0.99) and anti‐VP1/VP2 [−50 (−97;‐26) U/mL, P < 0.001] did not significantly increase in the placebo group.
Discussion
This is the first randomized placebo‐controlled trial evaluating the therapeutic effects of IVIg in patients with idiopathic chronic DCM and EMB proven B19V persistence, showing that IVIg (2 g/kg) did not result in any improvement of cardiac function, functional capacity or quality of life after 4 days of treatment.
The mode of action of IVIg is versatile with a broad range of activities: IVIg preparations are known to have anti‐infectious, anti‐inflammatory, and immunomodulating properties. 25 , 26 The administration of IVIg resulted in a significant increase of anti‐B19V antibodies (anti‐NS1 and anti‐VP1/VP2) in the treated patients, but did not result in a significant reduction of cardiac B19V load or inflammation. IVIg has no beneficial effects in the majority of patients with idiopathic chronic DCM and a cardiac B19V load of >200 copies/µg DNA. After the initiation of this study, several studies revealed that the EMB B19V load of DCM patients was comparable to controls with either diseased or healthy hearts. 5 Also, B19V load is not affected by immunosuppression in inflammatory DCM with significant B19V load, as recently published, 27 also indicating that B19V might be an innocent bystander. Adjudicating IVIg treatment solely based on cardiac function and B19V presence does not seem to be an effective strategy given the likely latent intracellular state of the virus in the majority of patients. 28 Therefore, additional determinants beyond viral load – e.g. active viral replication, location of the virus, co‐infection, inflammation and genetic background – might be crucial for B19V to yield a pathogenic potential in DCM. A better understanding of these mechanisms is crucial to select a subgroup of patients that still could benefit from IVIg therapy. 5 , 14 , 29 , 30 , 31
Our negative findings in chronic DCM are in line with the IMAC trial where IVIg on top standard therapy was given in patients with recent‐onset DCM and did not improve outcome. 32 DCM is the result of multiple underlying environmental, genetic and immunological insults and therefore likely does not follow a unidirectional treatment strategy. 33 Identifying downstream pathophysiological processes will be essential to develop future targeted treatment strategies for subsets of DCM patients.
The used total dosage (2 g/kg IVIg) within this study is known as a high‐dose therapy and is the same as previously studied in peripartum cardiomyopathy, 34 acute myocarditis in children, 35 recent‐onset DCM, 32 and our retrospective pilot study in idiopathic cardiomyopathy with EMB B19V persistence. 15 While the present study is unable to assess the possible beneficial effect of prolonged administration of IVIg (beyond 4 days or on a weekly/monthly basis), the lack of any improvement of either cardiac function, viral load, inflammation, or functional capacity makes a clinical relevant beneficial effect unlikely. We included chronic DCM patients and still observed a significant increase in systolic cardiac function in the total patient population, underscoring the fact that functional recovery may take longer than 6 months upon optimal heart failure therapy. 36 , 37
Limitations
The number of included patients in our trial is small. Moreover, given the limited information available on this topic before the initiation of this study, we decided to perform the power calculation solely based on the primary endpoint, which theoretically could have resulted in a type 2 error for the secondary endpoints. However, due to the randomized and double‐blind nature of the study and corresponding negative findings, we would not favour larger clinical trials with IVIg in B19V‐related DCM which are based solely on cardiac B19V load. The inclusion of patients with EMB B19V >200 copies/µg DNA in the current study was based on our previous results in post‐mortem samples of non‐cardiac diseased subjects. 15 After the initiation of this study, a load of >500 copies/µg DNA was suggested to be a clinically relevant threshold given its association with cardiac inflammation. 38 In a sub‐analysis of patients with EMB B19V >500 copies/µg DNA, we did not find a trend for a beneficial effect of IVIg on any of the endpoints. Nonetheless, a small effect cannot be excluded due to the limited number of patients in this sub‐analysis and as a consequence, insufficient power. Moreover, a sub‐analysis in patients with EMB B19V >500 copies/µg DNA and EMB proven inflammation (n = 7) could not be performed due to the lack of power. Lastly, due to the small sample size and over‐representation of males (78%) – in which DCM is known to be more prevalent – the effect of gender could not be evaluated within this trial. 39 , 40
Conclusion
Intravenous immunoglobulin therapy does not provide additional benefit on cardiac systolic function or functional capacity beyond standard medical therapy in patients with idiopathic chronic DCM and cardiac B19V persistence.
Funding
We acknowledge the support of the sponsor – Sanquin Plasma Products B.V. – of this investigator‐driven study. Members of the clinical operations and medical department of the sponsor supported the work of the researcher but did not make any scientific or research decisions. Statistical analyses were performed by an independent party (CRO Parexel). The researcher (cardiologist S.H.) was responsible for the study design, interpretation of the results, the development and writing of the report, and the decision to submit for publication, and had full access to all data. We acknowledge the support of the ERA‐Net‐CVD project MacroERA, 01KL1706, and IMI2‐CARDIATEAM (No 821 508), and the Netherlands Cardiovascular Research Initiative, an initiative with the support of the Dutch Heart Foundation, CVON2016‐Early HFPEF, 2015–10, CVON She‐PREDICTS, grant 2017–21, CVON Arena‐PRIME, 2017–18 for the DCM cohort and biobanking, allowing patient inclusion. Dr Hazebroek received a Kootstra Talent Post‐doc Fellowship grant (University of Maastricht). S. Heymans received funding from Sanquin Plasma Products B.V.
Conflict of interest: none declared.
Supporting information
Table S1. Laboratory measurements of inflammation and NT‐proBNP: placebo vs. IVIg.
Table S2. Heart failure medication at 6 months: placebo vs. IVIg.
Table S3. Baseline characteristics: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S4. Echocardiographic results: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S5. Endomyocardial biopsy results: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S6. Functional capacity and quality of life: placebo vs. IVIg.
Methods S1. Detailed study methods.
Contributor Information
Mark R. Hazebroek, Email: mark.hazebroek@mumc.nl.
Stephane R.B. Heymans, Email: s.heymans@maastrichtuniversity.nl.
References
- 1. Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005;112:1965–1970. [DOI] [PubMed] [Google Scholar]
- 2. D'Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Di Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001;85:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases ; 2648a–2648d . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 4. Arbustini E, Narula N, Dec GW, Reddy KS, Greenberg B, Kushwaha S, Marwick T, Pinney S, Bellazzi R, Favalli V, Kramer C, Roberts R, Zoghbi WA, Bonow R, Tavazzi L, Fuster V, Narula J. The MOGE(S) classification for a phenotype‐genotype nomenclature of cardiomyopathy: endorsed by the World Heart Federation. J Am Coll Cardiol 2013;62:2046–2072. [DOI] [PubMed] [Google Scholar]
- 5. Verdonschot J, Hazebroek M, Merken J, Debing Y, Dennert R, Brunner‐La Rocca HP, Heymans S. Relevance of cardiac parvovirus B19 in myocarditis and dilated cardiomyopathy: review of the literature. Eur J Heart Fail 2016;18:1430–1441. [DOI] [PubMed] [Google Scholar]
- 6. Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 2005;111:887–893. [DOI] [PubMed] [Google Scholar]
- 7. Pankuweit S, Portig I, Eckhardt H, Crombach M, Hufnagel G, Maisch B. Prevalence of viral genome in endomyocardial biopsies from patients with inflammatory heart muscle disease. Herz 2000;25:221–226. [DOI] [PubMed] [Google Scholar]
- 8. Bultmann BD, Klingel K, Sotlar K, Bock CT, Baba HA, Sauter M, Kandolf R. Fatal parvovirus B19‐associated myocarditis clinically mimicking ischemic heart disease: an endothelial cell‐mediated disease. Hum Pathol 2003;34:92–95. [DOI] [PubMed] [Google Scholar]
- 9. Bachelier K, Biehl S, Schwarz V, Kindermann I, Kandolf R, Sauter M, Ukena C, Yilmaz A, Sliwa K, Bock CT, Klingel K, Bohm M. Parvovirus B19‐induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PLoS One 2017;12:e0176311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt‐Lucke C, Zobel T, Schrepfer S, Kuhl U, Wang D, Klingel K, Becher PM, Fechner H, Pozzuto T, Van Linthout S, Lassner D, Spillmann F, Escher F, Holinski S, Volk HD, Schultheiss HP, Tschope C. Impaired endothelial regeneration through human parvovirus B19‐infected circulating angiogenic cells in patients with cardiomyopathy. J Infect Dis 2015;212:1070–1081. [DOI] [PubMed] [Google Scholar]
- 11. Duechting A, Tschope C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock CT. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol 2008;82:7942–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tschope C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kuhl U, Kandolf R, Schultheiss HP. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 2005;111:879–886. [DOI] [PubMed] [Google Scholar]
- 13. Hjalmarsson C, Liljeqvist JA, Lindh M, Karason K, Bollano E, Oldfors A, Andersson B. Parvovirus B19 in endomyocardial biopsy of patients with idiopathic dilated cardiomyopathy: foe or bystander? J Card Fail 2019;25:60–63. [DOI] [PubMed] [Google Scholar]
- 14. Schenk T, Enders M, Pollak S, Hahn R, Huzly D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 2009;47:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dennert R, Velthuis S, Schalla S, Eurlings L, van Suylen RJ, van Paassen P, Tervaert JW, Wolffs P, Goossens VJ, Bruggeman C, Waltenberger J, Crijns HJ, Heymans S. Intravenous immunoglobulin therapy for patients with idiopathic cardiomyopathy and endomyocardial biopsy‐proven high PVB19 viral load. Antivir Ther 2010;15:193–201. [DOI] [PubMed] [Google Scholar]
- 16. Maisch B, Hufnagel G, Kolsch S, Funck R, Richter A, Rupp H, Herzum M, Pankuweit S. Treatment of inflammatory dilated cardiomyopathy and (peri)myocarditis with immunosuppression and i.v. immunoglobulins. Herz 2004;29:624–636. [DOI] [PubMed] [Google Scholar]
- 17. Maisch B. Cardio‐immunology of myocarditis: focus on immune mechanisms and treatment options. Front Cardiovasc Med 2019;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lotze U, Egerer R, Gluck B, Zell R, Sigusch H, Erhardt C, Heim A, Kandolf R, Bock T, Wutzler P, Figulla HR. Low level myocardial parvovirus B19 persistence is a frequent finding in patients with heart disease but unrelated to ongoing myocardial injury. J Med Virol 2010;82:1449–1457. [DOI] [PubMed] [Google Scholar]
- 19. Heidendael JF, Den Boer SL, Wildenbeest JG, Dalinghaus M, Straver B, Pajkrt D. Intravenous immunoglobulins in children with new onset dilated cardiomyopathy. Cardiol Young 2018;28:46–54. [DOI] [PubMed] [Google Scholar]
- 20. Prasad AN, Chaudhary S. Intravenous immunoglobulin in children with acute myocarditis and/or early dilated cardiomyopathy. Indian Pediatr 2014;51:583–584. [DOI] [PubMed] [Google Scholar]
- 21. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–115. [PubMed] [Google Scholar]
- 22. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol 1993;71:1106–1107. [DOI] [PubMed] [Google Scholar]
- 23. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. J Stat Softw 2015;67:48. [Google Scholar]
- 24. Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 2004;291:2367–2375. [DOI] [PubMed] [Google Scholar]
- 25. Chaigne B, Mouthon L. Mechanisms of action of intravenous immunoglobulin. Transfus Apher Sci 2017;56:45–49. [DOI] [PubMed] [Google Scholar]
- 26. Nimmerjahn F, Ravetch JV. Anti‐inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 2008;26:513–533. [DOI] [PubMed] [Google Scholar]
- 27. Tschope C, Elsanhoury A, Schlieker S, Van Linthout S, Kuhl U. Immunosuppression in inflammatory cardiomyopathy and parvovirus B19 persistence. Eur J Heart Fail 2019;21:1468–1469. [DOI] [PubMed] [Google Scholar]
- 28. Verdonschot JA, Cooper LT, Heymans SR. Parvovirus B19 in dilated cardiomyopathy: there is more than meets the eye. J Card Fail 2019;25:64–66. [DOI] [PubMed] [Google Scholar]
- 29. Kuethe F, Lindner J, Matschke K, Wenzel JJ, Norja P, Ploetze K, Schaal S, Kamvissi V, Bornstein SR, Schwanebeck U, Modrow S. Prevalence of parvovirus B19 and human bocavirus DNA in the heart of patients with no evidence of dilated cardiomyopathy or myocarditis. Clin Infect Dis 2009;49:1660–1666. [DOI] [PubMed] [Google Scholar]
- 30. Moimas S, Zacchigna S, Merlo M, Buiatti A, Anzini M, Dreas L, Salvi A, Di Lenarda A, Giacca M, Sinagra G. Idiopathic dilated cardiomyopathy and persistent viral infection: lack of association in a controlled study using a quantitative assay. Heart Lung Circ 2012;21:787–793. [DOI] [PubMed] [Google Scholar]
- 31. Stewart GC, Lopez‐Molina J, Gottumukkala RV, Rosner GF, Anello MS, Hecht JL, Winters GL, Padera RF, Baughman KL, Lipes MA. Myocardial parvovirus B19 persistence: lack of association with clinicopathologic phenotype in adults with heart failure. Circ Heart Fail 2011;4:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre‐Amione G, Gass A, Janosko K, Tokarczyk T, Kessler P, Mann DL, Feldman AM, Car IMA. Controlled trial of intravenous immune globulin in recent‐onset dilated cardiomyopathy. Circulation 2001;103:2254–2259. [DOI] [PubMed] [Google Scholar]
- 33. Verdonschot JA, Hazebroek MR, Ware JS, Prasad SK, Heymans SR. Role of targeted therapy in dilated cardiomyopathy: the challenging road toward a personalized approach. J Am Heart Assoc 2019;8:e012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ Jr, MacGowan GA, Murali S, Rosenblum WD, Feldman AM, McNamara DM. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol 1999;34:177–180. [DOI] [PubMed] [Google Scholar]
- 35. Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, Baker AL, Perez‐Atayde AR, Newburger JW. Gamma‐globulin treatment of acute myocarditis in the pediatric population. Circulation 1994;89:252–257. [DOI] [PubMed] [Google Scholar]
- 36. Lupon J, Gavidia‐Bovadilla G, Ferrer E, de Antonio M, Perera‐Lluna A, Lopez‐Ayerbe J, Domingo M, Nunez J, Zamora E, Moliner P, Diaz‐Ruata P, Santesmases J, Bayes‐Genis A. Dynamic trajectories of left ventricular ejection fraction in heart failure. J Am Coll Cardiol 2018;72:591–601. [DOI] [PubMed] [Google Scholar]
- 37. Merlo M, Cannata A, Gobbo M, Stolfo D, Elliott PM, Sinagra G. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail 2018;20:228–239. [DOI] [PubMed] [Google Scholar]
- 38. Bock CT, Klingel K, Kandolf R. Human parvovirus B19‐associated myocarditis. N Engl J Med 2010;362:1248–1249. [DOI] [PubMed] [Google Scholar]
- 39. Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol 2013;38:7–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3:135–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Laboratory measurements of inflammation and NT‐proBNP: placebo vs. IVIg.
Table S2. Heart failure medication at 6 months: placebo vs. IVIg.
Table S3. Baseline characteristics: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S4. Echocardiographic results: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S5. Endomyocardial biopsy results: placebo vs. IVIg with baseline B19V EMB >500 copies/µg DNA.
Table S6. Functional capacity and quality of life: placebo vs. IVIg.
Methods S1. Detailed study methods.


