Abstract
Introduction
Counteracting impaired brain glucose metabolism with ketones may improve cognition in mild cognitive impairment (MCI).
Methods
Cognition, plasma ketone response, and metabolic profile were assessed before and 6 months after supplementation with a ketogenic drink containing medium chain triglyceride (ketogenic medium chain triglyceride [kMCT]; 15 g twice/day; n = 39) or placebo (n = 44).
Results
Free and cued recall (Trial 1; P = .047), verbal fluency (categories; P = .024), Boston Naming Test (total correct answers; P = .033), and the Trail‐Making Test (total errors; P = .017) improved significantly in the kMCT group compared to placebo (analysis of covariance; pre‐intervention score, sex, age, education, and apolipoprotein E4 as covariates). Some cognitive outcomes also correlated positively with plasma ketones. Plasma metabolic profile and ketone response were unchanged.
Conclusions
This kMCT drink improved cognitive outcomes in MCI, at least in part by increasing blood ketone level. These data support further assessment of MCI progression to Alzheimer's disease.
Keywords: acetoacetate, Alzheimer's disease, beta‐hydroxybutyrate, cognition, episodic memory, executive function, ketone, language, medium chain triglyceride, mild cognitive impairment
1. INTRODUCTION
Brain energy rescue is emerging as a potential strategy to reduce cognitive decline in mild cognitive impairment (MCI) and Alzheimer's disease (AD). This strategy is based upon three related observations: First, in those at increased risk of early‐ or late‐onset AD, brain glucose hypometabolism is present before the onset of cognitive symptoms. 1 , 2 , 3 Second, despite lower brain glucose uptake, brain ketone uptake remains normal in both MCI and AD. 4 , 5 Third, in MCI, the improvement in brain energy status by ketones correlates positively to improved cognitive outcomes, indicating a mechanistic link between improved brain energy status and improved cognitive function in this population. 6
One simple and safe way to increase blood ketone levels (acetoacetate [AcAc] and beta‐hydroxybutyrate [BHB]) and brain ketone uptake in MCI and AD is with a ketogenic medium chain triglyceride (kMCT) drink. 5 , 6 The first phase of the randomized controlled BENEFIC (Brain Energy, Functional Imaging, and Cognition) trial demonstrated that a drink providing 30 g/day of kMCT emulsified in a specific formulation improved brain energy in MCI versus a calorie‐matched long chain fatty acid placebo (n = 20). 6 That trial was powered specifically to address the change in brain energy status (glucose and ketones combined) by positron emission tomography (PET), with cognitive function as a secondary outcome. A second phase of the BENEFIC trial was initiated to increase the overall sample size to have adequate statistical power to detect an effect on cognition. In phase two, PET imaging was discontinued and a metabolic study was conducted to assess whether the plasma ketone response changed after 6 months on the kMCT drink or placebo. As a proof‐of‐concept study, the 6‐month duration was chosen to assess long‐term feasibility and tolerability of such an intervention in this population and to limit learning effects. All other aspects of the second phase were identical to the first phase including randomization, dose titration, calorie‐ and organoleptically matched kMCT and placebo formulation, inclusion/exclusion criteria, cognitive battery, and duration of treatment.
The main objective of the present paper is to report the complete cognitive outcomes of the BENEFIC trial. The secondary objectives are to report plasma ketones; free caprylic (C8) and capric acids (C10) levels; as well as the metabolic response, safety, and tolerability after the 6‐month intervention.
2. METHODS
2.1. Participants
The BENEFIC Trial was conducted with the written informed consent of all the participants and was approved by our institutional ethics committee (CIUSSS de l'Estrie–CHUS, Sherbrooke, Quebec, Canada). It was registered on ClinicalTrials.gov (NCT02551419). As reported previously, 6 participants were recruited locally and via our website. Inclusion criteria were male or female aged ≥55 years plus MCI based on the Peterson criteria: 7 (1) presence of a subjective memory complaint, (2) objective evidence of cognitive impairment as assessed by a neurocognitive battery, (3) absence of major depression (General Depression Scale score [GDS <10/30]), 8 and (4) full autonomy of daily living based on a score of ≤15/24 on the instrumental activities of daily living. 9 Exclusion criteria were diagnosis of a major cognitive disorder according to the 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders; 10 use of a cholinesterase inhibitor; alcohol or substance abuse; cancer within the past 2 years; smoking; uncontrolled diabetes (fasting plasma glucose >7 mM and/or glycated hemoglobin >6.5%); heart, liver, or renal disease; vitamin B12 deficiency; uncontrolled hypertension, dyslipidemia, or thyroid disease. Cognition was first assessed by the Montreal Cognitive Assessment (MoCA) and the Mini‐Mental State Exam (MMSE). Participants were eligible if they had a below normal score on either or both tests (MoCA: score of ≥18 to ≤26/30 11 and/or MMSE: score of ≥24 to ≤27/30 12 ). A detailed 90‐minute neurocognitive battery 6 was then administered to determine whether there was an objective deficit in one or more cognitive domains compared to appropriate normative data (≥1.5 standard deviation [SD] below the mean). Screening tests for all participants were reviewed by a collaborating physician and neuropsychologist prior to enrollment.
HIGHLIGHTS
Cognitive changes were assessed in a 6‐month randomized controlled trial of ketogenic medium chain triglyceride (kMCT) versus placebo in mild cognitive impairment (MCI).
The kMCT drink improved three domains—executive function, memory, and language.
Higher plasma ketones correlated positively with changes on four cognitive tests.
Plasma metabolic profile and ketone response were unchanged after 6 months of kMCT.
Efficacy, safety, and tolerability suggest testing kMCT to delay progression to Alzheimer's disease.
RESEARCH IN CONTEXT
Systematic review: All peer‐reviewed articles available on PubMed on the subject of ketones and Alzheimer's disease (AD) or mild cognitive impairment (MCI) were reviewed. Three other trials have reported using ketogenic medium chain triglyceride (kMCT) in mild to moderate AD with promising effect on cognition, but no other group has assessed a kMCT intervention in MCI.
Interpretation: This 6‐month randomized, placebo‐controlled trial demonstrated that a kMCT drink improved cognitive outcomes in MCI in the domains of episodic memory, executive function, and language. This improvement was in direct relation to plasma ketone level and brain ketone uptake. Our results show that rescuing brain energy with a kMCT drink can significantly improve cognitive outcomes in MCI.
Future directions: The effect of kMCT on MCI progression to AD should now be assessed with a sample size that would evaluate a global effect on cognition in a multi‐center trial.
2.2. Experimental design
Eligible participants were randomized as previously described. 6 Participants received their supply of kMCT or placebo at monthly visits and maintained a daily logbook to monitor compliance. Before each monthly visit, participants consumed their usual breakfast along with their assigned drink and came to the lab 1 to 2 hours later at which time a blood sample was drawn for metabolic and safety analyses. Neurocognitive tests were completed before and again during the final week of the intervention. Protocol compliance was set a priori at consuming ≥80% of the monthly supply of kMCT or placebo drink.
2.3. kMCT and placebo drinks
The kMCT and placebo drinks were the same as reported previously. 6 Briefly, the kMCT drink was a 12% emulsion of Captex 355 (60% C8, 40% C10; Abitec Corp, Columbus, Ohio, USA) in lactose‐free skim milk. The placebo contained high‐oleic acid sunflower oil as a calorie‐equivalent non‐ketogenic vegetable oil. Both drinks were prepared using a proprietary process (dairy pilot plant, Université Laval, Quebec City, Quebec, Canada). They were visually and organoleptically indistinguishable and were bottled in 250 mL high density polyethylene screw‐capped bottles (Nalgene, Rochester, New York, USA). Each lot underwent organoleptic evaluation and a microbiological testing for quality control before being provided to the participants. Participants were blinded to the drink's composition and instructed to take 125 mL of their assigned drink twice a day, usually with breakfast and again with supper (total of 250 mL/day) after a gradual titration in the first 2 weeks. The daily dose was titrated from 50 to 125 mL, twice a day, during the first 2 weeks. Compliance was measured by bottle count and daily logs. Between two and five extra bottles were provided each month to allow for spillage and disguise the monthly count for compliance.
2.4. Neurocognitive battery
Episodic memory was assessed using the French version of the 16‐item free and cued word learning and recall test (Rappel Libre/Rappel Indicé [RL/RI‐16]) 13 and the Brief Visual Memory Test‐Revised (BVMT‐R). 14 The Trail‐Making Test, Stroop Color and Word Interference test (Stroop), and the Verbal Fluency (VF) tests from the Delis‐Kaplan Executive Function System 15 provided information on executive function, attention, and processing speed, respectively. The Digit Symbol Substitution Tests and the forward and backward digit span from the Wechsler Adult Intelligence Scale 16 provided information on processing speed and working memory, respectively. The Boston Naming Test (BNT) 17 was used for language ability. To minimize a potential learning effect on the post‐supplementation test, two versions of validated word lists and stimulus pages were used in the RL/RI‐16 and BVMT‐R tests. Scores were age‐corrected or age‐, education‐, and sex‐corrected standard score from tables of normative scores from a similar population. 15 , 16 , 18 , 19 For clarity and to simplify reporting, only significant results or trends are presented in the tables.
2.5. Metabolic assessment
A subgroup of participants undertook two identical metabolic assessments, the first before starting the supplement and the second at the end of the 6‐month supplementation period. 20 Briefly, after a 12‐hour overnight fast, participants received a standardized breakfast and a 125 mL dose of the drink to which they had been randomized. Venous forearm blood samples were taken in ethylenediaminetetraacetic acid tubes at baseline and every 30 minutes during a 4‐hour study period to measure plasma C8 and C10 (the precursors to ketones formed in the liver) and ketones.
2.6. Laboratory methods
Plasma metabolites were analyzed as previously described. 21 Apolipoprotein E (APOE) genotyping was performed by real‐time polymerase chain reaction. 22 Homocysteine was measured by high‐performance liquid chromatography at the Dairy and Swine R & D Centre, Agriculture and Agri‐Food Canada, Sherbrooke, Quebec, Canada. 23 Plasma C8 and C10 levels were measured by gas chromatography‐mass spectrometry (GC‐MS; Agilent, Waldbronn, Germany) based on the method described previously. 24
2.7. Statistics
A sample size of n = 41/group was based on the neurocognitive outcome variable of the RL/RI‐16, VF, BNT, and Trail‐Making tests and was calculated to detect a moderate effect size of 0.3, with an alpha risk of 5% and 90% power (G × Power 3.1.9.2 25 ). Anticipating a 25% to 30% dropout during the 6‐month intervention, we planned to recruit 120 to 125 participants over the two phases of the trial combined.
Data are presented as mean ± SD. All statistical analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, Illinois, USA). An independent samples t‐test and chi‐square test of homogeneity for categorical data were run to determine whether there were differences between participants at baseline. An analysis of covariance was run to assess between‐group differences on post‐supplementation cognitive test scores, and blood parameters as dependent variables, with treatment group as a fixed factor, after accounting for the pre‐intervention score as a covariate in model 1. 26 Baseline characteristics predictive of cognitive outcomes were added as covariates in a model 2; that is, sex, age, education, APOEε4 status. For the few tests that did not respect the assumption of homogeneity of variances as assessed by Levene's test (P < .05), Quade's rank analysis of covariance was used. 27 Partial ƞ2 was used as an index of effect size with 0.01, 0.06, and 0.14 being considered small, moderate, or large effect sizes, respectively. 28 All outlier data were included in the analyses as they were estimated to not have an appreciable effect on the analysis. Pearson correlations were computed using delta scores (post‐ minus pre‐intervention) to assess the relationship between plasma ketones and cognitive measures.
Further analyses were performed with the same statistical approach for three separate sub‐groups: protocol compliant (≥80% doses taken), APOE ε4 (–), and APOE ε4(+); heterozygotes and homozygotes combined). Assumptions were checked for each dataset. Because of the small sample size of the ApoE4(+) subgroup, assumptions of homogeneity and normality of the variance were not fulfilled for most of the dependent variables, so a non‐parametric Mann‐Whitney U test was instead used to compare delta scores (POST‐PRE) between kMCT and placebo (P‐value).
3. RESULTS
For the overall trial, a total of 346 participants were screened, of whom 26 declined to participate and 198 were excluded. A total of 122 participants were enrolled, of whom 39 completed the kMCT arm and 44 completed the placebo arm. At baseline, both groups were of similar age, education, ratio of females to males, and APOEε4 status. MoCA and MMSE results were also similar (Table 1). Participants from both groups had a similar level of physical activity and a low index of depression. All participants had normal functional autonomy (data not shown). Blood pressure and general blood chemistry profile were not different between the two groups.
TABLE 1.
Participant characteristics at enrollment
| Placebo | kMCT | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | |
| Females/males | 26/17 | 19/20 | .286 | ||
| APOEε4 (+/–) | 9/32 | 10/28 | .650 | ||
| Age (y) | 72.9 | 6.9 | 71.4 | 7.2 | .344 |
| Education (y) | 12.7 | 3.6 | 13.2 | 3.3 | .470 |
| GDS (/30) | 5.8 | 4.5 | 6.3 | 5.3 | .640 |
| PASE (/793) | 130 | 58 | 147 | 74 | .250 |
| MoCA (/30) | 24.0 | 2.3 | 24.1 | 2.8 | .826 |
| MMSE (/30) | 27.5 | 1.9 | 27.6 | 2.4 | .914 |
| Body mass index (kg/m2) | 26.1 | 4.2 | 27.9 | 3.9 | .113 |
| Blood pressure (systolic; mm Hg) | 140 | 16 | 133 | 17 | .075 |
| Blood pressure (diastolic; mm Hg) | 80 | 10 | 78 | 11 | .473 |
| Plasma metabolites | |||||
| Total ketone (μM) | 252 | 197 | 241 | 190 | .841 |
| Glucose (mM) | 4.8 | 0.6 | 5.0 | 0.8 | .131 |
| Glycated hemoglobin (%) | 5.6 | 0.5 | 5.7 | 0.4 | .253 |
| Total cholesterol (mM) | 5.0 | 1.2 | 4.8 | 1.0 | .573 |
| Triglycerides (mM) | 1.1 | 0.5 | 1.2 | 0.6 | .410 |
| Thyroid stimulating hormone (mUI/L) | 2.1 | 1.2 | 2.3 | 0.8 | .374 |
| Vitamin B12 (pmol/L) | 372 | 216 | 363 | 139 | .852 |
| Homocysteine (μM) | 9.8 | 2.4 | 10.3 | 2.7 | .383 |
Abbreviations: GDS, Geriatric Depression Screening scale; kMCT, ketogenic medium chain triglyceride; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; PASE, Physical Activity Scale for the Elderly; SD, standard deviation.
P‐values are for differences between groups as measured by independent sample t‐tests or chi‐square test of homogeneity.
3.1. Cognitive scores
The raw scores of the first free recall trial of the RL/RI‐16 test improved significantly in the kMCT group (model 2; P = .047; partial ƞ2 = 0.046; Table 2 and Figure 1A). No significant changes were observed in either group on the BVMT‐R. VF (Categories) scores were significantly higher post‐intervention in the kMCT group in (+1.9 words) compared to placebo (model 1, –1.0 words; P = .005), with a moderate effect size (partial ƞ2 = 0.098; Table 2 and Figure 1B). The kMCT group had significantly fewer errors on all conditions of the Trail‐Making (model 1, P = .020; partial ƞ2 = 0.067) and Stroop tests (model 1, P = .042; partial ƞ2 = 0.053, Table 2). BNT scores improved post‐intervention in the kMCT group with +1.1 total correct responses, while the placebo group had 0.2 fewer total correct responses (Model 1; P = .018; partial ƞ2 = 0.069; Table 2 and Figure 1C). These differences between treatment groups remained significant after normalization (Table 3). There was no change in raw or normalized attention or processing speed scores in either group (data not shown).
TABLE 2.
Raw scores on the neurophysiological tests before (PRE) and after (POST) the intervention in domains in which significant differences were observed
| Placebo (n = 43) | kMCT (n = 39) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Model 1 P | Model 2 P | Partial ƞ2 | |
| Episodic memory | |||||||||||
| RL/RI‐16–trial 1 free recall (/16) | 6.4 | 2.2 | 6.6 | 2.2 | 6.5 | 2.0 | 7.5 | 2.3 | .054 | .047 | 0.046 |
| RL/RI‐16–total free recall (/48) | 23.8 | 6.1 | 23.6 | 6.8 | 24.4 | 6.0 | 25.7 | 6.6 | .118 | 0.031 | |
| RL/RI‐16–total recall (/48) | 43.1 | 5.1 | 40.6 | 6.4 | 43.3 | 4.9 | 42.6 | 4.6 | .070 | 0.041 | |
| BVMT‐R–trial 1 (/12) | 3.7 | 2.0 | 4.6 | 2.1 | 3.1 | 2.1 | 4.6 | 2.4 | .647 | 0.003 | |
| BVMT‐R–total (/36) | 15.9 | 6.2 | 18.1 | 6.3 | 16.2 | 6.8 | 18.8 | 7.3 | .734 | 0.002 | |
| Executive function | |||||||||||
| Verbal fluency–letters (total correct) | 29.1 | 8.1 | 29.4 | 8.0 | 31.6 | 10.4 | 32.6 | 11.9 | .402 | 0.009 | |
| Verbal fluency–categories (total correct) | 32.5 | 6.2 | 31.5 | 7.5 | 33.4 | 7.8 | 35.3 | 7.8 | .005 | .024 | 0.098 |
| Trail‐Making–switching (sec) | 130 | 49 | 131 | 59 | 124 | 56 | 120 | 53 | .664 | 0.002 | |
| Trail‐Making–total errors, all conditions | 1.7 | 2.4 | 2.5 | 3.7 | 2.7 | 4.1 | 1.8 | 2.5 | .020 | .017 | 0.067 |
| Stroop–inhibition (sec) | 85 | 28 | 85 | 31 | 79 | 25 | 77 | 21 | .535 | 0.005 | |
| Stroop–inhibition/switching (sec) | 99 | 38 | 102 | 50 | 87 | 29 | 84 | 26 | .257 a | 0.017 | |
| Stroop–total errors, all conditions | 9.0 | 8.1 | 7.4 | 5.4 | 5.9 | 5.4 | 3.9 | 5.3 | .042 | .113 | 0.053 |
| Language | |||||||||||
| Boston Naming Test–spontaneous responses | 46.1 | 5.6 | 46.7 | 6.5 | 47.6 | 5.6 | 48.8 | 5.6 | .317 | .253 | 0.013 |
| Boston Naming Test–total correct responses (/60) | 53.2 | 3.6 | 53.0 | 4.8 | 53.7 | 4.1 | 54.8 | 3.9 | .018 b | .033 b | 0.069 |
Abbreviations: BVMT‐R, Brief Visuospatial Memory Test–Revised; kMCT, ketogenic medium chain triglyceride; RL/RI‐16, 16‐item free/cued word learning and recall test; Stroop, Stroop color‐word interference test.
Notes: Two analysis of covariance models were used to assess the difference (P‐value) between kMCT and placebo after the 6‐month intervention (POST vs PRE): in Model 1, pre‐intervention score was the only covariate; in Model 2, in addition to pre‐intervention score, the covariates were sex, age, education, and APOEε4 status.
Quade transformation was used for data sets that did not respect the assumption of homogeneity of variances.
Results from multiple regressions are reported as significant mean differences at any given value of the covariate.
Partial ƞ2: measure of effect size.
FIGURE 1.
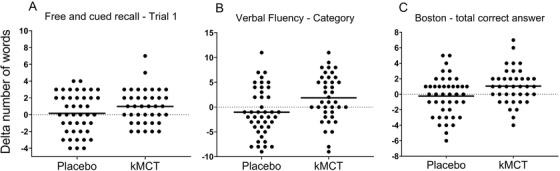
Change in cognitive scores. Change in raw scores from baseline (0) on the first trial of the RL/RI‐16 test (A) verbal fluency (categories) test (B) and Boston Naming Test (C‐ total correct responses), in the ketogenic medium chain triglyceride (kMCT) versus placebo group (P = .054, P = .005, P = .018, respectively)
TABLE 3.
Normalized Z scores on the neurophysiological tests before (PRE) and after (POST) the intervention in domains in which significant differences was observed
| PLACEBO (N = 43) | kMCT (N = 39) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | Model 1 | Model 2 | ||||||
| Z Score | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | P | Partial ƞ2 |
| Episodic memory | |||||||||||
| RL/RI‐16–trial 1 free recall | −0.84 | 1.09 | −0.74 | 1.13 | −0.77 | 0.89 | −0.26 | 1.04 | .042 | 0.051 | |
| RL/RI‐16–total free recall | −0.98 | 1.14 | −1.02 | 1.28 | −0.91 | 0.93 | −0.65 | 1.10 | .120 | 0.030 | |
| RL/RI‐16–total recall | −0.77 | 0.88 | −0.80 | 1.00 | −0.72 | 0.89 | −0.53 | 0.93 | .146 | 0.027 | |
| Executive function | |||||||||||
| Verbal fluency–letter | −0.56 | 0.83 | −0.52 | 0.80 | −0.33 | 1.05 | −0.21 | 1.18 | .410 | 0.009 | |
| Verbal fluency–categories | −0.12 | 0.83 | −0.23 | 0.93 | −0.05 | 0.93 | 0.22 | 0.95 | .005 | .018 | 0.098 |
| Trail‐Making–switching | −0.15 | 1.02 | −0.12 | 1.25 | −0.09 | 1.24 | 0.07 | 1.11 | .575 | 0.004 | |
| Trail‐Making–errors, switching | 0.26 | 0.60 | 0.03 | 0.93 | 0.08 | 0.95 | 0.25 | 0.66 | .043 | .047 | 0.052 |
| Stroop–inhibition | −0.51 | 1.17 | −0.40 | 1.21 | −0.22 | 1.12 | −0.14 | 0.99 | .953 | <0.001 | |
| Stroop–inhibition/switching | −0.70 | 1.31 | −0.58 | 1.19 | −0.21 | 1.11 | −0.12 | 1.06 | .369 a | 0.007 | |
| Stroop–errors inhibition | −0.07 | 1.08 | −0.02 | 0.97 | 0.18 | 0.83 | 0.45 | 0.73 | .046 | .092 | 0.051 |
| Stroop–errors inhibition/switching | −0.48 | 1.26 | −0.36 | 1.06 | 0.02 | 0.92 | 0.38 | 0.81 | .007 | .011 | 0.089 |
| Language | |||||||||||
| Boston–spontaneous responses | −1.85 | 1.29 | −1.64 | 1.31 | −1.58 | 1.23 | −1.28 | 1.26 | .416 | 0.008 | |
Abbreviations: kMCT, ketogenic medium chain triglyceride; RL/RI‐16, 16‐item free/cued word learning and recall test; Stroop, Stroop color‐word interference test.
Three analysis of covariance models were used to assess the difference (P‐value) between kMCT and placebo after the 6‐month intervention (POST vs PRE): in Model 1, the covariate was pre‐intervention score; in Model 2, the covariates were sex, education, APOEε4 status and pre‐intervention score.
Quade transformation was used for data sets that did not respect the assumption of homogeneity of variance.
Partial ƞ2: measure of effect size.
Subgroup analysis in the APOEε4(‒) and protocol‐compliant participants, also showed improvement in the kMCT group compared to placebo for VF (categories) and total correct responses on the BNT (P < .05). Improvement on some free recall tests was also seen post‐kMCT in these subgroups (Tables S1–S2 in supporting information). APOEε4(+) participants on kMCT (Table S3 in supporting information) had better immediate free recall (0.8 more words recalled while those on placebo had 0.9 fewer words recalled; P = .036), VF (categories; P = .048), and fewer total errors on the Trail‐Making Test (P = .017).
3.2. Metabolic and laboratory results
Post‐intervention, total plasma ketones were increased significantly in the kMCT group compared to placebo (P < .0001). There was no change in body mass index (BMI) or body weight in either group. After the intervention, glucose, cholesterol, and aspartate transaminase were significantly higher in the kMCT group but remained within the clinical reference range; no other changes in blood chemistry were observed (Table S4 in supporting information). Plasma C8 and C10 (Figure 3A) and ketone responses (Figure 3B) to the kMCT drink taken with a standard breakfast were robustly increased over 4 hours; pre‐ to post‐intervention, neither this response to oral kMCT nor baseline values (T0) changed for plasma C8, C10, or ketones. No change was observed on the placebo. The change in plasma total ketones correlated positively with the change in several cognitive tests of episodic memory, executive function, and language, with coefficients of correlation of r = +0.229 to +0.325 (P < .042 to .0028; Figure 2A, B, C). Additional significant correlations with the change in plasma ketone level were observed on the Trail‐Making Tests: fewer total errors (r = −0.233, P = .040), number‐letter switching (r = +0.268, P = .042), and switching versus motor speed condition contrast scale (r = +0.289, P = .0028; data not shown).
FIGURE 3.
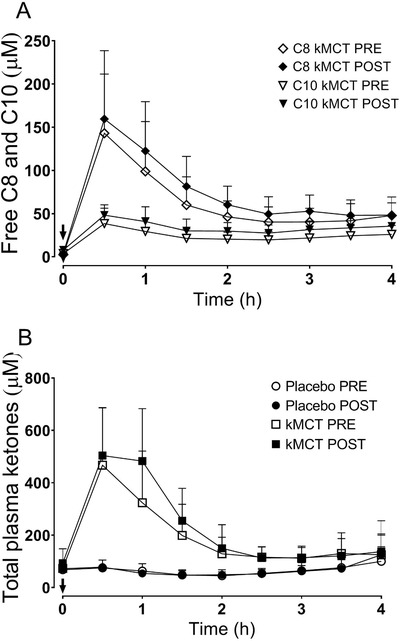
Plasma free caprylic acid (C8) and capric acid (C10) (A) and total ketone response (B) throughout the 4‐hour metabolic study day. A dose of 15 g of ketogenic medium chain triglyceride (kMCT) (A, B) or placebo drink (B) was consumed (arrow) before (◇C8 kMCT; ∇C10 kMCT; ○ Placebo; □ kMCT) and 6 months after supplementation (◆ C8 kMCT; ▾ C10 kMCT; ⬤ Placebo; ■ kMCT). For clarity, placebo data are not shown for C8 and C10 (A) but did not exceed the baseline values shown at T0 for C8 (3.9 μM) or C10 (7.9 μM). Data are means ± standard deviation
FIGURE 2.
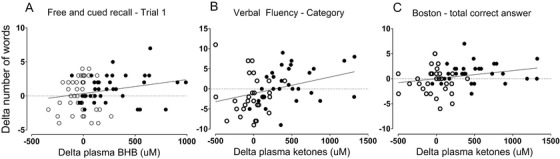
Plasma ketones and cognitive outcomes. Correlation between the change in plasma beta‐hydroxybutyrate (BHB) or change in plasma total ketones (BHB + acetoacetate) and three cognitive outcomes on the placebo (○) or ketogenic medium chain triglyceride (kMCT) (●): trial 1 of the RL/RI‐16 test (A; r = +0.232, P = .039), verbal fluency (categories) test (B; r = +0.325, P = .013), and Boston Naming Test (total correct answers) (C; r = +0.229, P = .042)
3.3. Compliance and adverse events
Of the 83 participants who completed the trial, protocol compliance was 89% ± 9% as measured by returned bottle count and did not differ between groups. In the kMCT group, 24 participants discontinued compared to 15 in the placebo group, for an average drop‐out rate of 32% over both groups. There were no serious adverse events in either group but among the participants that completed the study, 74% of the kMCT group and 40% of the placebo group reported at least one adverse event. Gastrointestinal‐related adverse events were responsible for 50% and 75% of the drop‐outs in the placebo and kMCT group, respectively. About one third of all adverse events resolved after the first month.
4. DISCUSSION
The BENEFIC Trial showed that performance on widely used tests of episodic memory, executive function, and language improved over 6 months in MCI when consuming 30 g/day of a kMCT drink relative to a matching placebo. Moderate to large effect sizes (partial ƞ2 of 0.06 to 0.14) were observed on four cognitive tests in the kMCT group, suggesting that these cognitive improvements were clinically relevant, especially on tests of executive function and language (Tables 2 and 3, 29 , 30 ).
Improved cognitive outcomes were directly and significantly correlated to increased plasma ketone levels (Figure 3) but also to increased ketone uptake by the brain, as shown in our previous study, 6 demonstrating a mechanistic link between mild ketosis, brain energy rescue by ketones, and improved cognitive outcomes. 1 We previously reported that in MCI and AD the brain retains a normal capacity to use additional ketones when they are available, 5 , 6 an observation that has recently been confirmed elsewhere. 31 Free (unesterified) C8 and C10 in blood are precursors to ketone formation by the liver but can also enter and be directly metabolized by the brain. 32 Hence, it is also possible that C8 and C10 have a direct effect on brain energetics and function without necessarily being first metabolized systemically to ketones.
In clinical AD or MCI trials, effects of two types of ketogenic intervention on cognition have been reported over the past decade: a kMCT supplement was tested in AD, 33 , 34 , 35 and a ketogenic diet was used in both MCI 31 , 36 and mild to moderate AD. 37 , 38 These trials showed some improvement in cognition but most were not powered to assess cognitive outcomes. We demonstrate here that providing 30 g/day of kMCT in two 15 g doses results in robust and sustained ketone production over 6 months with significantly improved cognition in MCI. This improvement was observed despite a variable ketone response that can possibly arise due to the high glycemic Western diet. Because the participants selected had normal glycemia, BMI, and cardiometabolic profile, we may have partially limited that variability in ketone response. Older people with pre‐diabetes or other metabolic disorders may have a mildly reduced response to an oral kMCT and might therefore benefit more from a multidomain approach to improve insulin sensitivity (low glycemic diet, exercise, etc.), a possibility that remains to be studied.
This kMCT drink significantly improved cognition in MCI independently of age, sex, education, and APOEε4 status (Tables 2 and 3, Tables S1–S3). The lack of effect of ApoEε4 status on cognition remains exploratory because this study was not adequately powered to assess this point. APOEε4 may impede the response to a ketogenic diet 33 or at least elicit a different response, as fewer cognitive domains were changed after the intervention in our APOEε4(+) participants (Table S2 and S3), but this remains to be clarified with a larger sample size.
No drugs are approved for MCI 39 and drugs used in AD do not delay cognitive decline in MCI. 40 , 41 Nevertheless, the current positive results with kMCT and those with exercise, 42 , 43 , 44 , 45 suggest that by improving brain energetics, cognitive outcomes can be improved in MCI. Performance on the free recall test is predictive of AD diagnosis. 29 , 30 , 46 , 47 For example, a score of 20–24/48 on total free recall is associated with a delay of 4.03 years to AD diagnosis while a score of 25 to 30 is associated with a 4.89 year delay. 29 Hence, with a free recall performance of 25.7 words on the kMCT drink and 23.6 on placebo (Table 2), the kMCT group would be predicted to develop AD several months later than the placebo group. The BENEFIC Trial was not powered to assess either the rate of progression from MCI to AD or a possible delay in onset of AD but it provides a rationale on which such future ketogenic intervention trials could be designed. A longer intervention with more sustained mild ketonemia and other favorable metabolic changes, that is, lower glycemia, may be necessary to achieve this goal. Slowing progression toward AD during the MCI phase by 1 or more years could result in meaningful improvements for the patient and substantial savings for society. 48
One of the strengths of our present results is the beneficial effect of kMCT in three cognitive domains—episodic memory, executive function, and language—an improvement that remained significant after adjustment for covariates well known to influence cognitive function (age, sex, education, APOEε4 status; Tables 2 and 3). Another strength is that our participants were recruited both directly from the community and from memory clinic referrals, so the results are broadly generalizable to the general population. The kMCT arm had a calorie‐ and organoleptically matched placebo, so the beneficial effect of this particular kMCT‐based drink on cognition was clearly due to it producing ketones not just to it being a generic source of fat calories (Tables 3 and 4; Figure 3). The plasma ketone response and metabolic profile did not change significantly during the trial (Figure 3, Table S4). There were no serious adverse events and protocol compliance was 89% for participants that completed the study, so it is clearly feasible to conduct a kMCT supplementation trial in MCI over a period long enough to monitor change in cognitive performance. After the 6‐month intervention, cardiometabolic measures were unchanged or remained within the normal reference range for age (Table S4), suggesting that concerns about saturated fat and weight gain or other aspects of cardiovascular health are not justified in this population in relation to consuming our kMCT (or the placebo) drink.
One limitation to this trial is that the overall drop‐out rate was 32% and 38% in the kMCT group. An improved formulation would undoubtedly reduce these adverse events but they may be difficult to completely eliminate for a 30 g/day dose of kMCT so an alternative ketone supplement would be worth testing. We also did not directly assess AD biomarkers of neuropathology so despite the clinical improvement, it is unclear whether the kMCT achieved disease modification. We did measure plasma amyloid beta (Aβ) but did not see any differences between groups or after the kMCT or placebo (data not shown). Brain PET and cerebrospinal fluid markers of Aβ and phosphorylated tau have been reported to decrease in MCI after just 8 weeks on a modified Mediterranean ketogenic diet. 31 Animal studies show that various ketogenic interventions reduce neuropathological load in transgenic AD mice. 49 , 50 Hence, it is plausible that some degree of disease modification could have occurred in the present trial but this awaits confirmation. There was no post‐intervention wash‐out period so whether cognitive function would have returned to pre‐intervention levels is unknown.
We conclude that this formulation of a kMCT drink improved four cognitive outcomes in MCI by increasing blood ketone levels and presumably improving brain energy supply because blood ketone levels and brain ketone metabolism are directly related. 6 It is safe and feasible for an MCI population to consume a 15 g kMCT supplement twice daily for 6 months. Assessment of whether a ketogenic intervention can delay progression of MCI to AD is now warranted.
Funding information
This research was supported by the Alzheimer Association USA (Part the Cloud program; Phase 1) and Nestlé Health Sciences (Phase 2). Abitec provided the Captex 355 and placebo oil. EMC is supported by a Postdoctoral Fellowship from the Alzheimer Society Research Program ‐ Alzheimer Society Canada and the Fonds de Recherche en Santé du Québec (FRQS). MR was funded by MITACS.
CONFLICTS OF INTEREST
Stephen C. Cunnane has consulted for or received travel honoraria or test products for research from Nestlé Health Science, Bulletproof, Cerecin, and Abitec. Stephen C. Cunnane is the founder and director of the consulting company, Senotec Ltd. JPG is an employee of the Société des Produits Nestlé S.A. There are no other conflicts to report.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Audrey Perreault, Etienne Croteau, Camille Vandenberghe, Mélanie Martineau (INAF, Université Laval, Quebec City), Isabelle Audet (Agriculture and Agri‐food Canada), Irina Breton and Isabelle Monard (Nestlé Research, Lausanne, Switzerland) provided valuable technical assistance.
Fortier M, Castellano C‐A, St‐Pierre V, et al. A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6‐month RCT. Alzheimer's Dement. 2021;17:543–552. 10.1002/alz.12206
REFERENCES
- 1. Cunnane SC, Trushina E, Morland C, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morbelli S, Brugnolo A, Bossert I, et al. Visual versus semi‐quantitative analysis of 18F‐FDG‐PET in amnestic MCI: an European Alzheimer's Disease Consortium (EADC) project. J Alzheimers Dis. 2015;44:815‐826. [DOI] [PubMed] [Google Scholar]
- 3. Ishibashi K, Onishi A, Wagatsuma K, Fujiwara Y, Ishii K. Longitudinal 18F‐FDG images in patients with Alzheimer disease over more than 9 years from a preclinical stage. Clin Nucl Med. 2020;45:e185‐e9. [DOI] [PubMed] [Google Scholar]
- 4. Castellano CA, Baillargeon JP, Nugent S, et al. Regional brain glucose hypometabolism in young women with polycystic ovary syndrome: possible link to mild insulin resistance. PLoS One. 2015;10:e0144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croteau E, Castellano CA, Richard MA, et al. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer's disease. J Alzheimers Dis. 2018;64:551‐561. [DOI] [PubMed] [Google Scholar]
- 6. Fortier M, Castellano CA, Croteau E, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15:625‐634. [DOI] [PubMed] [Google Scholar]
- 7. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303‐308. [DOI] [PubMed] [Google Scholar]
- 8. Yesavage JA, Brink TL, Rose TL. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37‐49. [DOI] [PubMed] [Google Scholar]
- 9. Hébert R, Guilbault J, Desrosiers J, Dubuc N. The functional autonomy measurement system (SMAF): a clinical‐based instrument for measuring disabilities and handicaps in older people. Can Geriatr J. 2001;4:141‐147. [Google Scholar]
- 10. American Psychiatric Association, & Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (DSM‐5): Washington, D.C., USA: American Psychiatric Association; 2013. [Google Scholar]
- 11. Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. ‘Mini mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 13. Van der Linden M, Coyette F, Poitrenaud J. GREMEM. L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI‐16). In: Van der Linden SAM, & Agniel A, eds. L'evaluation des troubles de la mémoire episodique (avec leur etalonnage). Marseille, France: Solal; 2004:25‐47. [Google Scholar]
- 14. Benedict R. Brief Visuospatial Memory Test‐Revised Professional Manual. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 15. Delis D, Kaplan E, Kramer J. In: Corporation P, ed. Delis‐Kaplan Executive Function System (D‐KEFS). San Antonio, TX (USA); 2001. [Google Scholar]
- 16. Wechsler D. Wechsler Memory Scale‐Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 17. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 18. Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston Naming Test. Clin Neuropsychol. 2007;21:617‐637. [DOI] [PubMed] [Google Scholar]
- 19. Dion M, Potvin O, Belleville S, et al. Normative data for the rappel libre/Rappel indice a 16 items (16‐item free and cued recall) in the elderly Quebec‐French population. Clin Neuropsychol. 2015;28(Suppl 1):S1‐S19. [DOI] [PubMed] [Google Scholar]
- 20. St‐Pierre V, Vandenberghe C, Lowry CM, et al. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front Nutr. 2019;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St‐Pierre V, Courchesne‐Loyer A, Vandenberghe C, Hennebelle M, Castellano CA, Cunnane SC. Butyrate is more ketogenic than leucine or octanoate‐monoacylglycerol in healthy adult humans. J Funct Foods. 2017;32:170‐175. [Google Scholar]
- 22. Koch W, Ehrenhaft A, Griesser K, et al. TaqMan systems for genotyping of disease‐related polymorphisms present in the gene encoding apolipoprotein E. Clin Chem Lab Med. 2002;40:1123‐1131. [DOI] [PubMed] [Google Scholar]
- 23. Simard F, Guay F, Girard CL, Giguère A, Laforest JP, Matte JJ. Effects of concentrations of cyanocobalamin in the gestation diet on some criteria of vitamin B12 metabolism in first‐parity sows. J Anim Sci. 2007;85:3294‐3302. [DOI] [PubMed] [Google Scholar]
- 24. Wan PJ, Dowd MK, Thomas AE, Butler BH. Trimethylsilyl derivatization/gas chromatography as a method to determine the free fatty acid content of vegetable oils. J Am Oil Chem Soc. 2007;84:701‐708. [Google Scholar]
- 25. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175‐191. [DOI] [PubMed] [Google Scholar]
- 26. Dimitrov D, Rumrill P. Pretest‐posttest designs and measurement of change. Work. 2003;20:159‐165. [PubMed] [Google Scholar]
- 27. Quade D. Rank analysis of covariance. J Am Statis Assoc. 1967;62:1187‐1200. [Google Scholar]
- 28. Cohen J. Statistical Power Analysis for the Behavioral Sciences, Rev. ed. Hillsdale, NJ, USA: Lawrence Erlbaum Associates, Inc; 1977. [Google Scholar]
- 29. Grober E, Veroff AE, Lipton RB. Temporal unfolding of declining episodic memory on the free and cued selective reminding test in the predementia phase of Alzheimer's disease: implications for clinical trials. Alzheimers Dement. 2018;10:161‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Auriacombe S, Helmer C, Amieva H, Berr C, Dubois B, Dartigues JF. Validity of the free and cued selective reminding test in predicting dementia: the 3C study. Neurology. 2010;74:1760‐1767. [DOI] [PubMed] [Google Scholar]
- 31. Neth BJ, Mintz A, Whitlow C, et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at‐risk for Alzheimer's disease: a Pilot Study. Neurobiol Aging. 2019;86:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928‐5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC‐1202 in mild to moderate Alzheimer's disease: a randomized, double‐blind, placebo‐controlled, multicenter trial. Nutr Metab. 2009;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ota M, Matsuo J, Ishida I, et al. Effects of a medium‐chain triglyceride‐based ketogenic formula on cognitive function in patients with mild‐to‐moderate Alzheimer's disease. Neurosci Lett. 2019;690:232‐236. [DOI] [PubMed] [Google Scholar]
- 35. Xu Q, Zhang Y, Zhang X, et al. Medium‐chain triglycerides improved cognition and lipid metabolomics in mild to moderate Alzheimer's disease patients with APOE4(−/−): a double‐blind, randomized, placebo‐controlled crossover trial. Clin Nutr. 2019;39(7):2092‐2105. [DOI] [PubMed] [Google Scholar]
- 36. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer's disease. Alzheimer's Dement. 2018;4:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brandt J, Buchholz A, Henry‐Barron B, Vizthum D, Avramopoulos D, Cervenka MC. Preliminary report on the feasibility and efficacy of the modified atkins diet for treatment of mild cognitive impairment and early Alzheimer's disease. J Alzheimers Dis. 2019;68:969‐981. [DOI] [PubMed] [Google Scholar]
- 39. Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673‐2734. [DOI] [PubMed] [Google Scholar]
- 40. Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48‐week randomized, placebo‐controlled trial. Neurology. 2009;72:1555‐1561. [DOI] [PubMed] [Google Scholar]
- 41. Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo‐controlled trial. Neurology. 2004;63:651‐657. [DOI] [PubMed] [Google Scholar]
- 42. Liu‐Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12‐month randomized controlled trial. Arch Intern Med. 2010;170:170‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagamatsu LS, Chan A, Davis JC, et al. Physical activity improves verbal and spatial memory in older adults with probable mild cognitive impairment: a 6‐month randomized controlled trial. J Aging Res. 2013;2013:861893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fiatarone Singh MA, Gates N, Saigal N, et al. The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double‐blind, double‐sham controlled trial. J Am Med Dir Assoc. 2014;15:873‐880. [DOI] [PubMed] [Google Scholar]
- 45. Baker LD, Frank LL, Foster‐Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grande G, Vanacore N, Vetrano DL, et al. Free and cued selective reminding test predicts progression to Alzheimer's disease in people with mild cognitive impairment. Neurol Sci. 2018;39:1867‐1875. [DOI] [PubMed] [Google Scholar]
- 47. Derby CA, Burns LC, Wang C, et al. Screening for predementia AD: time‐dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Association As. 2019 Alzheimer's disease facts and figures. Alzheimer's Dement. 2019;15:321‐387. [Google Scholar]
- 49. Zilberter M, Ivanov A, Ziyatdinova S, et al. Dietary energy substrates reverse early neuronal hyperactivity in a mouse model of Alzheimer's disease. J Neurochem. 2013;125:157‐171. [DOI] [PubMed] [Google Scholar]
- 50. Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D‐beta‐hydroxybutyrate protects neurons in models of Alzheimer's and Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:5440‐5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153‐162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


