Abstract
Background and Aims
Portal hypertension (PH) and sarcopenia are common in patients with advanced chronic liver disease (ACLD). However, the interaction between PH and sarcopenia and their specific and independent impact on prognosis and mortality has yet to be systematically investigated in patients with ACLD.
Methods
Consecutive patients with ACLD and hepatic venous pressure gradient (HVPG) ≥10 mm Hg with available CT/MRI imaging were included. Sarcopenia was defined by transversal psoas muscle thickness (TPMT) at <12 mm/m in men and <8 mm/m in women at the level of the third lumbar vertebrae. Hepatic decompensation and mortality was recorded during follow‐up.
Results
Among 203 patients (68% male, age: 55 ± 11, model for end‐stage liver disease [MELD]: 12 [9‐15]), sarcopenia was observed in 77 (37.9%) and HVPG was ≥20 mm Hg in 98 (48.3%). There was no correlation between TPMT and HVPG (r = .031, P = .66), median HVPG was not different between patients with vs without sarcopenia (P = .211). Sarcopenia was significantly associated with first/further decompensation both in compensated (SHR: 3.05, P = .041) and in decompensated patients (SHR: 1.86, P = .021). Furthermore, sarcopenia (SARC) was a significant predictor of mortality irrespective of HVPG (HVPG < 20‐SARC: SHR: 2.25, P = .021; HVPG ≥ 20‐SARC: SHR: 3.33, P = .001). On multivariate analysis adjusted for age, HVPG and MELD, sarcopenia was an independent risk factor for mortality (aHR: 1.99, 95% confidence interval: 1.2‐3.3, P = .007).
Conclusion
Sarcopenia has a major impact on clinical outcomes both in compensated and in decompensated ACLD patients. The presence of sarcopenia doubled the risk for mortality independently from the severity of PH.
Keywords: advanced chronic liver disease, cirrhosis, hepatic venous pressure gradient, muscle wasting, portal hypertension, sarcopenia
Key Points.
Studies evaluating the impact of sarcopenia on hepatic outcomes in patients with clinically significant portal hypertension (PH) are scarce.
In this study we could show that sarcopenia is a significant predictor of mortality irrespective of severity of PH.
Sarcopenia remained an independent risk factor for mortality even after adjusting for age, portal pressure and MELD.
1. INTRODUCTION
Sarcopenia has emerged as an important prognostic factor in patients prior and after liver transplantation 1 , 2 , 3 as it impacts mortality. 4 , 5 , 6 Screening and therapeutic interventions for sarcopenia are thus recommended by recent guidelines. 7 Since sarcopenia is not implemented in current prognostic scoring systems such as the Child‐Pugh score (CPS) or the model for end‐stage liver disease (MELD), 8 , 9 the MELD‐sarcopenia 10 has been developed and improved prognostication. 11 Even though sarcopenia is now recognized as a major determinant of patients’ outcome prior and after liver transplantation, several questions remain:
First, the reference standard for diagnosing sarcopenia in cirrhosis remains to be established: the skeletal muscle index (SMI) 1 , 3 , 12 has become a widely used CT‐based parameter for evaluating sarcopenia but requires specialized software and expertise. Nevertheless, several other studies have investigated the transversal‐psoas muscle thickness (TPMT), the largest transversal diameter of the musculus psoas normalized by height, a method that is readily available in daily clinical routine. Importantly, TPMT has been previously demonstrated to correlate with prognosis in patients with advanced chronic liver disease. 6 , 13 , 14 We have recently proposed a standardized TPMT sarcopenia cut‐off at the level of the third lumbar vertebrae that was even superior to SMI in predicting survival. 15
Furthermore, it remains to be shown if an improvement of sarcopenia results in a survival benefit. While studies have demonstrated a gain in muscle mass after structured physical exercise, 16 , 17 , 18 evidence for improved survival after overcoming sarcopenia in patients with cirrhosis is lacking—although recently reversal of sarcopenia after TIPS implantation has been reported to be associated with improved survival. 14 , 19
Finally, the interaction between sarcopenia and portal hypertension (PH) has not been well studied. Measurement of the hepatic venous pressure gradient (HVPG) represents the gold standard to diagnose clinically significant PH (CSPH). Once CSPH (HVPG ≥ 10 mm Hg) has developed, patients are at considerable risk for hepatic decompensation. 20 , 21 , 22 In general, CSPH is present in about 50%‐60% of patients with compensated cirrhosis without gastro‐oesophageal varices (GEV). 21 , 23 , 24 Vice versa, patients with GEV are usually found with a HVPG of ≥ 10 mm Hg. 23 , 25 An HVPG ≥ 20 mm Hg defines high‐risk PH that increases the risk of failure to control variceal bleeding, early rebleeding and bleeding‐related mortality. 23 , 26 , 27 Recently, Kang et al 28 found that CPS, MELD and HVPG were prognostic factors in non‐sarcopenic patients, but not in patients with severe sarcopenia.
Thus, the aim of our study was to systematically assess the relationship among sarcopenia, severity of PH and survival in patients with CSPH.
2. PATIENTS AND METHODS
2.1. Study population
Consecutive patients undergoing HVPG‐measurement between 1 January 2004 and 31 December 2017 were screened for the presence of clinically significant portal hypertension (CSPH), defined as HVPG ≥ 10 mm Hg 23 and available abdominal cross‐sectional imaging by computed‐tomography (CT) or magnetic resonance (MR) within ± 200 days of HVPG measurement. Inclusion criteria were as follows: (a) age > 18 years, (b) established diagnosis of cirrhosis, (c) available information on body weight and height, (d) CSPH and (e) available CT/MR images within ± 200 days of HVPG measurement. Exclusion criteria were any extrahepatic malignancy or hepatocellular carcinoma and any significant comorbidity/disease significantly impacting on life expectancy. Days until the date of last follow‐up or date of death/decompensation were calculated from the time of the CT/MR scan. Mortality, (further) hepatic decompensation defined as either large‐volume paracentesis, hepatic encephalopathy grade III‐IV (=hospital admission for HE) and variceal bleeding was recorded. Patients were censored at date of liver transplantation.
2.2. Imaging Analysis
All measurements were obtained on axial contrast‐enhanced CT and MR scans of the abdomen. CT scans were performed on multidetector CT scanners (minimal requirements of 16 rows) using various vendors and scanner types given the long time span of the study. Different iodinated contrast agents were body weight adapted administered with a iodine concentration between 250 and 400 mg/mL. MR scans were obtained using 1.5 and 3T MR scanners. Different Gadolinum‐based contrast agents were administered adapted to body weight with a Gadolinium concentration between 150 and 300 mg/mL. Multiplanar reconstructed images were transferred to the routinely used picture archiving and communication system (PACS; IMPAX v. 6, Agfa) for TPMT measurements. Transverse venous‐phase images of the abdomen were loaded. As previously described, 29 the third lumbar vertebral body, where both transverse processes were depictable, was identified. TPMT was defined as the transversal diameter of the right psoas muscles perpendicular to the largest axial psoas muscle diameter at the L3 endplate. Results were normalized to body height and shown as mm psoas muscle thickness per m body height (mm/m).
Two independent radiology residents with 3 and 5 years of training, respectively, were instructed by a senior board‐certified radiologist specialized in abdominal imaging (UA) to measure the TPMT. Mean values of both measurements where then taken into account for statistical analysis. Kappa statistics found an interobserver variability of 0.72 indicating “substantial agreement”.
2.3. Definition of cut‐offs for sarcopenia
Sarcopenia was defined at a TPMT‐L3 < 12 mm/m in men and < 8 mm/m in women, which we previously established and validated to correlate best with mortality. 15
2.4. HVPG measurement
The right internal jugular vein was accessed under ultrasound guidance and local anaesthesia with Seldinger technique using a catheter introducer set (8.5F, Arrow International). Then, a specialized balloon catheter (7F, Pejcl Medizintechnik) was used to cannulate a hepatic vein as described previously. 30 , 31 CSPH was defined as an HVPG ≥ 10 mm Hg. 23 , 25
2.5. Statistics
Continuous variables were reported as mean ± standard deviation (SD) or median (IQR). Categorical variables were reported as absolute numbers (proportion, %) of patients with the certain characteristic. Student t‐test was used for group comparisons for parametric data and Mann‐Whitney U test for non‐parametric data. Kruskal‐Wallis H‐test was used to compare medians between groups of 3 or more. Chi‐squared or Fisher's exact tests were used to assess significant differences in proportions between groups. Patients entered follow‐up on the day of the CT/MR scan (ie day 0 of transplant‐free survival) and were followed until the day of their last clinical visit, death or any (further) hepatic decompensation. Patients undergoing liver transplantation were censored at the day of surgery. The impact of potential risk factors (eg sarcopenia, MELD, etc) on transplant‐free survival was analysed by uni‐ and multivariate cox regression. In our model, we included a maximum of one covariate per 5‐10 outcomes (ie mortality) to adhere to statistical standards. Variables were chosen after their (a) clinical relevance (b) statistical significance in univariate analysis. Established risk factors for mortality (eg age) and variables with a P < .1 on univariate cox regression were subsequently included in semi‐parametric proportional hazard multivariate Cox models. To investigate the effect of sarcopenia on survival and (further) hepatic decompensation, we used Fine and Gray competing risks regression models (cmprsk: Subdistribution Analysis of Competing Risks; https://CRAN.R‐project.org/package=cmprsk) 32 that considered liver transplantation as a competing risk. Two‐sided P < .05 were considered as statistically significant. The ibm spss 24.0 statistic software (SPSS Inc) was used for statistical analyses.
2.6. Ethics
This study was approved by the ethics committee of the Medical University of Vienna (EK1493/2016 and EK 1262/2017) and performed in accordance with the Declaration of Helsinki.
3. RESULTS
3.1. Study population
Among n = 885 patients undergoing measurement of HVPG within the defined study period, n = 605 (68.2%) had CSPH, and among these n = 224 (37%) underwent concomitant cross‐sectional CT/MR imaging. After excluding n = 21 (9.4%) as a result of predefined inclusion/exclusion criteria, a final number of n = 203 patients were included in this study (Figure 1, Table 1).
Figure 1.
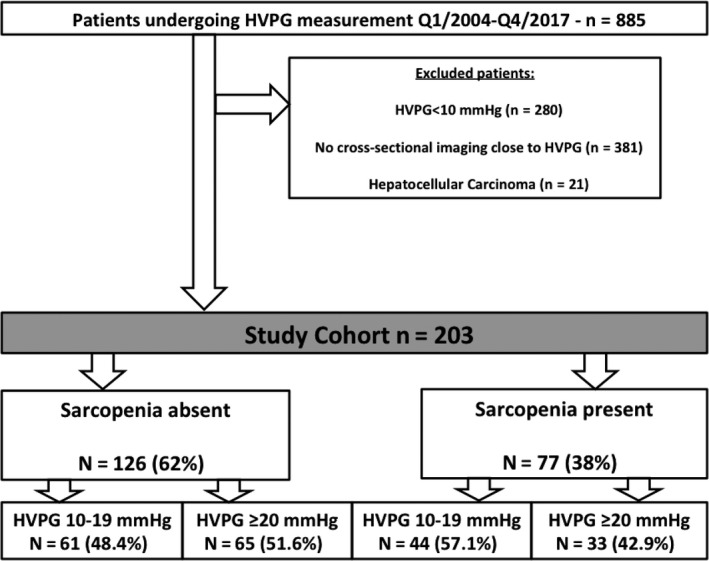
Study flow chart
Table 1.
Patient characteristics according to the presence of sarcopenia
| All patients (n = 203) | Sarcopenia absent (n = 126, 62.1%) | Sarcopenia present (n = 77, 37.9%) | P‐value | |
|---|---|---|---|---|
| TPMT (mm/m), mean ± SD | 12 ± 3.4 | 13.7 ± 2.9 | 9.2 ± 2.1 | <.001 |
| Age (years), mean ± SD | 55 ± 11 | 55 ± 10.8 | 54.5 ± 11.5 | .613 |
| Men (n, %) | 138 (68%) | 76 (60.3%) | 62 (80.5%) | .003 |
| Women (n, %) | 65 (32) | 50 (39.7) | 15 (19.5) | |
| Aetiology, n (%) | ||||
| ALD | 110 (54.2) | 66 (52.4) | 44 (57.1) | .802 |
| Viral hepatitis | 50 (24.6) | 34 (27) | 16 (20.8) | |
| NASH | 15 (7.4) | 9 (7.1) | 6 (7.8) | |
| Other | 28 (13.8) | 17 (13.5) | 11 (14.3) | |
| BMI (kg/m2), median (IQR) | 24.6 (22.4‐28.0) | 25.8 (23.1‐28.5) | 24.0 (21.3‐27.0) | .004 |
| BMI strata, n (%) | ||||
| <25 kg/m2 | 105 (51.7) | 54 (42.9) | 51 (66.2) | .005 |
| 25‐30 kg/m2 | 65 (32) | 47 (37.3) | 18 (23.4) | |
| >30 kg/m2 | 33 (16.3) | 25 (19.8) | 8 (10.4) | |
| HVPG (mm Hg), median (IQR) | 19 (16‐23) | 20 (16‐23) | 18 (16‐22.5) | .211 |
| HVPG strata, n (%) | ||||
| HVPG 10‐19 mm Hg | 105 (51.7) | 61 (48.4) | 44 (57.1) | |
| HVPG ≥ 20 mm Hg | 98 (48.3) | 65 (51.6) | 33 (42.9) | |
| Ascites, n (%) | ||||
| Moderate ascites | 76 (37.4) | 52 (41.3) | 24 (31.2) | .001 |
| Severe ascites | 56 (27.6) | 23 (18.3) | 33 (42.9) | |
| Hepatic encephalopathy, n (%) | ||||
| No | 144 (70.9) | 91 (72.2) | 53 (68.8) | .606 |
| Yes | 59 (29.1) | 35 (27.8) | 24 (31.2) | |
| Presence of oesophageal varices, n(%) a | 178 (89.4) | 114 (91.2) | 64 (86.5) | .296 |
| Previous variceal bleeding, n (%) | 56 (27.6) | 39 (31.0) | 17 (22.1) | .175 |
| Use of NSBB for bleeding prophylaxis, n (%) b | 166 (81.8) | 109 (86.5) | 57 (74.0) | .025 |
| Compensated, n (%) | 54 (26.6) | 41 (32.5) | 13 (16.9) | .014 |
| Decompensated, n (%) | 149 (73.4) | 85 (67.5) | 64 (83.1) | |
| MELD, median (IQR) | 12 (9‐15) | 11 (9‐15) | 13 (10‐17) | .067 |
| Albumin (g/L), mean ± SD | 33.2 ± 5.7 | 33.4 ± 5.8 | 32.8 ± 5.5 | .465 |
| Creatinine (mg/dL), median (IQR) | 0.80 (0.66‐0.99) | 0.81 (0.68‐0.95) | 0.79 (0.62‐1.02) | .803 |
| Sodium (mmol/L), median (IQR) | 137 (134‐140) | 137 (135‐140) | 136 (133‐139) | .035 |
| Bilirubin (mg/dL), median (IQR) | 1.66 (0.97‐2.98) | 1.55 (0.95‐2.62) | 1.76 (1.08‐3.94) | .212 |
| INR | 1.3 (1.2‐1.4) | 1.3 (1.2‐1.4) | 1.3 (1.2‐1.5) | .269 |
Information on the presence of varices is not available in n = 4 patients.
Defined as initiation or ongoing use of NSBB therapy after/during baseline HVPG measurement.
Mean age was 55 ± 11 years, 138 (68%) were man and liver disease aetiology was ALD in 110 (54.2%), viral hepatitis in 50 (24.6%), NASH in 15 (7.4%) and various other aetiologies in 28 (13.8%) patients.
Median MELD was 12, 9 , 10 , 11 , 12 , 13 , 14 , 15 138 (69%) and 62 (31%) had MELD < 15 and MELD ≥ 15 respectively. Median HVPG was 19 mm Hg. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 A total of 105 patients (51.7%) had HVPG values between 10 and 19 mm Hg and 98 (48.3%) had HVPG values ≥20 mm Hg. During follow‐up, 108 (53.2%) patients first/further decompensated (development or worsening of ascites: 43 [21.2%], HE III/IV: 25 [12.3%], and/or variceal bleeding: 12 [5.9%]) or first/further decompensated and died (n = 28; 13.9%) and 15 (7.4%) patients were transplanted. Overall, considering mortality only (transplant‐free survival) as the primary outcome, n = 69 (34%) of patients died during follow‐up.
3.2. Clinical characteristics according to the presence of sarcopenia
Sarcopenia was present in 77 (38%) patients. Sarcopenic patients were predominantly men (80.5% vs 60.3%, P = .003), showed lower BMI (24 [21.3‐27] vs 25.8 [23.1‐28.5], P = .004) and had higher MELD (13 [10‐17] vs 11 [9‐15], P = .067). Furthermore, patients with sarcopenia had more often decompensated disease (P = .014) and ascites (P = .001). Median HVPG values (P = .211) and proportion of HVPG ≥ 20 mm Hg patients (42.9% vs 51.6%, P = .227) were not different between sarcopenic and non‐sarcopenic patients respectively (Table 1).
3.3. The impact of sarcopenia on hepatic outcomes in all patients and stratified according to different stages of PH severity
Sarcopenia was associated with a significantly increased risk for mortality during follow‐up (SHR: 2.23, P < .001; Figure 2A). Sarcopenia was also a significant risk factor for (further) hepatic decompensation (SHR: 1.79, P = .003; Figure 3A).
Figure 2.
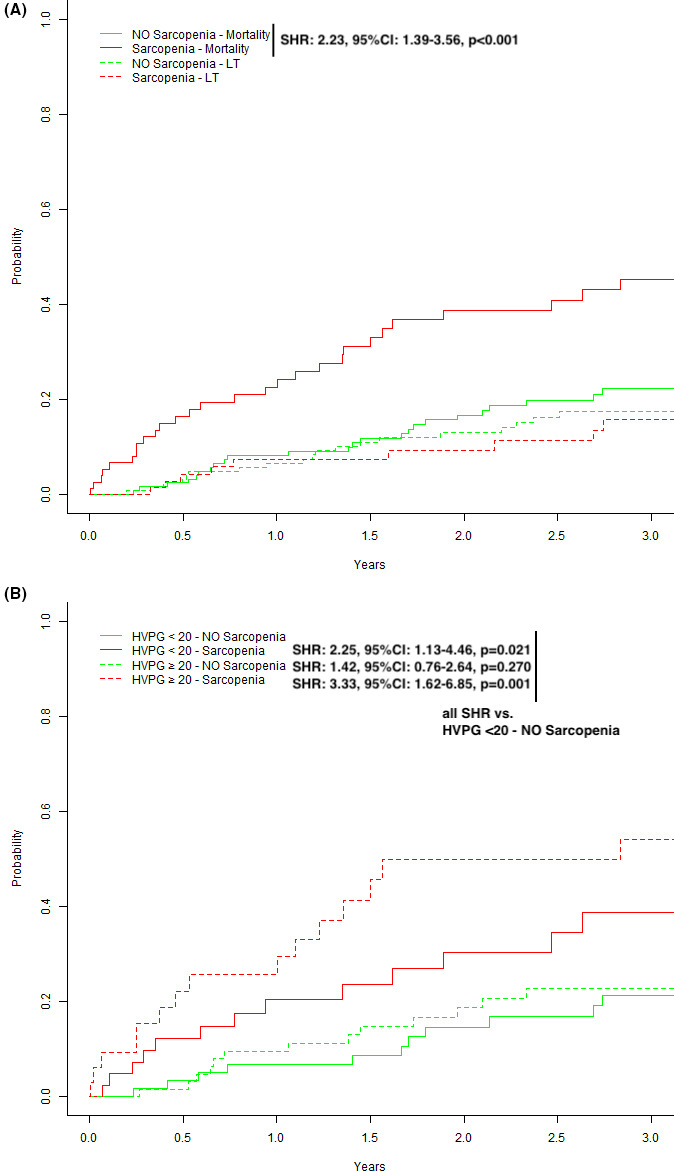
Competing risk analyses (event of interest: death, competing risk: liver transplantation) stratified for (A) the presence or absence of sarcopenia, (B) severity of portal hypertension (HVPG 10‐19 vs ≥ 20 mm Hg) and sarcopenia
Figure 3.
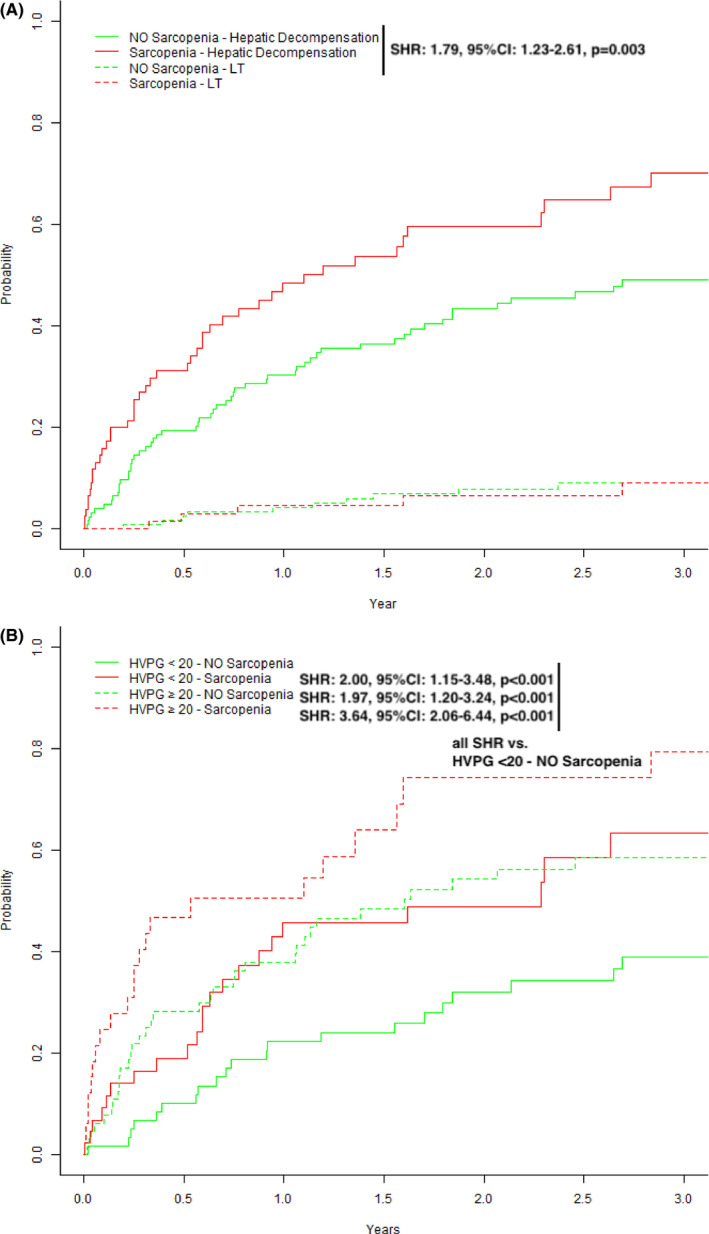
Competing Risk Analyses (event of interest: hepatic decompensation, competing‐risk: liver transplantation) stratified for (A) the presence or absence of sarcopenia and (B) severity of portal hypertension (HVPG 10‐19 vs ≥ 20 mm Hg) and sarcopenia
Presence of sarcopenia did not correlate with absolute HVPG values (r = .031, P = .66) and HVPG was not significantly different in sarcopenic vs non‐sarcopenic patients (18 [16‐23] vs 20 [16‐23] mm Hg, P = .211). When patients were stratified according to HVPG < 20/≥20 mm Hg ± presence or absence of sarcopenia (Table S4), the sarcopenic group showed predominantly male gender (P = .013), a lower BMI (0.023) and higher MELD scores (0.003). We then performed competing risk analyses according to HVPG strata and presence/absence of sarcopenia: patients with sarcopenia (HVPG < 20—Sarcopenia: SHR: 2.25, P = .021; HVPG ≥ 20—Sarcopenia: SHR: 3.33, P = .001) were found with a significantly increased risk for death as compared to non‐sarcopenic patients (HVPG ≥ 20—NO Sarcopenia: SHR: 1.42, P = .270) (Figure 2B). Similar results were seen when the outcome of interest was any (further) hepatic decompensation (Figure 3B).
3.4. The impact of sarcopenia in compensated and decompensated patients
Overall, 149 (73.4%) patients were decompensated at the time of study inclusion. The prevalence of decompensation was significantly higher in sarcopenic vs non‐sarcopenic patients (83.1% vs 67.5%, P = .014) These differences regarding the presence and severity of ascites were particularly pronounced—grade 3 ascites was observed in 42.9% of sarcopenic patients, but only in 18.3% of non‐sarcopenic patients (P = .001). Interestingly, hepatic encephalopathy was similarly frequent in patients with vs without sarcopenia (31.2% vs 27.8%; P = .606; Figure 4).
Figure 4.
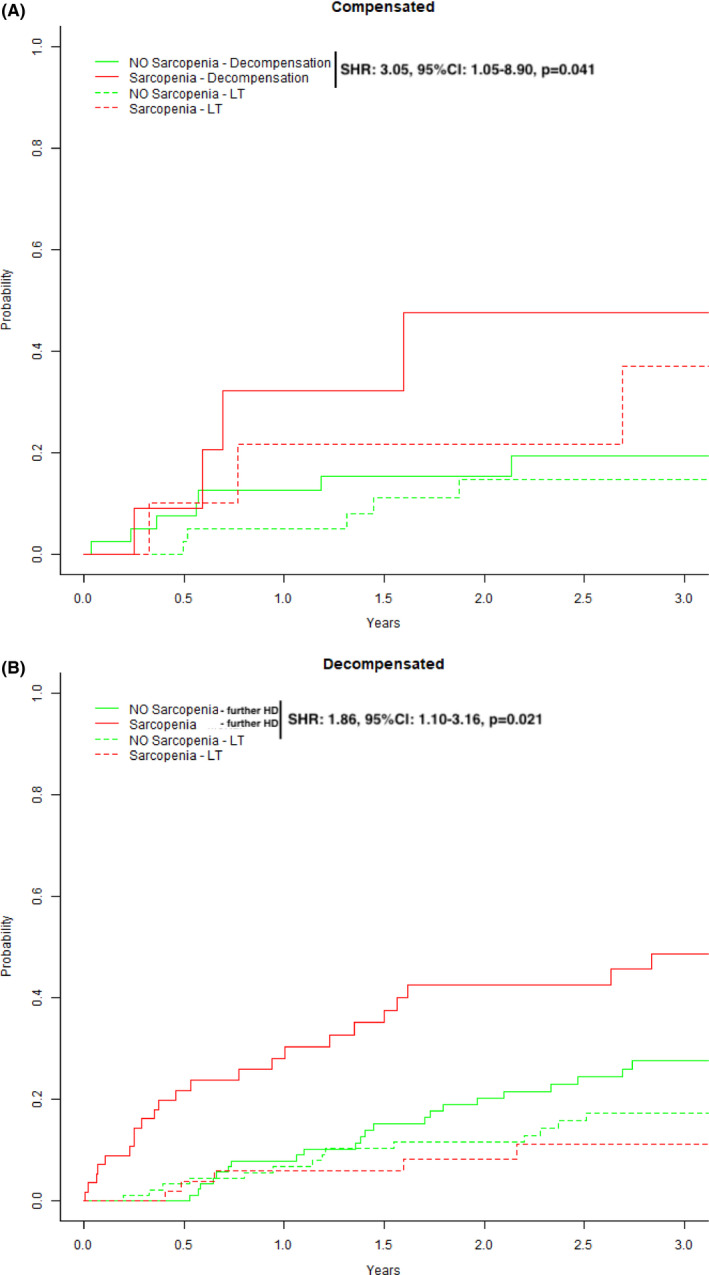
Competing risk analyses for (A) compensated patients stratified by the presence or absence of sarcopenia with first decompensation being the event of interest and liver transplantation (LT) representing a competing risk. In (B), decompensated patients were stratified by the presence or absence of sarcopenia with further hepatic decompensation being the event of interest and liver transplantation (LT) representing a competing risk
When stratifying by hepatic decompensation, we found that sarcopenia was a significant discriminating factor regarding outcomes both within compensated (Figure 4A: SHR: 3.05, P = .041, outcome of interest: first hepatic decompensation) and decompensated patients (Figure 4B: SHR: 1.86, P = .021, outcome of interest: further hepatic decompensation).
3.5. The impact of sarcopenia in subgroups of cirrhosis aetiologies
While the distribution of liver disease aetiologies was not different between sarcopenic vs non‐sarcopenic patients (Table 1, P = .802), we aimed to investigate whether sarcopenia plays a significant role regarding outcomes in the main aetiology of alcohol‐related liver disease (ALD; Figure S3).
A total of 74 (67.3%) of patients with ALD were abstinent, while 36 (32.7%) patients reported ongoing alcohol consumption. Among ALD patients, we found sarcopenia as a significant risk factor for mortality during follow‐up (SHR: 2.04, 95% CI: 1.12‐3.71, P = .020; Figure S3). Moreover, we performed an explorative analysis restricted to ALD patients that also included the interaction term abstinence × sarcopenia to investigate whether abstinence modifies the impact of sarcopenia on mortality. Interestingly, the interaction term did not yield a statistically significant result (aHR: 0.85, 0.23‐3.2, P = .813). Thus, abstinence did not seem to modify the prognostic impact of sarcopenia in ALD patients.
3.6. Sarcopenia is an independent risk factor for mortality inPH
When assessing potential risk factors for mortality in patients with PH, we found that age, male gender, presence of sarcopenia, serum albumin levels and MELD were associated with increased transplant‐free mortality on univariate analysis (Table 2).
Table 2.
Uni‐ and multivariate Cox regression analysis of independent risk factors for mortality
| Parameter | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | aHR | 95% CI | P‐value | |
| Age (per year) | 1.05 | 1.03‐1.08 | <.001 | 1.05 | 1.02‐1.07 | <.001 |
| Male gender (vs female) | 1.83 | 1.03‐3.24 | .039 | 1.67 | 0.92‐3.02 | .093 |
| BMI (per kg/m2) | 1.01 | 0.96‐1.06 | .630 | — | — | — |
| Sarcopenia present (vs absent) | 2.42 | 1.49‐3.93 | <.001 | 1.99 | 1.21‐3.28 | .007 |
| MELD (per point) | 1.09 | 1.027‐1.157 | .005 | 1.09 | 1.03‐1.17 | .005 |
| HVPG ≥ 20 mm Hg (vs 10‐19 mm Hg) | 1.48 | 0.92‐2.38 | .109 | 1.20 | 0.72‐2.00 | .478 |
| Albumin (per g/L) | 0.96 | 0.92‐0.99 | .019 | 0.99 | 0.94‐1.04 | .618 |
When adjusting for these factors, age (aHR 1.05, 95%CI: 1.02‐1.07, P < .001), MELD (aHR 1.09, 95%CI: 1.03‐1.17, P = .005) and sarcopenia (aHR 1.99, 95%CI: 1.21‐3.28, P = .007) remained as significant and independent risk factors for transplant‐free mortality.
4. DISCUSSION
In this study, we systematically investigated the impact of sarcopenia on mortality in ACLD patients with different severity of CSPH as stratified by HVPG. Furthermore we were able to validate the prognostic value of our previously established TPMT‐L3 sarcopenia cut‐off at <12 mm/m in men and <8 mm/m in women. 15 Importantly, sarcopenia was a significant independent risk factor for mortality after adjusting for age, hepatic dysfunction (MELD) and portal pressure (ie HVPG). Continuatively our data suggest that muscle wasting is one of the leading drivers of mortality in ACLD patients with CSPH. Ultimately multivariate regression analyses indicated that sarcopenia was independently associated with impaired transplant‐free survival after adjusting for traditional prognostic factors such as MELD.
In recent years, several studies linked sarcopenia to an impaired outcome in patients on the liver transplant waiting list—mostly in the setting of severely impaired hepatic dysfunction (ie end‐stage liver disease with high MELD). 4 , 5 , 6 , 36 However, sarcopenia has not been systematically assessed in patients with advanced (but not end‐stage) liver disease who might present with different severity of PH.
Kang et al 28 investigated the impact of sarcopenia in several subgroups of patients who were stratified by MELD, CPS and HVPG. In their study, MELD, CPS and HVPG were of prognostic value in non‐sarcopenic patients but not in patients with severe sarcopenia. 28 Importantly, patients with HVPG values between 10 and 19 mm Hg and presence of sarcopenia showed similar survival times to patients with HVPG ≥ 20 mm Hg and no sarcopenia. Vice versa patients with subclinical PH (HVPG 6‐9 mm Hg) with sarcopenia showed similar survival to patients with HVPG 10‐19 mm Hg without sarcopenia. This is in line with our data where patients with a HVPG between 10 and 19 mm Hg and presence of sarcopenia show similar survival times to patients with HVPG values ≥ 20 mm Hg but absence of sarcopenia. Importantly our data also show that among patients with a HVPG ≥ 20 mm Hg, the presence of sarcopenia was associated with a significantly shorter survival, even after adjusting of other risk factors in multivariate regression analysis (Table S5).
Ultimately, our study supports the use of TPMT as a readily available clinical method to assess sarcopenia that also provides critical prognostic information in patients with ACLD. The SMI has been used to diagnose sarcopenia in other cohorts, 1 , 2 , 7 however, its clinical applicability is limited by the requirement of specific radiologic software for computing the SMI values. In contrast, TPMT is easily and rapidly obtained and has been previously demonstrated to be of prognostic value in ACLD. 6 , 13 , 14 , 36 However, in previous studies, the TPMT was often measured at the level of the umbilicus that is not well‐standardized since in patients with cirrhosis and ascites the location of the umbilicus in relation to the axial skeleton varies substantially, as shown by Praktiknjo et al, 14 where the umbilicus was found at level of L4 in 40%, L5 in 20%, and L3 in 10% of patients. We therefore proposed a new standardized measurement of the TPMT at the level of the third lumbar vertebrae (TPMT‐L3) and found excellent prognostic capability. 15 In this study we validated the gender‐specific TPMT‐L3 cut‐off for sarcopenia—nevertheless it needs to be emphasized that this cut‐off has been initially computed and now internally validated by our group in partly overlapping patient populations, therefore external validation of our cut‐offs is needed prior to implementation of TPMT‐L3 into daily clinical routine for diagnosing sarcopenia.
As a result of the crucial impact of sarcopenia on survival in ACLD patients with CSPH, strategies for improving sarcopenia and reducing muscle wasting should be investigated. Exercise has been shown to improve muscle mass 16 , 17 and importantly also to lower HVPG, 37 , 38 but effects on survival have not been reported. Low levels of testosterone are often observed in patients with cirrhosis 34 , 39 , 40 , 41 and might be even lower in those with sarcopenia. 40 Thus, Sinclair et al 42 studied the effects of intramuscular testosterone supplementation and found an increase in muscle mass but no beneficial effects on mortality.
Our study has some limitations: First, it is a retrospective analysis, however, all patients undergoing measurement of HVPG at the Vienna Hepatic Hemodynamic Laboratory are thoroughly characterized in a prospective manner. Choosing a timeframe of ±200 days between HVPG measurement and CT scan may be criticized, however, we decided to apply this cut‐off since most patients are seen every 3‐6 months at our department and undergo imaging for HCC surveillance every 6 months. Therefore, we assumed that ±200 days is a realistic time frame for sarcopenia assessment on liver imaging (eg on a CT scan) to obtain prognostic information.
Second, the subgroup of patients undergoing cross‐sectional imaging by CT/MRI may not include a fully representative patient cohort, which may represent a selection bias in this cohort. Thirdly, we derived and used our own TPMT‐L3 cut‐off to define sarcopenia that was of excellent predictive capability similar to our previous study 15 but external validation is still required. Finally, owing to only a low number of patients with mild (ie subclinical) PH (HVPG 6‐9 mm Hg) and their intrinsic low risk of clinical events, we abstained from evaluating the distinct effects of sarcopenia in patients with mild PH.
In conclusion, sarcopenia diagnosed by TPMT‐L3 represents an independent risk factor for (further) decompensation and mortality in ACLD patients with CSPH, irrespective of MELD and severity of PH. Future studies should investigate if TPMT‐L3 can capture dynamic changes in sarcopenia that may be of particular prognostic relevance in patients with ACLD and CSPH.
Supporting information
Supplementary Material
Paternostro R, Bardach C, Hofer BS, et al. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int.2021;41:799–809. 10.1111/liv.14758
Handling Editor: Virginia Hernandez‐Gea
REFERENCES
- 1. Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis – aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765‐777. [DOI] [PubMed] [Google Scholar]
- 2. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duarte‐Rojo A, Ruiz‐Margain A, Montano‐Loza AJ, Macias‐Rodriguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end‐stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transplant. 2018;24(1):122‐139. [DOI] [PubMed] [Google Scholar]
- 4. Montano‐Loza AJ, Meza‐Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166‐173, 73.e1. [DOI] [PubMed] [Google Scholar]
- 5. Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transplant. 2012;18(10):1209‐1216. [DOI] [PubMed] [Google Scholar]
- 6. Durand F, Buyse S, Francoz C, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151‐1157. [DOI] [PubMed] [Google Scholar]
- 7. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70(1):172‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiesner R, Edwards E, Freeman R, et al. Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91‐96. [DOI] [PubMed] [Google Scholar]
- 9. Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver‐transplant waiting list. N Engl J Med. 2008;359(10):1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montano‐Loza AJ, Duarte‐Rojo A, Meza‐Junco J, et al. Inclusion of sarcopenia within MELD (MELD‐sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Translat Gastroenterol. 2015;6:e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Vugt JLA, Alferink LJM, Buettner S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2017;68(4):707‐714. [DOI] [PubMed] [Google Scholar]
- 12. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta‐analysis. PLoS One. 2017;12(10):e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huguet A, Latournerie M, Debry PH, et al. The psoas muscle transversal diameter predicts mortality in patients with cirrhosis on a waiting list for liver transplantation: a retrospective cohort study. Nutrition. 2018;51–52:73‐79. [DOI] [PubMed] [Google Scholar]
- 14. Praktiknjo M, Book M, Luetkens J, et al. Fat‐free muscle mass in magnetic resonance imaging predicts acute‐on‐chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67(3):1014‐1026. [DOI] [PubMed] [Google Scholar]
- 15. Paternostro R, Lampichler K, Bardach C, et al. The value of different CT‐based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39(12):2374‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(11):1920‐6.e2. [DOI] [PubMed] [Google Scholar]
- 17. Kruger C, McNeely ML, Bailey RJ, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep. 2018;8(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiraoka A, Michitaka K, Kiguchi D, et al. Efficacy of branched‐chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastro Hepatol. 2017;29(12):1416‐1423. [DOI] [PubMed] [Google Scholar]
- 19. Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastro Hepatol. 2013;25(1):85‐93. [DOI] [PubMed] [Google Scholar]
- 20. D'Amico G, Morabito A, D'Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563‐576. [DOI] [PubMed] [Google Scholar]
- 21. de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743‐752. [DOI] [PubMed] [Google Scholar]
- 22. Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut. 2013;62(11):1634‐1641. [DOI] [PubMed] [Google Scholar]
- 23. Garcia‐Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310‐335. [DOI] [PubMed] [Google Scholar]
- 24. Groszmann RJ, Garcia‐Tsao G, Bosch J, et al. Beta‐blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353(21):2254‐2261. [DOI] [PubMed] [Google Scholar]
- 25. Reiberger T, Puspok A, Schoder M, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129(Suppl 3):135‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abraldes JG, Villanueva C, Banares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol. 2008;48(2):229‐236. [DOI] [PubMed] [Google Scholar]
- 27. Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117(3):626‐631. [DOI] [PubMed] [Google Scholar]
- 28. Kang SH, Jeong WK, Baik SK, Cha SH, Kim MY. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle. 2018;9(5):860‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population‐based study. Lancet Oncol. 2008;9(7):629‐635. [DOI] [PubMed] [Google Scholar]
- 30. Ferlitsch A, Bota S, Paternostro R, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int 2015. 35(9):2115‐2120. [DOI] [PubMed] [Google Scholar]
- 31. Paternostro R, Reiberger T, Mandorfer M, et al. Plasma renin concentration represents an independent risk factor for mortality and is associated with liver dysfunction in patients with cirrhosis. J Gastroenterol Hepatol. 2017;32(1):184‐190. [DOI] [PubMed] [Google Scholar]
- 32. Team R . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 33. van Vugt JL, Levolger S, de Bruin RW, et al. Systematic review and meta‐analysis of the impact of computed tomography assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277‐2292. [DOI] [PubMed] [Google Scholar]
- 34. Sinclair M, Grossmann M, Angus PW, et al. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31(3):661‐667. [DOI] [PubMed] [Google Scholar]
- 35. Lucidi C, Lattanzi B, Di Gregorio V, et al. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38(5):851‐857. [DOI] [PubMed] [Google Scholar]
- 36. Kim TY, Kim MY, Sohn JH, et al. Sarcopenia as a useful predictor for long‐term mortality in cirrhotic patients with ascites. J Korean Med Sci. 2014;29(9):1253‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berzigotti A, Albillos A, Villanueva C, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293‐1305. [DOI] [PubMed] [Google Scholar]
- 38. Macias‐Rodriguez RU, Ilarraza‐Lomeli H, Ruiz‐Margain A, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Translat Gastroenterol. 2016;7(7):e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paternostro R, Heinisch BB, Reiberger T, et al. Erectile dysfunction in cirrhosis is impacted by liver dysfunction, portal hypertension, diabetes and arterial hypertension. Liver Int. 2018;38(8):1427‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paternostro R, Heinisch BB, Reiberger T, et al. Dysbalanced sex hormone status is an independent predictor of decompensation and mortality in patients with liver cirrhosis. Hepatol Res 2018;49(2):201‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sinclair M, Grossmann M, Gow PJ, Angus PW. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015;30(2):244‐251. [DOI] [PubMed] [Google Scholar]
- 42. Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65(5):906‐913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


