Sotagliflozin is a dual inhibitor of sodium–glucose co‐transporter (SGLT) 1 and 2, although it is approximately 20‐fold more selective for SGLT2. 1 SGLT1 contributes to glucose reabsorption in the gastrointestinal tract, whereas SGLT2 has this function in the proximal renal tubule. 2 The effects of sotagliflozin in heart failure were recently studied in the Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure trial (SOLOIST‐WHF). 3 SOLOIST‐WHF had a complex history including withdrawal of the major sponsor, a resulting shortfall in funding and the impact of the novel coronavirus disease pandemic. As a result, fewer patients were recruited than originally intended (1222 instead of 4000), the trial was terminated early, potential endpoints were not fully adjudicated, and the primary outcome was changed from that originally planned (first occurrence of either hospitalization for heart failure or cardiovascular death). Despite these tribulations, the investigators are to be congratulated in maintaining the integrity of the trial, bringing it to successful completion, and promptly analysing and reporting their findings. As a result, SOLOIST‐WHF fills an important gap in knowledge about the use of SGLT2 inhibitors to treat patients with heart failure and reduced ejection fraction (HFrEF) and offers a tantalising suggestion that these drugs may also be beneficial in patients with heart failure and preserved ejection fraction (HFpEF). 3
Unlike the EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction trial (EMPEROR‐Reduced) and the Dapagliflozin And Prevention of Adverse outcomes in Heart Failure trial (DAPA‐HF), SOLOIST‐WHF enrolled patients with worsening of existing heart failure requiring intravenous diuretic therapy, usually in the hospital setting (the proportion enrolled out of hospital has not been reported). 4 , 5 At randomization, participants were required to have a B‐type natriuretic peptide (BNP) level ≥150 pg/mL (≥450 pg/mL for patients with atrial fibrillation) or N‐terminal proBNP (NT‐proBNP) ≥600 pg/mL (≥1800 pg/mL for patients with atrial fibrillation). 3 There was no upper left ventricular ejection fraction (LVEF) limit, but randomization was stratified according to LVEF (<50% or ≥50%). Unlike DAPA‐HF and EMPEROR‐Reduced, patients were required to have a diagnosis of type 2 diabetes. 3 , 4 , 5 , 6 , 7 , 8 Patients were randomized when haemodynamically stable, prior to hospital discharge (n = 596) or within 3 days after discharge (n = 626). Key exclusion criteria were systolic blood pressure <100 mmHg, estimated glomerular filtration rate <30 mL/min/1.73 m2 and a digoxin level >1.2 ng/mL. The revised primary endpoint was total number of hospitalizations and urgent visits for heart failure (first and subsequent) and deaths from cardiovascular causes. 3
Not surprisingly, patients enrolled in SOLOIST‐WHF differed in several ways from those in DAPA‐HF and EMPEROR‐Reduced (Table 1 ). 3 , 4 , 5 , 6 , 7 , 8 They were older and more were women and the median LVEF was higher (all in keeping with the absence of an upper LVEF threshold for inclusion); the median estimated glomerular filtration rate was lower (in keeping with the greater proportion of older people and women and requirement for all patients to have type 2 diabetes). Because all patients had recent worsening heart failure, the proportion remaining in New York Heart Association class III and IV was greater than in the earlier trials and median NT‐proBNP was higher than in DAPA‐HF (although not EMPEROR‐Reduced, reflecting the inclusion criteria for that trial). 3 , 4 , 5 , 6 , 7 , 8
Table 1.
Key baseline characteristics of patients in DAPA‐HF, EMPEROR‐Reduced and SOLOIST‐WHF
| DAPA‐HF (n = 4744) | EMPEROR‐Reduced (n = 3730) | SOLOIST‐WHF (n = 1222) | |
|---|---|---|---|
| Age, years | 66 | 67 | 69 |
| Female sex, % | 23 | 24 | 34 |
| NYHA class III–IV, % | 32 | 25 | 50 |
| LVEF, % | 31 | 27 | 35 b |
| Prior HF hospitalisation (or equivalent), % | 47 | 31 a | 100 |
| Median NT‐proBNP, pg/mL | 1437 | ∼1900 | ∼1780 |
| Atrial fibrillation, % | 40 | 37 | 47 |
| Diabetes (history), % | 42 | 50 | 100 |
| eGFR, mL/min/1.73 m2 | 66 | 62 | 50 b |
| Beta‐blocker, % | 96 | 95 | 92 |
| MRA, % | 71 | 71 | 64 |
| RAS blocker (including ARNI), % | 94 | 89 | 91 |
| ARNI, % | 11 | 19 | 17 |
Values are means or proportions (%) unless otherwise stated.
ARNI, angiotensin receptor–neprilysin inhibitor; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; RAS, renin–angiotensin system.
Within the previous 12 months.
Median.
The findings of SOLOIST‐WHF are difficult to compare directly with DAPA‐HF and EMPEROR‐Reduced because of the different patient population enrolled, characteristics of the drug and the use of unadjudicated events. Hospitalized patients have higher event rates than ambulatory patients and participants with type 2 diabetes higher rates than those without diabetes. The adjudication process usually rejects a substantial proportion of suspected worsening heart failure events, so use of unadjudicated events also inflates the endpoint rate. 9 The single outcome not influenced by adjudication is all‐cause mortality and the rate of death from any cause in the placebo group of SOLOIST‐WHF was 16.3 per 100 person‐years of follow‐up, much higher than the rates of 9.5 and 10.7 per 100 person‐years in DAPA‐HF and EMPEROR‐Reduced, respectively. Although not the final, revised, primary outcome, the SOLOIST‐WHF investigators did report time to first occurrence of hospitalization for worsening heart failure or death from cardiovascular causes, which was the primary endpoint in EMPEROR‐Reduced and the first secondary endpoint in DAPA‐HF. The hazard ratio for this outcome was 0.71 [95% confidence interval (CI) 0.56–0.89] in SOLOIST‐WHF, compared with 0.75 (0.65–0.86) in EMPEROR‐Reduced and 0.75 (0.65–0.85) in DAPA‐HF. 3 , 4 , 5 , 10 This one outcome that can be compared across all three trials demonstrates remarkable consistency of benefit, despite the differences in populations enrolled. In SOLOIST‐WHF, the hazard ratio for the revised primary endpoint, total number of hospitalizations and urgent visits for heart failure (first and subsequent) and deaths from cardiovascular causes, was 0.67 (0.52–0.85). This was also examined post hoc in DAPA‐HF, where the rate ratio was 0.74 (0.64–0.86) [unpublished]. Breaking down this primary composite outcome in SOLOIST‐WHF into its components, showed that sotagliflozin led to large and statistically significant reductions in the total number of worsening heart failure events (hazard ratio 0.64, 95% CI 0.49–0.83); in a post hoc analysis of DAPA‐HF the rate ratio for this composite was 0.70 (0.58–0.84) [unpublished]. There was also a favourable trend to lower cardiovascular mortality in SOLOIST‐WHF, in keeping with the benefits observed in the earlier SGLT2 inhibitor trials. Due to the smaller sample size and shorter follow‐up (median of only 9.0 months) than planned, SOLOIST‐WHF was not powered to provide a statistically robust assessment of the effect of sotagliflozin on mortality (there were 141 deaths in SOLOIST‐WHF compared with 515 in EMPEROR‐Reduced and 605 in DAPA‐HF). However, when the results of all three trials are pooled, there is a clear reduction in both cardiovascular and all‐cause mortality (Figure 1 ).
Figure 1.
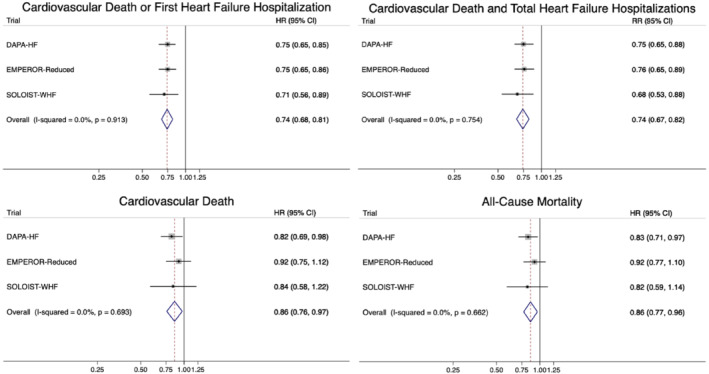
Meta‐analysis of DAPA‐HF, EMPEROR‐Reduced and SOLOIST‐WHF. The figure shows pooled treatment effect estimates calculated from the reported individual trial‐level estimates using a fixed‐effect meta‐analysis model. CI, confidence interval; HR, hazard ratio; RR, rate ratio.
The key subgroups of interest were LVEF <50% vs. ≥50% and treatment initiation before or after discharge from hospital, each of which showed a benefit consistent with that in the trial overall. Clearly, the LVEF subgroup is particularly notable as it hints at efficacy in HFpEF similar to that in HFrEF, although the number of patients with a LVEF ≥50% was modest (n = 256). In an additional LVEF subgroup analysis, the hazard ratio for the primary outcome was 0.69 (0.51–0.92) in patients with a LVEF <40% (n = 725) and 0.68 (0.45–1.03) in patients with a LVEF ≥40% (n = 494).
The analysis of safety was also crucial, especially as the patients studied were enrolled either in hospital or shortly after discharge. Concerns have been raised about administration of SGLT2 inhibitors in hospitalized patients and the risk of diabetic ketoacidosis. 11 , 12 Despite all patients in SOLOIST‐WHF having type 2 diabetes, in the safety analysis only 2 of 605 patients in the sotagliflozin group experienced diabetic ketoacidosis compared with 4 of 611 patients in the placebo group. Of interest, significantly more patients in the sotagliflozin group (n = 42) experienced diarrhoea than in the placebo group (n = 25), a difference not reported in DAPA‐HF or EMPEROR‐Reduced and one likely to reflect SGLT1 inhibition in the intestines.
In summary, SOLOIST‐WHF contributes three very important findings relevant to the use of SGLT2 inhibitors in clinical practice. First, it adds a third trial confirming the benefits of drugs in this class for patients with HFrEF, creating a particularly strong evidence base (Figure 1 ). Second, SOLOIST‐WHF answers the question about whether this treatment can be started in hospital following an episode of decompensation (and the answer is resoundingly yes). Third, SOLOIST‐WHF shows that use of a SGLT2 inhibitor in patients stabilized after an episode of decompensation is not associated with an increased risk of diabetic ketoacidosis. Here it is worth specifying how ‘stabilized’ was defined in the trial: a systolic blood pressure ≥100 mmHg, no treatment with intravenous inotropic therapy or intravenous vasodilators within 2 days prior to randomization, and no requirement for mechanical ventilation or oxygen therapy in the last 24 h. Patients also had to have discontinued intravenous diuretic and chronic oral loop diuretic prescribed and/or administered. Patients with diabetes, especially those on insulin, or an insulin secretagogue, may still be at risk of diabetic ketoacidosis if fluid and calorie intake is curtailed, insulin dose reduced, or both. 11 , 12 Finally, as alluded to earlier, SOLOIST‐WHF offers the first tantalising hint that SGLT2 inhibitors might also be beneficial in HFpEF, although we need to wait until later in 2021–22 for definitive proof, which will be provided by the two large ongoing dedicated HFpEF trials, i.e. EMPEROR‐Preserved (NCT03057951) and the Dapagliflozin Evaluation to improve the LIVEs of patients with pReserved ejection fraction heart failure (DELIVER, NCT03619213). Importantly, DELIVER also includes hospitalized and recently discharged patients, as in SOLOIST‐WHF. While waiting for the results of EMPEROR‐Preserved and DELIVER, the immediate priority for physicians is to implement this highly effective (and cost‐effective) treatment in patients with HFrEF, the majority of whom are eligible for it. 13 , 14 SGLT2 inhibitors can now be used in both inpatients and outpatients.
Conflict of interest: J.J.V.McM. is supported by British Heart Foundation Centre of Research Excellence (grant RE/18/6/34217); and reports payments to his employer for work on clinical trials, consulting and other activities, from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal‐Cor, GSK, KBP Biosciences, Novartis, Pfizer, and Theracos and personal lecture fees from Abbott, Hikma, Ionis, Sun Pharmaceuticals, and Servier. K.F.D. reports payments to his employer for working on the DAPA‐HF trial from AstraZeneca and personal lecture fees from AstraZeneca and Eli Lilly.
References
- 1. Deeks ED. Sotagliflozin: a review in type 1 diabetes. Drugs 2019;79:1977–1987. [DOI] [PubMed] [Google Scholar]
- 2. Ferrannini E. Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab 2017;26:27–38. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 5. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA‐HF Committees and Investigators . A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019;21:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMurray JJ, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA‐HF Committees and Investigators . The Dapagliflozin and Prevention of Adverse‐outcomes in heart failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402–1411. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F; EMPEROR‐Reduced Trial Committees and Investigators . Evaluation of the effect of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR‐Reduced trial. Eur J Heart Fail 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 9. Petrie MC, McMurray JJ. Do we need clinical events committees to adjudicate end points? Circ Heart Fail 2020;13:e007209. [DOI] [PubMed] [Google Scholar]
- 10. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 11. Ekanayake P, Hupfeld C, Mudaliar S. Sodium‐glucose cotransporter type 2 (SGLT‐2) inhibitors and ketogenesis: the good and the bad. Curr Diab Rep 2020;20:74. [DOI] [PubMed] [Google Scholar]
- 12. Musso G, Saba F, Cassader M, Gambino R. Diabetic ketoacidosis with SGLT2 inhibitors. BMJ 2020;371:m4147. [DOI] [PubMed] [Google Scholar]
- 13. McEwan P, Darlington O, McMurray JJ, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Qin L. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail 2020;22:2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaduganathan M, Greene SJ, Zhang S, Grau‐Sepulveda M, DeVore AD, Butler J, Heidenreich PA, Huang JC, Kittleson MM, Joynt Maddox KE, McDermott JJ, Owens AT, Peterson PN, Solomon SD, Vardeny O, Yancy CW, Fonarow GC. Applicability of US Food and Drug Administration labeling for dapagliflozin to patients with heart failure with reduced ejection fraction in US clinical practice: the Get With the Guidelines‐Heart Failure (GWTG‐HF) registry. JAMA Cardiol 2020. Nov 13. 10.1001/jamacardio.2020.5864 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


