Abstract
Our study aimed to provide a comprehensive overview of trends in incidence, survival, mortality and treatment of first primary invasive breast cancer (BC), according to age, stage and receptor subtype in the Netherlands between 1989 and 2017. Data from all women diagnosed with first primary stage I to IV BC (N = 320 249) were obtained from the Netherlands Cancer Registry. BC mortality and general population data were retrieved from Statistics Netherlands. Age‐standardised incidence and mortality rates were calculated with annual percentage change (APC) and average annual percentage change (AAPC) statistics. The relative survival (RS) was used as estimator for disease‐specific survival. The BC incidence for all BC patients combined significantly increased until 2013 from 126 to 158 per 100 000 person‐years, after which a declining trend was observed. Surgery became less extensive, but (neo‐)adjuvant systemic treatments and their combinations were given more frequently. The RS improved for all age groups and for most stages and receptor subtypes, but remained stable for all subtypes since 2012 to 2013 and since 2000 to 2009 for Stage IV BC at 15 years of follow‐up. Overall, the 5‐ and 10‐year RS increased from 76.8% (95% confidence interval [CI]: 76.1, 77.4) and 55.9% (95% CI: 54.7, 57.1) in 1989 to 1999 to 91.0% (95% CI: 90.5, 91.5) and 82.9% (95% CI: 82.2, 83.5), respectively, in 2010 to 2016. BC mortality improved regardless of age and overall decreased from 57 to 35 per 100 000 person‐years between 1989 and 2017. In conclusion, the BC incidence in the Netherlands has steadily increased since 1989, but the latest trends show promising declines. Survival improved markedly for most patients and the mortality decreased regardless of age.
Keywords: breast cancer, incidence, mortality, survival, treatment
Short abstract
What's new?
Studies that simultaneously capture incidence, survival, and mortality trends in breast cancer are scarce, and receptor subtype‐specific trends have remained largely unexplored. This study provides an up‐to‐date and comprehensive overview of first primary invasive breast cancer trends in the Netherlands in 1989 to 2017. Breast cancer incidence increased for all breast cancer patients combined until 2013, but the latest trends show a promising decline. Treatment strategies became more complex. Relative survival improved for all age groups and for most stages and receptor subtypes, and mortality decreased overall. The results may be useful in supporting healthcare management and informing current clinical practice.
Abbreviations
- AAPC
average annual percentage change
- APC
annual percentage change
- BC
breast cancer
- BCS
breast‐conserving surgery
- CI
confidence interval
- ER
oestrogen receptor
- ER+
oestrogen receptor positive
- ESP
European standard population
- ESR
European standardised rates
- HER2
human epidermal growth factor receptor 2
- HER2‐
human epidermal growth factor receptor 2 negative
- HER2+
human epidermal growth factor receptor 2 positive
- HR
hormone receptor
- ICD‐O
International Classification of Diseases for Oncology
- IHC
Immunohistochemistry
- IKNL
Netherlands Comprehensive Cancer Organisation
- NCR
Netherlands Cancer Registry
- PALGA
National Pathology Archive
- PR
progesterone receptor
- PR+
progesterone receptor positive
- PY
person‐years
- RS
relative survival
- TNM
tumour node metastasis
- UICC
Union for International Cancer Control
1. INTRODUCTION
Breast cancer (BC) is the most common cancer and leading cause of cancer‐related death among women in most countries worldwide. 1 It accounts for almost one in four cancers (24.2%) in women, with an estimated 2.1 million new cases globally in 2018. 1 The incidence of BC has been rising for decades in most developed countries and is expected to continue to rise. 1 Meanwhile, mortality rates have been steadily decreasing in most European, American and other high‐income countries, while weak‐to‐moderate increases in mortality have been observed in some lower‐to‐middle income countries. 2 , 3 , 4 Worldwide, BC is responsible for 15.0% of all cancer‐related deaths in women, with an estimated 627 000 deaths in 2018. 1 However, BC survival has improved significantly in recent decades for all age groups in most countries. 4
The rising trends in BC incidence are attributed to the increased presence of known risk factors, including early age at menarche, late age at menopause, low parity, nulliparity, not breastfeeding, use of oral contraceptives, hormone replacement therapy and older age at first childbearing. 5 , 6 Other factors that have been implicated to influence BC incidence include changes in lifestyle factors such as excessive alcohol intake, increasing prevalence of obesity and a decrease in physical activity. 5 , 7 , 8 Moreover, screening programmes could influence incidence, but can also influence stage distribution and improvements in BC survival and eventually mortality. 4 Improvement in survival could also be explained by earlier detection outside screening, improvements in treatment, access to appropriate healthcare and increasing disease awareness. 4 , 5
In the Netherlands, incidence, survival and mortality trends of BC are generally comparable to those observed globally, as shown by various studies. 9 , 10 , 11 , 12 , 13 However, studies describing and interpreting these endpoints simultaneously are scare, and many of the currently available trend studies in the Netherlands or elsewhere in Europe are no longer up to date. Additionally, receptor subtype‐specific trends have remained largely unexplored, while these subtypes have become increasingly important in recent years as targets of new personalised ([neo‐]adjuvant) treatment strategies. 13 , 14 , 15 Comprehensive trend analyses are useful for medical doctors to better inform patients about their disease and are of great interest to breast cancer researchers, policy makers and patient advocates. Therefore, this study aimed to provide an up‐to‐date and comprehensive overview of first primary invasive breast cancer trends in incidence, treatment, survival and mortality in the Netherlands between 1989 and 2017. Trend evaluation was performed for all BC patients combined and stratified by age group, stage and receptor subtype.
2. MATERIALS AND METHODS
2.1. Data sources
Data from all women aged ≥18 years, diagnosed with tumour, node and metastasis (TNM) Stages I to IV first primary invasive BC between 1989 and 2017, were obtained from the nationwide population‐based Netherlands Cancer Registry (NCR), hosted by the Netherlands Comprehensive Cancer Organisation (IKNL). The NCR contains records on pathologically confirmed cancers after notification by the National Pathology Archive (PALGA). Yearly linkage with the national discharge register data ensures high completeness. All tumours in the registry are coded according to the International Classification of Diseases for Oncology (ICD‐O). Patient‐, tumour‐ and treatment‐related characteristics were collected from medical records from all Dutch hospitals by trained tumour registrars from the NCR. Information on vital status and date of death is regularly obtained through linkage with the Dutch Municipal Personal Records database and was updated until January 31, 2018. Data on invasive BC mortality cases and data on the general Dutch female population were obtained from Statistics Netherlands. 16 , 17
2.2. Tumour stage, receptor subtype and treatment
The Union for International Cancer Control (UICC) TNM classification of malignant tumours was used to categorise BC stage. From 1989 to 2017, various editions have been introduced, ranging from the fourth to the eighth edition, and resulted in changes in the definition of tumour stage. 18 Most noticeably, going from the fifth to the sixth edition in 2003, a shift from Stage II to Stage III BC occurred as tumours with more than three positive lymph nodes were categorised as Stage III according to the sixth edition, whereas they were previously categorised as Stage II disease. All tumours were classified according to the TNM classification valid at the date of diagnosis. If pathological stage was missing, clinical stage was used.
Oestrogen receptor (ER) status and progesterone receptor (PR) status were determined by immunohistochemistry (IHC) and were actively registered by the NCR since 2005. Tumours were defined as ER/PR‐positive (ER+/PR+) when >10% of the tumour cells stained positive (from 2011 the threshold was ≥10%). Human epidermal growth factor receptor 2 (HER2) was introduced and registered since 2006. Tumours were defined HER2‐positive (HER2+) if IHC was 3+ (at least 10% of cells showed strong intensity membrane staining) or when confirmed positive with in situ hybridization (FISH/CISH). HER2‐negativity (HER2−) was declared by IHC when less than 10% of the cells showed membrane staining or when FISH/CISH test outcome was negative. Tumours with IHC 2+ without FISH/CISH confirmation available were considered unknown. For the analyses, we grouped receptor subtypes into: hormone receptor (HR)+/HER2− (eg, ER+ and/or PR+ and HER2−), HR+/HER2+ (ie, ER+ and/or PR+ and HER2+), HR−/HER2+ (ie, ER−/PR−/HER2+) and HR−/HER2− (ie, ER−/PR−/HER2−).
Treatment data on surgery, radiotherapy, chemotherapy and endocrine therapy were included in the NCR since 1989 on an aggregated level. Types of chemotherapy (eg, taxane‐based and/or anthracycline‐based) and endocrine therapy (eg, tamoxifen and/or aromatase inhibitors) were specified by the NCR since 2003. Targeted therapy was included in the NCR since 2005 and almost exclusively existed of trastuzumab (~99%). Treatment proportions were determined based on specific treatments received by patients at any time during their treatment process, irrespective of duration or whether it was completed. Type of endocrine therapy (tamoxifen and/or aromatase inhibitors) was specified based on the first administered treatment, as information on treatment in the NCR was only available up to 1 year after diagnosis.
2.3. Statistical analyses
Annual crude and age‐standardised incidence and mortality rates for the period 1989 to 2017 were calculated per 100 000 person‐years (PY) using the general population size, as obtained from Statistics Netherlands, as person‐time denominator. 19 Crude rates were calculated as 3‐year moving averages with 2‐year moving averages calculated at both ends of the study period and rates were age‐standardised (European Standardised Rates, ESR) to the 2013 European Standard Population 95+ (2013 ESP 95+). 20 , 21
Trend changes over time were evaluated with joinpoint regression analyses, with each model representing a series of connected straight lines on a log scale and with each joinpoint denoting a statistically significant change in trends. Annual percentage changes (APCs) were determined for each trend segment and provide an overview of all trend changes over time. The average annual percentage change (AAPC) provides a good summary measure of the overall trend and was determined over the whole period. 22 , 23 Both APCs and AAPCs were calculated from the slope coefficients of the underlying joinpoint models and were determined with the freely available Joinpoint Regression Program version 4.7.0.0 and based on the previously determined age‐standardised incidence and mortality rates. 24 Two‐sided significance was determined at an α = 0.05 level. Analyses were performed using the “Uncorrelated Error Model” and the “Grid Search Method” setting, with the number of points placed between observed X‐values set at 3. For model selection, the recommended Bayesian Information Criteria 3 method was used. 23 The minimum allowed number of joinpoints was set at zero. The maximum allowed number of joinpoints to be tested was based on the algorithmic recommendation table included in the Joinpoint help manual 4.7.0.0 (available at https://surveillance.cancer.gov/joinpoint), allowing a maximum of five joinpoints for overall, age‐ and stage‐specific rates and a maximum number of two joinpoints for the subtype‐specific rates. The parametric method was used to calculate 95% confidence intervals (CI). Further programme parameters were kept at their default settings.
The relative survival (RS) was used as an estimator of disease‐specific survival and is the ratio between the observed survival of the patients and the expected survival in the general Dutch population, matched by attained age, sex and calendar year. Expected survival was determined using nationwide lifetables of the general Dutch population adapted from Statistics Netherlands, containing survival probability data of women aged 0 to 99 years in 1989 to 2018. Outcomes were age‐standardised using the traditional method with cumulative weights based on the age distribution in the 2013 ESP 95+. 20 Used weights were 0.47, 0.14, 0.30 and 0.09 for the <40, 40 to 49, 50 to 74, and ≥75 age groups, respectively. 25 The RS was calculated using the Ederer II approach. 26 Brenner's period analysis was used to derive more up‐to‐date estimates of the RS by exclusively considering the survival time data of patients during a (recent) time period of interest by left‐truncating all observations at the start of the time period and right‐censoring them at its end. This is in contrast with the traditional cohort methodology, which provides outdated long‐term survival estimates based on patients that were diagnosed many years ago without consideration of ongoing improvements. A more detailed description of the period analysis methodology is provided elsewhere. 27 End of follow‐up was defined as year of death, year of emigration or 2016, whichever came first. We limited survival analyses to 2016 to avoid potential overestimation of long‐term survival outcomes following period analyses. 27
All data analyses were performed using the Stata Software Package, version 14.2, and are presented for all BC patients combined and stratified by age group (<40, 40‐49, 50‐74, and ≥75), stage and receptor subtype when sample size allowed. Women with unavailable treatment data (eg, due to not receiving any treatment or incomplete registration) were excluded. To overcome difficulties in trend recognition over time due to the changes in tumour stage classification, Stages II and III BC were analysed individually as well as grouped together. Cut‐off points for the age groups were based on the age at invitation to the current Dutch national mammographic screening programme (50‐74 years), with younger and older women grouped separately.
3. RESULTS
3.1. Study population
In total, 320 249 women were diagnosed with first primary invasive BC in the Netherlands between 1989 and 2017 and of all women who died (N = 2 027 353) during this period, 97 187 died from BC (4.8%). The median age at diagnosis was 61 years (range 18‐107 years). All population characteristics are presented in Table 1. Data on the yearly number of BC deaths are included in Supplementary Table S1.
TABLE 1.
Patient, tumour and treatment characteristics of all women diagnosed with first primary invasive breast cancer in the Netherlands between 1989 and 2017
| Period of diagnosis | 1989 to 1992 | 1993 to 1996 | 1997 to 2000 | 2001 to 2004 | 2005 to 2008 | 2009 to 2012 | 2013 to 2017 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Age at diagnosis | ||||||||||||||||
| Median | 61.7 | 61.0 | 60.1 | 59.2 | 59.7 | 60.5 | 61.4 | 60.5 | ||||||||
| Range | 21.8 to 100.3 | 20.4 to 107.3 | 18.7 to 104.2 | 18.7 to 100.4 | 18.9 to 102.1 | 19.2 to 103.1 | 18.5 to 103.8 | 18.5 to 107.3 | ||||||||
| <40 | 2364 | 7.3 | 2463 | 6.6 | 2519 | 6.2 | 2829 | 6.4 | 2682 | 5.7 | 2660 | 5.2 | 3378 | 5.1 | 18 895 | 5.9 |
| 40 to 49 | 6085 | 18.8 | 7028 | 18.8 | 7639 | 18.8 | 8423 | 18.9 | 8986 | 19.0 | 9160 | 17.9 | 10 880 | 16.3 | 58 201 | 18.2 |
| 50 to 74 | 17 547 | 54.1 | 20 492 | 54.8 | 22 223 | 54.7 | 24 949 | 56.0 | 27 385 | 57.9 | 30 789 | 60.3 | 41 930 | 62.7 | 185 315 | 57.9 |
| ≥75 | 6440 | 19.9 | 7444 | 19.9 | 8265 | 20.3 | 8333 | 18.7 | 8215 | 17.4 | 8450 | 16.5 | 10 691 | 16.0 | 57 838 | 18.1 |
| Tumour stage | ||||||||||||||||
| I | 9992 | 31.4 | 13 348 | 36.6 | 14 972 | 37.5 | 16 558 | 37.6 | 18 848 | 40.1 | 22 816 | 44.8 | 31 212 | 46.7 | 127 746 | 40.3 |
| II | 16 505 | 51.8 | 17 863 | 49.0 | 19 430 | 48.7 | 20 107 | 45.7 | 19 114 | 40.7 | 19 046 | 37.4 | 24 688 | 37.0 | 136 753 | 43.1 |
| III | 3282 | 10.3 | 3282 | 9.0 | 3401 | 8.5 | 5211 | 11.8 | 6814 | 14.5 | 6673 | 13.1 | 7255 | 10.9 | 35 918 | 11.3 |
| IV | 2083 | 6.5 | 1996 | 5.5 | 2103 | 5.3 | 2145 | 4.9 | 2236 | 4.8 | 2344 | 4.6 | 3633 | 5.4 | 16 540 | 5.2 |
| Unknown | 574 | 938 | 740 | 513 | 256 | 180 | 91 | 3292 | ||||||||
| Tumour size a | ||||||||||||||||
| T1 | 14 190 | 45.2 | 18 101 | 50.2 | 20 640 | 52.3 | 23 797 | 54.6 | 26 702 | 57.2 | 30 041 | 59.5 | 40 559 | 61.2 | 174 030 | 55.4 |
| T2 | 12 741 | 40.6 | 13 491 | 37.4 | 14 387 | 36.5 | 15 852 | 36.3 | 16 264 | 34.9 | 16 587 | 32.9 | 20 126 | 30.3 | 109 448 | 34.9 |
| T3 | 1617 | 5.1 | 1608 | 4.5 | 1616 | 4.1 | 1738 | 4.0 | 1886 | 4.0 | 2125 | 4.2 | 3346 | 5.0 | 13 936 | 4.4 |
| T4 | 2866 | 9.1 | 2835 | 7.9 | 2820 | 7.1 | 2232 | 5.1 | 1793 | 3.8 | 1737 | 3.4 | 2291 | 3.5 | 16 574 | 5.3 |
| Unknown | 1022 | 1392 | 1183 | 915 | 623 | 569 | 557 | 6261 | ||||||||
| Lymph node status a | ||||||||||||||||
| N0 | 17 679 | 56.8 | 21 621 | 60.2 | 23 032 | 59.0 | 24 682 | 57.1 | 27 959 | 60.1 | 31 518 | 62.3 | 44 087 | 66.3 | 190 578 | 60.9 |
| N1 | 12 708 | 40.8 | 13 640 | 38.0 | 15 427 | 39.5 | 15 247 | 35.3 | 12 959 | 27.8 | 13 849 | 27.4 | 17 742 | 26.7 | 101 572 | 32.5 |
| N2 | 659 | 2.1 | 590 | 1.6 | 528 | 1.4 | 2125 | 4.9 | 3440 | 7.4 | 3052 | 6.0 | 2545 | 3.8 | 12 939 | 4.1 |
| N3 | 104 | 0.3 | 49 | 0.1 | 59 | 0.2 | 1165 | 2.7 | 2199 | 4.7 | 2183 | 4.3 | 2159 | 3.2 | 7918 | 2.5 |
| Unknown | 1286 | 1527 | 1600 | 1315 | 711 | 457 | 346 | 7242 | ||||||||
| Histological grade b | ||||||||||||||||
| Grade 1 | 1022 | 8.1 | 1611 | 10.3 | 4248 | 16.1 | 6988 | 19.4 | 9196 | 22.7 | 10 295 | 24.3 | 14 031 | 24.5 | 47 391 | 20.6 |
| Grade 2 | 4207 | 33.5 | 5824 | 37.3 | 11 179 | 42.3 | 16 202 | 45.0 | 18 032 | 44.5 | 19 275 | 45.6 | 28 176 | 49.2 | 102 895 | 44.6 |
| Grade 3 | 7330 | 58.4 | 8163 | 52.3 | 10 997 | 41.6 | 12 833 | 35.6 | 13 249 | 32.7 | 12 723 | 30.1 | 15 027 | 26.3 | 80 322 | 34.8 |
| Unknown | 19 877 | 21 829 | 14 222 | 8511 | 6791 | 8766 | 9645 | 89 641 | ||||||||
| ER‐status | ||||||||||||||||
| Positive | 37 675 | 81.7 | 41 961 | 83.9 | 55 964 | 84.7 | 135 600 | 83.6 | ||||||||
| Negative | 8433 | 18.3 | 8073 | 16.1 | 10 116 | 15.3 | 26 622 | 16.4 | ||||||||
| Unknown | 1160 | 1025 | 799 | 2984 | ||||||||||||
| PR‐status | ||||||||||||||||
| Positive | 29 126 | 65.9 | 33 193 | 67.5 | 45 736 | 69.3 | 108 055 | 67.8 | ||||||||
| Negative | 15 104 | 34.1 | 16 011 | 32.5 | 20 269 | 30.7 | 51 384 | 32.2 | ||||||||
| Unknown | 3038 | 1855 | 874 | 5767 | ||||||||||||
| HER2‐status | ||||||||||||||||
| Positive | 6435 | 15.3 | 6892 | 14.3 | 8766 | 13.7 | 22 093 | 14.3 | ||||||||
| Negative | 35 617 | 84.7 | 41 291 | 85.7 | 55 143 | 86.3 | 132 051 | 85.7 | ||||||||
| Unknown | 5216 | 2876 | 2970 | 11 062 | ||||||||||||
| Receptor subtype c | ||||||||||||||||
| HR+/HER2− | 30 594 | 73.4 | 35 865 | 74.8 | 48 453 | 75.9 | 114 912 | 74.9 | ||||||||
| HR+/HER2+ | 3929 | 9.4 | 4481 | 9.3 | 5843 | 9.2 | 14 253 | 9.3 | ||||||||
| HR−/HER2+ | 2384 | 5.7 | 2329 | 4.9 | 2908 | 4.6 | 7621 | 5.0 | ||||||||
| HR−/HER2− | 4765 | 11.4 | 5287 | 11.0 | 6652 | 10.4 | 16 704 | 10.9 | ||||||||
| Unknown | 5596 | 3097 | 3023 | 11 716 | ||||||||||||
| Surgery + radiotherapy d | ||||||||||||||||
| BCS + RT | 8480 | 26.1 | 12 749 | 34.1 | 15 315 | 37.7 | 19 608 | 44.0 | 22 796 | 48.2 | 25 494 | 49.9 | 36 977 | 55.3 | 141 419 | 44.2 |
| BCS + no RT | 486 | 1.5 | 781 | 2.1 | 1028 | 2.5 | 847 | 1.9 | 712 | 1.5 | 754 | 1.5 | 1256 | 1.9 | 5864 | 1.8 |
| Mastectomy + RT | 4433 | 13.7 | 5653 | 15.1 | 6041 | 14.9 | 6063 | 13.6 | 5910 | 12.5 | 6276 | 12.3 | 8579 | 12.8 | 42 955 | 13.4 |
| Mastectomy + no RT | 7609 | 23.5 | 13 662 | 36.5 | 14 591 | 35.9 | 14 105 | 31.7 | 13 518 | 28.6 | 13 715 | 26.9 | 13 215 | 19.8 | 90 415 | 28.2 |
| Surgery (unspecified) + RT | 3855 | 11.9 | 500 | 1.3 | 32 | 0.1 | 13 | 0.0 | 5 | 0.0 | 2 | 0.0 | 3 | 0.0 | 4410 | 1.4 |
| Surgery (unspecified) + no RT | 4416 | 13.6 | 610 | 1.6 | 82 | 0.2 | 11 | 0.0 | 3 | 0.0 | 4 | 0.0 | 8 | 0.0 | 5134 | 1.6 |
| No surgery + RT | 730 | 2.3 | 574 | 1.5 | 412 | 1.0 | 395 | 0.9 | 302 | 0.6 | 263 | 0.5 | 295 | 0.4 | 2971 | 0.9 |
| No surgery + no RT | 2427 | 7.5 | 2898 | 7.7 | 3145 | 7.7 | 3492 | 7.8 | 4022 | 8.5 | 4551 | 8.9 | 6546 | 9.8 | 27 081 | 8.5 |
| Chemotherapy | ||||||||||||||||
| Taxane‐containing CT e | 4 | 0.0 | 8 | 0.0 | 68 | 0.2 | 159 | 0.4 | 3589 | 7.6 | 5947 | 11.6 | 3175 | 4.7 | 12 950 | 4.0 |
| Anthracycline‐containing CT f | 6 | 0.0 | 26 | 0.1 | 125 | 0.3 | 3673 | 8.2 | 3299 | 7.0 | 2016 | 3.9 | 1195 | 1.8 | 10 340 | 3.2 |
| Taxane‐ and anthracycline‐ containing CT g | 0 | 0.0 | 0 | 0.0 | 6 | 0.0 | 127 | 0.3 | 2297 | 4.9 | 10 521 | 20.6 | 22 024 | 32.9 | 34 975 | 10.9 |
| CT (other/unspecified) h | 4329 | 13.3 | 5483 | 14.6 | 9849 | 24.2 | 11 245 | 25.3 | 8804 | 18.6 | 4029 | 7.9 | 306 | 0.5 | 44 045 | 13.8 |
| No CT | 28 097 | 86.6 | 31 910 | 85.3 | 30 598 | 75.3 | 29 330 | 65.9 | 29 279 | 61.9 | 28 546 | 55.9 | 40 179 | 60.1 | 217 939 | 68 |
| Endocrine therapy i | ||||||||||||||||
| Tamoxifen first | 18 | 0.1 | 38 | 0.1 | 143 | 0.4 | 9649 | 21.7 | 21 043 | 44.5 | 26 104 | 51.1 | 29 774 | 44.5 | 86 769 | 27.1 |
| Aromatase inhibitors first | 0 | 0.0 | 1 | 0.0 | 2 | 0.0 | 863 | 1.9 | 2335 | 4.9 | 3999 | 7.8 | 8950 | 13.4 | 16 150 | 5.0 |
| Ovarian ablation j | 9 | 0.0 | 91 | 0.2 | 194 | 0.5 | 184 | 0.4 | 118 | 0.2 | 113 | 0.2 | 186 | 0.3 | 895 | 0.3 |
| ET (other/unspecified) k | 9708 | 29.9 | 11 436 | 30.6 | 14 630 | 36.0 | 9145 | 20.5 | 0 | 0.0 | 0 | 0.0 | 4 | 0.0 | 44 923 | 14.0 |
| No endocrine therapy | 22 701 | 70.0 | 25 861 | 69.1 | 25 677 | 63.2 | 24 693 | 55.4 | 23 772 | 50.3 | 20 843 | 40.8 | 27 965 | 41.8 | 171 512 | 53.6 |
| Systemic therapy | ||||||||||||||||
| Chemotherapy only | 3829 | 11.8 | 4670 | 12.5 | 6683 | 16.4 | 6490 | 14.6 | 4448 | 9.4 | 4633 | 9.1 | 5598 | 8.4 | 36 351 | 11.4 |
| Endocrine therapy only | 9225 | 28.4 | 10 719 | 28.6 | 11 604 | 28.5 | 11 191 | 25.1 | 11 747 | 24.9 | 14 436 | 28.3 | 20 616 | 30.8 | 89 538 | 28.0 |
| Chemotherapy and endocrine therapy | 510 | 1.6 | 847 | 2.3 | 3365 | 8.3 | 8605 | 19.3 | 9843 | 20.8 | 12 965 | 25.4 | 14 105 | 21.1 | 50 240 | 15.7 |
| Targeted therapy l | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 115 | 0.3 | 3751 | 7.9 | 5075 | 9.9 | 7220 | 10.8 | 16 161 | 5.0 |
| No systemic therapy | 18 872 | 58.2 | 21 191 | 56.6 | 18 994 | 46.7 | 18 133 | 40.7 | 17 479 | 37.0 | 13 950 | 27.3 | 19 340 | 28.9 | 127 959 | 40.0 |
| Total | 32 436 | 100.0 | 37 427 | 100.0 | 40 646 | 100.0 | 44 534 | 100.0 | 47 268 | 100.0 | 51 059 | 100.0 | 66 879 | 100.0 | 320 249 m | 100.0 |
Abbreviations: AI, aromatase inhibitor; BCS, breast‐conserving surgery; CT, chemotherapy; ET, endocrine therapy; ER, oestrogen receptor; HER2, human epidermal growth factor receptor2; HR, hormone receptor; NCR, Netherlands Cancer Registry; PR, progesterone receptor; RT, radiotherapy.
Note: The various elements included in the table were collected by the NCR at different moments in time. ER status and PR status were routinely collected and included in the NCR since 2005 and HER2 status since 2006. Specification of chemotherapy and endocrine therapy regimens in the NCR has been done since 2003. Target therapy was routinely collected and included in the NCR since 2005 (mainly trastuzumab). Treatment data in the NCR are collected up to 1 year after initial cancer diagnosis. The data may still include 162 in situ BC cases due to discrepancies in registration within the dataset. Percentages may not total to 100% due to rounding.
Tumour size and lymph node status are based on the pathological stage, but clinical stage was used if pathological stage was unavailable.
Including 502 first primary BCs that were defined as “undifferentiated” in the NCR.
HR+ = ER+ and/or PR+, HR− = ER− and PR−.
Patients that received both BCS and mastectomy were included in the mastectomy group.
The chemotherapy regimen contains taxanes, but no anthracyclines.
The chemotherapy regimen contains anthracyclines, but no taxanes.
The chemotherapy regimen contains both taxanes and anthracyclines.
All other chemotherapy regimens (eg, cyclophosphamide/cisplatin‐containing regimes) and/or chemotherapy not further specified.
The NCR codes aromatase inhibitors specifically; tamoxifen is coded as endocrine treatment. Both treatments were included when provided as initial treatment.
Ovarian ablation includes LHRH agonist treatment, radiotherapy and/or surgical removal of the ovaries to reduce oestrogen production in premenopausal women.
All other endocrine treatments (eg, fulvestrant) and/or not further specified.
Patients received targeted therapy either alone or in combination with CT, ET or both.
Total numbers provided do not correspond with those for the ER, PR, HER2 and the receptor subtype groups due to their inclusion since 2005 to 2009.
3.2. Incidence
The BC incidence for all patients combined significantly increased from 126 to 153 per 100 000 PY (AAPC = 0.7% [95% CI: 0.6, 0.9]) between 1989 and 2017 (Figure 1A and Table S2). Age‐specific results showed an increase in BC incidence from 15 to 20 (AAPC = 1.0% [95% CI: 0.5, 1.5]) in women aged <40 years, 150 to 176 (AAPC = 0.5% [95% CI: 0.2, 0.7]) for 40 to 49 years, and 237 to 315 per 100 000 PY (AAPC = 1.1% [95% CI: 0.8, 1.3]) in women aged 50 to 74 years at time of diagnosis. In women aged ≥75, the incidence decreased from 300 to 269 per 100 000 PY (AAPC = −0.3% [95% CI: −0.5, −0.2]) between 1989 and 2017.
FIGURE 1.
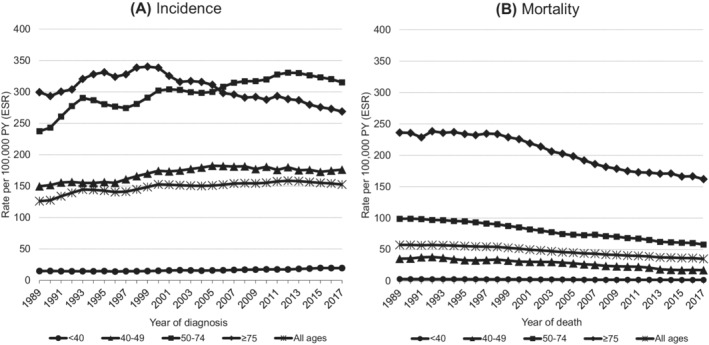
All ages combined and age‐specific first primary invasive breast cancer incidence (A) and mortality (B) trends (3‐year moving averages) in the Netherlands in the period 1989 to 2017. Rates were adjusted for age (European Standard Rates, ESR) by direct standardisation according to the 2013 European Standard Population 95+ and calculated per 100 000 person‐years (PY)
In some subperiods, significant declines in BC incidence were observed for all BC patients combined; in the period 1993 to 1997, the incidence declined from 145 to 141 (APC = −1.3% [95% CI: −2.1, −0.5]) and in the period 2013 to 2017 from 158 to 153 per 100 000 PY (APC = −0.8% [95% CI: −1.1, −0.5]). In women aged 40 to 49 years, the BC incidence significantly declined from 2006 onward from 182 to 176 per 100 000 PY (APC = −0.4% [95% CI: −0.6, −0.2]) and in women aged 50 to 74 years it declined from 330 to 315 per 100 000 PY (APC = −1.1% [95% CI: −1.6, −0.7]) between 2013 and 2017. In women aged ≥75, BC incidence decreased since 1998 from 339 to 269 per 100 000 PY (APC = −1.2% [95% CI: −1.3, −1.1]) in 2017 (Table S2).
3.3. Tumour stage
The incidence rates of Stage I BC for all patients combined increased from 36 to 72 per 100 000 PY (AAPC = 2.6% [95% CI: 2.1, 3.0]) between 1989 and 2017. In the same period, the combined incidence of Stages II and III BC decreased from 80 to 72 per 100 000 PY (AAPC = −0.3% [95% CI: −0.5, −0.1]). The incidence of Stage IV BC remained stable around 8 per 100 000 PY (AAPC = −0.2% [95% CI: −0.6, 0.2]) (Figure S1 and Table S3).
Prior to the shift from the fifth to sixth edition of the TNM classification, the incidence of Stages II and III combined increased from 80 to 84 per 100 000 PY (AAPC = 0.5% [95% CI: 0.2, 0.7]) between 1989 and 2003 and declined from 84 to 72 per 100 000 PY (AAPC = −1.1% [95% CI: −1.3, −0.8]) after the shift in 2003 to 2017. Similar declines after the shift were observed for Stages II and III individually (Table S4).
The incidence of Stage I BC increased for all age groups between 1989 and 2017, with the largest increase observed in women aged 50 to 74 years, increasing from 69 to 176 per 100 000 PY (AAPC = 3.5% [95% CI: 3.0, 3.9]). The combined incidence of Stages II and III BC increased in women aged <40 and 40 to 49 years, whereas it decreased in women aged 50 to 74 and ≥75 years. In women aged 40 to 49 years, the incidence of Stage IV BC increased, whereas it remained stable for the other age groups (Figure S2 and Tables S3 and S4).
3.4. Receptor subtype
Between 2006 and 2017, the incidence of HR+/HER2− BC increased from 104 to 112 per 100 000 PY (AAPC = 0.7% [95% CI: 0.5, 0.9]) and from 12 to 13 per 100 000 PY (AAPC = 1.0% [95% CI: 0.8, 1.3]) for HR+/HER2+ BC for all ages combined. Meanwhile, the incidence of HR−/HER2+ BC declined from 8 to 7 per 100 000 PY (AAPC = −0.9% [95% CI: −1.7, −0.2]) and from 16 to 15 per 100 000 PY (AAPC = −0.3% [95% CI: −0.6, −0.0]) for HR−/HER2− BC (Figure S3 and Table S5).
HR+/HER2− BC incidence decreased slightly from 123 to 121 per 100 000 PY (AAPC = −0.3% [95% CI: −0.6, −0.0]) in women aged 40 to 49 years, whereas it significantly increased among women aged <40 and 50 to 74 years between 2006 and 2017. The incidence of HR+/HER2+ BC increased for women aged <40, 40 to 49 and 50 to 74 years. No changes in incidence of HR+/HER2− and HR+/HER2+ BC were observed since 1989 among women aged ≥75 years. Concurrently, the incidence of HR−/HER2+ BC decreased from 15 to 13 (AAPC = −1.8% [95% CI: −2.3, −1.3]), and HR−/HER2− BC decreased from 29 to 27 per 100 000 PY (AAPC−0.7 [95% CI: −1.1, −0.3]) in women aged 50 to 74 years. The HR‐negative BC incidence remained stable for the remaining age groups (<40, 40‐49 and ≥75 years) regardless of HER2‐status (Figure 2 and Table S5).
FIGURE 2.
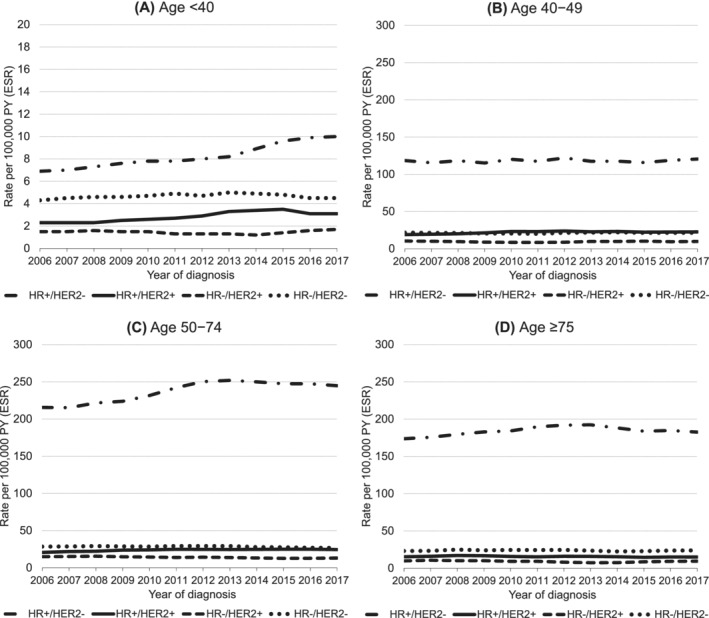
Incidence trends in the Netherlands stratified by receptor subtype between 2006 and 2017 in women diagnosed with first primary invasive breast cancer. Rates were adjusted for age (European Standard Rates, ESR) by direct standardisation according to the 2013 European Standard Population 95+ and calculated per 100 000 person‐years (PY). HR + = ER+ and/or PR+, HR− = ER− and PR−. Information on ER/PR and HER2‐status was routinely collected by the Dutch cancer registry since 2005 and 2006, respectively. Note the different scaling in A
3.5. Treatment strategies
3.5.1. Surgery and radiotherapy
The proportion of women with BC that underwent surgery remained stable around 90% since 1989. Breast‐conserving surgery (BCS) became the preferred surgical intervention since 2003 with 60.1% of all surgically treated patients undergoing BCS in 2017 (Figure 3). Radiotherapy use increased from 55.4% in 1989 to 70.1% in 2017 and was almost exclusively given in combination with surgical treatment (up to 99.6% in 2013 to 2017) (Figure 3 and Table 1). The most commonly provided local treatment was BCS followed by radiotherapy, with 55.3% of BC patients receiving this combination in 2013 to 2017 (Table 1).
FIGURE 3.
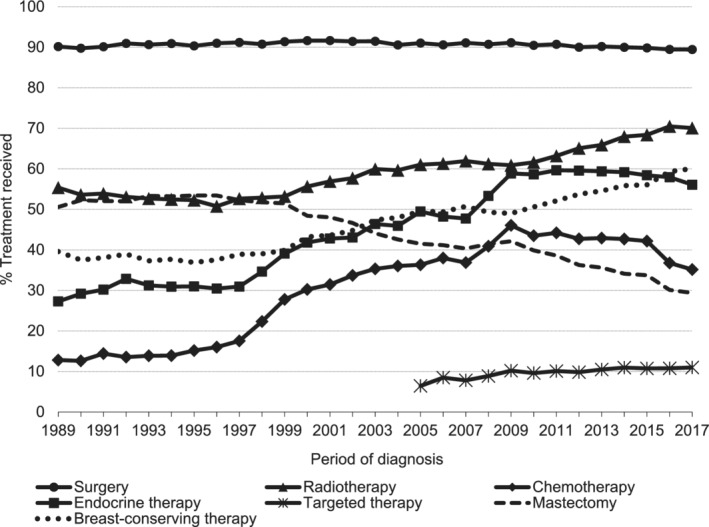
Proportion of treatment received by patients with first primary invasive breast cancer in the Netherlands between 1989 and 2017. Targeted therapy (mainly trastuzumab) was routinely collected by the NCR since 2005. Cumulative proportion was calculated per treatment strategy and based on treatment received (yes/no). Proportions of mastectomy and breast‐conserving surgery were calculated based on the proportion of patients receiving surgery. Patients that received both surgical treatments were included in the mastectomy group
3.5.2. Systemic treatment
The use of any systemic treatment increased from 41.8% in 1989 to 1992 to 71.1% in 2013 to 2017. Most women received endocrine therapy only (28.4% in 1989‐1992 and 30.8% in 2013‐2017). The proportion of women that received both chemotherapy and endocrine therapy increased from 1.6% in 1989 to 1992 to 25.4% in 2009 to 2012, but slightly declined to 21.1% in 2013 to 2017. The use of targeted therapy (mainly trastuzumab) increased from 7.9% in 2005 to 2008 to 10.8% in 2013 to 2017 (Figure S4). Trends in systemic treatment use over time according to age, stage and receptor subtype are included in Figure S5.
3.5.3. Chemotherapy
The overall proportion of women that received chemotherapy increased from 12.8% in 1989 to 46.0% in 2009, and decreased to 35.1% in 2017 (Figure 3). Chemotherapy use likewise decreased since 2009 for most age groups and stages, and for the HR+/HER2− subtype, but remained stable in women aged ≥75 years (2%‐3%) and in women with Stage IV BC (41‐43%), as shown in Figure S6. Among all women receiving chemotherapy, the proportion treated with both taxane‐ and anthracycline‐containing regimens increased from 5.7% in 2003 to 2005 to 79.3% in 2015 to 2017 (Figure S7a).
3.5.4. Endocrine therapy
Endocrine therapy use increased from 27.3% in 1989 to 59.6% in 2011, and slightly decreased to 56.1% in 2017 (Figure 3). Most patients received tamoxifen as initial endocrine therapy. Use of tamoxifen for all BC patients combined was stable at 88.2% to 91.8% between 2003 to 2005 and 2009 to 2011, and subsequently decreased to 74.5% in 2015 to 2017. The use of aromatase inhibitor as initial endocrine therapy increased from 11.2% to 25.0% between 2003 to 2005 and 2015 to 2017 (Figure S7b). Endocrine therapy use increased among women of all ages and for most BC stages (Stages I−III), as shown in Figure S8.
3.6. Relative survival
The RS at 5 and 10 years of follow‐up for all BC patients combined was 76.8% (95% CI: 76.1, 77.4) and 55.9% (95% CI: 54.7, 57.1) in 1989 to 1999, respectively, and increased to 91.0% (95% CI: 90.5, 91.5) and 82.9% (95% CI: 82.2, 83.5) in 2010 to 2016. Between 2000 to 2009 and 2010 to 2016, the 15‐year RS increased from 66.0% (95% CI: 65.2, 66.7) to 75.4% (95% CI: 74.6, 76.2) and the 20‐year RS increased from 53.5% (95% CI: 52.2, 54.8) to 68.1% (95% CI: 67.1, 69.1) (Figure 4).
FIGURE 4.
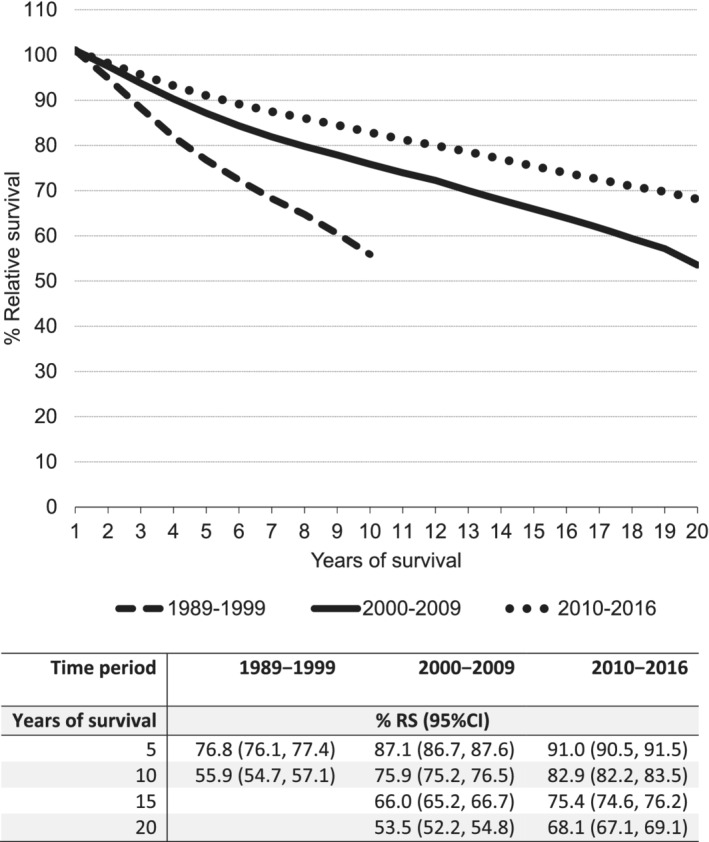
Age‐standardised relative survival (RS) outcomes with corresponding 95% confidence intervals of first primary invasive breast cancer in the Netherlands between 1989 and 2017. Relative survival was adjusted for age by direct standardisation according to the 2013 European Standard Population 95+
The RS improved for all age groups and most stages between 1989 to 1999 and 2010 to 2016, but the 15‐year RS remained stable for Stage IV BC between 2000 to 2009 (RS = 4.6% [95% CI: 3.1, 6.4]) and 2010 to 2016 (RS = 7.2% [95% CI: 4.6, 10.5]). The survival of all receptor subtypes improved between 2006 to 2011 and 2012 to 2013, but no further improvements were observed in the subsequent period 2014 to 2016 (Figure 5 and Table S6).
FIGURE 5.
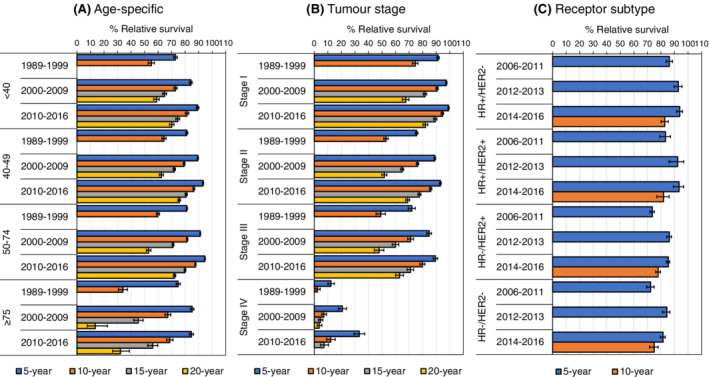
Age‐specific (A) and age‐standardised stage (B) and receptor subtype‐specific (C) relative survival outcomes with corresponding 95% confidence intervals of first primary invasive breast cancer patients in the Netherlands diagnosed between 1989 and 2016. Relative survival was adjusted for age by direct standardisation according to the 2013 European Standard Population 95+. HR + = ER+ and/or PR+, HR− = ER− and PR−. Information on ER/PR and HER2‐status was routinely collected by the Dutch cancer registry since 2005 and 2006, respectively. For Stage IV BC, the 20‐year relative survival in 2010 to 2016 could not be estimated due to low patient numbers
The RS improved for all women aged <40, 40 to 49 and 50 to 74 years with Stages I to III BC between 1989 to 1999 and 2010 to 2016 for all years of follow‐up. The RS at 10 and 15 years of follow‐up remained stable for those with Stage IV BC since 2000 to 2009 and likewise did not improve since 2000 to 2009 in women aged ≥75 years with any stage BC (Figure S9 and Table S7). The 5‐year RS of all receptor subtypes remained stable since 2012 to 2013 irrespective of age (Figure S10 and Table S8). Survival outcomes were overall slightly lower in women aged ≥75 years in comparison with other age groups and deteriorated with advancing stage for all age groups (Figure 5, Figure S9, and Tables S6 and S7).
3.7. Mortality
The BC mortality for women of all ages decreased from 57 to 35 per 100 000 PY (AAPC = −1.8% [95% CI: −1.9, −1.7]) between 1989 and 2017. Similar trends were observed for all age groups, as shown in Figure 1B and Table S2.
4. DISCUSSION
This study provides a comprehensive overview of first primary invasive BC incidence, survival, mortality and treatment trends stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017, using population‐based data on 320 249 women with first primary invasive BC from the NCR. BC incidence in the Netherlands has steadily increased between 1989 and 2013. However, in recent years, the latest time trends (APCs) revealed noticeable declines in BC incidence for the entire patient population, in women aged 40 to 49 and 50 to 74 years, and in women with Stage I disease. In women aged ≥75 years, BC incidence has been declining since 1998. Systemic treatment increasingly involved a combination of chemotherapy, endocrine therapy and targeted therapy. The RS improved markedly over time for all years of follow‐up for most patients, but remained stable for all receptor subtypes since 2012 to 2013 and since 2000 to 2009 in women with Stage IV BC at 15 years of follow‐up. BC mortality steadily decreased in women of all age groups since 1989.
4.1. Incidence
The rising trends in BC incidence are consistent with those found in previous Dutch and global (trend) studies 1 , 4 , 5 , 9 , 10 , 11 , 12 and can be attributed in part to changes in the prevalence of known risk and lifestyle factors that have been shown to influence BC incidence. 4 , 5 In a recent case‐control study, the increasingly common use of both oral contraceptives (for more than 10 years) and hormone replacement therapy (for more than 3 years) has been shown to increase the risk of BC (relative risk = 3.2 [95% CI: 1.4, 7.4]) in women aged <55 years. 6 Together with the increased alcohol consumption among younger people this might explain the rising BC incidence in women aged <40 years in this study. 7 The worldwide rise in overweight and obesity in recent decades is also likely to have contributed to the increase in BC incidence in both premenopausal and postmenopausal women. 8 In the United States, decreases in BC incidence in 2002 and 2003 were attributed to the declining use of hormone replacement therapy in postmenopausal women following unfavourable publicity. 28 However, similar trends were not observed in the Netherlands until 2005 and are likewise not observed now. 29
The observed trends in BC incidence are probably also influenced by the population‐based mammography screening programme, which has been operational in the Netherlands since 1989 and for which women aged 50 to 74 years are invited biennially. Screening is intended to favourably change the stage at diagnosis and leads to a strong temporary increase in BC incidence due to the detection of (mainly) slow growing tumours followed by a decline in more advanced BC stages. 4 , 5 This corresponds with the observed increase in the incidence of Stage I BC and the decline in incidence of Stage II/III BC, which was most prominent in women aged 50 to 74 years. The decline in BC incidence observed since 1998 in women aged ≥75, who are no longer offered screening (compensatory drop), might also reflect screening practices. 30
The decline in BC incidence shown by the latest trends (2013‐2017 for all patients combined) might be associated with the transition from screen‐film to digital mammography between 2003 and 2010. In the period when digital mammography was implemented an increase in BC incidence was observed in women aged 50 to 74 (2004‐2013, APC = 1.2% [95% CI: 1.0, 1.5]) and in women with Stage I BC (2005‐2012, APC = 3.4% [95% CI: 3.0, 3.8]), whereas no rise in incidence was observed prior to digital mammography implementation. A similar pattern was observed in women aged 50 to 74 year with HR+/HER2+ BC. In all cases, incidence rates either decreased or remained stable in the subsequent period, which might suggest a temporal increase after implementation of digital mammography. 31 However, in our study, the relation to screening was not directly taken into account in the analyses since mode of detection was not registered in the NCR until 2011. A recent study based on actual screening attendance did show that the incidence of Stages III and IV BC was significantly higher in nonscreened vs screened women (94 vs 38 per 100 000 PY, respectively; odds ratio [OR] = 2.86, 95% CI: [2.72, 3.00]). 32 In our data, 56% of all women aged 50 to 74 years were diagnosed through screening between 2011 and 2017. Thus, screening has at least partially affected the BC incidence. Alternatively, the observed decline in BC incidence in women aged 40 to 49 years might partly relate to the increase in prophylactic bilateral mastectomies, which significantly lowers the BC incidence in unaffected high risk women with BRCA mutations (85%‐100%) 33 and recently showed a significant increase in uptake in women (mean age 41.8 years) who received genetic testing after 2008 (32.7% in the Netherlands). 34
4.2. Treatment strategies
Therapeutic approaches of BC in the Netherlands have changed drastically since 1989. BCS with adjuvant radiotherapy became the preferred treatment over mastectomy after the publication of landmark trials. 35 , 36 The steep increase in both adjuvant chemotherapy and endocrine therapy use between 2007 and 2009 can be explained by the broadening of their indications following the 2008 revision of the Dutch evidence‐based guidelines and the introduction of the decision tool “Adjuvant! Online”, which was developed to predict the potential benefit of systemic treatment for individual BC patients. 37
The decline in chemotherapy use after 2009 is likely also related to changes in the Dutch evidence‐based guidelines for the management of BC (www.oncoline.nl), which now recommends endocrine therapy instead of chemotherapy in postmenopausal women with Grade 2 tumours >1.1 cm and ER/PgR >50%. Possibly also related to the decline in chemotherapy use is the increased use of the 70‐gene signature (70‐GS, “MammaPrint”) and other measures used to assess tumour aggressiveness (Ki67 immunohistochemistry, PgR status, etc.), together with a growing focus on shared decision‐making and a more reluctant attitude of clinicians towards the use of chemotherapy in low‐risk patients. 38 , 39
4.3. Survival and mortality
Advances in treatment and more personalised therapeutic guidelines likely also contributed to the improvements in BC survival and mortality. 3 , 4 , 5 The sharp increase in the proportion of women that received both taxane and anthracycline containing regimens from 2003‐2005 to 2015‐2017 may provide some explanation for the observed improvements in survival, as use of combination chemotherapy has been shown to improve survival in metastatic BC since the late 1960s. 14 Improvements in survival and mortality may also relate to more personalised therapy (adjuvant endocrine therapy and anti‐HER2 therapy) facilitated since the beginning of this century by the use of information on tumour biology (HR and HER2‐status), which has improved treatment allocation to patients that will more likely benefit based on their tumour characteristics, even for Stage IV disease. 40 The gains in survival and mortality may also in part be attributed to the changed composition of women who receive endocrine therapy, following changes in the Dutch national guidelines. Before 1999, endocrine therapy was given to all postmenopausal women with N+ BC and was provided, irrespective of menopausal status, to all women with N+ and ER+ BC. The similar survival of women with either HER2‐positive or HER2‐negative BC, irrespective of HR‐status, likely relates to the use of trastuzumab, which was recommended in the Netherlands since 2005. 15 When not treated with trastuzumab, the overall survival of HER2‐positive BC is poorer compared to HER2‐negative BC 41 .
Stage at diagnosis has also remained one of the most important determinants for BC survival, with survival becoming increasingly worse with advancing stage. Improvements in stage‐specific survival have been described previously 9 , 11 and may partly be explained by stage migration, due to advances in detecting distant metastases, but also evolutions in TNM classification. 42 In clinical practice, the impact of stage migration has been observed after implementation of FDG‐PET in lung cancer care, which resulted in an increase in Stage IV classification. 43 Improvements in the detection of distant metastases at time of BC diagnosis likewise resulted in stage migration. 44 It is therefore possible that stage migration contributed to the observed improvements in stage‐specific survival observed here. Poorer adherence to treatment guidelines in older patients, together with the fact that these women are no longer included in population screening, may be responsible for the higher Stage II to IV rates at diagnosis in women aged ≥75 years and might to some extent explain the lower survival observed in these women compared to the younger age groups. 45
Decreases in BC mortality have been observed previously in most European, North American and other high‐income countries. 3 , 4 , 5 In the south‐eastern region of the Netherlands, mortality rates declined annually with 2% between 1995 and 2004. 9 In the current study, a similar annual decline was observed for the entire Netherlands between 1989 and 2017. The declines in BC mortality and improvements in survival have mainly been related to advances in early diagnosis. 3 , 4 , 5 Worldwide, early detection (mainly due to the more widespread use of mammography screening) has been suggested to be causal in the decline in BC mortality in high‐income countries. 2 , 4 Findings in the Netherlands have led to the same conclusions. 12 , 46 , 47 Projections from a simulation study based on six distinct models on BC mortality trends in the United States further showed that screening was on average associated with 44% (model range: 35%–60%) and 37% (model range: 26%–51%) of the observed decline in overall BC mortality among women aged 30 to 79 years in 2000 and in 2012, respectively. The remaining decline in mortality in 2012 was on average attributed to chemotherapy; 31% (model range: 22%–37%), endocrine therapy; 27% (model range: 18%–36%) and trastuzumab; 4% (model range: 1%–6%). 48 However, the data do not support the viewpoint that screening has a substantial effect on BC mortality, as declines in BC mortality in the Netherlands have been present since the late 1980s, prior to the implementation of a nationwide screening programme. 49 Moreover, in this study, declines in mortality were slightly higher in women aged <40 and 40 to 49 years than in older women, where organised screening is expected to influence the mortality. Also, declines in this study were already observed in the period shortly after screening implementation, which is not expected due to the usual time lag before screening effects become apparent. 50 Advances in treatment are therefore more likely to have caused this effect. 49
4.4. Strengths and limitations
The major strength of this study was the use of a large population‐based dataset from the NCR spanning almost three decades of BC data. Data of all new BC patients were collected by trained registrars, leading to high completeness and ruling out selection bias. This study is among the first to include a detailed description on BC trends according to receptor subtype in Europe, which is another major strength. Nevertheless, data on receptor subtype were still limited and consequently, we could not detect clear trends based on receptor subtype. Furthermore, we did not have information available on risk and lifestyle factors and were therefore not able to directly assess trends in incidence according to these factors. We experienced some difficulties in the assessment of trends due to the changing definition of tumour stage. In particular, the change from the fifth to sixth TNM classification resulted in a noticeable shift from Stage II to III disease, which complicated trend recognition and comparisons over time. We tried to address this shortcoming by combining both stages for analyses and by assessing preshift and postshift time trends separately with joinpoint regression analyses. Finally, we did not have information available on the BC‐specific survival and therefore we used RS as an estimator. Nonetheless, the RS is an appropriate method to use in population‐based studies on survival in the absence of cause of death information and does not suffer from misclassification.
5. CONCLUSION
This study provides a comprehensive overview of first primary invasive BC trends in the Netherlands since 1989. The incidence of BC for the entire patient population has steadily increased between 1989 and 2013, but has been declining since. Whether this declining trend continues, should be confirmed by future trend studies covering subsequent time periods. Meanwhile, the RS improved for all age groups and for most stages and receptor subtypes, and the mortality of first primary invasive BC has decreased substantially since 1989. The observed trends in BC incidence, mortality and survival likely result from the combined effect of preventive measures, earlier diagnosis (population screening and better disease awareness), advances in treatment, national implementation of personalised treatment guidelines and changes in the exposure to known risk factors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was reviewed and approved by the Privacy Review Board of the Netherlands Cancer Registry.
Supporting information
Appendix S1: Supporting information
ACKNOWLEDGEMENTS
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry as well as staff for scientific advice. In particular, the authors would personally like to thank Linda de Munck and Janneke Verloop for their helpful contribution during the conduction of this study.
van der Meer DJ, Kramer I, van Maaren MC, et al. Comprehensive trends in incidence, treatment, survival and mortality of first primary invasive breast cancer stratified by age, stage and receptor subtype in the Netherlands between 1989 and 2017. Int. J. Cancer. 2021;148:2289–2303. 10.1002/ijc.33417
Adri C. Voogd, Marjanka K. Schmidt and Sabine Siesling contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings are available from the Netherlands Cancer Registry upon reasonable request (data request study number K18.244, w w w .iknl.nl).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Hashim D, Boffetta P, La Vecchia C, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27:926‐933. [DOI] [PubMed] [Google Scholar]
- 3. Bosetti C, Bertuccio P, Levi F, Chatenoud L, Negri E, La Vecchia C. The decline in breast cancer mortality in Europe: an update (to 2009). The Breast. 2012;21:77‐82. [DOI] [PubMed] [Google Scholar]
- 4. Youlden DR, Cramb SM, Dunn NAM, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36:237‐248. [DOI] [PubMed] [Google Scholar]
- 5. Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6:229‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brinton LA, Brogan DR, Coates RJ, Swanson CA, Potischman N, Stanford JL. Breast cancer risk among women under 55 years of age by joint effects of usage of oral contraceptives and hormone replacement therapy. Menopause. 2018;25:1195‐1200. [DOI] [PubMed] [Google Scholar]
- 7. Heath AK, Muller DC, van den Brandt PA, et al. Nutrient‐wide association study of 92 foods and nutrients and breast cancer risk. Breast Cancer Res. 2020;22:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picon‐Ruiz M, Morata‐Tarifa C, Valle‐Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louwman WJ, Voogd AC, Van Dijck JAAM, et al. On the rising trends of incidence and prognosis for breast cancer patients diagnosed 1975–2004: a long‐term population‐based study in southeastern Netherlands. Cancer Causes Control. 2008;19:97‐106. [DOI] [PubMed] [Google Scholar]
- 10. van der Waal D, Verbeek ALM, den Heeten GJ, Ripping TM, Tjan‐Heijnen VCG, Broeders MJM. Breast cancer diagnosis and death in The Netherlands: a changing burden. European J Public Health. 2015;25:320‐324. [DOI] [PubMed] [Google Scholar]
- 11. Vondeling GT, Menezes GL, Dvortsin EP, et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otten JDM, Broeders MJM, Fracheboud J, Otto SJ, de Koning HJ, Verbeek ALM. Impressive time‐related influence of the Dutch screening programme on breast cancer incidence and mortality, 1975‐2006. Int J Cancer. 2008;123:1929‐1934. [DOI] [PubMed] [Google Scholar]
- 13. Sukel MPP, van de Poll‐Franse LV, GAP N, et al. Substantial increase in the use of adjuvant systemic treatment for early stage breast cancer reflects changes in guidelines in the period 1990–2006 in the southeastern Netherlands. Eur J Cancer Clin Oncol. 2008;44:1846‐1854. [DOI] [PubMed] [Google Scholar]
- 14. Zurrida S, Veronesi U. Milestones in breast cancer treatment. Breast J. 2015;21:3‐12. [DOI] [PubMed] [Google Scholar]
- 15. de Munck L, Schaapveld M, Siesling S, et al. Implementation of trastuzumab in conjunction with adjuvant chemotherapy in the treatment of non‐metastatic breast cancer in The Netherlands. Breast Cancer Res Treat. 2011;129:229‐233. [DOI] [PubMed] [Google Scholar]
- 16. Statistics Netherlands (CBS) . Statline: Bevolking; geslacht, leeftijd en burgerlijke staat; 2018. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7461bev/table?dl=1B894. Accessed March 15, 2019.
- 17. Statistics Netherlands (CBS) . Statline: Overledenen; belangrijke doodsoorzaken (korte lijst), leeftijd, geslacht; 2018. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7052_95/table?dl=1BAAC. Accessed March 18, 2019.
- 18. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. West‐Sussex: Wiley‐Blackwell; 2017:272. [Google Scholar]
- 19. Consonni D, Coviello E, Buzzoni C, Mensi C. A command to calculate age‐standardized rates with efficient interval estimation. Stata J. 2012;12:688‐701. [Google Scholar]
- 20. Pace M, Lanzieri G, Glickman M, Zupanič T. Revision of the European Standard Population: report of Eurostat's task force. Luxembourg: Publications Office of the European Union; 2013. [Google Scholar]
- 21. Naing NN. Easy way to learn standardization: direct and indirect methods. Malaysian J Med Sci. 2000;7:10‐15. [PMC free article] [PubMed] [Google Scholar]
- 22. Clegg LX, Hankey BF, Tiwari R, Feuer EJ, Edwards BK. Estimating average annual per cent change in trend analysis. Stat Med. 2009;28:3670‐3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335‐351. [DOI] [PubMed] [Google Scholar]
- 24. Joinpoint Regression Program . Version 4.7.0.0. February 26. Statistical Research and Applications Branch: National Cancer Institute; 2019. [Google Scholar]
- 25. Pokhrel A, Hakulinen T. Age‐standardisation of relative survival ratios of cancer patients in a comparison between countries, genders and time periods. Eur J Cancer. 2009;45:642‐647. [DOI] [PubMed] [Google Scholar]
- 26. Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. 2015;15:186‐215. [Google Scholar]
- 27. Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up‐to‐date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer. 2004;40:326‐335. [DOI] [PubMed] [Google Scholar]
- 28. Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast‐cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670‐1674. [DOI] [PubMed] [Google Scholar]
- 29. Soerjomataram I, Coebergh JWW, Louwman MWJ, Visser O, van Leeuwen FE. Does the decrease in hormone replacement therapy also affect breast cancer risk in The Netherlands? J Clin Oncol. 2007;25:5038‐5039. [DOI] [PubMed] [Google Scholar]
- 30. Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ. 2009;339:b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sankatsing VDV, Fracheboud J, de Munck L, et al. Detection and interval cancer rates during the transition from screen‐film to digital mammography in population‐based screening. BMC Cancer. 2018;18:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Munck L, Fracheboud J, de Bock GH, den Heeten GJ, Siesling S, MJM B. Is the incidence of advanced‐stage breast cancer affected by whether women attend a steady‐state screening program? Int J Cancer. 2018;143:842‐850. [DOI] [PubMed] [Google Scholar]
- 33. Alaofi RK, Nassif MO, Al‐Hajeili MR. Prophylactic mastectomy for the prevention of breast cancer: review of the literature. Avicenna J Med. 2018;8:67‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Metcalfe K, Eisen A, Senter L, et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br J Cancer. 2019;121:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Dongen JA, Voogd AC, Fentiman IS, et al. Long‐term results of a randomized trial comparing breast‐conserving therapy with mastectomy: European Organization for Research and Treatment of cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143‐1150. [DOI] [PubMed] [Google Scholar]
- 36. Fisher B, Anderson S, Bryant J, et al. Twenty‐year follow‐up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233‐1241. [DOI] [PubMed] [Google Scholar]
- 37. Struikmans H, Nortier JW, Rutgers EJ, et al. Guideline ’Treatment of breast cancer 2008’ (revision). Ned Tijdschr Geneeskd. 2008;152:2507‐2511. [PubMed] [Google Scholar]
- 38. Kuijer A, van Bommel ACM, Drukker CA, et al. Using a gene expression signature when controversy exists regarding the indication for adjuvant systemic treatment reduces the proportion of patients receiving adjuvant chemotherapy: a nationwide study. Genet Med. 2016;18:720‐726. [DOI] [PubMed] [Google Scholar]
- 39. van Steenhoven JEC, Kuijer A, Schreuder K, et al. The changing role of gene‐expression profiling in the era of de‐escalating adjuvant chemotherapy in early‐stage breast cancer. Ann Surg Oncol. 2019;26:3495‐3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Abreu FB, Schwartz GN, Wells WA, Tsongalis GJ. Personalized therapy for breast cancer. Clin Genet. 2014;86:62‐67. [DOI] [PubMed] [Google Scholar]
- 41. Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146:264‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon: stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312:1604‐1608. [DOI] [PubMed] [Google Scholar]
- 43. Schuurman MS, Groen HJM, Pruim J, Janssen‐Heijnen MLG, Pukkala E, Siesling S. Temporal trends and spatial variation in stage distribution of non‐small cell lung cancer in The Netherlands. Screening. 2014;4:9‐10. [Google Scholar]
- 44. Polednak AP. Increase in distant stage breast cancer incidence rates in US women aged 25–49 years, 2000–2011: the stage migration hypothesis. J Cancer Epidemiol. 2015;2015:710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bastiaannet E, Liefers GJ, de Craen AJM, et al. Breast cancer in elderly compared to younger patients in The Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124:801‐807. [DOI] [PubMed] [Google Scholar]
- 46. Otto SJ, Fracheboud J, Verbeek ALM, et al. Mammography screening and risk of breast cancer death: a population‐based case–control study. Cancer Epidemiol Biomarkers Prev. 2012;21:66‐73. [DOI] [PubMed] [Google Scholar]
- 47. van der Waal D, Ripping TM, Verbeek ALM, Broeders MJM. Breast cancer screening effect across breast density strata: a case–control study. Int J Cancer. 2017;140:41‐49. [DOI] [PubMed] [Google Scholar]
- 48. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000‐2012. JAMA. 2018;319:154‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Botha JL, Bray F, Sankila R, Parkin DM. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718‐1729. [DOI] [PubMed] [Google Scholar]
- 50. Lee SJ, Boscardin WJ, Stijacic‐Cenzer I, Conell‐Price J, O'Brien S, Walter LC. Time lag to benefit after screening for breast and colorectal cancer: meta‐analysis of survival data from the United States, Sweden, United Kingdom, and Denmark. BMJ. 2013;346:e8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting information
Data Availability Statement
The data that support the findings are available from the Netherlands Cancer Registry upon reasonable request (data request study number K18.244, w w w .iknl.nl).


