Abstract
Objective
Improving shared decision‐making (SDM) enables more tailored cancer treatment decisions. We evaluated a Time Out consultation (TOC) with the general practitioner (GP), between cancer diagnosis and treatment decision, which aims at supporting SDM and improving continuity of primary care. This study aims to evaluate the effects of a TOC on perceived SDM, information provision and self‐efficacy.
Methods
This randomised controlled trial included newly diagnosed patients with curable cancer (breast, lung, colorectal, gynaecologic and melanoma) from four Dutch hospitals. Primary outcome is perceived SDM and secondary outcomes are information provision and self‐efficacy.
Results
One hundred fifty‐four patients (control n = 77, intervention n = 77) – female: 75%, mean age: 61 (SD ± 11.9). In the intervention group, 80.5% (n = 62) had a TOC, of which 82.3% (n = 51) took place after treatment decision. Perceived SDM was lower in the intervention group (−8.9 [95% CI: 0.6–17.1]). Among those with a TOC before treatment decision (n = 11), perceived SDM was comparable to the control group (66.5 ± 27.2 vs. 67.9 ± 26.1).
Conclusion
Even though patients are motivated to have a TOC, implementing a TOC between diagnosis and treatment decision is challenging. Effects of a timely TOC could not be established. Non‐timely TOC decreased perceived SDM. Planning of the TOC should be optimised, and future research should establish if adequately timed TOC results in improved SDM in cancer patients.
Keywords: cancer, decision‐making, general practitioners, neoplasms, physicians, primary health care, psycho‐oncology
1. BACKGROUND
Cancer is the second leading cause of death globally. In 2018, over 17 million people worldwide were diagnosed with cancer, a number that is expected to reach 21 million patients by the year 2030. 1 As cancer mainly affects the elderly, the increase is to a large extent caused by ageing.
Cancer treatment should be personalised. This means that, besides tailoring treatment choice to tumour characteristics, for every patient, the treatment option should be chosen which best fits a patient's preferences and circumstances. This is increasingly complex because of several reasons. First, the spectrum of treatment modalities for cancer expanded in recent years. Second, comorbidity is common among cancer patients and may interfere with cancer treatment.2, 3, 4 Furthermore, treatment decisions become more complex at higher age, due to comorbidities, declining life expectancy, and changing life perspectives and priorities. Consequently, personalised treatment decisions require a balanced decision‐making process between patients and healthcare professionals, with thorough weighing of curative treatment options in the light of patient preferences and personal context.
Although many general practitioners (GPs) do participate in care before diagnosis of cancer, structural guidance and care by the GP starting from the moment of diagnosis onwards is uncommon. 5 In view of their position, this seems to be a missed opportunity. GPs are well equipped to support the patient during their cancer care pathway: they usually have a longstanding and personal relationship with their patients and work with an integral and personalised approach, including psychosocial support. In that regard, of all caregivers involved, GPs are probably best positioned to balance treatment options in the perspective of the patient's medical history and personal preferences.6, 7 It is therefore that professional and patient organisations advocate a structured and expanded role for the GP in the cancer care pathway, starting from the moment cancer is diagnosed. 6
Personalised cancer care requires active involvement of the patient in treatment decision by shared decision‐making (SDM). For successful SDM in complex decisions, several steps are required, that is, creating awareness of choice, explanation of treatment options, consideration of the treatment options provided and making an informed choice. 8 Research suggests that SDM improves knowledge and understanding of treatment options,9, 10, 11 creates more realistic expectations, 9 and better matches patient's preferences and subsequent treatment decisions. 9 Moreover, patients feel better informed, 12 are more determined on their personal values 12 and experience better communication with their practitioner.9, 10, 11 Adequate SDM may also improve medication adherence, 11 mental health‐related quality of life 13 and reduce healthcare costs. 14 Several large studies have demonstrated that patients want to be involved in decision‐making.15, 16, 17 Additionally, a recent survey in the Netherlands among 4700 patients treated for cancer showed that the majority of patients prefer their GP to be involved, as the GP can help to create awareness of choice and can prepare the patient for the treatment decision in the hospital. 18
So far, the effectiveness of GP involvement in SDM for cancer treatment decisions has not been evaluated. In the randomised controlled GRIP trial, we evaluate the effects of providing structural follow‐up care from primary care during cancer treatment in hospital. This follow‐up care starts with a Time Out consultation (TOC) between patient and GP immediately after cancer diagnosis. Here we report the effects of a TOC after a cancer diagnosis for patients treated with curative intent, on patient‐perceived SDM, information provision and perceived self‐efficacy.
2. METHODS
2.1. Design
The GRIP trial is a multicentre randomised controlled trial following the patient from cancer diagnosis until 3 months after the completion of primary treatment with a maximum of 1‐year follow‐up. The study was conducted in four Dutch hospitals between April 2015 and May 2017 in the region of Utrecht, the Netherlands. In addition to the usual hospital care, patients randomised to the GRIP intervention group were offered structured follow‐up guidance from primary care consisting of two components: (1) a TOC with the GP and (2) structured follow‐up during cancer treatment by a primary care oncology nurse and the GP. For full exploration and understanding of the effects of the first component (TOC), we report these effects in this paper separately. As follow‐up care was delivered after, and independently from the TOC, we expect no interference. The GRIP study protocol was published previously. 19 The study protocol was assessed by the Medical Ethical Committee of the University Medical Center Utrecht and was considered non‐eligible for full ethical review according to Dutch law (METC number: 15‐075/C). This study was performed in accordance with the Helsinki Declaration 1975. The GRIP trial is registered in the ‘Netherlands Trial Register’ (Trial number: NTR5909).
2.2. Patient and public involvement
The Dutch Federation of Cancer Patient Organizations (NFK) was part of the GRIP project group. NFK contributed to the definition of research priorities, participated in the intervention and study design, including the choice of outcome measures (SDM). NFK also contributed to the writing of the manuscript.
2.3. Study population and setting
Patients were eligible for participation if they were aged 18 or over, newly diagnosed with either breast cancer, colorectal cancer, gynaecological cancer, lung cancer or melanoma, and scheduled for curative treatment (usually stages I–III and, in rare cases, stage IV). Patients were excluded in case of major psychiatric diseases, personality disorders, inability to fill in questionnaires, or if the patient's GP worked outside the study area, did not agree to participate, or if the patient already started cancer treatment.
2.4. Recruitment and randomisation
After diagnosis, eligible patients were approached for participation by their treating physician or oncology nurse in the treating hospital. If patients consented, they were contacted by the researchers by phone the (working) day after diagnosis to verify eligibility and provide further study information. Upon confirmation of willingness to participate, patients were randomised. Equally allocated (1:1) randomisation was performed by using an online computerised randomisation module provided by an independent data centre of the UMC Utrecht. Minimisation was applied to ensure balance between groups regarding treating hospital and cancer type. Due to the nature of the intervention, patients, healthcare providers and researchers extracting data from the electronic medical record (EMR) could not be fully blinded for the intervention. All participants gave verbal and written consent for participation.
2.5. Usual care
All patients received cancer care as usual in the hospital, which is to a large extent protocolised. Protocols for curative treatment vary according to cancer type and patient and disease characteristics. In general, additional investigations are required such as determination of laboratory values and imaging, and multidisciplinary team discussions on treatment options. In one or more consultations with the medical specialist, the diagnosis is explained to the patient, information about cancer and treatment options is given, and the final treatment decision is made.
Involvement of the GP following primary cancer diagnosis varies between hospitals, specialists and GPs. In general, the GP is informed about the diagnosis by phone or by mail through electronic data interchange after the multidisciplinary team reaches consensus on the diagnosis and treatment. Thereafter, contact between the GP and the patient depends on the individual initiative of either the GP or the patient.
2.6. Intervention: the time out consultation
All patients received usual care as described previously. In the same call as in which patients were included, patients in the intervention group were asked to schedule a TOC with their GP immediately after randomisation to prepare for the final treatment decision. The TOC was a 20‐min consultation with the GP. The aim of the TOC was to improve the SDM process and improve continuity of primary care. For this consultation, the GP was instructed to give psychosocial guidance, including discussing the impact of diagnosis and consequences. Furthermore, the GP was instructed to check patient's understanding of information, to create awareness that a choice of treatment exists, and to stimulate the use of the ‘three‐questions’ model during the specialist consultation on the final treatment decision. The three‐questions model is developed to support patient involvement and information exchange when discussing therapeutic options in medical care. 20 The three questions are: What are my options? What are the possible benefits and harms of those options? How likely are the benefits and harms of each option to occur for me? 20
Participating GPs received information on the GRIP study by their GP cooperative organisations. The GPs of patients who were randomised to the intervention group were trained by phone on the content of the GRIP intervention by the researcher after the patient consented to participate. During this telephone contact, the researcher provided the necessary instructions to perform a TOC. In addition, information on the steps GPs were expected to take was given by email and through a website.
2.7. Outcomes
To report the primary outcome (perceived level of SDM) and secondary outcomes (received information and perceived self‐efficacy), patients filled in three validated questionnaires 2 weeks after inclusion (T1) online or, if preferred, on paper, which was after the TOC and after the treatment decision had been made. Only perceived self‐efficacy was measured at both baseline (i.e., after randomization) (T0) and T1. Non‐responders were sent two automatic reminders by mail after 2 and 5 days, and were contacted by phone by the researcher if non‐response maintained.
2.8. Primary outcome
The perceived level of SDM was measured using the Shared Decision‐Making Questionnaire (SDM‐Q‐9), which contains nine items with a six‐point Likert scale and focuses on the decision process in the hospital. 21 A score was calculated, which ranged from 0 to 100 and a higher score indicated higher perceived SDM. During the trial (26 January 2016), the following question was added to get more specific information about the role of the GP in SDM: ‘My GP helped me make my choice of treatment’, since the original questionnaire did not evaluated the coaching physician. The question was analysed separately for 84 patients (see Table 2).
TABLE 2.
Results of perceived shared decision‐making, provided information assessment and self‐efficacy
| Intervention (n = 74) | TOC before treatment decision (n = 11) | TOC after treatment decision (n = 51) | No TOC (n = 12) | Control (n = 74) | Estimated mean difference between study groups (95% CI) | |
|---|---|---|---|---|---|---|
| Perceived shared decision‐making | ||||||
| T1 mean score (±SD) | 59.2 (±27.9) | 66.5 (±27.2) | 55.7 (±28.7) | 67.2 (±23.8) | 67.9 (±26.1) | −8.9 (−17.1; −0.6) |
| −8.4 b (−16.8; 0.0) | ||||||
| GP involved in treatment decision a | n = 40 | n = 6 | n = 27 | n = 7 | n = 44 | |
| T1 percentage agreement | ||||||
| ‐ Completely disagree | 70.0% | 50.0% | 66.7% | 100% | 68.2% | |
| ‐ Strongly disagree | 12.5% | 0.0% | 18.5% | 0.0% | 6.8% | |
| ‐ Somewhat disagree | 0.0% | 0.0% | 0.0% | 0.0% | 4.5% | |
| ‐ Somewhat agree | 2.5% | 0.0% | 3.7% | 0.0% | 6.8% | |
| ‐ Strongly agree | 7.5% | 16.7% | 7.4% | 0.0% | 6.8% | |
| ‐ Completely agree | 7.5% | 33.3% | 3.7% | 0.0% | 6.8% | |
| Information assessment of patients | ||||||
| T1 mean score (±SD) | ||||||
| ‐ Disease | 58.1 (±22.6) | 57.6 (±24.3) | 56.4 (±21.9) | 66.0 (±24.2) | 59.9 (±21.7) | −1.4 (−8.7; 5.9) |
| ‐ Medical tests | 73.4 (±24.0) | 82.8 (±21.3) | 71.7 (±24.7) | 72.2 (±23.0) | 75.5 (±22.2) | −2.2 (−9.8; 5.5) |
| ‐ Treatments | 41.9 (±21.0) | 49.4 (±25.1) | 38.1 (±17.7) | 51.2 (±26.7) | 45.1 (±20.5) | −3.1 (−9.9; 3.7) |
| ‐ Other services | 27.8 (±25.8) | 26.5 (±20.7) | 24.1 (±21.5) | 44.4 (±39.5) | 28.0 (±25.0) | −0.5 (−8.7; 7.6) |
| ‐ Places of care | 27.9 (±33.6) | 18.2 (±22.9) | 28.8 (±32.7) | 33.3 (±44.9) | 22.5 (±28.7) | 4.2 (−6.0; 14.5) |
| ‐ Self‐help | 40.1 (±35.7) | 42.4 (±42.4) | 38.6 (±32.9) | 44.4 (±43.4) | 43.7 (±32.6) | −4.3 (−15.5; 6.9) |
| ‐ Satisfaction with information | 75.2 (±23.4) | 75.8 (±26.2) | 74.5 (±23.7) | 77.8 (±21.7) | 75.2 (±23.4) | −0.5 (−8.2; 7.2) |
| ‐ Helpfulness of information | 79.3 (±21.9) | 81.8 (±22.9) | 77.8 (±22.8) | 83.3 (±17.4) | 76.6 (±21.9) | 2.3 (−4.9; 9.6) |
| Perceived Efficacy in Patient–Physician Interactions | ||||||
| T1 mean score (±SD) | 22.3 (±2.4) | 22.8 (±2.4) | 22.1 (±2.5) | 22.7 (±2.2) | 22.1 (±2.9) | 0.4 c (−0.4; 1.1) |
| 0.3 d (−0.5; 1.1) | ||||||
| Mean difference (±SD) T1 − T0 within groups (95% CI) | 1.1 (0.4; 1.8) | 1.5 (‐0.7; 3.8) | 1.0 (0.1; 1.9) | 1.2 (‐1.0; 3.4) | 0.5 (−0.1; 1.2) | |
Abbreviations: PEPPI, Perceived Self‐Efficacy in Patient–Physician Interactions; SD, standard deviation; TOC, Time Out consultation; T0, baseline measurement; T1, assessment after 2 weeks.
Question was added after the trial started.
Added correction comorbidities (none; ≥1 comorbidities).
Added correction PEPPI at baseline.
Added correction PEPPI at baseline and comorbidities (none, ≥1 comorbidities).
2.9. Secondary outcomes
Received information was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Group information questionnaire, we used the Dutch version of the EORTC‐info25 which contains two extra questions on alternative medicine and a patient held record. The 27‐item cancer‐specific questionnaire had a four‐point Likert scale. 22 This questionnaire assessed the amount of information received on multiple cancer‐related themes (diagnosis, medical tests, treatments, other services, places of care and self‐help), the satisfaction and usefulness of received information. With the items a score was calculated, which ranged from 0 to 100. A higher score indicates a better perceived information provision.
Self‐efficacy is defined as ‘the individual's capacity to produce desired effects’. 23 Perceived self‐efficacy was measured using the Perceived Self‐Efficacy in Patient–Physician Interactions (PEPPI‐5) questionnaire, which contains 10 items with a five‐point Likert scale. 24 With these items, a score was calculated which ranged from 5 to 25. A higher score indicates higher perceived self‐efficacy.
2.10. Intervention adherence
Adherence to the protocol for the consistency, content and planning of the TOC were assessed using the free text in the GP's EMR in the intervention group. EMR data are registered for each GP consultation as part of usual care. Performance of the content of TOC according to protocol was confirmed if the free text referred to components of the TOC intervention (TOC content or references to the GRIP study). Timing of the TOC according to protocol was defined as a TOC between diagnosis and treatment decision. Dates from the primary care and hospital EMR were used. Consultations in the control arm were evaluated for contamination, defined as a consultation in which the patient was empowered for SDM or the treatment has been discussed before filling out the SDM questionnaire. All GP consultations within 2 weeks of inclusion were registered in both groups.
2.11. Data collection
Patient characteristics were obtained from the questionnaires collected directly after inclusion (baseline). Data extraction at baseline, including the number of GP contacts (year prior to inclusion), was performed in the free text and coded routine care data from the EMR of each GP practice. GP characteristics at T0 and rurality were collected from public Dutch online databases for GP experience.25, 26
Comorbidities, date of diagnosis, cancer stage and treatment decision were extracted from the EMR in the hospital. For comorbidities we registered if a patient had pulmonary disease, cardiovascular disease, endocrine disease, neurological pain, neurological disability, psychological disease, skin disease, locomotor disease, urogenital disease, sensory disease and/or a neoplasm. The moment of treatment decision was defined as the moment the patient agreed with or chose the treatment.
2.12. Sample size
The sample size was based on the primary outcomes of the GRIP study, that is, satisfaction with care and healthcare utilisation at 3 months after the end of therapy (excluding hormone therapy) or maximal of 1 year after T0 if treatment duration exceeded 9 months. We assumed a medium effect size (0.5) to be a relevant difference between the two study groups. Using a power of 0.8 and an alpha less than 0.05, at least 64 patients per study group were required. Accounting for an estimated dropout of 15%, 75 participants in each group were needed. 19
2.13. Statistical analysis
The study population was described descriptively. Intervention effects compared to usual care were analysed following the intention‐to‐treat principle. Additionally, outcomes were described stratified for patients with a TOC before treatment decision (conform protocol), a TOC after treatment decision and no TOC.
Paired sample t‐test was used to calculate mean changes and 95% confidence intervals of self‐efficacy from baseline to T1 within groups. ANOVA was used to calculate between‐groups differences (i.e., intervention vs. control group) at T1 (for all outcomes), adjusted for stratification factors (i.e., hospital and cancer type) and baseline measurements if present. Additional adjustment for comorbidity (none vs. any) was done because of potentially relevant group differences at baseline.
All analyses were performed with IBM SPSS 25.0.0.2 and statistical significance was set at p < 0.05.
3. RESULTS
3.1. Study population
In total, 396 patients were approached for participation in the treating hospital (Figure 1). Sixty‐five patients could not be included, 60 because they did not meet inclusion criteria and 5 because they could not be contacted. Of those invited to participate, 177 patients declined, with main reasons: ‘too much of a burden shortly after diagnosis; and ‘no extra guidance needed’. Finally, 154 patients were randomised to either the intervention (n = 77) or the usual care control group (n = 77) (Table 1). The 154 patients were registered with 119 different GPs from 79 different GP centres.
FIGURE 1.
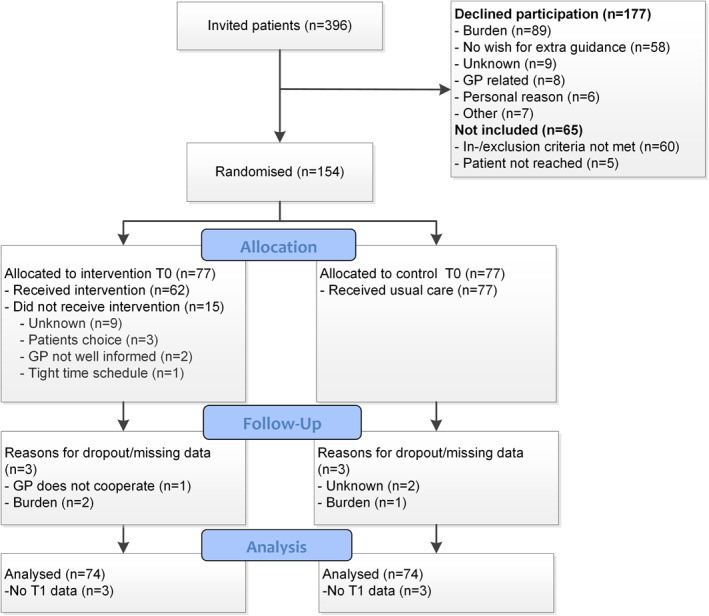
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of the GRIP study after 2 weeks (T1)
TABLE 1.
Baseline characteristics of the study participants, intervention patients divided into groups based on TOC timing
| Intervention (n = 77) | TOC before treatment decision (n = 11) | TOC after treatment decision (n = 51) | No TOC (n = 12) a | Control (n = 77) | |
|---|---|---|---|---|---|
| Female N (%) | 57 (74.0) | 8 (72.7) | 37 (72.5) | 10 (83.3) | 58 (75.3) |
| Age mean (±SD) | 61.8 (11.4) | 62.4 (8.7) | 61.4 (11.0) | 61.3 (15.6) | 59.3 (12.2) |
| Cancer type N (%) | |||||
| Breast | 38 (49.4) | 6 (54.5) | 24 (47.1) | 8 (66.7) | 40 (51.9) |
| Colorectal | 20 (26.0) | 4 (36.4) | 14 (27.5) | 2 (16.7) | 18 (23.4) |
| Melanoma | 13 (16.9) | ‐ | 9 (17.6) | 2 (16.7) | 11 (14.3) |
| Lung | 3 (3.9) | ‐ | 3 (5.9) | ‐ | 2 (2.6) |
| Gynaecologic | 3 (3.9) | 1 (9.1) | 1 (2.0) | ‐ | 6 (7.8) |
| Hospital setting N (%) | |||||
| Academic | 22 (28.6) | 6 (54.5) | 13 (25.5) | 2 (16.7) | 24 (31.2) |
| Non‐academic | 55 (71.4) | 5 (45.5) | 38 (74.5) | 10 (83.3) | 53 (68.8) |
| Cancer stage b N (%) | |||||
| 0 | 2 (2.6) | ‐ | 2 (3.9) | ‐ | 2 (2.6) |
| I | 34 (44.2) | 4 (36.4) | 21 (41.2) | 7 (58.3) | 34 (44.2) |
| II | 22 (28.6) | 2 (18.2) | 15 (29.4) | 4 (33.3) | 27 (35.1) |
| III | 18 (23.4) | 5 (45.5) | 12 (23.5) | 1 (8.3) | 14 (18.2) |
| IV | 1 (1.3) | ‐ | 1 (2.0) | ‐ | ‐ |
| Education | |||||
| Low | 32 (41.6) | 5 (45.5) | 20 (39.2) | 5 (41.7) | 25 (32.5) |
| Middle | 13 (16.9) | 1 (9.1) | 10 (19.6) | 2 (16.7) | 18 (23.4) |
| High | 32 (41.6) | 5 (45.5) | 21 (41.2) | 5 (41.7) | 34 (44.2) |
| Number of comorbidities (N %) | |||||
| None | 25 (32.5) | 5 (45.5) | 15 (29.4) | 5 (41.7) | 39 (50.6) |
| ≥1 | 52 (67.5) | 6 (54.5) | 36 (70.6) | 7 (58.3) | 38 (49.4) |
| Number of GP contacts (year prior inclusion) median (Q1–Q3) | 7 (4.0–0.0) | 7 (3.0–10.0) | 6 (3.0–9.0) | 8 (6.0–12.3) | 6 (3.5–11.0) |
| Perceived self‐efficacy (PEPPI‐5) mean (±SD) | 21.0 (±3.3) | 21.3 (±2.4) | 21.2(±3.0) | 21.5 (±3.8) | 21.5 (±3.0) |
| GP years of working experience median (Q1–Q3) | 17 (12.0–25.5) | 26 (10.0–34.0) | 16 (12.0–22.0) | 20 (12.3–27.5) | 16 (10.5–24.5) |
| GP setting N (%) | |||||
| Urban c | 51 (66.2) | 7 (63.6) | 36 (70.6) | 6 (50) | 45 (58.4) |
| Between rural and urban d | 14 (18.2) | 1 (9.1) | 9 (17.6) | 3 (25) | 15 (19.5) |
| Rural e | 12 (15.6) | 3 (27.3) | 6 (11.8) | 3 (25) | 17 (22.1) |
Abbreviations: SD, standard deviation; Q1, Interquartile range at 25%; Q3, Interquartile rage at 75%; TOC, Time Out consultation; PEPPI, Perceived Self‐Efficacy in Patient–Physician Interactions.
Excluding lost to follow‐up, n = 3.
Stage based on clinical TNM classifications.
≥1000 addresses per km2.
1000–1500 addresses per km2.
≤1000 addresses per km2.
Patients in the intervention and control group were comparable with respect to baseline characteristics, except for the proportion of patients with comorbidities, which was higher in the intervention group (67.5%) as compared to the control group (49.4%) (Table 1). The majority of patients had either breast (51%) or colorectal (25%) cancer. Most patients (75%) were female, and the mean age was 61 (SD ± 11.9 years).
Most GPs of the study population worked in an urban setting (62%) and had a median work experience of 16 years (IQR 11–25.3).
3.2. Implementation of Time Out consultation
In the intervention group, 80.5% (n = 62) of the patients had a TOC (a GP consultation that included the elements of the TOC). However, only 17.7% (n = 11) had the TOC scheduled according to protocol, that is, between diagnosis and final treatment decision.
The median time from diagnosis to TOC was 7 days (IQR 6–12) in the 11 patients in whom the TOC could be scheduled according to protocol and 16 days (IQR 11–23) if the TOC was planned after the treatment decision. The median time from diagnosis to treatment decision was 13 days (IQR 8–14) for those with a TOC before treatment decision, 5 days (IQR 1–7) for those with a TOC after the treatment decision and 5 days (IQR 0.50–9.75) for patients without a TOC. In the intervention group, 22% (n = 17) of the patients received the diagnosis and treatment decision on the same day, and 51% (n = 39) within 7 days. In the control group, the median number of days between diagnosis and treatment decision was 6 days (IQR 2–10).
GP consultations (including non‐TOC) within 2 weeks after diagnosis took place in 53.2% (n = 41) of the patients in the intervention group and in 33.8% (n = 26) of the control group. Potential contamination (i.e., a GP having seen an intervention patient before seeing a patient from the control arm) occurred in two patients in the control arm.
3.3. Perceived shared decision‐making
Perceived SDM was significantly lower in the intervention group compared to usual care (between‐group difference: 8.9 [95% CI: 0.6–17.1]) (Table 2). Additional adjustment for comorbidity yielded a comparable non‐significant between‐group difference (8.4 [95% CI: −0.0–16.8]). In the 11 intervention patients with a TOC planned according to protocol, perceived SDM was comparable to the control group 66.5 (±27.2) versus 67.9 (±26.1), respectively.
3.4. Received information
Levels of perceived information provision in the two study arms did not differ for all topics: ‘Disease’, ‘Medical tests’, ‘Treatment’, ‘Other services’, ‘Places of care’, ‘Self‐help’, ‘Satisfaction with the amount of information’ and ‘Helpfulness of information’ (Table 2).
3.5. Self‐efficacy
Self‐efficacy in the intervention group improved significantly from baseline to T1, with a mean difference of 1.1 (95% CI, 0.4‐1.8). For the control group this within mean difference was 0.5 (95% CI, ‐0.1‐1.2). No significant between group difference was found: 0.4 (95% CI, ‐0.4‐1.1) (Table 2).
4. DISCUSSION
This study aimed to evaluate the effects of a TOC with a GP shortly after a cancer diagnosis for patients scheduled to be treated with curative intent, on perceived SDM, received information and perceived self‐efficacy. Although the TOC was well accepted by patients (80.5% did make an appointment with the GP after diagnosis), only one‐fifth was adequately planned, that is, before a treatment, decision was made in the treating hospital. Therefore, we could not adequately evaluate if there is a benefit from the TOC on the SDM process. A GP consultation post the treatment decision resulted in lower SDM. Moreover, 34% of the patients in the control group had contact with their GP in 2 weeks after diagnosis and 20% indicated that their GP was involved in treatment decision‐making.
It appeared to be challenging to plan a TOC preceding the treatment decision. This can be explained by the fact that current time interval between diagnosis and therapy decision is (too) short. For 22% of the patients, who were mainly patients with breast cancer or melanoma, the treatment decision was made on the day of the diagnosis. For half of all patients, a decision was made within 7 days. The assumption that a short time to decision hampers TOC planning according to protocol is supported by the observation that the time between diagnosis to therapy decision was short (median 5 days) for those patients who had the TOC after treatment decision. Also, participating clinicians report that the current cancer care pathway is focused on rapid diagnostics 27 and early start of treatment. Delayed TOC planning in this study may also be partly related to the time required for patients to consider study participation. Finally, delayed TOC planning may also be related to the pragmatic design of our study: instead of the research team or the hospital scheduling the TOC for the patient, we decided to leave this responsibility to the patient, thus reflecting current daily care practice. In the short and stressful period between diagnosis and therapy choice, scheduling a TOC may not have been feasible for the majority of patients.
Our results show that perceived SDM was lower if a TOC was planned after the treatment decision. The most likely explanation is that patients perceive SDM more negatively if they are informed and coached on the added value and possibility of SDM, after the possibility to actually apply SDM has already passed.
Compared to the literature, the number of patient‐initiated GP contacts after diagnosis was high. In previous studies, which aimed to involve the GP in cancer care, the uptake of interventions was generally between 27% and 60%, as compared to more than 80% in our intervention group.28, 29, 30 Even though we did not find a beneficial effect on the SDM process, the TOC may have an effect on the second aim of the TOC: continuity of primary care. On the short term, patients visited their GP more often in the intervention arm compared to the control arm. Results on continuity of primary care along the cancer care continuum will be published elsewhere.
4.1. Study limitations
This study has several strengths and limitations. The present study contributes evidence from a pragmatic, well‐powered randomised controlled trial to the scarce knowledge on SDM interventions for curative cancer treatment involving the GP. Another strength is the full access to the free text and coded routine care data from the EMR of each general practitioner practice; therefore, adherence could be assessed. However, we did not record the TOC, and therefore, we were not able to assess the compliance to the detailed intervention delivery across GPs. Therefore, future studies should consider the option to record the TOC. In addition, future studies could evaluate the role of the GP in monitoring and evaluation of cancer treatment. Another limitation is that breast cancer patients are over‐represented, which might make the results less generalisable to the total cancer patient population. 31 Over‐representation of breast cancer is often seen in cancer research, 32 probably due to the high incidence of breast cancer, and the fact that the breast cancer care path is usually highly structured, which facilitates recruitment. Also, our study focuses on cancer patients treated with curative intent and findings cannot be generalised to those treated with palliative intent, because the SDM process and the added value of the GP may well be different. This is supported by a recent non‐controlled study, which suggested that patients and health care workers (GPs and treating physicians) experienced improvement in the SDM process after implementing a similar TOC, among palliatively treated cancer patients. 33 One reason for a potential difference in effect is that curatively treated patients might not always experience having a treatment choice.34, 35 In addition, 66 (19.3%) of the eligible patients were not included in our study because they expressed ‘no wish for extra guidance’ or ‘GP‐related’ reasons. This selection resulted in a study population whose wish for additional contacts with their GP may be relatively strong. Furthermore, patients and healthcare providers could not be blinded due to the nature of the intervention, which might have influenced the outcomes, but the healthcare providers were not actively informed about group allocation. We did not collect information on whether the specialists in the hospital treated patients in both groups. We assume that randomisation accounts for it, but we cannot exclude an imbalance. Moreover, we were not able to assess which actor or actors delayed the planning of the TOC. In addition, we cannot exclude that the GP provided contradicting information on the treatment decision. Last, during the development of the intervention, we involved the NFK and the participating general practitioners, but hospital care professionals had less input in the development of the intervention, which may have hampered implementation of the TOC.
4.2. Clinical implications
The clinical implications of this study are not easy to define. Our study demonstrated that in the present cancer care continuum, it is logistically difficult to adequately plan a TOC in primary care between diagnosis and treatment. This seems mainly due to the urgency to start treatment after a cancer diagnosis. Besides hampering TOC implementation, this perceived urgency may impede the potential to reflect on the optimal therapy choice by obstructing the deliberation process. This study also showed that the majority of patients were motivated to consult the GP in preparation for the final treatment decision with the specialist. Hence, to evaluate the effects of a TOC, the planning of the TOC needs to be optimised. An important message from this study is that to ensure that the TOC is effectively incorporated in the decision process, the hospital team should initiate the planning of the TOC with the GP. Moreover, to optimally involve the GP in shared decision‐making in cancer care, the GP should participate in multidisciplinary team meetings.
5. CONCLUSION
In conclusion, planning a TOC in primary care between diagnosis and treatment decision for cancer patients treated with curative intent was challenging due to the short time between diagnosis and treatment choice. Although patients' acceptance was high, given the high uptake of the intervention, the majority of TOC in our study was planned after the treatment decision has already been made. Effects of a timely TOC could therefore not be established. Non‐timely TOC decreased perceived SDM. Planning of the TOC should be optimised, and future research should establish if adequately timed TOC result in improved shared decision‐making in cancer patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Niek J. de Wit, Elsken van der Wall, Anne M. May and Charles W. Helsper designed the GRIP trial. Thijs van Dalen, Marc A.M.T. Verhagen, Arjen J. Witkamp, Ron Koelemi, Annebeth E. Flinterman, Eleonora B.L. van Dorst, Kim A.B.M. Pruissen‐Peeters, Leon M.G. Moons, Franz M.N.H. Schramel, Marcel T.M. van Rens and Miranda F. Ernst conducted the selection and inclusion of patients. Ietje A.A. Perfors and Eveline A. Noteboom performed the trial execution, the data analysis and drafted the paper, under supervision of Niek J. de Wit, Elsken van der Wall, Anne M. May and Charles W. Helsper. All authors read, revised and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank all included patients for their participation and all primary care and hospital employees who contributed or assisted in conducting the GRIP trial. In addition, we thank the medical students who assisted in the data collection of the GRIP trial. The GRIP study is financially supported by the Danone Ecosystem Fund. The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review,or approval of the manuscript; and decision to submit the manuscript for publication.
DATA AVAILABILITY
The data generated in this study are available from the corresponding author on reasonable request.
References
- 1. Worldwide Cancer Incidence Statistics: Cancer Research UK. 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer/incidence. [Cited October 2, 2019]. [Google Scholar]
- 2. Signaleringscommissie . Nazorg bij kanker: de rol van de eerstelijn. Amsterdam: KWF Kankerbestrijding; 2011. https://docplayer.nl/10326502‐Nazorg‐bij‐kanker‐de‐rol‐van‐de‐eerste‐lijn‐signaleringscommissie‐kanker‐van‐kwf‐kankerbestrijding.html [Google Scholar]
- 3. Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88(3):653‐663. [DOI] [PubMed] [Google Scholar]
- 4. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337‐350. [DOI] [PubMed] [Google Scholar]
- 5. Hurtaud A, Aubin M, Ferrat E, et al. Continuity of care in general practice at cancer diagnosis (COOC‐GP study): a national cohort study of 2853 patients. Br J Gen Pract. 2019;69(679):e88‐e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231‐1272. [DOI] [PubMed] [Google Scholar]
- 7. Meiklejohn JA, Mimery A, Martin JH, et al. The role of the GP in follow‐up cancer care: a systematic literature review. J Cancer Surviv. 2016;10(6):990‐1011. [DOI] [PubMed] [Google Scholar]
- 8. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. Br Med J. 2007;335(7609):24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coulter A, Ellins J. Patient‐Focused Interventions: A Review of the Evidence. London: Health Foundation; 2006. https://www.health.org.uk/sites/default/files/PatientFocusedInterventions_ReviewOfTheEvidence.pdf [Google Scholar]
- 12. Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014(1):CD001431. [DOI] [PubMed] [Google Scholar]
- 13. Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7:CD006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kennedy AD, Sculpher MJ, Coulter A, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. J Am Med Assoc. 2002;288(21):2701‐2708. [DOI] [PubMed] [Google Scholar]
- 15. Brom L, Hopmans W, Pasman HRW, Timmermans DR, Widdershoven GA, Onwuteaka‐Philipsen BD. Congruence between patients’ preferred and perceived participation in medical decision‐making: a review of the literature. BMC Med Inform Decis Mak. 2014;14(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh JA, Sloan JA, Atherton PJ, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16(9):688. [PMC free article] [PubMed] [Google Scholar]
- 17. Berger AM, Buzalko RJ, Kupzyk KA, Gardner BJ, Djalilova DM, Otte JL. Preferences and actual chemotherapy decision‐making in the greater plains collaborative breast cancer study. Acta Oncol. 2017;56(12):1690‐1697. [DOI] [PubMed] [Google Scholar]
- 18. Kankerpatiënt wil meer steun huisarts bij maken van keuzes. NFK (Dutch Federation of Cancer Patient Organisations). 2019. https://nfk.nl/nieuws/kankerpatient-wil-meer-steun-huisarts-bij-maken-van-keuzes. [Cited October 2, 2019]. [Google Scholar]
- 19. Perfors IAA, Helsper CW, Noteboom EA, van der Wall E, de Wit NJ, May AM. Randomised controlled trial protocol (GRIP study): examining the effect of involvement of a general practitioner and home care oncology nurse after a cancer diagnosis on patient reported outcomes and healthcare utilization. BMC Cancer. 2018;18(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shepherd HL, Barratt A, Trevena LJ, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross‐over trial. Patient Educ Counsel. 2011;84(3):379‐385. [DOI] [PubMed] [Google Scholar]
- 21. Kriston L, Scholl I, Hölzel L, Simon D, Loh A, Härter M. The 9‐item Shared Decision Making Questionnaire (SDM‐Q‐9). Development and psychometric properties in a primary care sample. Patient Educ Counsel. 2010;80(1):94‐99. [DOI] [PubMed] [Google Scholar]
- 22. Arraras JI, Greimel E, Sezer O, et al. An international validation study of the EORTC QLQ‐INFO25 questionnaire: an instrument to assess the information given to cancer patients. Eur J Cancer. 2010;46(15):2726‐2738. [DOI] [PubMed] [Google Scholar]
- 23. Flammer A. Self‐efficacy. In: Smelser Smelser NJ, Baltes B, eds. International Encyclopedia of the Social & Behavioral Sciences. Elsevier; 2015:504‐508. [Google Scholar]
- 24. ten Klooster PM, Oostveen JCM, Zandbelt LC, et al. Further validation of the 5‐item perceived efficacy in patient–physician Interactions (PEPPI‐5) scale in patients with osteoarthritis. Patient Educ Counsel. 2012;87(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 25. Vektis Openbaar AGB register. https://www.agbcode.nl/Webzoeker/Zoeken. [Cited October 2, 2019]. [Google Scholar]
- 26. CBS Stedelijkheid per postcode. https://www.cbs.nl/nl-nl/maatwerk/2016/41/stedelijkheid-woz-en-uitkeringen-per-postcode-2014. [Cited October 2, 2019]. [Google Scholar]
- 27. Schramel FMNH, Epping A, Van De Groep J. De snelheidsduivel. Medisch Contact. 2005:1356‐1357. [Google Scholar]
- 28. Drury M, Yudkin P, Harcourt J, et al. Patients with cancer holding their own records: a randomised controlled trial. Br J Gen Pract. 2000;50(451):105‐110. [PMC free article] [PubMed] [Google Scholar]
- 29. Bergholdt SH, Hansen DG, Larsen PV, Kragstrup J, Søndergaard J. A randomised controlled trial to improve the role of the general practitioner in cancer rehabilitation: effect on patients’ satisfaction with their general practitioners. BMJ Open. 2013;3(7):e002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luker K, Beaver K, Austin L, Leinster SJ. An evaluation of information cards as a means of improving communication between hospital and primary care for women with breast cancer. J Adv Nurs. 2000;31(5):1174‐1182. [DOI] [PubMed] [Google Scholar]
- 31. Hudson SV, Ohman‐Strickland PA, Bator A, et al. Breast and prostate cancer survivors’ experiences of patient‐centered cancer follow‐up care from primary care physicians and oncologists. J Cancer Surviv. 2016;10(5):906‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glynn RW, Chin JZ, Kerin MJ, Sweeney KJ. Representation of cancer in the medical literature‐a bibliometric analysis. PLoS One. 2010;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noteboom EA, de Wit NJ, van Asseldonk I, et al. Off to a good start after a cancer diagnosis: implementation of a time out consultation in primary care before cancer treatment decision. J Cancer Survivorship. 2020;14(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beaver K, Jones D, Susnerwala S, et al. Exploring the decision‐making preferences of people with colorectal cancer. Health Expect. 2005;8(2):103‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirpara DH, Cleghorn MC, Sockalingam S, Quereshy FA. Understanding the complexities of shared decision‐making in cancer: a qualitative study of the perspectives of patients undergoing colorectal surgery. Can J Surg. 2016;59(3):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are available from the corresponding author on reasonable request.


