ABSTRACT
Objective
To evaluate whether there is a differential benefit of planned Cesarean delivery (CD) over planned vaginal delivery (VD) in women with a twin pregnancy and the first twin in cephalic presentation, depending on prespecified baseline maternal and pregnancy characteristics, and/or gestational age (GA) at delivery.
Methods
This was a secondary analysis of the Twin Birth Study, which included 2804 women with a twin pregnancy and the first twin (Twin A) in cephalic presentation between 32 + 0 and 38 + 6 weeks' gestation at 106 centers in 25 countries. Women were assigned randomly to either planned CD or planned VD. The main outcome measure was composite adverse perinatal outcome, defined as the occurrence of perinatal mortality or serious neonatal morbidity in at least one twin. The baseline maternal and pregnancy characteristics (markers) considered were maternal age, parity, history of CD, use of antenatal corticosteroids, estimated fetal weight (EFW) of Twin A, EFW of Twin B, > 25% difference in EFW between the twins, presentation of Twin B, chorionicity on ultrasound, method of conception, complications of pregnancy, ruptured membranes at randomization and GA at randomization. Separate logistic regression models were developed for each marker in order to model composite adverse perinatal outcome as a function of the specific marker, planned delivery mode and the interaction between these two terms. In addition, multivariable logistic regression analysis with backward variable elimination was performed separately in each arm of the trial. The association between planned mode of delivery and composite adverse perinatal outcome, according to GA at delivery, was assessed using logistic regression analysis.
Results
Of the 2804 women initially randomized, 1391 were included in each study arm. None of the studied baseline markers was associated with a differential benefit of planned CD over planned VD in the rate of composite adverse perinatal outcome. GA at delivery was associated differentially with composite adverse perinatal outcome in the treatment arms (P for interaction < 0.001). Among pregnancies delivered at 32 + 0 to 36 + 6 weeks, there was a trend towards a lower rate of composite adverse perinatal outcome in those in the planned‐VD group compared with those in planned‐CD group (29 (2.2%) vs 48 (3.6%) cases; odds ratio (OR) 0.62 (95% CI, 0.37–1.03)). In pregnancies delivered at or after 37 + 0 weeks, planned VD was associated with a significantly higher rate of composite adverse perinatal outcome, as compared with planned CD (23 (1.5%) vs 10 (0.7%) cases; OR, 2.25 (95% CI, 1.06–4.77)).
Conclusion
The perinatal outcome of twin pregnancies with the first twin in cephalic presentation may differ depending on GA at delivery and planned mode of delivery. At 32–37 weeks, planned VD seems to be favorable, while, from around 37 weeks onwards, planned CD might be safer. The absolute risks of adverse perinatal outcomes at term are low and must be weighed against the increased maternal risks associated with planned CD. © 2019 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: gestational age, mode of delivery, multiple pregnancy, twins
CONTRIBUTION —
What are the novel findings of this work?
In women with a twin pregnancy, we identified gestational age at delivery to be a strong prognostic factor for the composite outcome of perinatal mortality and severe neonatal morbidity, an association that was dependent upon the planned mode of delivery.
What are the clinical implications of this work?
Women with a twin pregnancy and the first twin in cephalic presentation should be counseled that planned vaginal delivery, compared with planned Cesarean delivery, seems to have a favorable effect on perinatal outcome from 32 to 37 weeks' gestation, while, from around 37 weeks onwards, Cesarean delivery might be safer for both babies; however, the absolute risks are very low.
INTRODUCTION
Among the many challenges in the management of women with twin pregnancy, mode of delivery is the last decision to be made before birth. In order to answer the question of whether delivery should be performed by planned Cesarean delivery (CD) or by planned vaginal delivery (VD), the Twin Birth Study (TBS) randomized 2804 women with a twin pregnancy and the first twin presenting in cephalic position between 32 + 0 and 38 + 6 weeks' gestation. The study showed that planned CD, in comparison with planned VD, did not influence the risk of perinatal mortality and morbidity (2.2% vs 1.9%; odds ratio (OR), 1.16 (95% CI, 0.77–1.74); P = 0.49) 1 .
While the results of the TBS were reassuring, women were included over a wide gestational‐age (GA) range. The impact of GA at delivery on perinatal outcome is much stronger at early GAs of 32 to 33 weeks than at beyond 37 weeks. The relatively high prevalence of perinatal mortality and morbidity at early GAs, in combination with the increased rate of preterm birth in women with a twin pregnancy, may mask the actual effect of mode of delivery at term 2 , 3 , 4 .
The TBS incorporated a subgroup analysis for GA at randomization (32 + 0 to 33 + 6 weeks, 34 + 0 to 36 + 6 weeks and 37 + 0 to 38 + 6 weeks), and found no statistically significant association between GA at randomization and planned mode of delivery. However, delivery often occurred weeks after randomization. The subgroup of women randomized after 37 weeks seemed to benefit from planned CD in terms of composite adverse perinatal outcome (seven events in 500 fetuses (1.4%) in the planned‐VD group compared with two events in 477 fetuses (0.4%) in the planned‐CD group; OR = 3.3) 1 .
The aims of this re‐analysis of the TBS data were two‐fold. First, we aimed to investigate whether baseline maternal and pregnancy characteristics can be used to guide the decision between planned CD and planned VD. Second, we aimed to evaluate the impact of GA at delivery on the association between planned delivery method and the primary outcome of the trial, which was a composite of perinatal mortality and severe neonatal morbidity.
METHODS
Study design and participants
This was a secondary analysis of data collected in the TBS (trial registration number ISRCTN74420086, NCT00187369; protocol http://sites.utoronto.ca/miru/tbs/Protocol_march_2005.pdf), which was a multicenter open‐label randomized controlled trial conducted between 2003 and 2011 that had recruited 2804 women with a twin pregnancy from 106 participating centers in 25 countries. The background, methods, baseline characteristics of the participating women and the results have been reported previously 1 . In short, women were eligible for the study if they had a twin pregnancy between 32 + 0 and 38 + 6 weeks' gestation, the first twin (Twin A) was in cephalic presentation and both fetuses were alive with an estimated weight between 1500 g and 4000 g, confirmed by ultrasound within 7 days before randomization. Women with a pregnancy as early as 32 weeks were enrolled, because many women with a twin pregnancy wish to begin planning the method of delivery at this stage and because many twin births are preterm.
After providing informed consent, women were assigned randomly to planned CD or planned VD, using a computerized randomization program with stratification according to parity (0 vs ≥ 1) and GA. The trial profile is illustrated in Figure 1. Participants with an indication for delivery underwent either elective CD (for those in the planned‐CD group) or labor induction (for those in the planned‐VD group). Delivery was planned between 37 + 5 weeks and 38 + 6 weeks, as evidence at the time suggested that perinatal outcome is optimal during this GA window. The primary outcome of the TBS was a composite of perinatal mortality or serious neonatal morbidity. Serious neonatal morbidity was defined as one or more of the following: birth trauma; 5‐min Apgar score < 4; abnormal level of consciousness; seizures on at least two occasions before 72 h of age; need for assisted ventilation with the use of an endotracheal tube, inserted within 72 h after birth and remaining in place for at least 24 h; septicemia or meningitis within 72 h after birth; necrotizing enterocolitis; bronchopulmonary dysplasia; Grade‐III or ‐IV intraventricular hemorrhage; or cystic periventricular leukomalacia 1 .
Figure 1.
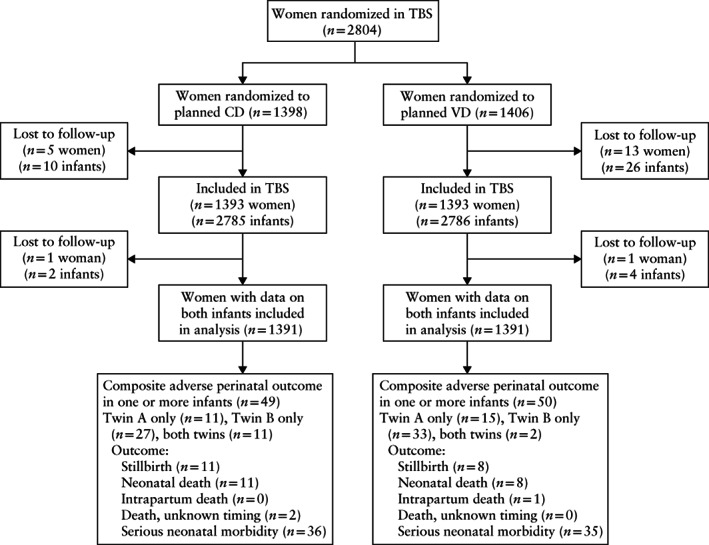
Flowchart summarizing randomization of women with twin pregnancy in Twin Birth Study (TBS) to planned Cesarean delivery (CD) or planned vaginal delivery (VD).
The research ethics committee at each participating center approved the study, and the Research Ethics Board at the Sunnybrook Health Sciences Centre approved all secondary analyses. For the purpose of this analysis, we chose to use the binary outcome of composite adverse perinatal outcome, defined as the occurrence of perinatal mortality or serious neonatal morbidity in at least one twin. At the time at which the trial was conducted, there was no core outcome set for multiple pregnancy.
We selected a set of 13 baseline maternal and pregnancy characteristics (markers) that have been suggested as prognostic factors for outcome after planned CD or planned VD. These were maternal age, parity, history of CD, use of antenatal corticosteroids, estimated fetal weight (EFW) of Twin A, EFW of Twin B, > 25% difference in EFW between the twins (Twin B > Twin A), presentation of Twin B (cephalic vs non‐cephalic), chorionicity on ultrasound, method of conception, complications of pregnancy (presence of at least one of the following complications: pre‐eclampsia, other hypertensive disorders, chorioamnionitis, placental abruption, placenta previa, antepartum hemorrhage, diabetes or other serious maternal or obstetrical complication), ruptured membranes at randomization and GA at randomization.
In the original study, it was calculated that a sample size of 2800 pregnancies would be needed in order to detect a reduction from 4% to 2% in the risk of the composite primary outcome of fetal or neonatal death or serious neonatal morbidity with a policy of planned CD, with 80% power and a two‐sided type‐I error rate of 0.05, allowing for a 10% rate of crossover between groups. We estimated a power of about 76% for investigating the effect of the interaction between GA at delivery and management on composite adverse perinatal outcome, which is a realistic and acceptable estimate for a subgroup analysis (Appendix S1).
Statistical analysis
To evaluate if the decision regarding the planned mode of delivery can potentially be guided by the selected markers, we developed a series of logistic regression models, each including one of the abovementioned markers, a management indicator (planned VD vs planned CD) and a marker‐by‐management interaction term. The dependent variable of the models was composite adverse perinatal outcome for either of the twins. Furthermore, we used multivariable logistic regression modeling including all markers, using a stepwise backward elimination approach (with Akaike information criterion) twice (once in the planned‐CD group and once in the planned‐VD group), in order to investigate whether we could identify a subgroup of women whose predicted risk of composite adverse perinatal outcome would be lower following planned CD than it would be following planned VD.
We developed a logistic regression model to evaluate the association between GA at delivery of Twin A (as a continuous variable) and the outcome of the planned delivery method. The model predicts the occurrence of composite adverse perinatal outcome as a function of GA at delivery, planned mode of delivery and the interaction between these two terms. For all values of GA at delivery, we calculated the risk of composite adverse perinatal outcome separately for planned VD and planned CD and calculated the risk difference; CIs for the risk differences were calculated using bootstrapping.
We also compared composite adverse perinatal outcome between the planned‐VD and planned‐CD groups in the subgroup delivered at 32 + 0 to 36 + 6 weeks and in the subgroup delivered at or after 37 + 0 weeks. ORs with 95% CIs for the association between planned VD, as compared with planned CD, and composite adverse perinatal outcome and its components were calculated using a logistic regression model for any fetus and separately for Twin A and Twin B.
All analysis in this study was exploratory and performed based on an intention‐to‐treat principle. We used R for Windows (Version 3.0.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of 1393 women who were planned to deliver by CD, 1252 (89.9%) eventually delivered both twins by CD and 140 (10.1%) delivered one or both twins vaginally (11 (0.7%) women had a combined delivery); 92 (6.6%) women delivered vaginally at the woman's request, and data were missing for one woman. Of 1393 women who were planned to deliver vaginally, 783 (56.2%) delivered both twins vaginally, 144 (10.3%) delivered by Cesarean section at the woman's request and the remaining 466 (33.5%) delivered at least one of the twins by Cesarean section because of maternal or fetal indications; 59 women (4.2%) had a combined delivery. The reasons for CD in both groups are summarized in Table S1.
Of 1391 women with information on both fetuses allocated to planned CD, 49 (3.5%) experienced composite adverse perinatal outcome; 11 (0.8%) cases occurred in Twin A only, 27 (1.9%) in Twin B only and 11 (0.8%) in both twins. Among the 1391 women allocated to planned VD with information on both fetuses, 50 (3.6%) experienced composite adverse perinatal outcome; 15 (1.1%) cases occurred in Twin A only, 33 (2.4%) in Twin B only and 2 (0.1%) in both twins (Figure 1). This resulted in a relative risk (RR) of composite adverse perinatal outcome in the planned‐VD vs planned‐CD group of 0.98 (95% CI, 0.66–1.44).
Baseline characteristics in guiding management
Table 1 summarizes the distribution of the 13 investigated markers in each arm of the trial. Generally, the distribution of the studied markers was similar between the trial arms. The association between each marker and composite adverse perinatal outcome following planned VD or planned CD is also presented in Table 1. In both the planned‐VD and planned‐CD trial arms, higher GA at randomization was associated significantly with a lower risk of composite adverse perinatal outcome (OR, 0.84 (95% CI, 0.71–0.98) and OR, 0.73 (95% CI, 0.61–0.87), respectively) (Figure S1).
Table 1.
Baseline characteristics of 2786 twin pregnancies in Twin Birth Study and association with composite adverse perinatal outcome, according to randomization to planned vaginal delivery or planned Cesarean delivery
| Characteristic | Planned vaginal delivery (n = 1393 women and 2786 infants) | Planned Cesarean delivery (n = 1393 women 2785 infants) | P for interaction* | ||
|---|---|---|---|---|---|
| n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | ||
| Maternal age | 1.00 (0.96–1.05) | 1.01 (0.96–1.06) | 0.948† | ||
| < 25 years | 12/365 (3.3) | 16/371 (4.3) | |||
| 25–29 years | 14/396 (3.5) | 11/390 (2.8) | |||
| 30–34 years | 14/357 (3.9) | 9/366 (2.5) | |||
| 35–39 years | 9/222 (4.1) | 10/216 (4.6) | |||
| > 39 years | 1/53 (1.9) | 3/50 (6.0) | |||
| Parity | 0.108 | ||||
| Nulliparous | 18/537 (3.4) | Reference | 22/536 (4.1) | Reference | |
| Parous | 32/856 (3.7) | 1.12 (0.63–2.06) | 27/857 (3.2) | 0.76 (0.43–1.36) | |
| Previous Cesarean delivery | 0.62 | ||||
| Yes | 4/97 (4.1) | 1.17 (0.35–2.95) | 6/100 (6.0) | 1.85 (0.69–4.15) | |
| No | 46/1296 (3.5) | Reference | 43/1293 (3.3) | Reference | |
| Gestational age at randomization | 0.84 (0.71–0.98) | 0.73 (0.61–0.87) | 0.095† | ||
| 32 + 0 to 33 + 6 weeks | 25/478 (5.2) | 24/475 (5.1) | |||
| 34 + 0 to 36 + 6 weeks | 18/665 (2.7) | 23/679 (3.4) | |||
| 37 + 0 to 38 + 6 weeks | 7/250 (2.8) | 2/239 (0.8) | |||
| Method of conception | 0.273 | ||||
| Spontaneous | 38/1170 (3.2) | Reference | 42/1156 (3.6) | Reference | |
| Assisted reproduction | 12/223 (5.4) | 1.69 (0.83–3.20) | 7/237 (3.0) | 0.81 (0.33–1.71) | |
| EFW Twin A | 0.999 (0.998–1.000) | 0.999 (0.998–1.00) | 0.618† | ||
| < 2000 g | 21/409 (5.1) | 20/440 (4.5) | |||
| 2000–2499 g | 22/578 (3.8) | 21/563 (3.7) | |||
| 2500–2999 g | 7/351 (2.0) | 7/323 (2.2) | |||
| ≥ 3000 g | 0/53 (0.0) | 1/65 (1.5) | |||
| EFW Twin B | 0.999 (0.998–0.999) | 0.999 (0.998–1.00) | 0.922† | ||
| < 2000 g | 24/412 (5.8) | 22/419 (5.3) | |||
| 2000–2499 g | 21/602 (3.5) | 19/607 (3.1) | |||
| 2500–2999 g | 5/313 (1.6) | 7/311 (2.3) | |||
| ≥ 3000 g | 0/65 (0.0) | 1/54 (1.9) | |||
| Difference in EFW > 25%, Twin B > Twin A | 0.982 | ||||
| Yes | 0/15 (0.0) | — | 1/10 (10.0) | 3.08 (0.17–16.87) | |
| No | 50/1376 (3.6) | Reference | 48/1380 (3.5) | Reference | |
| Presentation of Twin B | 0.292 | ||||
| Cephalic | 23/783 (2.9) | Reference | 28/792 (3.5) | Reference | |
| Non‐cephalic | 27/610 (4.4) | 1.53 (0.87–2.72) | 21/601 (3.5) | 0.99 (0.55–1.75) | |
| Chorionicity on ultrasound | 0.194 | ||||
| Dichorionic | 42/970 (4.3) | 2.06 (0.98–5.06) | 32/961 (3.3) | 1.01 (0.52–2.12) | |
| Monochorionic | 7/326 (2.1) | Reference | 11/334 (3.3) | Reference | |
| Antenatal corticosteroids used | 0.735 | ||||
| Yes | 23/452 (5.1) | 1.81 (1.02–3.19) | 24/452 (5.3) | 2.06 (1.16–3.65) | |
| No | 27/941 (2.9) | Reference | 25/941 (2.7) | Reference | |
| Pregnancy complications | 0.182 | ||||
| None | 47/1289 (3.6) | Reference | 39/1253 (3.1) | Reference | |
| ≥ 1 | 3/104 (2.9) | 0.79 (0.19–2.21) | 10/140 (7.1) | 2.39 (1.11–4.72) | |
| Membranes ruptured at randomization | 0.589 | ||||
| Yes | 3/76 (3.9) | 1.11 (0.26–3.11) | 5/83 (6.0) | 1.84 (0.62–4.36) | |
| No | 47/1313 (3.6) | Reference | 44/1307 (3.4) | Reference | |
| Gestational age at delivery | 0.68 (0.40–1.20) | 0.19 (0.10–0.38) | < 0.001 | ||
| 32 + 0 to 36 + 6 weeks | 29/1292 (2.2) | 48/1338 (3.6) | |||
| 37 + 0 to 38 + 6 weeks | 23/1490 (1.5) | 10/1444 (0.7) | |||
P for interaction obtained using logistic regression analysis.
Odds ratios (OR) calculated using generalized estimation equation method, which takes into account correlation between twins. When number of events in one cell is zero, stable OR cannot be calculated. EFW, estimated fetal weight.
We did not observe a statistically significant interaction with planned mode of delivery for any of the studied markers (Table 1) and they therefore did not show any potential to aid in the decision between planned CD and planned VD in the studied population of women.
Multivariable logistic regression analysis of the association between the studied markers and composite adverse perinatal outcome was performed with stepwise backward variable selection. In the planned‐VD group, none of the variables remained in the model. In the planned‐CD group, the only variable that remained in the model was GA at randomization. The full models are summarized in Table S2. We could not identify any combination of baseline characteristics that would aid in the identification of women who were more likely to benefit from planned CD over planned VD.
Association between GA at delivery and risk of composite adverse perinatal outcome
Median GA at randomization was 34.9 weeks (interquartile range (IQR) 33.3–36.4 weeks) and median GA at delivery was 37.0 weeks (IQR, 35.9–37.9 weeks). Median time from enrollment to delivery was 1.3 weeks (IQR, 0.3–3.0 weeks). In the study group overall, GA at delivery was associated significantly with the risk of composite adverse perinatal outcome (OR, 0.67 (95% CI, 0.60–0.76)); in other words, for each additional week of gestation, the risk of composite adverse perinatal outcome decreased by 33%.
While women allocated to planned CD delivered on average only 0.1 week earlier than did those allocated to planned VD (mean ± SD, 36.7 ± 1.5 weeks vs 36.8 ± 1.5 weeks), the association between GA at delivery and composite adverse perinatal outcome differed between the trial arms (Table S3). Figure 2a shows the probability of composite adverse perinatal outcome as a function of GA at delivery. In women allocated to planned CD, the risk of composite adverse perinatal outcome was higher at earlier GAs (32–36 weeks) at delivery. The difference became smaller with more advanced GA, and the lines for the two management options cross at 36 weeks. After 36 weeks, planned CD is associated with a lower risk of composite adverse perinatal outcome. A risk difference plot showed that the benefit from planned VD is smaller when delivery is between 32 and 35.5 weeks (Figure 2b). From 37 weeks onwards, the upper limit of the 95% CI of the risk difference line crosses zero.
Figure 2.
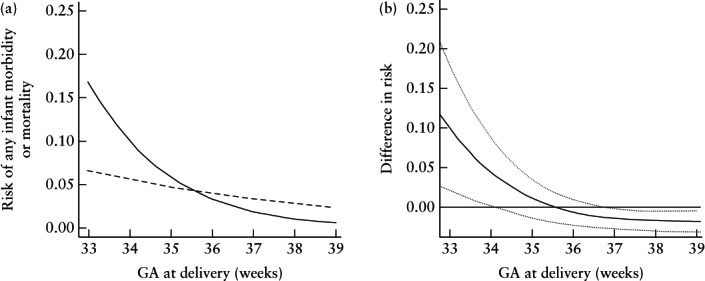
(a) Risk of composite adverse perinatal outcome in twin pregnancies randomized to planned Cesarean delivery (CD) ( ) and those randomized to planned vaginal delivery (VD) (
) and those randomized to planned vaginal delivery (VD) ( ), according to gestational age (GA). (b) Difference (with 95% CI) in model‐based estimated risks of composite adverse perinatal outcome (of one or both twins) between planned‐CD and planned‐VD groups, according to GA at delivery. Values above 0 represent better outcome in planned‐VD group, and those below zero represent better outcome in planned‐CD group.
), according to gestational age (GA). (b) Difference (with 95% CI) in model‐based estimated risks of composite adverse perinatal outcome (of one or both twins) between planned‐CD and planned‐VD groups, according to GA at delivery. Values above 0 represent better outcome in planned‐VD group, and those below zero represent better outcome in planned‐CD group.
We classified the study groups into two subgroups according to a GA at delivery of 32 + 0 to 36 + 6 weeks or at or after 37 + 0 weeks. In Table 2, the rate of composite adverse perinatal outcome and its components are shown according to planned mode of delivery and GA at delivery. The association between GA at delivery and composite adverse perinatal outcome is presented separately for Twin A and Twin B in Figure S2. With increasing GA at delivery, the risk of composite adverse perinatal outcome in both Twin A and Twin B was lower in the planned‐CD group. In the planned‐VD group, the risk of composite adverse perinatal outcome in Twin A differed only slightly according to GA at delivery, whereas the risk of composite adverse perinatal outcome in Twin B differed according to GA between 33 and 35 weeks, was stable between 35 and 37 + 5 weeks, and varied again between 38 and 39 weeks.
Table 2.
Association between planned mode of delivery and adverse perinatal outcome in 5564 twins, according to gestational age at delivery
| Delivery at 32 + 0 to 36 + 6 weeks | Delivery at ≥ 37 + 0 weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Fetuses (n) | Planned VD (n = 1292) | Planned CD (n = 1338) | OR (95% CI) | Fetuses (n) | Planned VD (n = 1490) | Planned CD(n = 1444) | OR (95% CI) |
| Composite adverse perinatal outcome | 2630 | 29 (2) | 48 (4) | 0.62 (0.37–1.03) | 2931 | 23 (2) | 10 (1) | 2.25 (1.06–4.77) |
| Twin A | 1315 | 10 (2) | 20 (3) | 0.51 (0.24–1.10) | 1466 | 7 (1) | 1 (0) | 6.85 (0.84–55.80) |
| Twin B | 1315 | 19 (3) | 28 (4) | 0.69 (0.38–1.26) | 1465 | 16 (2) | 9 (1) | 1.74 (0.76–3.96) |
| Perinatal death* | 2630 | 8 (1) | 16 (1) | 0.52 (0.21–1.29) | 2931 | 9 (1) | 6 (0) | 1.46 (0.50–4.23) |
| Twin A | 1315 | 3 (0) | 7 (1) | 0.44 (0.11–1.71) | 1466 | 4 (1) | 1 (0) | 3.90 (0.44–34.95) |
| Twin B | 1315 | 5 (1) | 9 (1) | 0.57 (0.19–1.72) | 1465 | 5 (1) | 5 (1) | 0.97 (0.28–3.36) |
| Birth trauma* | ||||||||
| Long‐bone fracture* | 2606 | 0 (0) | 0 (0) | — | 2916 | 4 (1) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 0 (0) | — | 1455 | 4 (1) | 0 (0) | — |
| Other bone fracture* | 2606 | 1 (0) | 1 (0) | 1.03 (0.06–16.46) | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 1 (0) | 1 (0) | 1.03 (0.06–16.50) | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| Peripheral nerve injury at 72 h of age or at discharge* | 2606 | 0 (0) | 0 (0) | — | 2916 | 1 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 1 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| Subdural hematoma* | 2606 | 0 (0) | 0 (0) | — | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| 5‐min Apgar score < 4* | 2606 | 2 (0) | 2 (0) | 1.03 (0.15–7.31) | 2916 | 5 (0) | 0 (0) | — |
| Twin A | 1305 | 2 (0) | 1 (0) | 2.06 (0.19–22.80) | 1461 | 1 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 1 (0) | — | 1455 | 4 (1) | 0 (0) | — |
| Abnormal level of consciousness* | 2606 | 2 (0) | 8 (1) | 0.13 (0.02–1.05) | 2916 | 6 (0) | 5 (0) | 1.95 (0.49–7.79) |
| Twin A | 1305 | 0 (0) | 2 (0) | — | 1461 | 2 (0) | 2 (0) | — |
| Twin B | 1301 | 2 (0) | 6 (0) | 0.17 (0.02–1.42) | 1455 | 4 (1) | 3 (0) | 1.29 (0.29–5.80) |
| Coma* | 2606 | 1 (0) | 0 (0) | — | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 1 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| Stupor or decreased response to pain* | 2606 | 0 (0) | 2 (0) | — | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 2 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| Hyperalert, drowsy or lethargic* | 2606 | 1 (0) | 6 (0) | — | 2916 | 6 (0) | 3 (0) | — |
| Twin A | 1305 | 0 (0) | 2 (0) | — | 1461 | 2 (0) | 0 (0) | — |
| Twin B | 1301 | 1 (0) | 4 (1) | — | 1455 | 4 (1) | 3 (0) | — |
| Neonatal seizures within 72 h of birth* | 2606 | 2 (0) | 3 (0) | 0.51 (0.05–5.67) | 2916 | 3 (0) | 2 (0) | — |
| Twin A | 1305 | 0 (0) | 1 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 2 (0) | 2 (0) | 0.51 (0.05–5.68) | 1455 | 3 (0) | 2 (0) | — |
| > 2 seizures* | 2606 | 1 (0) | 1 (0) | — | 2916 | 1 (0) | 2 (0) | — |
| Twin A | 1305 | 0 (0) | 1 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 1 (0) | 0 (0) | — | 1455 | 1 (0) | 2 (0) | — |
| 2 seizures* | 2606 | 1 (0) | 0 (0) | — | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 1 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| 1 seizure | 2606 | 0 (0) | 2 (0) | — | 2916 | 2 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 2 (0) | — | 1455 | 2 (0) | 0 (0) | — |
| Ventilation via endotracheal tube within 72 h of age* | 2606 | 29 (2) | 37 (3) | 0.64 (0.31–1.32) | 2916 | 14 (1) | 3 (0) | 0.65 (0.11–3.88) |
| Twin A | 1305 | 6 (1) | 15 (2) | 0.34 (0.09–1.26) | 1461 | 3 (0) | 0 (0) | — |
| Twin B | 1301 | 23 (4) | 22 (3) | 0.82 (0.38–1.77) | 1455 | 11 (1) | 3 (0) | 0.65 (0.11–3.87) |
| At or after 24 h* | 2606 | 15 (1) | 24 (2) | — | 2916 | 2 (0) | 3 (0) | — |
| Twin A | 1305 | 3 (0) | 9 (1) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 12 (2) | 15 (2) | — | 1455 | 2 (0) | 3 (0) | — |
| Before 24 h | 2606 | 14 (1) | 13 (1) | — | 2916 | 12 (1) | 0 (0) | — |
| Twin A | 1305 | 3 (0) | 6 (1) | — | 1461 | 3 (0) | 0 (0) | — |
| Twin B | 1301 | 11 (2) | 7 (1) | — | 1455 | 9 (1) | 0 (0) | — |
| Sepsis within 72 h of birth* | 2606 | 2 (0) | 1 (0) | 2.06 (0.19–22.73) | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 1 (0) | 0 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 1 (0) | 1 (0) | 1.03 (0.06–16.50) | 1455 | 0 (0) | 0 (0) | — |
| Necrotizing enterocolitis* | 2606 | 2 (0) | 1 (0) | 2.06 (0.19–22.73) | 2916 | 1 (0) | 0 (0) | — |
| Twin A | 1305 | 2 (0) | 1 (0) | 2.06 (0.19–22.80) | 1461 | 1 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 0 (0) | — | 1455 | 0 (0) | 0 (0) | — |
| Cystic periventricular leukomalacia* | 2606 | 0 (0) | 2 (0) | — | 2916 | 0 (0) | 0 (0) | — |
| Twin A | 1305 | 0 (0) | 1 (0) | — | 1461 | 0 (0) | 0 (0) | — |
| Twin B | 1301 | 0 (0) | 1 (0) | — | 1455 | 0 (0) | 0 (0) | — |
Data are given as n or n (%) unless specified otherwise.
Component of composite adverse perinatal outcome. Reported odds ratios (OR) are for dichotomized outcome, i.e. yes vs no. ORs for all infants calculated using generalized estimation equation method, which takes into account correlation between twins. When number of events in one cell is zero, stable OR cannot be calculated. There were no infants with spinal cord injury, basal or depressed skull fracture, subdural hematoma, meningitis, Grade‐III or ‐IV intraventricular hemorrhage or bronchopulmonary dysplasia in either group. CD, Cesarean delivery; VD, vaginal delivery.
There were 2630 twins delivered at 32 + 0 to 36 + 6 weeks (1292 in the planned‐VD group, 1338 in the planned‐CD group). Of these fetuses, 29 (2.2%) experienced composite adverse perinatal outcome in the planned‐VD group compared with 48 (3.6%) in the planned‐CD group (OR, 0.62 (95% CI, 0.37–1.03)). No significant differences were found in any of the individual components of composite adverse perinatal outcome between the two planned modes of delivery.
There were 2934 twins born at or after 37 weeks (1444 in the planned‐CD group, 1490 in the planned‐VD group). Of these fetuses, 23 (1.5%) in the planned‐VD group experienced composite adverse perinatal outcome compared with 10 (0.7%) in the group randomized to planned CD (OR, 2.25 (95% CI, 1.06–4.77)). No significant differences were observed in any of the individual components of composite adverse perinatal outcome between the two planned modes of delivery.
DISCUSSION
In this study, we identified GA at birth as a strong prognostic factor for perinatal outcome, depending on the planned mode of delivery. In pregnancies delivered before 37 weeks, a trend towards a lower rate of composite adverse perinatal outcome in those randomized to planned VD was noted, while, from around 37 weeks onwards, fewer cases of composite adverse perinatal outcome were noted in pregnancies randomized to planned CD. The effect was more pronounced with increasing GA.
In June 2017, the French JUMODA trial, which was a prospective cohort study in 5915 women with a twin pregnancy after 32 weeks and the first twin in cephalic position, was published. The JUMODA trial found a higher rate of adverse neonatal events in twins born at 32–37 weeks after planned CD compared with after planned VD (32 + 0 to 34 + 6 weeks: propensity score matching OR, 1.72 (95% CI, 1.09–2.70); 35 + 0 to 36 + 6 weeks: propensity score matching OR, 2.52 (95% CI, 1.05–6.04)). These results are remarkably similar to our findings 5 . Retrospective studies on the effect of GA on neonatal outcomes related to the planned mode of delivery for women with twin pregnancy have reported similar results; before 36–37 weeks, the optimal mode of delivery for twins is planned VD and, beyond term, planned CD may result in better outcome 3 , 6 , 7 , 8 , 9 .
In our analysis, it was impossible to detect the exact GA at which planned CD leads to better perinatal outcome, as compared with planned VD. In previous retrospective studies, this point was found to be from 36 weeks onwards 3 , 6 , 9 , 10 while, in the JUMODA study, this was from 37 weeks onwards 5 .
It is unlikely that a randomized trial of the size needed to validate the findings at a GA of at or after 37 weeks will ever be performed, especially since it is unlikely that women with twin pregnancy will deliver at a GA much more advanced than ≥ 37 weeks.
The positive effect of planned CD over planned VD shown at later GAs might be overcome by earlier planned delivery than was advised in the TBS11. The authors of a recently published meta‐analysis recommend delivery for monochorionic twins before 36 + 6 weeks and for dichorionic twins before 37 + 6 weeks 11 .
Our analysis focused on short‐term outcomes. The protective effect of VD at an earlier GA for twins seemed to arise partly because of the lower need for assisted ventilation. Children born after planned VD suffer less frequently from respiratory problems than do those born after planned CD, and it is very likely that this effect is more pronounced for neonates born more prematurely. Recent studies, performed mainly in women with a singleton pregnancy, suggest the use of antenatal corticosteroids for planned CD at 34–37 weeks 12 . We found no difference in the proportion of women taking antenatal corticosteroids between the two study groups. The difference in the rate of adverse perinatal outcome between the modes of delivery might have been smaller if antenatal corticosteroids had been given more frequently before planned CD at 34–37 weeks' gestation. The use of antenatal corticosteroids was associated with a higher risk of composite adverse perinatal outcome in both trial arms. This may be due to confounding, as antenatal corticosteroids may be a surrogate marker for high‐risk delivery.
It is important to keep in mind that clinical decisions should be made based on long‐term outcomes. The long‐term outcomes of the TBS have been published separately; no difference in the rate of adverse neurodevelopmental outcome was found between children born after planned CD and those born after planned VD (5.99% vs 5.83%; OR, 1.04 (95% CI, 0.77–1.41); P = 0.79) 13 . The rate of adverse outcome after 2 years was higher than anticipated in both study groups, probably owing to a relatively high percentage of premature twins in both groups 13 . These outcomes have, however, not been analyzed according to GA at delivery.
Perinatal outcome alone should not influence the decision of mode of delivery. In the TBS, no differences were seen in short‐term and serious long‐term maternal morbidity and mortality between the planned‐CD and planned‐VD groups, but the study might be too small to detect a difference in the incidence of rare, but serious, complications 1,14 . CD is, based on retrospective studies, associated with a small, but increased, incidence of maternal complications, as compared with VD. A scarred uterus increases the chance of morbidly adherent placenta and uterine rupture in future pregnancies 15 .
In the planned‐VD group, 44% of women went on to deliver at least one twin by CD. Reassessment of eligibility for VD at the time of labor was part of the study protocol and, in cases of maternal or fetal complications, CD was indicated. Therefore, the high CD rate in the planned‐VD group cannot be seen as a deviation from protocol or crossover. In the planned‐VD group, 10% of women requested CD, which is comparable with the 7% of women who requested VD in the planned‐CD group, and which would be in the range of an acceptable crossover rate. We emphasize that the aim of the study was to compare the strategy of planned mode of delivery (CD vs VD). The results of the trial and this analysis can be used only for future counseling of women by comparing planned mode of delivery. However, it is not possible to predict which women will have an emergency CD during labor despite planned VD or which will have contractions and deliver vaginally despite planned CD.
Many more twins at the most advanced GAs, i.e. the group with a possible benefit of planned CD, will have been induced compared with those at lower GAs. Our analysis was based on the intention‐to‐treat approach. We did not adjust for any intervention after randomization, such as induction of labor.
Apart from GA at delivery, none of the other examined variables or any combination of them could identify with acceptable precision women who would benefit from one delivery method over the other. The presentation of the second twin did not demonstrate potential in aiding the decision of planned CD or planned VD in the studied population of women. Thus, there is still a need to identify other factors to help identify women and children who could benefit from one planned delivery method over the other.
In conclusion, based on a post‐hoc analysis of the TBS, we identified GA at delivery as a strong prognostic factor for perinatal outcome, an association that was dependent upon the planned mode of delivery. Thus, women with a twin pregnancy and the first twin in cephalic position should be counseled that planned VD seems to have a favorable effect on perinatal outcome at a GA of 32–37 weeks, while, from around 37 weeks onwards, CD might be safer for both babies, but the absolute risks are very low and must be weighed against the increased maternal risks associated with planned CD.
Supporting information
Appendix S1 Power calculation
Figure S1 Association between gestational age at randomization and risk of composite adverse perinatal outcome in either twin in twin pregnancies randomized to planned Cesarean delivery (CD) and those randomized to planned vaginal delivery (VD).
Figure S2 Association between gestational age at delivery and risk of composite adverse perinatal outcome in Twin A and Twin B separately, in twin pregnancies randomized to planned Cesarean delivery (CD) and those randomized to planned vaginal delivery (VD).
Table S1 Mode of delivery and reason for Cesarean delivery, according to randomization to planned vaginal or planned Cesarean delivery
Table S2 Multivariable models for baseline characteristics in prediction of composite adverse perinatal outcome, according to randomization to planned vaginal or planned Cesarean delivery
Table S3 Modeling of risk of composite adverse perinatal outcome as function of management (planned Cesarean delivery (CD) vs vaginal delivery (VD)), gestational age at delivery and interaction between the two
ACKNOWLEDGMENTS
We thank all the participants in the Twin Birth Study and the staff at the Centre for Mother, Infant, and Child Research for their hard work and dedication. The Twin Birth Study was supported by a grant (63164) from the Canadian Institute of Health Research. P.T. and M.H.Z. were supported by a grant from The Netherlands Organization for Scientific Research (NWO ‐ grant number 401.16.080). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Twin Birth Study Collaborative Group
Barrett JF, Allen AC, Armson BA, Asztalos EV, Farrell S, Gafni A, Hanigsberg JE, Hannah ME, Hutton EK, Joseph KS, Leduc L, Mason D, Ohlsson A, Okun N, Ross S, Sanchez JJ, Willan AR, Bracken M, Crowley P, Donner A, Duley L, Ehrenkranz R, Thorpe K, Castaldi JL, Bertin MS, Partida Y, Galimberti D, Messina A, Voto LS, Voto GN, Prieto MJ, Buraschi F, Sexer H, Palermo M, Varela DM, Savransky R, Dunaievsky A, Andina E, Laterra C, Susacasa S, Frailuna MA, Almansa SR, Barrere MB, García H, Rivero M, Gomez EE, Schinini J, Ahlbom M, Aguirre JD, Martín Rde L, Videla A, Mesas W, Arias C, Castagnola MC, Gorostiaga RA, Curioni M, Mohedano M, Dip V, Roque A, Duhalde EM, Dodd J, Deussen A, Crowther C, Gardener G, Chaplin J, Wilkins D, Mahomed K, Green A, Baade R, Haran M, Hanafy A, Davis G, Roberts L, Tucker S, Duncan C, Watson D, Lawrence A, Laubach M, Breugelmans R, Calderon I, Martins A, Magalhães C, Cecatti JG, Surita FG, Rosado L, Vidal AC, de Souza GR, Maia Filho NL, Santana DS, de Sa RA, Marcolino L, Marques CC, Zanella PL, Milan C, Bollis MD, Steibel G, Ayub AC, Moreira S, Lima AC, Scavuzzi A, de Souza AS, de Moraes Filho OB, Carvalho SA, Bornia RG, da Silva NR, Spinola R, Lopes LM, Sass N, Korkes H, Chalem E, Yokota EJ, Silveira MR, Wood S, Miller L, McLeod L, Armson BA, Fanning C, Mueller V, Gregorovich S, Moore E, Gratton R, Kennedy L, Scheufler P, Reid D, Klam S, Daitchman R, Farquharson D, Harrison K, Kulkarni R, Scarfone R, Laplante J, Carson G, Williams S, Rosman D, Nemtean D, Olatunbosun F, Pierson K, Crane J, Hutchens D, Okun N, Zaltz A, Brown M, Ornstein M, Visram S, Bordin J, Siurna H, Petruskavich S, Gagnon A, Lee JM, Fernandez A, Kaye S, Haslauer KA, Cundiff G, Gomez R, Kusanovic JP, Neculman KS, Valenzuela LL, Leiva EM, Cabrera JG, Ravanal MM, Orrego RS, Matijevic R, Makhlouf A, Saber O, Abdelradey T, Kirss F, Rull K, Vaas P, Hopp H, Nonnenmacher A, Michaelis S, Hasbargen U, Delius M, Antsaklis A, Drakakis P, Major T, Bartha T, Salim R, Harel L, Chayen B, Siev S, Hallak M, Mei‐Dan E, Gonen R, Wolff L, Sadan O, Mansour‐Schwake D, Petchinkin L, Hakim M, Perlitz Y, Ben‐Ami M, Pansky S, Simms‐Stewart D, Wilson M, El‐Zibdeh M, AlFaris L, Heres M, Sluis A, Roumen FJ, Rinkens J, Willekes C, Alleman S, van Zandvoort SG, Porath MM, Verhoeven C, Mol BW, Radfar F, Khan S, Preis K, Swiatkowska‐Freund M, Krasomski G, Kesiak M, Krekora M, Zych K, Wilczynski J, Breborowicz G, Dera A, ur Rahman S, Al‐Jassim AA, Stamatian F, Caracostea G, Gojnic M, Fazlagic A, Perovic M, Vasiljevic B, Stefanovic T, Gonce A, Rodriguez SH, Massanes M, Moratonas EC, Rodriguez CR, Martinez SA, Llurba E, Franch AS, de la Calle M, Dans F, Sancha M, Lopez S, Palomo ML, del Valle MD, Martín MN, Delgado CL, Fournier MC, Ojutiku D, Masuku M, Goodsell K, Southam D, Tuffnell D, Germaine T, Palethorpe R, Farrar D, Wright J, Al‐Taher H, Meehan H, Bricker L, Dower M, Houghton G, Pascall A, Longworth H, Sau A, Thornton J, Fisher J, Houda M, Simm A, Bugg G, Deshpande R, Davis Y, Holloway F, Welch R, Hollands H, Young P, Hinshaw K, Bargh A, Edmundson D, Cameron H, Alonso J, Austt AG, Ortiz A, Burgis J, Brown S, Gregg A, Borowski K, Fleener D, Deaver J, Sumersille M, Aronoff C, Bland K, Kontopoulos E, Rivero Y, Lovett SM, Zatinsky S, Diogo M, Coonrod DV, Jimenez BF, Chan S, Hewson SA, Hoac T, Kowal C, Mangoff K, Mergler S, Shi M.
Contributor Information
M. H. Zafarmand, Email: m.h.zafarmand@amc.uva.nl.
the Twin Birth Study Collaborative Group:
JF Barrett, AC Allen, BA Armson, EV Asztalos, S Farrell, A Gafni, JE Hanigsberg, ME Hannah, EK Hutton, KS Joseph, L Leduc, D Mason, A Ohlsson, N Okun, S Ross, JJ Sanchez, AR Willan, M Bracken, P Crowley, A Donner, L Duley, R Ehrenkranz, K Thorpe, JL Castaldi, MS Bertin, Y Partida, D Galimberti, A Messina, LS Voto, GN Voto, MJ Prieto, F Buraschi, H Sexer, M Palermo, DM Varela, R Savransky, A Dunaievsky, E Andina, C Laterra, S Susacasa, MA Frailuna, SR Almansa, MB Barrere, H García, M Rivero, EE Gomez, J Schinini, M Ahlbom, JD Aguirre, L Martín Rde, A Videla, W Mesas, C Arias, MC Castagnola, RA Gorostiaga, M Curioni, M Mohedano, V Dip, A Roque, EM Duhalde, J Dodd, A Deussen, C Crowther, G Gardener, J Chaplin, D Wilkins, K Mahomed, A Green, R Baade, M Haran, A Hanafy, G Davis, L Roberts, S Tucker, C Duncan, D Watson, A Lawrence, M Laubach, R Breugelmans, I Calderon, A Martins, C Magalhães, JG Cecatti, FG Surita, L Rosado, AC Vidal, GR de Souza, NL Maia Filho, DS Santana, RA de Sa, L Marcolino, CC Marques, PL Zanella, C Milan, MD Bollis, G Steibel, AC Ayub, S Moreira, AC Lima, A Scavuzzi, AS de Souza, OB de Moraes Filho, SA Carvalho, RG Bornia, NR da Silva, R Spinola, LM Lopes, N Sass, H Korkes, E Chalem, EJ Yokota, MR Silveira, S Wood, L Miller, L McLeod, BA Armson, C Fanning, V Mueller, S Gregorovich, E Moore, R Gratton, L Kennedy, P Scheufler, D Reid, S Klam, R Daitchman, D Farquharson, K Harrison, R Kulkarni, R Scarfone, J Laplante, G Carson, S Williams, D Rosman, D Nemtean, F Olatunbosun, K Pierson, J Crane, D Hutchens, N Okun, A Zaltz, M Brown, M Ornstein, S Visram, J Bordin, H Siurna, S Petruskavich, A Gagnon, JM Lee, A Fernandez, S Kaye, KA Haslauer, G Cundiff, R Gomez, JP Kusanovic, KS Neculman, LL Valenzuela, EM Leiva, JG Cabrera, MM Ravanal, RS Orrego, R Matijevic, A Makhlouf, O Saber, T Abdelradey, F Kirss, K Rull, P Vaas, H Hopp, A Nonnenmacher, S Michaelis, U Hasbargen, M Delius, A Antsaklis, P Drakakis, T Major, T Bartha, R Salim, L Harel, B Chayen, S Siev, M Hallak, E Mei‐Dan, R Gonen, L Wolff, O Sadan, D Mansour‐Schwake, L Petchinkin, M Hakim, Y Perlitz, M Ben‐Ami, S Pansky, D Simms‐Stewart, M Wilson, M El‐Zibdeh, L AlFaris, M Heres, A Sluis, FJ Roumen, J Rinkens, C Willekes, S Alleman, SG van Zandvoort, MM Porath, C Verhoeven, BW Mol, F Radfar, S Khan, K Preis, M Swiatkowska‐Freund, G Krasomski, M Kesiak, M Krekora, K Zych, J Wilczynski, G Breborowicz, A Dera, S Ur Rahman, AA Al‐Jassim, F Stamatian, G Caracostea, M Gojnic, A Fazlagic, M Perovic, B Vasiljevic, T Stefanovic, A Gonce, SH Rodriguez, M Massanes, EC Moratonas, CR Rodriguez, SA Martinez, E Llurba, AS Franch, M de la Calle, F Dans, M Sancha, S Lopez, ML Palomo, MD Del Valle, MN Martín, CL Delgado, MC Fournier, D Ojutiku, M Masuku, K Goodsell, D Southam, D Tuffnell, T Germaine, R Palethorpe, D Farrar, J Wright, H Al‐Taher, H Meehan, L Bricker, M Dower, G Houghton, A Pascall, H Longworth, A Sau, J Thornton, J Fisher, M Houda, A Simm, G Bugg, R Deshpande, Y Davis, F Holloway, R Welch, H Hollands, P Young, K Hinshaw, A Bargh, D Edmundson, H Cameron, J Alonso, AG Austt, A Ortiz, J Burgis, S Brown, A Gregg, K Borowski, D Fleener, J Deaver, M Sumersille, C Aronoff, K Bland, E Kontopoulos, Y Rivero, SM Lovett, S Zatinsky, M Diogo, DV Coonrod, BF Jimenez, S Chan, SA Hewson, T Hoac, C Kowal, K Mangoff, S Mergler, and M. Shi
REFERENCES
- 1. Barrett JF, Hannah ME, Hutton EK, Willan AR, Allen AC, Armson BA, Gafni A, Joseph KS, Mason D, Ohlsson A, Ross S, Sanchez JJ, Asztalos EV. A randomized trial of planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med 2013;14: 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith GCS, Fleming KM, White IR. Birth order of twins and risk of perinatal death related to delivery in England, Northern Ireland, and Wales 1994–2003: retrospective cohort study. BMJ 2007; 334: 576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith GCS, Pell JP, Dobbie R. Birth order, gestational age, and risk of delivery related perinatal death in twins: retrospective cohort study. BMJ 2002; 325: 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith GC, White IR. Planned cesarean or vaginal delivery for twin pregnancy. N Engl J Med 2014; 370: 279. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz T, Prunet C, Azria E, Bohec C, Bongain A, Chabanier P, D'Ercole C, Deruelle P, De Tayrac R, Dreyfus M, Dupont C, Gondry J, Graesslin O, Kayem G, Langer B, Marpeau L, Morel O, Parant O, Perrotin F, Pierre F, Poulain P, Riethmuller D, Rozenberg P, Rudigoz RC, Sagot P, Sénat MV, Sentilhes L, Vayssière C, Venditelli F, Verspyck E, Winer N, Lecomte‐Raclet L, Ancel PY, Goffinet F; JUmeaux MODe d'Accouchement (JUMODA) Study Group and the Groupe de Recherche en Obstétrique et Gynécologie (GROG) . Association Between Planned Cesarean Delivery and Neonatal Mortality and Morbidity in Twin Pregnancies. Obstet Gynecol 2017; 129: 986–995. [DOI] [PubMed] [Google Scholar]
- 6. Dong Y, Luo ZC, Yang ZJ, Chen L, Guo YN, Branch W, Zhang J, Huang H. Is Cesarean Delivery Preferable in Twin Pregnancies at > =36 Weeks Gestation? PLoS One 2016; 11: e0155692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roberts CL, Algert CS, Nippita TA, Bowen JR, Shand AW. Association of prelabor cesarean delivery with reduced mortality in twins born near term. Obstet Gynecol 2015; 125: 103–110. [DOI] [PubMed] [Google Scholar]
- 8. Wenckus DJ, Gao W, Kominiarek MA, Wilkins I. The effects of labor and delivery on maternal and neonatal outcomes in term twins: a retrospective cohort study. BJOG 2014; 121: 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmann E, Oldenburg A, Rode L, Tabor A, Rasmussen S, Skibsted L. Twin births: Cesarean section or vaginal delivery? Acta Obstet Gynecol Scand 2012; 91: 463–469. [DOI] [PubMed] [Google Scholar]
- 10. Smith GCS, Shah I, White IR, Pell JP, Dobbie R. Mode of delivery and the risk of delivery‐related perinatal death among twins at term: A retrospective cohort study of 8073 births. BJOG 2005; 112: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 11. Cheong‐See F, Schuit E, Arroyo‐Manzano D, Khalil A, Barrett J, Joseph KS, Asztalos E, Hack K, Lewi L, Lim A, Liem S, Norman JE, Morrison J, Combs CA, Garite TJ, Maurel K, Serra V, Perales A, Rode L, Worda K, Nassar A, Aboulghar M, Rouse D, Thom E, Breathnach F, Nakayama S, Russo FM, Robinson JN, Dodd JM, Newman RB, Bhattacharya S, Tang S, Mol BW, Zamora J, Thilaganathan B, Thangaratinam S; Global Obstetrics Network (GONet) Collaboration . Prospective risk of stillbirth and neonatal complications in twin pregnancies: systematic review and meta‐analysis. BMJ 2016; 354: i4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nada AM, Shafeek MM, El Maraghy MA, Nageeb AH, Salah El Din AS, Awad MH. Antenatal corticosteroid administration before elective caesarean section at term to prevent neonatal respiratory morbidity: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol 2016; 199: 88–91. [DOI] [PubMed] [Google Scholar]
- 13. Asztalos EV, Hannah ME, Hutton EK, Willan AR, Allen AC, Armson BA, Gafni A, Joseph KS, Ohlsson A, Ross S, Sanchez JJ, Mangoff K, Barrett JF. Twin Birth Study: 2‐year neurodevelopmental follow‐up of the randomized trial of planned cesarean or planned vaginal delivery for twin pregnancy. Am J Obstet Gynecol 2016; 214: 371.e1–371.e19. [DOI] [PubMed] [Google Scholar]
- 14. Hutton EK, Hannah ME, Ross S, Joseph KS, Ohlsson A, Asztalos EV, Willan AR, Allen AC, Armson BA, Gafni A, Mangoff K, Sanchez JJ, Barrett JF; Twin Birth Study Collaborative Group . Maternal outcomes at 3 months after planned caesarean section versus planned vaginal birth for twin pregnancies in the Twin Birth Study: a randomised controlled trial. BJOG 2015; 122: 1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavender T, Justus Hofmeyr G, Neilson JP, Kingdon C, Gyte GM. Caesarean section for non‐medical reasons at term. Cochrane Database Syst Rev 2012; 3: CD004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Power calculation
Figure S1 Association between gestational age at randomization and risk of composite adverse perinatal outcome in either twin in twin pregnancies randomized to planned Cesarean delivery (CD) and those randomized to planned vaginal delivery (VD).
Figure S2 Association between gestational age at delivery and risk of composite adverse perinatal outcome in Twin A and Twin B separately, in twin pregnancies randomized to planned Cesarean delivery (CD) and those randomized to planned vaginal delivery (VD).
Table S1 Mode of delivery and reason for Cesarean delivery, according to randomization to planned vaginal or planned Cesarean delivery
Table S2 Multivariable models for baseline characteristics in prediction of composite adverse perinatal outcome, according to randomization to planned vaginal or planned Cesarean delivery
Table S3 Modeling of risk of composite adverse perinatal outcome as function of management (planned Cesarean delivery (CD) vs vaginal delivery (VD)), gestational age at delivery and interaction between the two


