Abstract
Background and Objective
Some children with asthma have low lung growth, putting them at increased risk for COPD later in life. However, it is currently not clear who will experience this adverse growth pattern. We therefore investigated the predictive role of blood eosinophils as a type 2 inflammation marker in lung growth, focusing on the presence and severity of asthma.
Methods
We investigated blood eosinophils and lung function growth (percentage of predicted values) using linear mixed models in children and adolescents from two longitudinal cohorts. One cohort was hospital‐based and consisted of asthmatic children at their first outpatient clinic visit after referral by the general practitioner (n = 133, mean age 9.8), while the second was a general population‐based birth cohort (PIAMA, asthma n = 52 and non‐asthma n = 433, mean age 8.1). The hospital‐based cohort had not been treated with inhaled corticosteroids (ICS) before referral.
Results
Subjects in the hospital‐based asthma cohort had more severe asthma compared with the asthmatic subjects in the population‐based cohort, defined by lower lung function levels and a higher prevalence of bronchial hyper‐responsiveness. In the asthma cohort, higher blood eosinophil numbers were associated with less growth in FEV1 (estimated change in lung function per 1 unit increase in ln blood eosinophils (B): −0.66%/year (95% confidence interval (CI): −1.11 to −0.20, p < .01)) and FVC (B: −0.40%/year (95% CI: −0.75 to −0.05), p = .025)) during follow‐up in adolescence (min 7, max 17 years). These associations were not observed in the general population‐based birth cohort, regardless of asthma status during follow‐up (age 8–16).
Conclusions and Clinical Relevance
Blood eosinophil counts in children with asthma not treated with ICS at referral were predictive of lower growth in FEV1 and FVC during follow‐up in adolescence. Our findings indicate that this association is dependent on the degree of asthma severity. Future studies should address whether anti‐eosinophilic treatments preserve lung function growth in children with asthma.
Keywords: asthma, eosinophils, lung function growth, pediatrics
1. INTRODUCTION
Children with asthma are more likely to reach lower levels of maximally attained lung function in early adulthood than their healthy peers, 1 , 2 making them more susceptible to COPD later in life. 2 , 3 Four different patterns of lung function growth and decline until early adulthood in asthmatics have been described. These lung function trajectories were defined as ‘normal growth and normal decline’, ‘normal growth and early decline’, ‘reduced growth and normal decline’, and ‘reduced growth and early decline’. 2 However, limited information is known about the determinants of these patterns. So far, initial lung function level at first measurement, airway hyper‐responsiveness, sex and the age at which the plateau is reached were reported to differ between the four established lung function trajectories. 2
In asthma, local airway inflammation is usually eosinophilic in nature. 4 As a result, high numbers of eosinophils in the airways and in blood are a characteristic feature of this type 2–mediated inflammation. 4 , 5 , 6 The ratio of eosinophils circulating in the blood to local tissue inflammation is approximately 1:100. 7 The role of severe type 2 inflammation in lung function growth in asthma is unknown. Furthermore, longitudinal research has shown that numbers of blood eosinophils are associated with maximal lung growth and pulmonary function at different stages of development and that this association cannot be completely explained by bronchial hyper‐responsiveness (BHR) or asthma, suggesting that blood eosinophils are also a risk factor for lower lung function growth and possibly COPD later in life in the general population. 8 , 9 , 10
Treatment of airway inflammation, as reflected by BHR, with inhaled corticosteroids (ICS) is the cornerstone of asthma therapy, 11 but its use has not been shown to preserve lung growth. 12 However, the emergence of novel medication such as monoclonal antibodies targeting the IL‐5 receptor pathway provides additional treatment opportunities in preventing and mitigating the effects of eosinophilic‐mediated, type 2 high inflammation. 13
The predictive role of blood eosinophils as a marker of type 2 inflammation in lung function growth requires further exploration, 14 focusing on the role of asthma status and severity. We have therefore investigated the association of blood eosinophil counts with lung function growth in asthmatic children with varying degrees of asthma severity and in non‐asthmatic children from two longitudinal cohorts.
2. METHODS
2.1. Study design and participants
This study was performed using data from a longitudinal hospital‐based asthma cohort 15 and PIAMA (Prevention and Incidence of Asthma and Mite Allergy), a Dutch population–based birth cohort. 16 The asthma cohort consisted of 406 Dutch children who were referred to a paediatric pulmonologist in the University Medical Center Groningen (UMCG) by their general practitioner between 1972 and 1976. All children were between 8 and 12 years and had asthma not treated by ICS at referral. Approximately 25% of these children were not treated at all for their asthma at that time. The other 75% used medication that was common in the 1970s (thiazinamium, promethazine, disodium cromoglycate). During this initial visit, a standardized assessment was performed, including spirometry, medical history, physical examination, blood eosinophil measurement, histamine bronchoprovocation test and skin prick testing. All subjects included in this asthma cohort had BHR as determined by a histamine‐induced bronchoprovocation test. Subjects were classified as having BHR if the provocative concentration of histamine sufficient to produce a 10% drop in FEV1 (PC10) was 16 mg/mL or less. 17 After this initial visit, treatment was optimized and subjects had routine check‐ups by the paediatric pulmonologist at least once a year. Spirometric data during these routine check‐ups were retrieved from medical records. This asthma cohort has been previously described elsewhere (METc 2012‐173). 15 , 18
For the PIAMA cohort, pregnant women were recruited in 1996/97 and a total of 3963 newborns were included into the study. Data have been collected during pregnancy, at 3 months post‐partum, annually till age 8 and thereafter at ages 11, 14, 17 and 20. Lung function measurements were performed in subsets of children at ages 8, 12 and 16. This included a methacholine bromide provocation test at age 8. BHR was defined as a decrease in FEV1 of 20% or more with a cumulative dose equal to or lower than 0.61 mg methacholine bromide. The study was approved by the respective Medical Ethics Committees, and informed (parental) consent was obtained from all participants (METC protocol number 07‐337/K).
2.2. Inclusion criteria
In the asthma cohort, all subjects had lung function testing including bronchial responsiveness and a blood eosinophil count at baseline (n = 406). For the cross‐sectional analysis, only children with doctor‐diagnosed asthma confirmed by the presence of BHR were included (n = 205). For the longitudinal analysis, subjects in the asthma cohort were required to have a minimum of three lung function measurements during a follow‐up period of 2 years or longer (n = 133). In the PIAMA cohort, subjects included in the cross‐sectional analysis were required to have a blood eosinophil measurement, lung function measurement at age 8 and data on asthma status (n = 797). For the longitudinal analysis, in addition to blood eosinophil measurement, data on asthma status and a lung function measurement at age 8, subjects were required to have at least one lung function test at ages 12 or 16 (n = 485).
2.3. Clinical assessment
Spirometry in the asthma cohort was performed with a water‐sealed spirometer (Lode spirograph D75). Lung function measurements (FEV1 and FVC) were performed at ages 8, 12 and 16 in the PIAMA cohort. At age 8, Jaeger Masterscreen pneumotachographs (Viasys Healthcare) were used and EasyOne spirometers (Medical Technologies Inc) were used at age 12. At age 16, both spirometers were used. Calibration of the values from EasyOne spirometers at age 16 was performed by using a regression equation derived from a separate experiment of 49 healthy volunteers. 19 For both cohorts, the reference equations from the Global Lung Function Initiative were used to calculate percentage of predicted values according to age, height and sex. 20 Lung function testing was performed according to American Thoracic Society/European respiratory society criteria 21 in both cohorts.
In the asthma cohort, a positive asthma diagnosis was based on the paediatric pulmonologist's assessment of clinical symptoms together with exclusion of specific respiratory diseases such as cystic fibrosis. 17 In the PIAMA cohort, asthma was defined as fulfilling two of the following three criteria following the MeDALL definition, as agreed by international experts: ever had a doctor‐diagnosed asthma, wheeze within the last 12 months and steroid use for respiratory problems in the last 12 months. 22
In the asthma cohort, eosinophils were collected at the first visit by means of venous blood samples. Quantification of eosinophilic granulocytes was performed by counting in a Bürker chamber (Scherf; Cecchinato) in combination with an eosin stain solution (10 ml eosin 1%, 10 mL formol 40% and 80 mL of water). 15 In the PIAMA cohort, samples of 2 mL were collected in EDTA tubules. Eosinophil counts were determined by means of an automatic cell counter XE‐21000 (Sysmex Corp).
In the asthma cohort, intracutaneous skin tests were performed with allergen extracts for house dust mite, grass pollens, mould, weeds, animal dander and feather (Diephuis Laboratory). A positive result was defined as a wheal size of ≥5 mm. 18 In the PIAMA cohort, a positive skin prick test was defined as at least one skin prick test with a wheal size ≥3 mm from the following seven tests: house dust mites Dermatophagoides pteronyssinus and Dermatophagoides farinae, cat dander, dog dander, mixed grass, mixed trees and alternia (ALK). Sensitization was defined as at least one positive skin prick test at baseline in both cohorts.
2.4. Statistical analysis
Data are presented as mean, standard deviation (s.d.) for normally distributed continuous variables. For non‐normally distributed variables, median and interquartile range are provided. Frequency distributions are presented for categorical variables. To compare groups, Pearson's chi‐square test was used for categorical variables and the independent‐sample t test was used for continuous variables with normal distribution. For non‐normally distributed data, the Mann–Whitney U test was used for group comparison. Blood eosinophils were natural log (ln)‐transformed to establish a normal distribution. Lung function growth was analysed based on the change in percentage of predicted values at all included ages. By using percentage of predicted values, we correct for the sex‐specific association between changes in height and age and lung function. Furthermore, we plotted the mean (±sd) absolute (in L) and relative (in % predicted) FEV1 levels at all measurement points in both cohorts.
For the cross‐sectional analyses, linear regression was used to assess the association between blood eosinophils and lung function at age 8 in PIAMA and between blood eosinophils and lung function at initial visit in the asthma cohort, respectively. To estimate the association between blood eosinophils at age 8 and lung function growth between ages 8 and 16 for PIAMA and the association between blood eosinophils at baseline and lung function growth during all subsequent follow‐up visits for the asthma cohort, we used linear mixed models on all available lung function measurements with random subject intercepts. For both cross‐sectional and longitudinal analyses, the PIAMA cohort was stratified based on asthma status at age 8. In these models, we included ln‐transformed eosinophils at age 8 and the interaction between follow‐up time and ln‐transformed eosinophils at age 8. The beta‐coefficient of this interaction is presented in the tables and indicates the association between baseline blood eosinophils and lung function growth. The following confounders were chosen for the asthma cohort, sex and allergic sensitization. For the PIAMA cohort, confounders were selected a priori 19 and were collected by questionnaires and clinical testing: sex, parental education, parental atopy, respiratory infections within 3 weeks of lung function measurement, allergic sensitization and BHR. To address the role of ICS, we performed a sensitivity analysis in which we investigated associations between eosinophils and lung function levels and growth in the asthmatic PIAMA population that did not use ICS. Statistical analysis was performed using SPSS (IBM SPSS Version 25.0), and statistical significance was set at a 5% level.
3. RESULTS
3.1. Cohort characteristics
In the asthma cohort, 205 and 133 subjects were included in the cross‐sectional and longitudinal analysis, respectively. Subjects included in the longitudinal analysis were seen by the paediatric pulmonologist with a median of seven times (min 3, max 37 visits) during the follow‐up period and median follow‐up after initial presentation was 3 years in the asthma cohort (min 2, max 7.5 years). In the asthma cohort, all subjects were treated by a paediatric pulmonologist. Amongst the asthmatic group in the PIAMA cohort, only two subjects were treated by a paediatric pulmonologist, pulmonologist or a special care nurse in the last 12 months at the 8‐year examination point. Mean age at baseline was 10.1 years (s.d. 1.4) in the asthma cohort. In the PIAMA cohort, 797 participants were investigated in the cross‐sectional analysis at age 8.1 (s.d. 0.3), of which 10.3% (n = 82) had asthma (Table S1). A total of 494 subjects from the PIAMA cohort were included in the longitudinal analysis and 52 (10.7%) of these subjects had asthma (Table 1, Figure 1). For a full comparison of included and excluded subjects, please see Tables S2–S5.
TABLE 1.
Longitudinal cohort characteristics
| Baseline characteristics of subjects included in longitudinal analysis | PIAMA | Asthma cohort | |
|---|---|---|---|
| Asthma | Non‐asthma | Asthma | |
| Subjects | 52 (10.7%) | 433 (89.5%) | 133 (100%) |
| Age | 8.0 (0.3) | 8.1 (0.3) | 9.8 (1.3) |
| Male | 28 (53.8%) | 206 (47.6%) | 101 (75.9%)** |
| FEV1% predicted, % | 102.0 (12.9)* | 106.8 (10.6) | 75.0 (14.1)** |
| FVC% predicted, % | 103.2 (11.3) | 104.6 (10.6) | 89.8 (12.5)** |
| FEV1/FVC% predicted, % | 98.2 (8.4)* | 101.5 (6.5) | 82.8 (10.1)** |
| Blood eosinophils (10^9/L)† | 0.47 (2.1)* | 0.30 (2.0) | 0.39 (2.4) |
| BHR | 72.3%* | 39.3% | 100%** |
| Positive skin prick test | 66.7%* | 18.1% | 99.2%** |
| Maternal atopy | 78.8%* | 64.9% | ‐ |
| Paternal atopy | 40.4% | 32.9% | ‐ |
| Low parental education | 9.6% | 7.6% | ‐ |
| Intermediate parental education | 28.8% | 31.7% | ‐ |
| High parental education | 61.5% | 60.6% | ‐ |
| Respiratory infection | 34.6% | 22.8% | ‐ |
Baseline PIAMA defined as visit at age 8. Asthma cohort baseline defined as presentation to paediatric pulmonologist. Parental education based on highest attained educational level of father or mother, low: primary school, lower vocational or lower secondary education, intermediate: vocational education or intermediate/higher secondary education, high: higher vocational education and university. Respiratory infection defined as cold or airway infection in past 3 weeks.
Geometric mean (geometric SD).
Significantly different from non‐asthma group.
Significantly different from PIAMA asthma group.
FIGURE 1.
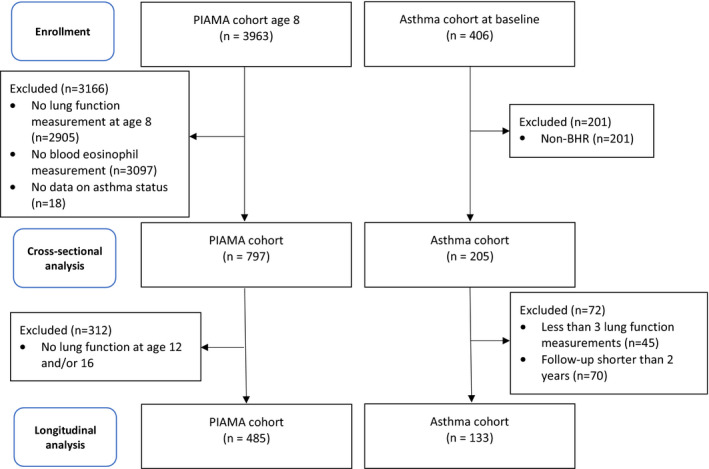
Flow chart of included subjects
3.2. Cross‐sectional analysis
In the PIAMA cohort, asthma at age 8 was significantly associated with a lower percentage predicted FEV1 (mean 103.0 vs. 106.8) (p < .01) and FEV1/FVC (mean 99.1 vs. 101.9) (p < .01) at age 8 in comparison with non‐asthmatic subjects (Table S1). Asthma at age 8 was associated with higher blood eosinophil numbers in the PIAMA cohort (p ≤ .01). Subjects in the asthma cohort had more severe asthma compared with the asthmatic subjects in the population‐based PIAMA cohort, defined by lower lung function levels and a higher prevalence of bronchial hyper‐responsiveness.
3.3. Blood eosinophils and baseline lung function
Blood eosinophils were not associated with lung function in the cross‐sectional analyses (Table 2).
TABLE 2.
Cross‐sectional analysis of the association of blood eosinophils (ln‐transformed) and lung function
| FEV1%pred | FVC%pred | FEV1/FVC%pred | ||||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Cross‐sectional analysis | ||||||
| Asthma cohort | −0.17 (−2.49, 2,15) | .88 | −0.25 (−2.26, 1.8) | .81 | −0.08 (−1.76, 1.60) | .93 |
| PIAMA non‐asthmatic | −0.13 (−1.34, 1.08) | .84 | 0.11 (−1.16, 1.38) | .86 | −0.20 (−0.97, 0.57) | .60 |
| PIAMA asthmatic | −0.80 (−5.34, 3.74) | .72 | 0.04 (−4.73, 4.81) | .99 | −0.56 (−4.14, 3.02) | .76 |
| PIAMA asthmatic—no ICS | −0.44 (−5.24, 4.37) | .86 | −0.01 (−5.07,5.06) | .99 | −0.15 (−3.86,3.56) | .94 |
PIAMA: adjusted for sex, parental education and atopy, respiratory infections within 3 weeks of measurement, bronchial hyper‐responsiveness (BHR) and sensitization at age 8. Asthma cohort was adjusted for sex and sensitization. Reference equations for lung function measurement obtained from the Global Lung Function Initiative. 20 β: Change in % predicted lung function for every 1‐unit increase in ln (blood eosinophils). For unadjusted analysis, see Table S6. ICS—inhaled corticosteroid: defined as use within the last 12 months at the 8‐year measurement point.
3.4. Blood eosinophils and lung function growth
Figure 2 shows that lung function levels in the asthma cohort (both as absolute (in L) and relative (%predicted) level) were lower in the asthma cohort, but the growth rates were similar compared with the PIAMA asthmatic and non‐asthmatic groups. In the asthma cohort, higher blood eosinophil numbers at baseline were associated with less annual growth of FEV1 during the follow‐up period (Table 3, B: −0.66%/year (95% CI: −1.11 to −0.20), p < .01). Blood eosinophils were also inversely related to annual growth in FVC (B: −0.40%/year (95% CI: −0.75 to −0.05), p = .025). In the PIAMA non‐asthmatic group, blood eosinophils were significantly associated with a higher growth of FEV1 (B: 0.23%/year (95% CI: 0.01 to 0.44), p = .03) but not with FEV1/FVC and FVC growth. In the PIAMA asthmatic group, blood eosinophil numbers at age 8 were not associated with lung function growth from age 8 to 16. The regression estimates for the main effects of blood eosinophils and time, and for the blood eosinophils × time interactions are shown in Table S8.
FIGURE 2.
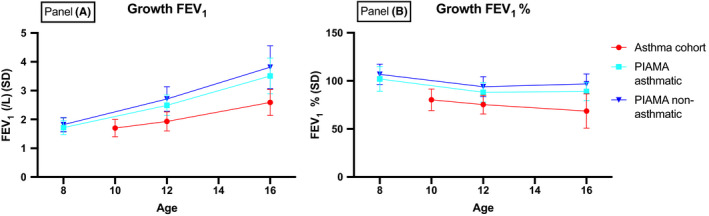
Absolute (Panel A) and relative FEV1 growth (Panel B) in (1) the asthma cohort; (2) asthma patients from the PIAMA birth cohort; and (3) healthy, non‐asthmatic controls from the PIAMA cohort. Data are presented as mean (standard deviation). FEV1, %: FEV1 as percentage of predicted
TABLE 3.
Longitudinal analysis of blood eosinophils (ln‐transformed) and lung function growth
| Longitudinal analysis | FEV1%pred | FVC%pred | FEV1/FVC%pred | |||
|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Asthma cohort | −0.66 (−1.11, −0.20) | <.01 | −0.40 (−0.75, −0.05) | .025 | −0.33 (−0.68, 0.02) | .07 |
| PIAMA non‐asthmatic | 0.21 (0.00, 0.40) | .04 | 0.10 (−0.00, 0.31) | .33 | 0.10 (−0.02, 0.22) | .11 |
| PIAMA asthmatic | 0.00 (−0.91, 0.91) | .99 | −0.26 (−1.14, 0.61) | .55 | 0.19 (−0.60, 0.99) | .63 |
| PIAMA asthmatic—no ICS | 0.03 (−0.89, 0.96) | .94 | −0.27 (−1.20, 0.67) | .57 | 0.23 (−0.62, 10.09) | .59 |
PIAMA: adjusted for sex, parental education and atopy, respiratory infections within 3 weeks of measurement, bronchial hyper‐responsiveness (BHR) and sensitization at age 8. Asthma cohort was adjusted for sex and sensitization. Reference equations for lung function measurement obtained from the Global Lung Function Initiative. 20 β: Change in % predicted lung function for every 1‐unit increase in ln (blood eosinophils). For unadjusted analysis, please see Tables S6 and S7. ICS—inhaled corticosteroid: defined as use within the last 12 months at the 8‐year measurement point.
3.5. ICS sensitivity analysis
In the cross‐sectional and longitudinal asthma population in the PIAMA cohort, 11% and 12.3% used ICS during the last year, respectively. Analysis of the association between blood eosinophils at baseline and lung function level and growth including only asthmatic PIAMA subjects who did not use ICS showed no significant findings (Tables 2 and 3).
4. DISCUSSION
4.1. Main findings
Higher blood eosinophil numbers were associated with lower growth of FEV1 and FVC during follow‐up in adolescence in a hospital‐based cohort of children with asthma and BHR. These associations were not observed in a general population‐based birth cohort, regardless of the presence or absence of asthma.
4.2. Blood eosinophils and lung function growth in asthmatics
We demonstrated that higher numbers of blood eosinophils predicted less lung function growth during follow‐up in children presenting with asthma. These findings are in alignment with a previous study finding of a weak correlation between blood eosinophils in asthmatic children and persistence of asthma symptoms in adulthood. 23 These children were between the age of 5 and 15 and were all asthmatic. Lung function measurements at baseline were comparable to those of our asthma cohort. Interestingly, the degree of airflow obstruction in this study was also similar to that of our asthma cohort. It is therefore tempting to speculate that the inverse association between blood eosinophils and lung function growth is dependent on the degree of airflow obstruction. A direct comparison between studies was not possible as we investigated growth in actual lung function as opposed to future risk of asthma symptoms. The greater degree of airflow obstruction in the hospital‐based cohort to our opinion reflects the more severe asthma. Furthermore, the fact that all these children were referred to a paediatric pulmonologist indicates a greater degree of disease severity when compared to the PIAMA cohort. Our findings support the idea that blood eosinophils may reflect increased disease activity expressed by eosinophilic inflammation, which may in turn lead to lower lung function growth. The number of blood eosinophils has been associated with asthma exacerbations, 24 , 25 and asthma exacerbations have been associated with increased lung function decline in patients with asthma. 26 This may suggest that eosinophils have an underlying effect on lung growth that is mediated at least to a certain degree, by the number and severity of exacerbations. Although we did not have specific data pertaining to exacerbations, we speculate that eosinophilic inflammation may reflect asthma severity, and affect lung growth, potentially through exacerbations.
Given the interrelation of blood eosinophilia and airway remodelling in asthma, 27 anti‐eosinophilic medication may be warranted in children with more severe asthma to preserve lung function growth. Clinical trials investigating drugs blocking the IL‐5 pathway have reported a reduction in exacerbations and an improved lung function in asthmatic children. 28 , 29 Furthermore, use of anti‐IL‐5 treatment in mild asthma has been associated with a reduction in airway remodelling markers, detected by bronchial biopsies. 27 The role of eosinophilia in airway remodelling is further supported by a case study of three subjects with severe adolescent asthma, which found that mepolizumab was associated with a long‐term improvement in lung function. 30 However, clinical trials investigating the effects of anti‐eosinophilic medication on lung growth are required to confirm this association.
Children from the asthma cohort did not use ICS at baseline, while 12.3% of patients with asthma included in the longitudinal analysis in the PIAMA cohort used ICS in the last 12 months before blood eosinophils were collected. This is due to the availability of ICS during presentation and follow‐up of the asthma cohort in the 1970s. This raises the question whether the use of ICS could explain the differences observed between patients with asthma in the PIAMA cohort and in the asthma cohort during follow‐up. Blood eosinophils were not associated with baseline lung function or lung function growth in the asthmatic PIAMA subjects not using ICS. Furthermore, previous studies have not shown that ICS is associated with preserved lung growth. 12 It is therefore unlikely that the observed differences were mediated by corticosteroid use.
4.3. Blood eosinophils and longitudinal lung function in general population
No association was seen between blood eosinophils at baseline and lung function growth during adolescence in subjects with asthma from the general population–based birth cohort. In the non‐asthmatic group, blood eosinophils were significantly associated with a higher growth of FEV1 but not with FEV1/FVC and FVC growth. A comparable study, performed in a Danish birth cohort, found that blood eosinophils did not affect lung function, independent of asthma status. 31 Although children in that study were considerably younger, it reinforces the notion that blood eosinophils may not be a predictor of lung function growth in the general population.
4.4. Blood eosinophils and baseline lung function
Numbers of blood eosinophils were not associated with lung function measurements at baseline in any cohort, regardless of asthma status. Our findings are contrary to those of Ulrik et al., 8 , 23 who found that the peripheral eosinophil numbers were inversely correlated with FEV1 in adolescence and early adulthood, regardless of asthma or the presence of BHR. This study differs from ours in that it included subjects in early adulthood. More research is required to disentangle the impact or role of BHR and allergic sensitization on the association between blood eosinophils and lung function growth. There is growing evidence that allergic comorbidity may play a role in specific lung function trajectories for asthmatic and non‐asthmatic individuals, 32 , 33 and further studies have to address this interesting question.
4.5. Strengths and limitations
An important strength of this study was the ability to perform objective lung function measurements in a cohort from the age of 8 to 16. Lung function growth analysis included data on relevant exposures that could also impact lung growth, present as early as in utero. Another strength was the number of subjects in the asthmatic cohort. A limitation of this study was that eosinophil collection was only performed in a subset of the original PIAMA cohort, possibly resulting in selection bias. Included subjects in the PIAMA cohort were more likely to have an atopic mother and to be asthmatic themselves (Tables S2, S3). Blood eosinophil levels did however not differ significantly between included and excluded subjects. Eosinophil analysis was only performed at baseline. This may not provide an actual reflection of longitudinal blood eosinophil numbers. Another possible limitation is that blood eosinophils likely do not always reflect eosinophils in pulmonary tissue, especially in subjects with a low blood eosinophil count. 34 Furthermore, due to a time gap of >20 years in data collection between the two cohorts, differences in lifestyle and treatment of asthma may reduce comparability of established associations. Our findings should be validated in a similar cohort of asthmatic children with varying degree of asthma severity who do or do not receive current ICS treatment. Studies using bronchial biopsies to investigate the association between eosinophils, airway remodelling and impact on lung function growth should be performed to ascertain causality.
5. CONCLUSION
In conclusion, we have shown that blood eosinophils in children presenting with untreated asthma were predictive of a lower growth in FEV1 and FVC during follow‐up in adolescence. This association was not seen in children in the general population or in children with a milder form of asthma. Blood eosinophils likely represent a single mechanism in a multifactorial pathological pathway leading to reduced lung function, and more research is needed on the association between eosinophils and lung function before age 8 and on maximum attained lung function in early adulthood.
CONFLICT OF INTEREST
Dr. G. H. Koppelman reports grants from Lung Foundation of the Netherlands, during the conduct of the study; grants from Lung Foundation of the Netherlands, TEVA, the Netherlands, GSK, Ubbo Emmius Foundation, TETRI Foundation, Vertex, outside the submitted work; GHK has participated in advisory board meetings to GSK and PURE‐IMS.
AUTHOR CONTRIBUTION
GHK, JMV and HJLK designed the research. GHK and JMV supervised the research. HJLK performed the statistical analysis. GHK, JMV, HJLK and UG analysed the data. HJLK wrote the paper.
Supporting information
Table S1‐S8
ACKNOWLEDGEMENTS
The PIAMA study has received funding from the Netherlands Organization for Health Research and Development, the Netherlands Organization for Scientific Research, the Lung Foundation Netherlands (previously Asthma Fund), the Netherlands Ministry of Spatial Planning, Housing, and the Environment, and the Netherlands Ministry of Health, Welfare, and Sport.
Judith M. Vonk and Gerard H. Koppelman shared senior author.
DATA AVAILABILITY STATEMENT
The supporting and/or analyzed datasets during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194(5):607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374(19):1842‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bui DS, Burgess JA, Lowe AJ, et al. Childhood lung function predicts adult chronic obstructive pulmonary disease and asthma‐chronic obstructive pulmonary disease overlap syndrome. Am J Respir Crit Care Med. 2017;196(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 4. Kulkarni N, Ragazzo V, Costella S, et al. Eosinophilic airway inflammation is increased in children with asthma and food allergies. Pediatr Allergy Immunol. 2012;23(1):28‐33. [DOI] [PubMed] [Google Scholar]
- 5. Kartasamita CB, Rosmayudi O, Demedts M. Total serum IgE and eosinophil count in children with and without a history of asthma, wheezing, or atopy in an urban community in Indonesia. J Allergy Clin Immunol. 1994;94(6):981‐988. [DOI] [PubMed] [Google Scholar]
- 6. Steerenberg PA, Janssen NAH, De Meer G, et al. Relationship between exhaled NO, respiratory symptoms, lung function, bronchial hyperresponsiveness, and blood eosinophilia in school children. Thorax. 2003;58(3):242‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gleich GJ. Current understanding of eosinophil function. Hospital Pract. 1988;23(3):137‐160. [DOI] [PubMed] [Google Scholar]
- 8. Ulrik CS. Eosinophils and pulmonary function: an epidemiologic study of adolescents and young adults. Ann Allergy Asthma Immunol. 1998;80(6):487‐493. [DOI] [PubMed] [Google Scholar]
- 9. Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51(4):1702536. [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Mensinga TT, Schouten JP, Rijcken B, Weiss ST. Determinants of maximally attained level of pulmonary function. Am J Respir Crit Care Med. 2004;169(8):941‐949. [DOI] [PubMed] [Google Scholar]
- 11. Woolcock AJ, Salome CM, Keena VA. Reducing the severity of bronchial hyperresponsiveness. Am Rev Respir Dis. 1991;143(3_pt_2):S75‐S77. [DOI] [PubMed] [Google Scholar]
- 12. Tonascia J, Adkinson NF, Bender B, et al. Long‐term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343(15):1054‐1063. [DOI] [PubMed] [Google Scholar]
- 13. Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti‐IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;(9):23‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castro‐Rodriguez JA, Saglani S, Rodriguez‐Martinez CE, Oyarzun MA, Fleming L, Bush A. The relationship between inflammation and remodeling in childhood asthma: A systematic review. Pediatr Pulmonol. 2018;53(6):824‐835. [DOI] [PubMed] [Google Scholar]
- 15. Roorda RJ, Gerritsen J, Van Aalderen WMC, Knol K. Skin reactivity and eosinophil count in relation to the outcome of childhood asthma. Eur Respir J. 1993;6(4):509‐516. [PubMed] [Google Scholar]
- 16. The PIAMA Research Group . PIAMA. [Internet]. Utrecht, The Netherlands: The PIAMA Research Group. https://piama.iras.uu.nl/english. Accessed June 1, 2019. [Google Scholar]
- 17. Roorda RJ, Gerritsen J, van Aalderen WMC, et al. Follow‐up of asthma from childhood to adulthood: influence of potential childhood risk factors on the outcome of pulmonary function and bronchial responsiveness in adulthood. J Allergy Clin Immunol. 1994;93(3):575‐584. [DOI] [PubMed] [Google Scholar]
- 18. Grol MH, Gerritsen J, Vonk JM, et al. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: A 30‐year follow‐up study. Am J Respir Crit Care Med. 1999;160(6):1830‐1837. [DOI] [PubMed] [Google Scholar]
- 19. Milanzi EB, Wijga AH, Gehring U, et al. Air pollution exposure and lung function until age 16 years: the PIAMA birth cohort study. Eur Respir J. 2018;56(6):7‐7. [DOI] [PubMed] [Google Scholar]
- 20. Stanojevic S, Wade A, Stocks J. Reference values for lung function: past, present and future. Eur Respir J. 2010;36(1):12‐19. [DOI] [PubMed] [Google Scholar]
- 21. American Thoracic Society and European Respiratory Society . Am J Respir Crit Care Med. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. 2019;200(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hohmann C, Pinart M, Tischer C, et al. The development of the MeDALL core questionnaires for a harmonized follow‐up assessment of eleven European birth cohorts on asthma and allergies. Int Arch Allergy Immunol. 2014;163(3):215‐224. [DOI] [PubMed] [Google Scholar]
- 23. Ulrik CS. Peripheral eosinophil counts as a marker of disease activity in intrinsic and extrinsic asthma. Clin Exp Allergy. 1995;25(9):820‐827. [DOI] [PubMed] [Google Scholar]
- 24. Price DB, Rigazio A, Campbell JD, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet. Respir Med. 2015;3(11):849‐858. [DOI] [PubMed] [Google Scholar]
- 25. Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195(3):302‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flood‐Page P, Menzies‐Gow A, Phipps S, et al. Anti‐IL‐5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112(7):1029‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Folqué MM, Lozano J, Riggioni C, et al. ‘Real‐life’ experience in asthmatic children treated with omalizumab up to six‐years follow‐up. Allergol Immunopathol (Madr). 2019;47(4):336‐341. [DOI] [PubMed] [Google Scholar]
- 29. Corren J, Kavati A, Ortiz B, et al. Efficacy and safety of omalizumab in children and adolescents with moderate‐to‐severe asthma: a systematic literature review. Allergy Asthma Proc. 2017;38(4):250‐263. [DOI] [PubMed] [Google Scholar]
- 30. Hoshi M, Matsunaga M, Nogami K, et al. Three cases of severe adolescent asthma treated with mepolizumab: lung function trajectories. Asia Pac. Allergy. 2020;10(2):2‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185(11):1183‐1189. [DOI] [PubMed] [Google Scholar]
- 32. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763‐770. [DOI] [PubMed] [Google Scholar]
- 33. Alduraywish S, Luzak A, Lodge C, et al. The role of early life food sensitization in adolescent lung function: results from 2 birth cohort studies. J Allergy Clin Immunol Pract. 2019;7(6):1825‐1834.e12. [DOI] [PubMed] [Google Scholar]
- 34. Ullmann N, Bossley CJ, Fleming L, Silvestri M, Bush A, Saglani S. Blood eosinophil counts rarely reflect airway eosinophilia in children with severe asthma. Allergy Eur J Allergy Clin Immunol. 2013;68(3):402‐406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S8
Data Availability Statement
The supporting and/or analyzed datasets during the current study are available from the corresponding author on reasonable request.


