Abstract
Tendon and ligament injuries caused by trauma and degenerative diseases are frequent and affect diverse groups of the population. Such injuries reduce musculoskeletal performance, limit joint mobility, and lower people’s comfort. Currently, various treatment strategies and surgical procedures are used to heal, repair, and restore the native tissue function. However, these strategies are inadequate and, in some cases, fail to re-establish the lost functionality. Tissue engineering and regenerative medicine approaches aim to overcome these disadvantages by stimulating the regeneration and formation of neo-tissues. Design and fabrication of artificial scaffolds with tailored mechanical properties are crucial for restoring the mechanical function of tendons. In this review, we present the tendon and ligament structure, their physiology, and performance. On the other hand, we focus on the requirements for the development of an effective reconstruction device. We also describe the most common fiber-based scaffolding systems for tendon and ligament tissue regeneration like strand fibers, woven, knitted, braided, and braid-twisted fibrous structures, as well as electrospun and wet-spun constructs, discussing critically the advantages and limitations of their utilization. Finally, we point out the potential of multi-layered systems as the most effective candidates for tendon and ligaments tissue engineering.
The literature of fiber-based systems for tendon and ligament repair, healing and regeneration is reported. Fibrous scaffolds including strand fibers, woven, knitted, braided and braid-twisted fibrous constructs, electrospun and wet-spun structures, as well as multi-layered systems are critically reviewed and described, pointing out advantages and drawbacks of each approach.
Keywords: fibrous scaffolds, fiber-based fabrication technologies, tendon tissue engineering, ligament tissue engineering
Graphical abstract
1. Introduction
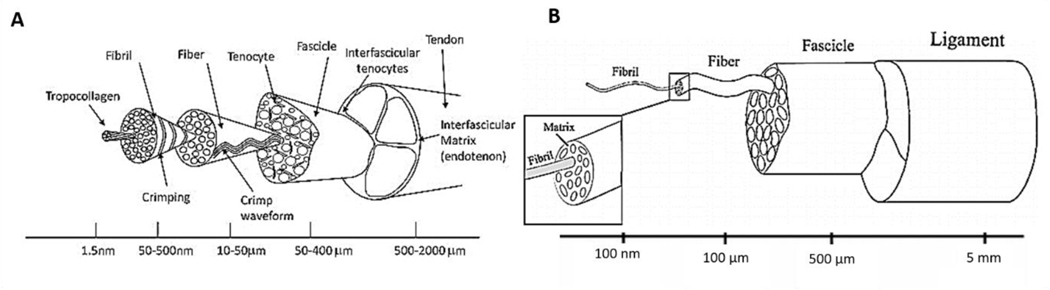
Tendons and ligaments are fibrous connective tissues, having the function of transmitting loads in between muscle and bone, and from one bone to another, respectively. The tendon and ligament structures are designed to carry loads and to guarantee sufficient biomechanical performances, bearing and reinforcing the joints as well as preventing bone luxation [1]. Tendon and ligaments are typically avascular and are mainly composed of extracellular matrix (ECM) composed of collagen type I, III, V, and X (75%), proteoglycans (e.g decorin) (20%), tenascin C, scleraxis, elastin (1%) and water. Progenitor cells, fibroblasts and tenocytes, represent the cellular component of the tissues. The structure of tendons and ligaments expands along their axes and shows a high hierarchical organization. The different organization levels are composed of: collagen molecules, collagen fibrils, collagen fibril bundles, collagen fibril fascicles, proteoglycans, and elastin [2–4] (Figure 1A-B). The highly organized structure and the tissue components influence their mechanical behavior [5,6].
Figure 1.
Physiology and mechanical characteristics of tendon and ligament tissue. A) Tendon hierarchical structures. Reproduced with permission [5]. Copyright 2015, Elsevier Inc.
B) Ligament hierarchical structure. Reproduced with permission [6]. Copyright 2010, Elsevier Ltd.
1.1. Tendon and ligament mechanical properties
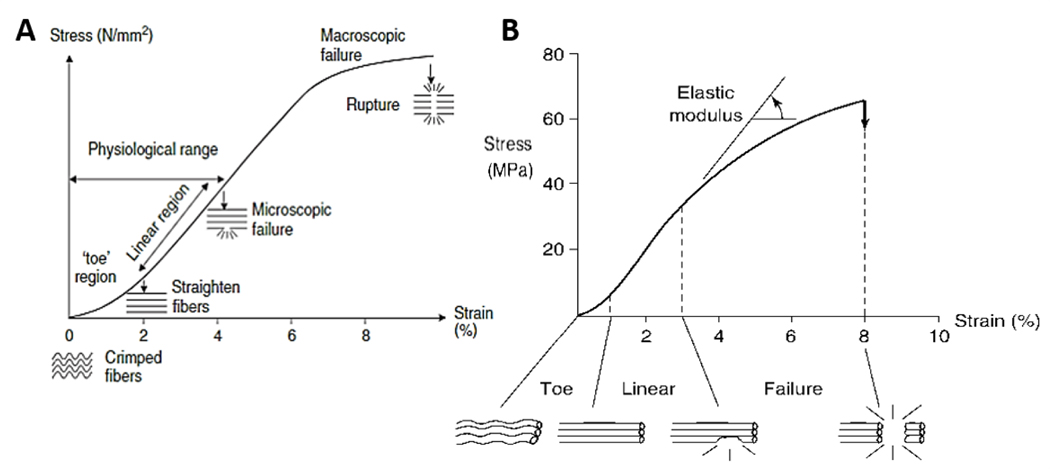
James et al. studied the mechanical properties of tendons, individuating a triphasic behavior of the tissue when strain is applied (Figure 2A) [7,8]. The first region (non-linear or toe region) exhibits low values of stress. In this phase, the force applied to the tissue is transferred to the collagen fibrils, inducing their contraction and rearrangement. The second region (linear region) is characterized by a linear increase of stress and strain, due to the straightening and stretching of collagen molecules. The third phase displays a flexion of the curve (microscopic failure). In this region, the stress values slightly diminish until the defibrillation of collagen fibers occurs, resulting in the macroscopic tissue rupture [1,7]. Ligament tissue showed a similar behavior when subjected to tensile load (Figure 2B) [9]. Depending on the type, the normal healthy tendon or ligament works between 7% and 40% failure load. Prosthetic implants for tendon and ligament replacement or reinforcement are intended to match or exceed the mechanical properties of the native tissue. However, some mechanical parameters such as strength, stiffness, and elongation obtained for existing devices, still do not correspond to the physiological values [2].
Figure 2.
Mechanical characteristics of tendon and ligament tissue. A) Representative stress-strain curve of tendon subjected to mechanical tensile test. Reproduced with permission [8]. Copyright 2005, Elsevier Ltd. B) Representative stress-strain curve of ligament tissue failed in tension. Reproduced with permission [9]. Copyright 1978, The American College of Sports Medicine.
1.2. Injuries and healing process
Tendon and ligament injuries in the young population are derived mainly from sport activities including football, basketball, and handball, while fall and lift of heavy weights are responsible for injuries in elderly population [10]. In addition to injuries, chronic deterioration of the tissues often affects the oldest part of the population. Both tissue injuries and degenerative processes, result in reduced musculoskeletal functions, limiting people’s activities. Annually, just in the United States, approximately 300.000 surgical repairs of shoulders, foot, and ankles [2], and around 350.000 anterior cruciate ligament (ACL) reconstructive surgeries are performed [11,12], costing around $30 billion [13]. When the injury occurs, the characteristic cascade of wound healing takes place: the tissue inflammation phase is followed by cellular growth, with subsequent ECM restoration [14,15]. The first stage is characterized by the formation of a blood clot on the injured area due to the migration of monocytes, leukocytes, and macrophages [2]. The proliferation phase provides the migration and deposition of fibroblasts to form the vascular granular tissue. The cells start to produce and deposit collagen (mostly type I and III) during the remodeling phase, allowing the synthesis of ECM, resulting in the re-growth of the tissue with low organization level of the fibers [16,17].
1.3. Treatment strategies
Historically, since the 1980s, the tendon and ligament injury treatments included several approaches based on non-operative treatments as well as various repair surgeries. Surgical procedures involve the implantation of natural or biological grafts [11], autografts, allografts, and synthetic grafts [18,19]. Autografts and allografts have been found to possess good mechanical strength as well as correct architectural structure and chemical composition to promote cell proliferation and to stimulate new tissue growth [1,20]. The main concerns about autografts are the donor site morbidity and limited graft availability. The use of cadaver allografts carries a high probability of bacterial infection, late biological incorporation, and transmission of blood-borne diseases [21,22]. The failure of the grafts has higher incidence for elderly patients, people with tears of a large size or with severe muscle atrophies and fatty infiltrations, and patients with systemic diseases [3]. For all these reasons, there is a need for the development of new solutions. The fabrication and implantation of scaffolds derived from biological sources or synthetic scaffolds composed of biodegradable polymers are drawing noticeable attention for the injury care of tendons and ligaments.
In this article, we review the literature of fiber-based scaffolds for tendon and ligament engineering. Fibrous constructs including strand fibers, woven, knitted, braided and braid-twisted, electro- and wet-spun structures are critically described and analyzed, focusing on advantages and drawbacks of each approach [23,24]. Finally, the potential of engineered multi-layered systems as solutions for tendon and ligament regeneration is presented.
2. Tendon and ligament tissue engineering
Tissue engineering (TE), is a multidisciplinary approach which combines engineering, material science, chemistry, and biology to successfully regenerate damaged tissues, recreating their architecture, and restoring their functions. In the end of 1993, Robert Langer and Joseph Vacanti introduced the concept of TE, describing the fundamental elements of tissue engineered systems defined as “a structural scaffold, a cell source, biological modulators and mechanical stimuli” [25]. According to this definition, TE of functional tendon and ligament requires the combination of porous three-dimensional (3D) matrix structures, which can enhance tissue regeneration as well as encapsulation of isolated cells and growth factors [21,26]. In this frame, the design of a proper scaffold degradation rate and matching the tissue mechanical properties are considered the main challenges. Ideally, the substitute should support the tissue functionality, and favor tissue formation while degrading over time, permitting the total regeneration of the injured site [27,28]. Moreover, the definition of the main factors which guide healing, as well as the detection of the mechanical, biological, and chemical patterns of stimuli are also considered among the most crucial aspects for a proper engineered tendon and ligament tissue design [27,29,30].
2.1. Role of mechanical, biological and chemical stimulation in tendon and ligament regeneration
The dynamic mechanical stimuli play a fundamental role in tendon and ligament development, homeostasis, repair, healing, and maintenance of strength [31–33]. The absence of the stimulation pathways induces a loss in the mechanical properties of the tissues [34–36]. Timing, direction, and magnitude of the mechanical stimulation affect cell integrin-mediated focal adhesion and cytoskeleton orientation, influencing cellular growth, differentiation, and ECM production [37–43]. Moreover, mechanical factors such as substrate stiffness, surface topography, and geometry are expected to significantly affect cellular activity and functions [21,44–46].
Biological stimulation is mainly promoted by various types of cells. Fibroblasts are the principal cell component of tendon/ligament tissues and are involved in the synthesis, secretion, deposition, and maintenance of the ECM [47–49]. Li et al. demonstrated that fibroblast cells cultured on scaffold’s surface showed a sufficient ECM deposition, proteoglycans production, and promoted adequate native tissue hierarchical organization [50]. The constant secretion of ECM can also improve the scaffold mechanical characteristics in terms of tensile properties, as well as its flexibility in the axial direction promoting mechanotransduction [41,51]. Mesenchymal stem cells (MSCs) might also be used for tissue regeneration purposes. Many studies have demonstrated their potential for differentiation into several cell types such as fibroblasts, osteoblasts, chondrocytes, myoblasts, and tenocytes [52–54]. Moreover, the administration of MSCs is reported to successfully promote healing and regeneration of various musculoskeletal tissues (e.g. bones and ligaments) [11,49,55].
Growth factors (GF) stimulate tendon and ligament cells, influencing tissue homeostasis, repair, and healing [11,22,56]. They possess the ability to affect cellular growth and migration, synthesis of different collagen types, as well as ECM protein secretion [57,58]. GFs such as basic Fibroblast Growth Factors (bFGFs), Bone Morphogenetic Proteins (BMPs), Transforming Growth Factor beta (TGF-β), and platelet-derived growth factors (PDGFs) are the most commonly used, due to their capability to promote the tendon/ligament regeneration [59–65].
2.2. Scaffolds used in tendon and ligament tissue engineering
The engineered scaffold functions as a support structure. Ideally, this kind of construct should be biocompatible, degradable, provoke the least possible inflammatory reaction and should be easy to implant [29]. It should act as a tissue growth support and vehicle for the cells and growth factors, while the adhesion of surrounded tissue should be avoided [66]. The scaffold is also supposed to recapitulate the mechanical features of the healthy tissue, guaranteeing the transmission of forces and strengthening the joint, without risk of failure [2]. In order to design a proper scaffold for tendon and ligament engineering, various natural materials, biodegradable polymers, and composite biomaterials have been considered [67–76]. Commercial materials used to produce and fabricate scaffolds for this application include synthetic degradable, synthetic non-degradable, biological, and naturally derived materials [2]. Synthetic biomaterials have the great advantage of improving tissue repair by sharing mechanical loads with the host tissue. The possibility of utilization of a large variety of polymers and fabrication methods, as well as flexible designs, led researchers to optimize dimensions, mechanical properties, biological characteristics, sterility, and degradation rates of biomaterials to better recapitulate the complexity of tendons and ligaments [77]. Synthetic biomaterials carry a lower risk of disease transmission in comparison to their natural counterparts when implanted in the host body [11]. Degradable synthetic polymers including poly(caprolactone) (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PLG) and their copolymers and composites, polydioxanone (PDO), poly (glycerol sebacate) (PGS), and polyurethane (PU) have been explored as promising candidate materials for the production of tendon and ligament engineered scaffolds [47,78–82]. Nevertheless, they do not show the optimal support for cellular adhesion, growth, and infiltration, or new tissue formation. To improve the biological response of cells cultured on the scaffolds, some research groups introduced the use of biomaterials derived from natural sources like collagen, alginate, chitosan, hyaluronic acid, gelatin, fibrin, heparin, and silk fibroin, which are reported to be more suitable for cell attachment and proliferation [52,57,60,83–88]. Even though they are able to improve the tissue healing process, their application is limited due to the poor suture retention and insufficient mechanical properties [21,89] (except for silk fibroin which matched the tensile characteristics of native ligament [86]). In order to overcome these disadvantages, researchers started to consider synthetic polymers as reinforcement of naturally derived scaffolds, exploring and designing different composites and multi-layered structures [46,90–93]. In the literature, many strategies are discussed for the design and fabrication of the scaffolds for tendon and ligament regeneration purposes (Table 1). This review is focused on fiber-based approaches for scaffold fabrication, which mimics the fibrillar architecture of the native tissue and potentially direct cellular organization and ECM deposition.
Table 1.
Summary of selected fiber-based systems for tendon and ligaments TE.
| Fabrication method | Scaffold Material | In vitro | In vivo | Clinical trial | Stimulation | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Strand fibers | Carbon, silk, and collagen, UHMWPE | • MSCs: cell adhesion, viability and growth • Tendon stem cells: cell infiltration, expression of tenogenic markers |
• Calve model: milder pain and exudation as well as earlier restoration of tendon movements and weight bearing • Sheep model: ingrowth of fibroblastic tissue, collagen deposition, and alignment • Rabbit model: reduction in strength and volume after 8 weeks • Rat model: collagen formation |
• Simple to implant • Well tolerated • Fibers bond directly to the bone without fibrotic interposition • Debris, giant cell presence, arthrofibrosis |
-- | • Restoration of continuity across the defect of the tendon • Discomfort and slight loss of movement of the knee • Carbon particles appeared in the regional lymph nodes • Carbon particles were found in the synovium, hyaline cartilage and menisci • Comparable or greater initial ultimate tensile stress than human ACL • Lobulation and hypertrophy |
• [104], [105], [108], [109] [110], [111], [113], [114] |
| Weaving | PET, collagen I, silk fibroin and LAP, PCL, chitosan, cellulose nanocrystals, PLA | • Pre-osteoblasts: cytocompatibility, osteogenic differentiation • Tendon-derived cells: cell elongations, alignment, expression of key tenogenic markers • MSCs: cell alignment, tenogenic differentiation • MSCs/tenocytesumbilical vein endothelial cells co-culture: expression of key tenogenic markers |
• Rabbit model: tissue around the scaffold was highly cellular and collagen fibrils were deposited • Rabbit model: increase of stiffness, collagen I deposition, tenomodulin expression • Rat model: formation of mature collagen fibers, promotion of bone and fibrocartilage tissue formation; enhancement of biomechanical properties |
• Laxity • Improvement of Lysholm and Tegner score • Enhancing of knee stability • Signs of pivot shift • Unimodal distribution of collagen fibrils • Cases of failures and ruptures |
• Mechanical stretching | • Signs of degenerative change • No development of functional tissue • Synovial reaction • Unsatisfactory long-term results • Sufficient mechanical properties • Poor cell infiltration • Osteointegration • Dynamic mechanical stretching improves collagen expression and tenogenic differentiation |
• [75], [117], [119], [122], [123], [124], [125], [128], [129], [130], [131], [132], [133] [134] |
| Knitting | Hyaluronan, PCL, PLCL, PLGA and silk fibroin | • MSCs: cell proliferation, cell elongation; expression of CD44, collagen I and III, laminin, fibronectin, and actin; orientation along the direction of microfiber alignment; deposition of ECM secretion (collagen I and III), expression of specific tenogenic markers |
• Rabbit model: tendon-like ECM expression; collagen fiber remodelling, neovascularization, expression of tenogenic markers |
-- | -- | • Expression of important protein for scaffold interaction and typical ligament proteins • Toe region profile and elastic modulus similar to ligaments • Sufficient biomechanical properties • Tissue regeneration and remodelling • Neovascularization |
• [52], [78], [135], [136], [137] |
| Braiding | Gore-Tex, PP, PLGA, Suture threads, GelMA and alginate, PGA, PLGA, PLLA, and fibronectin, silkworm gut | • Fibroblasts and primary ACL cells: cell attachment and proliferation • Tendon-derived cells: cell migration and alignment on the fiber axis; high expression of specific tenogenic genes • MSCs: cell adhesion, growth and tenogenic differentiation |
• Goat model: improvement of mechanical properties; inflammatory reaction |
• Pain • Increasing in degenerative changes • Improvements in Lysholm scores, activity scores, and arthrometry values • Operative complication • Improvement of stability • Decreasing of pivot shift |
• Mechanical stretching | • Deterioration over time • Effusions and pain • Mechanical properties comparable to native tendon/ligament • Integration of the scaffold • Normal joint laxity • Production of a collagen-rich matrix • Potential clinical efficacy (combined with stem cells therapies) • Braiding angle affects the mechanical properties |
• [115], [116], [141], [142], [144], [145], [147], [148], [102], [140], [149], [150], [152], [153] |
| Braid-twisting | PLLA, PEGDA | • Fibroblasts: cell proliferation, deposition of ECM |
• Rabbit model: smaller cross-sectional area, Sharpey’s fiber presence, formation of fibrocartilage |
-- | • Biochemical stimulation using BMP2 | • Great porosity • Mimicking the biomechanical response and the mechanical characteristics of native ACL • Osteointegration • Resistance to fatigue |
• [139], [154], [155], [156], [157] |
| Electrospinning | PCL, PA6 and silica particles, PEO, PLCL and hyaluronic acid, silk fibroin, PLGA, PDO, PLLA and dextran, collagen I, PLGA, PU, poly(trimethylene carbonate), zinc oxide, alginate, gelatin, chitosan, cellulose nanocrystals, cellulose acetate | • Fibroblasts: cell spreading, proliferation, and matrix deposition, aligned scaffolds guide parallel orientation of cells and higher collagen production, expression of integrin • MSCs: cells proliferation, spreading and infiltration, tenogenic differentiation, ECM deposition • Human primary tendon-derived cells: cell attachment |
• Rat model: cellular infiltration and colonization, improvement of glycosaminoglycans expression and higher of collagen organization • Rabbit model: no improvement on ultimate stress nor Young’s modulus values, reinforcement of tissue mechanical strength; antiadhesion effect • Rodent model: treated junctions have higher Young’s Modulus |
-- | • Biochemical stimulation using bFGFs, insulin, BMP-13 • Mechanical stretching |
• Scaffold implantation did not have negative effects • Sufficient mechanical properties for tendon repair • Restoring biomechanical strength • Aligned fibrous architectures showed anisotropic and significantly higher mechanical characteristics compared to randomly oriented fibers • Aligned fibers can mimic native tendon native architecture • Adhesion prevention • Positive effect on tendon and ligaments healing • Aligned cells on the nanofiber structure are significantly affected by stretching in axial direction • Regulation of mechanical properties and biological response (e.g. cell growth, differentiation, and matrix deposition) can be performed by varying the fiber diameter • Deposition of tendon-mimetic ECM • The fiber orientation can influence cell proliferation, differentiation, and immunomodulation |
• [41], [45], [46], [47], [56], [62], [67], [68], [69], [71], [72], [73], [80], [79], [91], [162], [165], [170], [171], [172], [173], [174], [175], [177], [178], [179], [180], [181], [182], [183], [225] |
| Wet-spinning | Chitosan, hyaluronan, alginate, and GelMA | • Fibroblasts: cell adhesion and proliferation, collagen I expression • MSCs: cell proliferation and alignment, collagen I and III production, specific tenogenic markers expression |
• Rat model: increasing of mechanical properties and collagen I deposition |
-- | • Mechanical stretching • Biochemical stimulation using BMP-12 |
• Great biological response • Stabilization of the joint • Combination of biochemical and mechanical stimulation promotes cell tenogenic • Larger size of yarns leads to higher mechanical properties (i.e. values of ultimate stress) |
• [187], [188], [189], [190] |
| Multi-layering | PCL, gelatin, chitosan, PLLA, PEO, tendon-derived ECM, fibronectin, PBS, PLGA, heparin/fibrin, PA6, Alginate, PDO, PGS, PLGA, PU, PLCL, polyethylene glycol, poly-L-lysine, silk, collagen, hyaluronic acid, bioactive glasses | • MSCs: cell elongation in the direction of the fiber scaffold, expression of tenogenic phenotype, good metabolic activity, orientation on fiber direction • Tenocytes: adhesion, viability and proliferation, ECM deposition • Tendon stem/progenitor cells: spindle-shape morphology, cell aligment, ECM deposition • Fibroblasts: cell vialibily and proliferation |
• Rat model: improvement of the structural and mechanical properties of tendon injury repair, immunologic compatibility; tenogenic gene expression of MSCs • Canine model: cells remained viable in the tendon repair environment, mild immunoresponse • Sheep model: no excessive inflammation nor tissue adhesion • Rabbit model: formation of collagen large fibrils and aligned fibers, increase of biomechanical properties |
• Biochemical stimulation using TGF-β3, PDGF-BB, BMP-12, bFGF • Mechanical stretching |
• Yield strain enhances during the culture time • Tendon ECM used as scaffold material might favour the differentiation into tenogenic lines • Provide stable and integral constructs easy to be handle during surgical procedures and in vivo implantations • Scaffold may release cells and growth factors in vivo at the same time • Mechanical and biochemical stimulation improve cell growth, align orientation, and tenogenic differentiation • Adhesion prevention • ECM deposition • Cell growth and infiltration • Vascularization • Cell loading enhances tissue regeneration • Sufficient mechanical properties • Tendon healing • Chemotaxis |
• [74], [76], [90], [176], [192], [193], [194], [195], [196], [197], [198], [199], [200],[201], [202], [203], [204] |
3. Fiber-based engineered scaffolds for tendons and ligaments
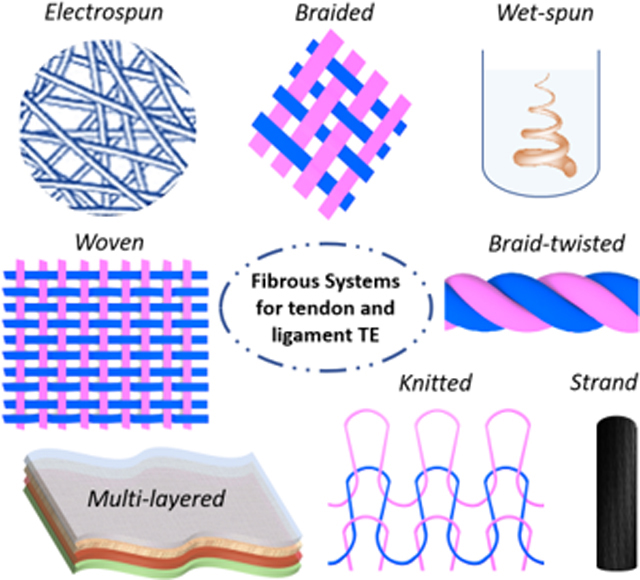
Fibrous scaffolds produced using various fiber-based fabricating techniques are recognized to be suitable for replacing anisotropic tissues and to promote their healing [94–99]. The specific structure enables them to mimic the collagen organization, to guarantee mechanical support and tissue infiltration during the regeneration process [100–103]. In this article, we have mainly focused on the analysis of the utilization of fiber-based architectures including strand fibers, woven, knitted, braided, and braid-twisted fibrous structures as well as electrospun, wet-spun and multi-layered fibrous constructs.
3.1. Strand fibers
In the late 1970s, strand carbon fibers were one of the first approaches used for tendon and ligament replacement. Since carbon compounds are present in native tissues, grafts made of carbon elements, such as carbon fibers, were explored due to their promising biocompatibility. Carbon fibers showed high tensile strength and low elongation rate. Moreover, their characteristics, such as the capability to induce infiltration, orientation, and production of collagen as well as biocompatibility and inertness made carbon fibers a considerable candidate for tissue repair purposes [104]. Several studies on carbon fibrous grafts for tendon/ligament showed that carbon fibers do not inhibit tissue growth, become stronger over time and they may perform as a scaffold for tissue regeneration [18,105,106].
In the 1980s, carbon fibers became commercially available from four different companies and clinically used in 20 countries [107]. Kumar et al. confirmed repair and regeneration of a tendon tissue defect after 30 days from carbon fibrous graft implantation. Collagen and blood vessel organization were conformed similar to the normal healing process, proving that carbon biologically acts as an inert material [104]. However, long-term follow up showed fragmentation and erosion degradation phenomena in the fibers, which provoked migration of the carbon particles in the synovium, hyaline cartilage, and menisci. Accumulation of the particles near lymph nodes, slight inflammatory reaction, synovitis, and hypertrophy were also reported [108,109]. Moreover, discomfort and the loss of knee mobility, and a consistent number of failure determined the limitation of carbon fiber use for tendon/ligament regeneration [108,110]. Even though carbon fibers were produced ‘naked’ in the beginning, many polymers and co-polymers have been utilized as their coatings or protection agents. Synthetic polymers including PCL, PLA, PGA, and their derived copolymers, as well as natural polymers such as gelatin have been considered for this purpose [18,107,111]. Carbon fibers were incorporated into 3D printed polymeric structures in order to improve the cell colonization. Positive results demonstrated the scaffold’s potential application in tendon/ligament repair [111].
Lately, strand fibers made of collagen via self-assembly or crosslinking technique have been investigated for reproducing the characteristic collagen fascicle structure of tendons and ligaments. Collagen was selected due to its great porosity and capacity to mimic the proper microenvironment and structural features of the native tissues, as well as its ability to act as elastic energy storage in the tissue structure during locomotion [112]. However, a study of Panas-Perez et al. showed that collagen strand fibers had low mechanical properties and degraded within 2 months after implantation, increasing the risk of premature failure. In order to increase the mechanical characteristics and the degradation rate, they introduced silk fibroin and fabricated a composite scaffold made of 14% silk and 86% collagen fibers (v/v). The composite scaffold demonstrated ultimate tensile stress comparable to native ligament and slow degradation rate but not adequate physical resorption [113]. Additionally, immunogenicity caused by bovine collagen and toxicity of chemical crosslinking agents have led researchers to consider other alternatives. Recently, ultrahigh molecular weight polyethylene (UHMWPE) strand fibers were incorporated into a hydrogel network for tendon reinforcement. The authors reported the matching of mechanical properties with the native tissue, as well as infiltration and expression of tenogenic genes by tendon stem cells. Additionally, collagen ingrowth was also observed in a rat model in vivo [114].
Strand fibers have the advantage of supporting collagen production, infiltration, and orientation. However, fragmentation and degradation phenomena of carbon fibers induced discomfort, loss of knee mobility, and failure; while not sufficient mechanical properties and fast degradation rate of fibers composed of collagen provoked their failure. Thus, the use of strand fibers as scaffold for tendon and ligament regeneration is now limited.
3.2. Woven fibrous structures
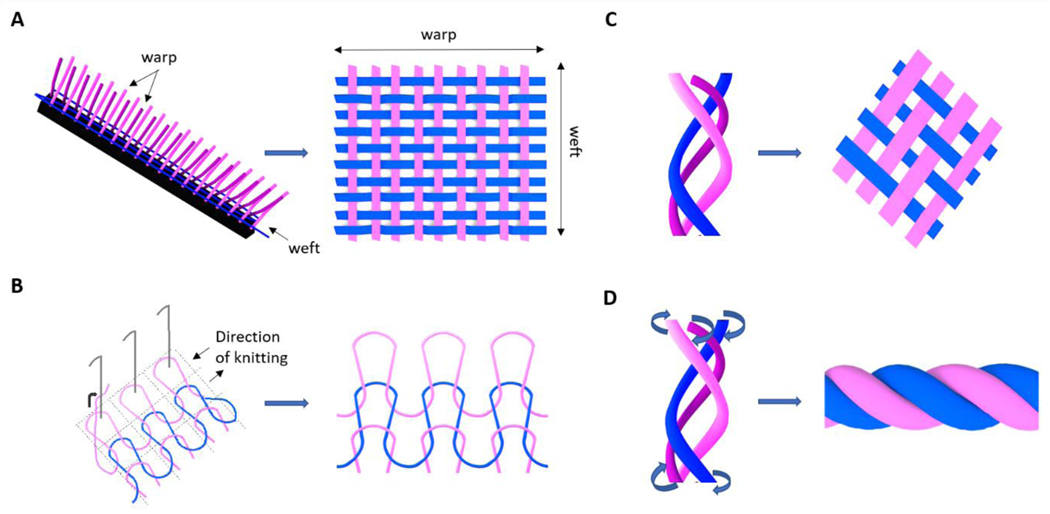
Common textile-based technologies such as weaving, knitting, braiding and braid-twisting have also been applied for the fabrication of 3D fibrous architectures for tendon/ligament applications (Figure 3). In this frame, woven structures are obtained by interlacing two orthogonal sets of fibers, namely warp and weft, in a methodic and repetitive pattern. More specifically, weft fibers perpendicularly cross warps fibers at each weaving step, resulting in a regular 3D fiber-based structure (Figure 3A). The use of non-degradable synthetic constructs is one of the oldest strategies for tendon/ligament repair. Commercial woven fibrous devices made of poly(ethylene terephthalate) (PET) (e.g. Leeds-Keio ligament or Ligament Advanced Reinforcement System) and stretched poly(tetrafluoroethylene) (Gore-Tex) have been widely implanted as tissue reinforcements and replacements [115–120]. Conditionally approved by the Food and Drugs Administration (FDA), they have the capacity to replace and protect the injured tissue, preventing osteoarthritis and allowing the ingrowth of new tissue without risk of cross-infection, while restoring the joint stability [121]. The Leeds-Keio (LK) ligament (Neoligaments Ltd, UK), a cylindrical woven structure made of fibers of PET, was introduced and applied in the clinical practice in the 1980s and 1990s, counting over 50000 implantations all over the word. The graft provided strength, relatively low stiffness, and biological inertness, giving immediate stability and promoting the ingrowth of collagen fibers [122]. Zaffagnini et al. studied intact LK ligaments after 20 years of implantation. Fibroblast migration and deposition of collagen tissue was proven by histologic and ultrastructural results. It has been reported, that the grafts were fully covered by autologous tissue, while collagen fibrils were physiologically orientated in the load direction. [123] However, long-term implantation outcome was only successful in about 60% of the patients [124]. An increase in laxity, loss of stability, and pivot shifting are reported in 50% of unsuccessful cases, while the device failure rupture occurred in about 30%. Denti et al. demonstrated that the loss of stability is not time-dependent, and showed comparable results of short-term and long-term analysis of the implanted grafts [125]. Shroven et al. demonstrated that the high rate of laxity led to a decrease in LK graft functions and tensile mechanical properties during the implantation time [126]. For all these disadvantages, recent studies doubted the efficiency of LK in the neoligament formation [117]. Another artificial non-degradable scaffold made of PET fibers for tendon and ligament replacement purposes is the Ligament Advanced Reinforcement System (LARS, France)[127]. Its intra-articular structure is characterized by longitudinal fibers which possess resistance to fatigue and permit cell infiltration. The extra-articular part is instead characterized by woven fibers which offer strength and resist to elongation. Left knee and right knee have clockwise and counterclockwise designs, respectively [119]. LARS artificial ligament showed a sufficient mechanical resistance of tension, flexion, and torsion loads. Moreover, it is not susceptible to torsional fatigue, wear, and tear events [128]. In their study, Gao et al. indicated a clinical outcome of LARS failure of 4,4% and a very low complication rate without significant evidence of synovitis, concluding good short and long term results of LARS implantation [119]. It has been also shown that LARS artificial ligament could induce the deposition of autologous collagen and neo tissue formation [129]. Since LARS and LK are both made of the same material, their biocompatibility is similar, thus the autologous tissue ingrowth rate is not significantly different. Zaffagnini et al. demonstrated through in vitro and in vivo studies the suitability of both LARS and LK surfaces for fibroblast adhesion and proliferation [123]. Even though the woven fibrous devices protected the injured tissues from axial splitting and abrasive wear, guaranteeing sufficient extensibility and tissue infiltration, they generally failed due to the long-term mechanical phenomena such as creep, fatigue, stress shielding and permanent deformation. Moreover, the contact with bone sharp edges might induce abrasions, generating debris which could result in joint synovitis [18,19]. Recently, more biocompatible materials (e.g. collagen) have been proposed for the production of woven scaffolds [130,131]. Learn et al. proposed a type I collagen woven system for tendon repair. The scaffold was seeded with MSCs and tested in vivo in a rabbit model. Results showed an increment in stiffness, collagen deposition, and specific tenogenic protein expression (i.e. tenomodulin) [132].
Figure 3.
Schematic illustration of fiber textile-based technologies and resulted 3D fibrous architectures: A) weaving; B) knitting; C) braiding; D) braid-twisting. Partially based on [97].
On the other hand, weaving textile technique using electrospun fibrous threads have also gained the interest of researches [133,134]. In this frame, Laranjera et al. fabricated a novel woven scaffold made of PLC, chitosan and cellulose nanocrystals electrospun fibers, showing its potential for tendon tissue engineering. The biological response was tested using two different cells’ sources: human adipose-derived stem cells and human tendon-derived cells. Results showed cell elongation and alignment, while the expression of specific tenogenic markers was detected. Moreover, cell infiltration and ECM oriented deposition were reported [134]. In the same manner, Dong et al. proposed the use of silk fibroin and laponite (LAP) wet-spun fibers to fabricate a woven platform for ligament applications. The system showed compatibility and osteogenic differentiation of mouse pre-osteoblasts in vitro, as well as osteointegration and improvement of mechanical properties in a rat model in vivo [75].
Woven structures provided strength and stability while promoting ingrowth of collagen fibers. However, these constructs do not allow optimal cell infiltration which is essential for efficient regeneration of the tissue. Moreover, fatigue and permanent deformation phenomena as well as laxity and pivot shifting represent big disadvantages of this approach.
3.3. Knitted fibrous structures
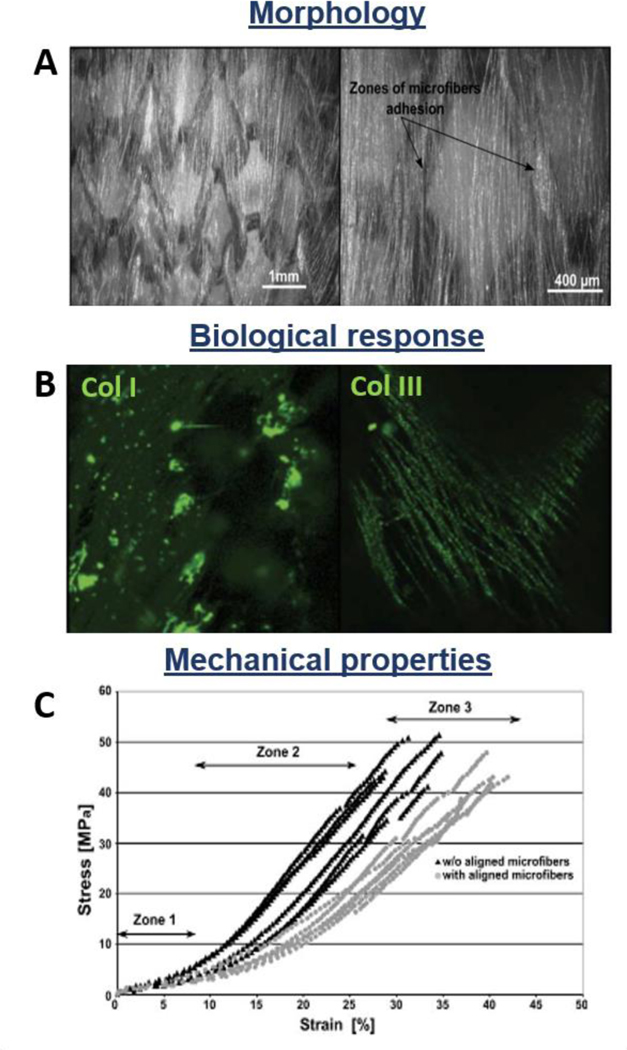
The knitting textile-based technology provides two sets of fibers interlocked in a series of connected loops, creating a 3D structure with knitted fibrous architecture (Figure 3B). The most interesting characteristic of knitted fibrous structures is their ability to reproduce the tendon/ligament toe region during tensile mechanical tests. Additionally, knitting technology allows the fabrication of less dense structures which can be tailored and customized for the desired size. The low-density structure shows internal interconnected pores, useful for nutrient and gas transportation, and tissue ingrowth. However, the high porosity does not permit a homogeneous and controlled distribution of the seeded cells. To solve this problem, different techniques such as cell encapsulation in hydrogel, elaboration of natural polymer fiber network, cell sheet TE, silk microporous sponge, and nanofiber deposition by electrospinning technique were developed [78,135,136]. Cristino et al. seeded MSCs on a hyaluronan-based knitted commercial scaffold for ligament regeneration (HYAFF). They reported homogeneous cell distribution, as well as cluster of differentiation 44 (CD44), collagen I and III, laminin, fibronectin, and actin expression, concluding that knitted HYAFF scaffold is not immunogenic and enables the new-tendon/ligament tissue ingrowth [52]. Cai et al. proposed a PLC and silk fibroin/PLCL knitted system for tendon engineering. In vitro biological performances were evaluated seeding rabbit bone marrow stem cells, showing cell elongation, proliferation and expression of tenogenic key markers. On the other hand, the scaffold was tested in vivo in a rabbit model, demonstrating sufficient biomechanical properties, as well as tissue repair and regeneration [137]. Moreover, Vaquette et al. designed a novel poly(lactide-co-caprolactone) PLCL knitted scaffold coated by aligned electrospun microfibers, to obtain a better spatial reproduction of cell microenvironment. Scanning Electron Microscope (SEM) images of the construct are reported in Figure 4A. Cells seeded on the composite scaffolds appeared uniformly distributed with spontaneous orientation along the aligned microfibers. Immunostaining proved cell proliferation and ECM secretion (mainly collagen I and III) (Figure 4B) [78]. Furthermore, stress-strain curves showed the characteristic triphasic profile, mimicking the mechanical behavior of the native tendon (Figure 4C). Sahoo et al. proposed a poly(lactic-co-glycolic acid) (PLGA) knitted scaffold coated with PLGA electrospun nanofibers to recapitulate the native nanofibrous architecture of tendon/ligament ECM, as well as to guarantee cell adhesion on a large biocompatible surface area [138]. The scaffold was seeded with the bone marrow stromal cells which expressed high level of specific tenogenic genes as collagen I, decorin, and biglycan, proving its potential as system for tendon/ligament TE. In another study, a silk knitted structure was combined with a collagen sponge to fabricate ACL replacements. In vivo results in a rabbit model showed collagen fibers formation, neovascularization and expression of specific tenogenic markers of stem/progenitor cells deposited on the construct [135].
Figure 4.
Morphological, biological, and mechanical properties of knitted fibrous structures: PLCL knitted scaffold coated by aligned electrospun microfibers. A) SEM images of scaffold structure; B) Collagen type I and III immunostaining after 14 days of culture; C) Stress-Strain curves of constructs coated and uncoated by aligned microfibers. Reproduced with permission [78]. Copyright 2010, Wiley Periodicals, Inc.
Even though the mechanical properties of basic knitted scaffolds seemed to be promising, the high porosity of this structure causes inadequate tissue ingrowth which restricts their use for tendon and ligament replacement [78].
3.4. Braided fibrous structures
The braiding textile technology creates an intertwined fibrous structure composed of three or more fibers, which diagonally interlace and overlap in a regular arrangement. The result is a 3D fiber-based construct with a braided morphological structure (Figure 3C), which can support high axial loads and provide extension and shear resistance. It possesses similar tendon/ligament stress-strain characteristics, as well as good resistance against abrasion, fatigue and catastrophic failure [139]. By optimizing the braiding angle, it is possible to fabricate structures with controlled geometry, pore diameter, porosity, yarn density, and mechanical properties [140,141]. The oldest braided fibrous devices were applied in the 1980s and were made of synthetic non-degradable polymers such as polypropylene (PP) (e.g. Ligament Augmentation Device) and Gore-Tex ligament. Kennedy introduced the concept of a cylindrical diamond-braided graft made of PP: Ligament Augmentation Device (LAD) (3M, St. Paul, Minn., USA). It consists of 9 tows (or strands) of high-tenacity (8gm/denier) PP yarn (180 fibers each) braided into a flat 6mm construct [142]. Normally, LAD was implanted in association with biological grafts, like the patella tendon, to enhance knee stability. The LAD has the function to protect the biological graft from rapid degeneration and loss of strength [18]. LAD has also the capability to share forces with the biological graft, allowing it to bear a portion of the load, as well as to prevent potential long-term fatigue failure and laxity. The device could carry 28–45% of the load applied on the graft depending on the type of tendon/ligament [143]. McPherson et al. evaluated the host body response to Kennedy LAD after 12 months from the implantation, showing a significant collagen deposition within the yarns with oriented collagen fibers in the longitudinal direction 2 years after implantation. Moreover, they showed that the augmented anterior cruciate ligament reconstruction was stronger after 2 years and just a limited local inflammatory response and a low immunogenic response were reported [144]. Kdolsky et al. investigated the clinical long-term outcomes in terms of laxity of LAD implantation, examining the functionality and stability of the graft after 5 years from the implantation. They found that 97% of the patients had a standard knee laxity. Within this group, 75% of the cases resulted in excellent recovery and patients could practice normal sport activities. On the other hand, 3% of the devices failed, mostly because of the long-term fatigue phenomena. However, it has been reported that LAD, as a foreign synthetic device, might provoke inflammatory reactions, infections, synovitis or eosinophilic, lymphocytic and plasma cell response in the host body, delaying the regeneration of the neo-tissue. After 12 months from the surgery, the histological evaluation of the graft showed the presence of giant cells and macrophages in the site where LAD was implanted [144]. Additionally, histological analysis showed that 25% of the patients suffered from chronic inflammation of synovium [145].
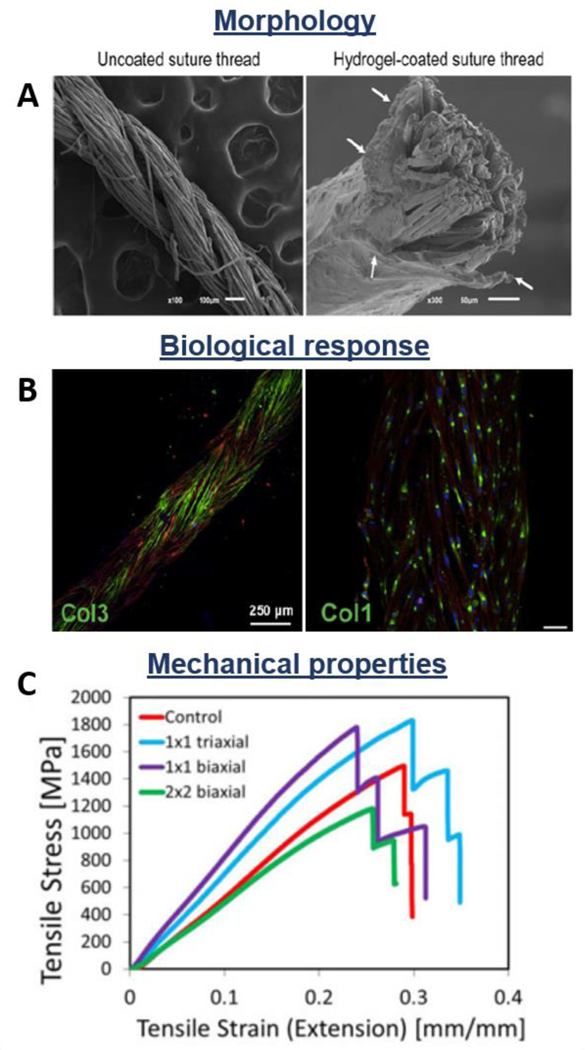
Another braided non-degradable synthetic device is the Gore-Tex ligament (W.L.Gore and Co., Flagstaff, Ariz.). It is a low-density braided poly(tetrafluoroethylene) (PTFE) structure composed of three multifilament beams [146]. The first PTFE scaffold for ligament replacement was inserted in 1982 to rapidly mobilize the knee, aiming to permanently substitute the injured tissue [147]. Roolker et al. reported clinical outcomes of Gore-Tex prosthesis after 5 years of implantation showing that PTFE devices led in 40% of the cases to better knee stability without pain. However, X-ray images indicated degenerative changes in 81% of the patients [115]. In another study, Ahlfeld et al. showed that the device improved knee stability in 87% of the patients, decreasing the pivot shifting. Pain decreased in 67% of the cases, but only 30% of the patients had total pain alleviation. Moreover, in 70% of the cases chondromalacia or articular cartilage defects occurred, while 10% of the patients were not capable to perform normal daily activities [148]. Dahlstedt et al. examined and compared the Gore-Tex graft and the Kennedy LAD, reporting the outcomes after 36 months from the implantation. The LAD group showed better results than the Gore-Tex group, which was affected from higher effusion and pain level. However, in both cases, the histology demonstrated the presence of giant cells and microscopic foreign particles, while no significant difference in terms of compliance was registered [116]. Nowadays, these braided non-degradable synthetic fibrous devices are not considered optimal for the replacement of tendons and ligaments, due to the inflammatory reaction, synovitis, and long-term fatigue phenomena they provoke. For these reasons, their clinical use has been limited, creating the need for a new generation of braided scaffolds which are made of degradable polymers such as PGA, PLGA, PCL, and PLA. The studies showed interesting results in terms of cell attachment, infiltration, and ECM production [149–151]. In another study, Yu et al. investigated the effect of air plasma treatment and fibronectin adsorption on PLLA braided constructs, revealing enhanced adhesion and proliferation of mesenchymal stem cells seeded on treated matrices [152]. Pagán et al. proposed a braided fibrous structure made of natural-derived materials (i.e. silkworm gut), showing great biocompatibility in vitro as well as sufficient mechanical properties for tendon and ligaments applications [153]. Cooper et al. examined the influence of different geometry on braid-twisted scaffold properties, showing that circular braided geometry had significantly higher maximum tensile load compared to the rectangular shape [141]. Additionally, Costa-Almeida et al. utilized the branding fabrication method for producing load-bearing hydrogel-based composite fibers made of braided suture threads coated with cell-laden hydrogel for tendon regeneration (Figure 5A) [102]. The hydrogel component was loaded with human tendon-derived cells which migrated through the hydrogel structure and aligned on the suture thread. Authors reported a higher expression of tendon specific markers such as scleraxis and tenascin C, as well as matrix remodeling related genes (i.e. matrix metalloproteinase-1 and −2). Fluorescence images show that the encapsulated cells produced a collagen-rich matrix, proving the potential of the system as scaffolds for tendon TE (Figure 5B). Moreover, the effect of braiding pattern on the mechanical properties of the constructs was investigated, concluding the 1×1 biaxial structure ensures the higher tensile modulus (Figure 5C).
Figure 5.
Morphological, biological, and mechanical properties of braided fibrous structures: Suture threads coated with cell-laden hydrogel. A) SEM images of coated and uncoated threads; B) Collagen type III and I deposition; C) Tensile stress-strain curves of differently braided constructs. Reproduced with permission [102]. Copyright 2017, John Wiley & Sons, Ltd.
Braided constructs made of degradable polymers are considered potential candidates for tendon and ligament TE due to their capability of matching the mechanical behavior of native tissues, while acting as an efficient cell vehicle. However, these structures can assess 3D cell encapsulation just if coated with hydrogel materials, and cannot properly recapitulate the tendon and ligament anisotropic features.
3.5. Braid-twisted fibrous structures
The combination of branding technology with fiber twisting results in braid-twisted constructs (Figure 3D). More specifically, this technique provides the twisting (e.g. clockwise or counterclockwise twisted) of the fibers which are subsequently subjected to the braiding process. Considering the large number of fiber bundles which compose tendons and ligaments, some research groups developed braid-twisted scaffolds to properly mimic the physiological hierarchical organization and the biomechanical properties of the tissues. The introduction of twisted fibers led to a significant improvement of the ultimate strain, ultimate tensile strength, and a wider toe region. Moreover, braid-twisted structures have higher porosity and quantity of pores, as well as better abrasion resistance compared to braided constructs [139,154]. The braid-twisted scaffolds are fabricated using synthetic biopolymers, like polyesters, as well as natural polymers (e.g. silk fibroin) to support cells’ attachment, growth, and proliferation [86,155]. Altman et al. were the first to design and produce twisted fibrous matrices for tendon and ligament regeneration. They developed a structure consisting of 6 silk fibroin parallel twisted cords made of fiber bundled strands. Even though the silk-fiber matrix was 80% less stiff compared to the parallel fibroin fiber structure, in vivo studies demonstrated that it was able to stabilize the injured joint [86]. To better reproduce the viscoelastic properties of the tendon/ligament tissues, Freeman et al. investigated the addition of 10% poly(ethylene glycol) diacrylate (PEGDA) hydrogel in PLLA braid-twisted scaffolds [139]. The incorporation of the hydrogel resulted in a decrease of pore sizes and porosity and in a loss of ultimate tensile stress values. However, they demonstrate that PEGDA/PLLA twist-braided scaffolds maintained the typical stress-strain behavior and matched the viscoelasticity of the native tissue in a better manner [139,156]. The effect of braiding and twisting angle was further investigated by evaluating the ultimate tensile strength and toe region length. Authors concluded that scaffolds made of 4 braids with 60° twisted fibers and 72° twisted fiber bundles are the most suitable for tendon and ligament engineering purposes [154]. Mengsteab et al. evaluated the in vivo response in terms of osteointegration of PLLA braid-twisted scaffold in an ACL reconstruction rabbit model. BMP-2 was injected in the bone tunnel through a saline solution in order to enhance osteogenesis. Results revealed osteointegration (as demonstrated by the presence of Sharpey’s fibers), reduced bone tunnel cross-sectional area and formation of fibrocartilage, showing that the system is a potential candidate for ligament tissue engineering [157].
The new generation of braid-twisted scaffolds shows good abrasion resistance and sufficient mechanical properties, as well as great porosity and larger number of pores, which promotes cell infiltration, proliferation, and tissue ingrowth. However, these constructs do not assist 3D cell loading and oriented distribution.
3.6. Electrospun nanofibrous meshes
Since the early 2000s, the electrospinning technique has been considered for biomedical applications. It soon became an interesting approach for the development and production of scaffolds for TE purposes [158–160]. The method permits the fabrication of very thin fibers when a polymer solution is flowing through a needle onto a collecting plate under electric field conditions [161]. Scaffolds made of nanofibers possess important advantages compared to differently fabricated scaffolds, including high surface area to volume ratio, small pore dimension, and large porosity. Studies have reported that electrospun nanofibers can reproduce the tissue ECM and offer a sufficient biomimetic environment for cell adhesion, growth, and differentiation, favoring a better tissue regeneration [158,162–164]. Electrospinning parameters such as polymer concentration to be dissolved in the solvent solution, flow rate, voltage, working distance or ambience conditions can be manipulated to control fiber diameter, porosity, and mechanical strength of the electrospun scaffold. It has been demonstrated that nanofibrous constructs have greater mechanical properties than bulk polymer material and enhance cell proliferation, metabolic activity, and matrix expression [165,166].
Fibrous scaffolds made through electrospinning show fiber diameters ranging from a few microns to less than 100 nm, aiming to reproduce ECM structures, like collagen fibrils which size ranges from 20 nm to 40 mm in vivo [167]. Considering those advantages, electrospun nanofibers have been explored for the fabrication of constructs for engineering of musculoskeletal tissues, including bones, cartilage, tendons, and ligaments [168–171]. The introduction of biomaterials might accelerate new tissue formation and, thanks to their biodegradability, a second surgery for removing the construct will be prevented. Synthetic polymers are normally used to fabricate scaffolds for tendon and ligament regeneration via electrospinning technique. Many studies proved their biocompatibility, testing the fibroblast adhesion, proliferation and infiltration on PCL [158,172], PLA [80], PLCL [173], PLGA [174], PDO [175] and PU [41] surfaces.
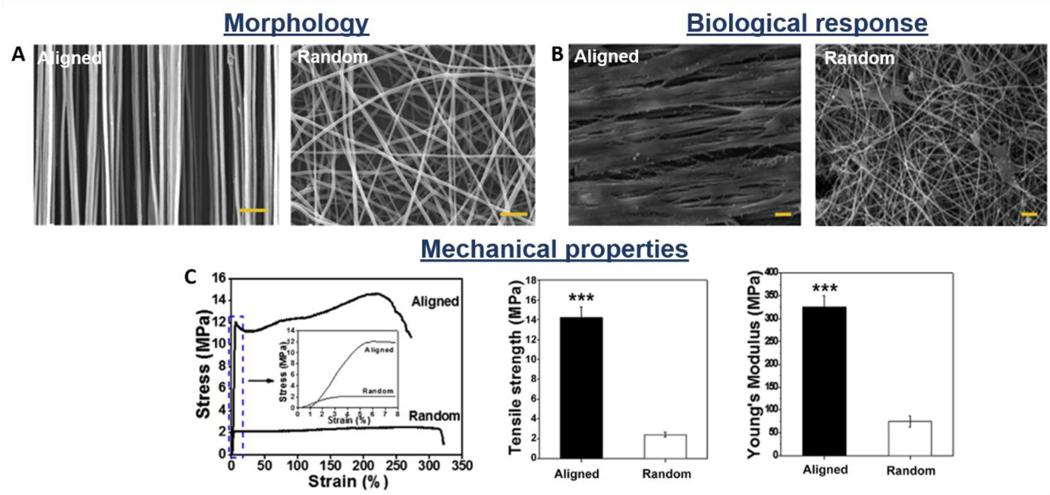
Nanofiber organization and alignment can be controlled during the fabrication process, in order to tailor appropriate scaffolds. Normally electrospun fibers are collected on a steady collector to obtain randomly oriented nanofibers, which have isotropic mechanical properties. However, for applications which require anisotropic mechanical properties, aligned fibers were collected on a rapidly rotating mandrel [173]. The scaffolds composed of random nanofibers possess isotropic mechanical features which are considered convenient for the engineering of isotropic tissues or drug delivery systems. Nevertheless, because the mechanical function of tendon and ligaments is highly influenced by the collagen fiber anisotropic orientation, aligned nanofibrous scaffolds appear to be more appropriate in this case [174]. Several studies demonstrated that the scaffold fiber organization could modulate the cellular response and regulate the scaffold properties. It has been shown that aligned fibers guide the cells into a preferential, longitudinal orientation, which mimics the parallel arrangement of the cells in the native tissue [47]. The cells are led to attach along the nanofibers long axis, allowing better cell alignment, distribution, differentiation, and matrix deposition compared to the cells cultured on the random fiber structures, which are typically polygonal and randomly oriented [176,177]. This is also due to the great porosity (higher than 80%) and high permeability of the structures, which affects the nutrient distribution, favoring the cell proliferation and tissue growth (Figure 6A-B). Sean et al. demonstrated through nuclei staining that the number of attached cells on scaffolds made of PLGA aligned nanofibers was significantly higher compared to random fibrous structures [46]. Additionally, numerous studies reported that aligned scaffolds significantly improve the tensile mechanical properties compared to random structures (Figure 6C) [176]. PLGA aligned scaffolds have tensile Young modulus that is 318% and tensile strength that is 324% higher compared to randomly arranged ones [174]. PCL and PLCL aligned scaffolds showed the same behavior, having 214% and 400% higher tensile Young modulus and 189% and 300% higher tensile strength, respectively [45,47,173]. However, the produced electrospun scaffolds appear to be significantly weaker, when compared to the tendon/ligament native tissues.
Figure 6.
Morphological, biological, and mechanical properties of synthetic electrospun nanofibrous scaffolds: influence of fiber orientation (aligned vs. random). A) SEM images; B) Morphology of mesenchymal stem cells seeded on the scaffolds’ surface: SEM images; C) Stress-strain catachrestic curves and derived tensile strength and Young’s modulus values. Reproduced with permission [176]. Copyright 2015, Elsevier Ltd.
In order to establish a stiffer alternative, Shang et al. proposed a new method to obtain thicker electrospun aligned nanofibrous scaffolds which are normally very thin and have relatively weak mechanical properties. The scaffolds were obtained using an improved electrospinning fabrication method with a grounded water bath which allows for the fabrication of about 200 μm aligned nanofibrous scaffolds. A significant increase in mechanical properties and structural stability of the constructs were reported [178]. Following the same purpose, other studies reported the fabrication of 3D electrospun yarn bundles and nanoyarn-reinforced scaffolds [47,69,73,173]. There devices showed higher tensile mechanical properties and were significantly stronger and stiffer than two-dimensional structures. Due to larger pores and greater porosity, the 3D scaffolds favor cell adhesion and migration, providing higher cell proliferation and infiltration (of approximately 500 um [173]). Furthermore, Erisken et al. and El Khatib et al. investigated the effect of the scaffold’s structural properties, such as the fiber diameter, on cell response in vitro [79,179]. The influence, regulation, and material-cell interactions were investigated evaluating the modulation on cell behavior on different scaffolds, which have fibers with diameters of 320nm, 680nm, and 1.80μm. It was reported that the scaffolds with fiber diameter of 320nm show the highest cell adhesion, as well as collagen and glycosaminoglycans (GAGs) expression, resulting in a better ECM production. On the other hand, the scaffolds with fiber diameter of 680nm promote the expression of collagen I, III, V, and tenomodulin (phenotypic markers of tendon fibroblasts), favoring cells to preserve their phenotype. Based on the obtained data, the authors underlined the importance and difficulty of selecting the best fiber diameter for the fabrication of scaffolds for tendon/ligament regeneration [79].
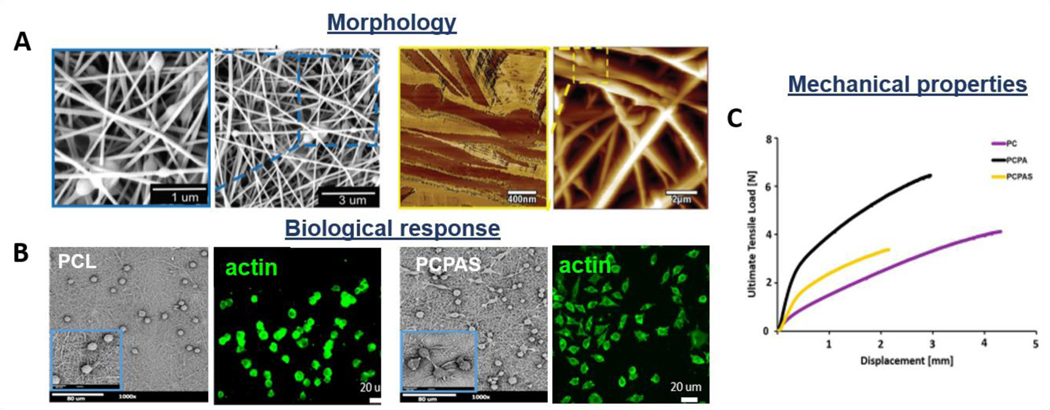
The architecture and structure of electrospun scaffolds allow the incorporation of natural components into the synthetic system to improve its biological responses and tissue healing [71,72]. In order to enhance the biocompatibility of synthetic scaffolds, the components of the ECM, e.g. collagen, can be added [68,180]. Even though collagen has high biocompatibility, its low mechanical properties have suggested that it should be combined with synthetic polymers, which have superior mechanical behavior. The co-spinning of synthetic materials with matrix proteins was considered by Sean et al., who electrospun a solution of collagen I with PLGA and PU producing a 3D scaffold. It has been demonstrated that the addition of collagen into the polymeric structure enhanced cell response, while did not significantly decrease the mechanical properties of the scaffold. They concluded that collagen might blend with other synthetic polymers to fabricate scaffolds with sufficient mechanical and biological properties for the required applications [46]. Due to the similar composition and chemical structure of collagen, silk fibroin derived materials were also investigated. Advantages such as good biocompatibility, biodegradability, no immunological activity, and high mechanical properties made this natural polymer an excellent candidate for the fabrication of tendon and ligament scaffolds [181]. Jin et al. investigated electrospun silk fibroin-based fibrous scaffold, showing that silk electrospun mat has sufficient mechanical properties and that its surface encourages human bone marrow stromal cells adhesion, migration and proliferation over 14 days [182]. Electrospun composite scaffolds have also been investigated to enhance the polymeric matrix’s response [43,177]. A study performed by Rinoldi et al. reported that the incorporation of silica nanoparticles into a bead-on-string fibrous structure could improve the wettability of the surface, favoring cell spreading and proliferation (Figure 7A-C) [165].
Figure 7.
Morphological, biological, and mechanical properties of bead-on-string composite electrospun nanofibrous scaffolds: incorporation of silica nanoparticles into a polymeric matrix. A) SEM and AFM images, B) Spreading of L929 cells seeded onto PCL and silica composite mat (PCPAS): SEM and acting staining images; C) Representative stress-strain curves of synthetic (PC, PCPA) vs. composite structures (PCPAS). Reproduced with permission [165]. Copyright 2018, The Royal Society of Chemistry.
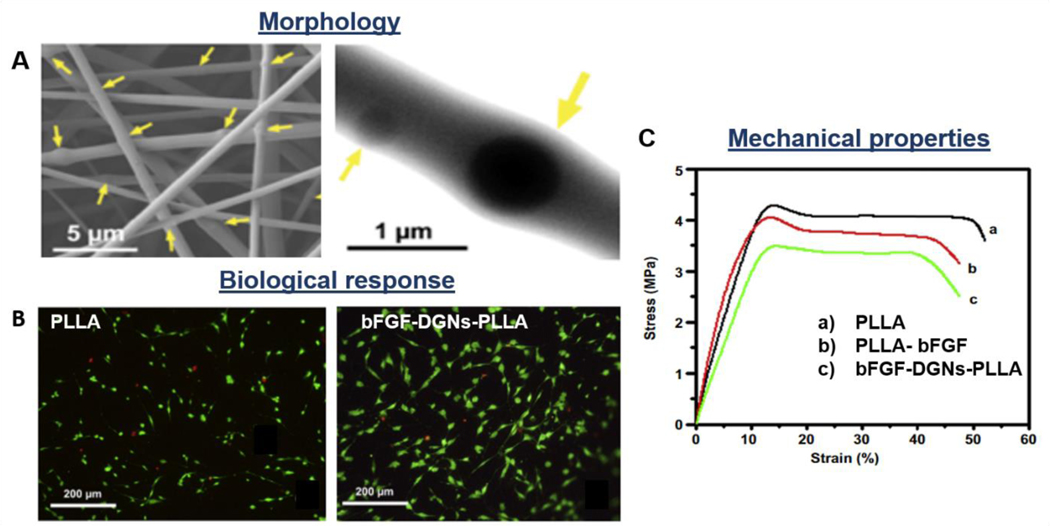
In order to modulate the cellular behavior and tissue growth, approaches such as seeding of stem cells on the scaffold surface [176] or the encapsulation of growth factors into the synthetic fibers, were developed [56,67,183]. bFGFs are generally selected as representative growth factors that influence and enhance the tendon/ligament repair and healing, cellular differentiation, spreading, proliferation, and ECM production. Considering the mentioned growth factors’ advantages, Liu et al. incorporated bFGFs into dextran glassy nanoparticles and electrospun PLLA-dextran composite fibers (Figure 8A). The loaded growth factors were maintaining their bioactivity for 30 days, releasing about 48.71 +/− 13.53% and enhancing fibroblastic viability (Figure 8B), collagenase production, capillary endothelial cell proliferation and tissue regeneration [56]. However, the incorporation of particles into the polymeric fibers may lead to a decrease in mechanical properties, due to the introduction of discontinuity, inhomogeneities, and defects in the fiber architecture (Figure 7C, 8C). In another study, Zhao et al. tested bFGF-loaded PLGA electrospun fibrous membranes with a core-shell structure. It has been shown that bFGF–PLGA scaffold enhances collagen organization and fibrocartilage formation. Moreover, the system showed higher mechanical properties such as ultimate load-to-failure and stiffness when compared to PLGA scaffolds. The results demonstrated that bFGF bioactivity was preserved for 21 days, accelerating significantly tissue healing and remodeling [62].
Figure 8.
Morphological, biological, and mechanical properties of PLLA-dextran-bFGF composite electrospun nanofibrous scaffolds: loading of growth factors into fibrous constructs. A) SEM images; B) Live and dead images of fibroblasts seeded on PLLA and PLLA composite scaffolds; C) Representative stress-strain curves of synthetic vs. composite structures. Reproduced with permission [56]. Copyright 2013, Elsevier Ltd.
Additionally, electrospun nanofibers have been successfully applied as tendon biomimetic sheath or physical barrier, to prevent adhesion of surrounded tissues [66]. The local release of bioactive agents loaded into the membranes can further improve the anti-adhesion effect and enhance tendon repair, as deeply described by Alimohammadi et al.
Overall, electrospun nanofibrous meshes have the great advantage of possessing nano/micro-size diameter of the fibers, large porosity, and very high surface area to volume ratio. The nanofibrous structure is a promising alternative to produce a scaffold for tendon and ligament regeneration, providing an adequate micro-environment for bio-signaling, supporting sufficient cell attachment and proliferation, as well as avoiding the adhesion of surrounded tissues. However, poor cell infiltration and lack of cell biding sites have still been considered the main limitations of those systems.
3.7. Wet-spun fibrous constructs
The wet-spinning technique provides a polymer solution flowing through a syringe into a liquid crosslinking bath, resulting in fiber formation. This technique can be utilized for the fabrication of randomly oriented fibers as well as parallel aligned fibrous systems [184,185]. A wide range of materials can be processed using the wet-spinning method, including natural-derived polymers [186] as well as native ECM components [187]. The possibility of fabricating cell-laden highly biocompatible fibrous constructs is most probably the greatest advantage of this technique which avoids the use of toxic solvent during the spinning process.
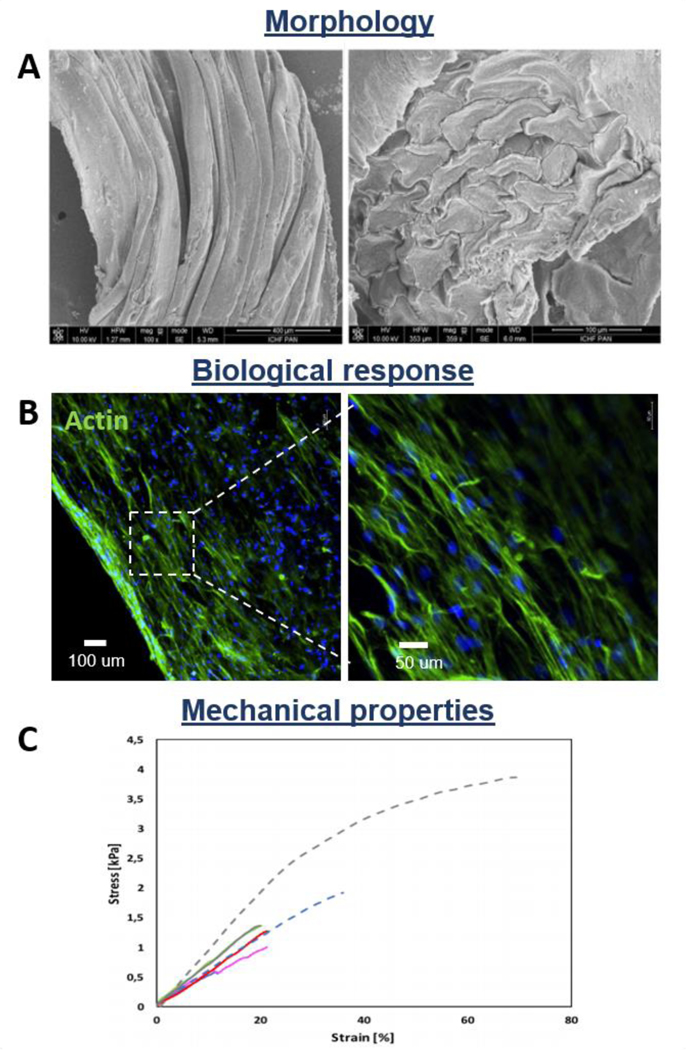
Nowotny et al. applied the wet spinning technique to fabricate chitosan fibrous scaffolds for tendon regeneration [188]. Data revealed that aligned architecture of the yarns significantly influences human MSCs attachment and orientation in the direction of the fiber axis. The results are supported by a study of Rinoldi et al. who biofabricated aligned hydrogel yarns loaded with MSCs via wet-spinning method, collecting the fibers on a rotating drum to form a fibrous hydrogel bundle for tendon TE (Figure 9A-C). The highly aligned orientation of human bone marrow MSCs on the fiber axis direction was observed (Figure 9B) [187]. Moreover, authors investigated the influence of the mechanical static stretching and BMP-12 treatment on MSCs cultured within the constructs, showing an enhancement of specific tenogenic matrix proteins expression.
Figure 9.
Morphological, biological, and mechanical properties of wet-spun fibrous constructs: cell-laden highly aligned hydrogel yarns. A) SEM images of fiber bundle (left) and cross-section (right); B) Morphology of MSCs cultured for 14 days into mechanically stimulated fibers (actin staining); C) Representative stress-strain curves of hydrogel yarns loaded with cells cultured in different conditions vs samples without cells, tested at day 0 (dash line) and day 14 of culture (continuous line). Reproduced with permission [187]. Copyright 2019, WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
In another study, Funakoshi et al. applied the wet spinning technique to fabricate chitosan-based hyaluronan hybrid polymer fibrous scaffolds for ligament regeneration (Figure 10A). The results revealed that hyaluronan presence significantly enhanced the mechanical properties of the construct as well as fibroblasts adhesion, proliferation, and collagen I production (Figure 10B) [189]. However, even though it is shown that the cells produced ECM proteins onto this kind of scaffolds, the mechanical properties of these constructs decrease during the culture time, probably due to material degradation phenomena or because of the additional matrix remodelling effect exerted by the cells (Figure 9C, 10C). Fibers fabricated via wet-spinning technique have a direct relation between yarns dimension and ultimate stress values [188]; however, do not always show sufficient mechanical characteristics to match tendon/ligament performance [186]. For this reason, wet-spun fibers have been often braided (Figure 11A), in order to form constructs with mechanical properties comparable to the native tissues. In vitro test showed collagen I and III deposition on chitosan-hyaluronan wet-spun scaffolds (Figure 11B), while in vivo implantation in a rat model resulted in low toxicity and inflammation response, mechanically stabilizing the joint during the healing process (Figure 11C) [190].
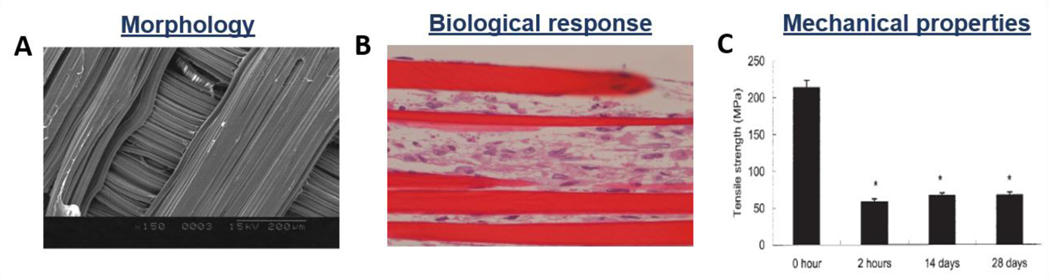
Figure 10.
Morphological, biological, and mechanical properties of wet-spun fibrous constructs: chitosan-hyaluronan fibrous scaffolds. A) SEM images of fibers structures; B) Light micrograph of fibroblasts proliferated in the wet-spun structure after 14 days of culture; C) Tensile strength of samples after different incubation times. Reproduced with permission [189]. Copyright 2005, Wiley Periodicals, Inc.
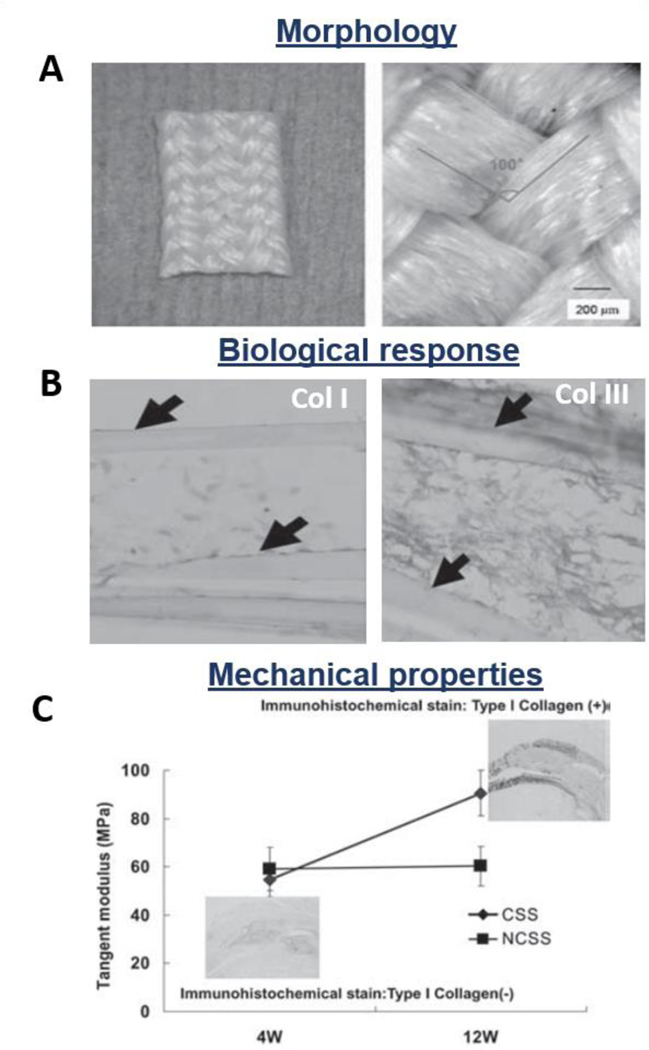
Figure 11.
Morphological, biological, and mechanical properties of wet-spun fibrous constructs: chitosan-hyaluronan 3D wet-spun fibers. A) SEM images of the final construct (after braiding); B) In vitro collagen I and III deposition; C) In vivo mechanical properties of rabbit rotator cuff defect models treated with the proposed scaffolds (CSS: cell seeded scaffold, NCSS: non-seeded scaffold). Reproduced with permission [190]. Copyright 2007, SAGE Publications.
Even though the wet-spinning technique has the ability of processing natural-derived materials, allowing the possibility of encapsulating cells and directing their organization, the mechanical properties of the wet-spun constructs are still considered insufficient for tendon applications.
3.8. Multi-layered scaffolds
Lately, multilayered approaches have gained attention as novel methods for scaffold design [191,192]. Different layer-by-layer techniques such as simultaneous wet electrospinning and cell-seeding, alternating layers of nanofibers and microfibers, varying polymers, or arranging electrospun layers coated with hydrogels have been developed [193–195]. Systems combining electrospun fibrous mats with woven/knitted constructs in multi-layered scaffolds have been recently explored for tendon and ligament engineering [78,138,196,197]. Rashid et al. developed a system composed of an inner layer of aligned electrospun PCL fibers and two outer layers of i) PDO woven structure and ii) electrospun PDO fibrous meshes. In vivo testing in a sheep model showed infiltration of cells (mostly tendon fibroblasts) and the development of blood vessels onto the electrospun layer. Non-excessive inflammatory reaction nor adhesion of surrounded tissue were reported [196]. On the other hand, knitted silk constructs were coated with PU/collagen electrospun matrix in order to improve the biological response of the knitted structure [198].
Recently, the electrospinning technique has also been combined with 3D printing technology, creating fibrous meshes layered with printed structures [76]. In this frame, Touré et al. directly electrospun PCL/PGS nanofibers onto 3D printed PCL/PGS construct loaded with bioactive glasses for tendon/ligament applications. Results revealed that the presence of bioactive glasses improved the biocompatibility in vitro, while sufficient mechanical properties (i.e. Young Modulus) were reported [76].
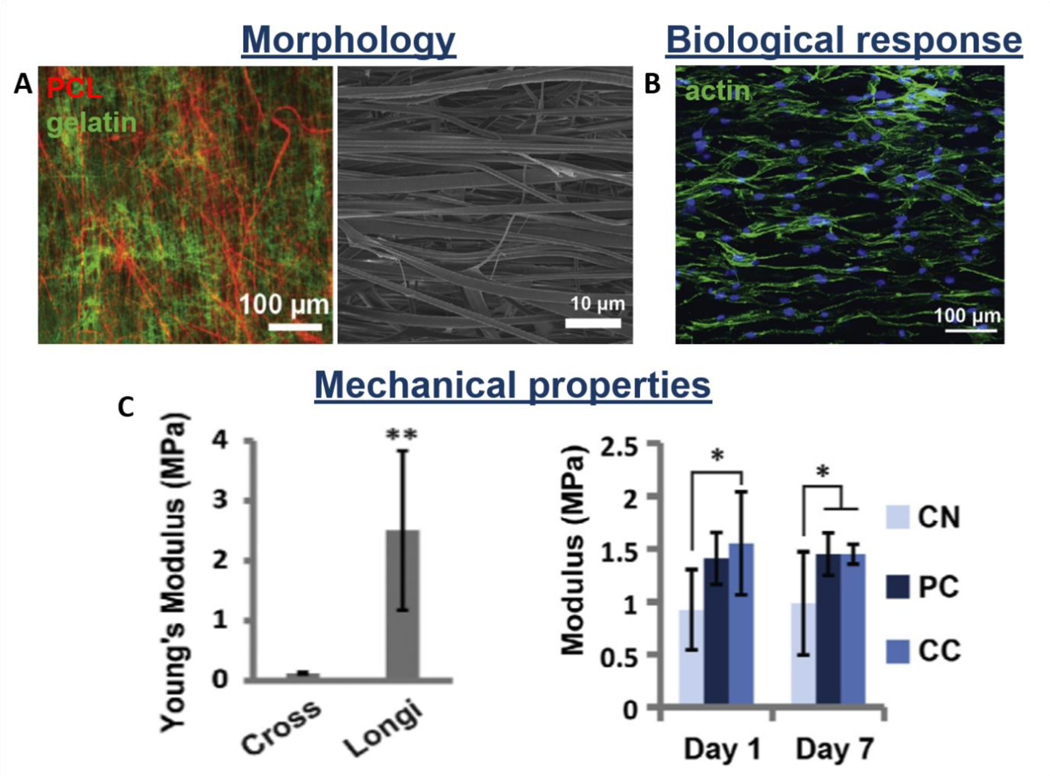
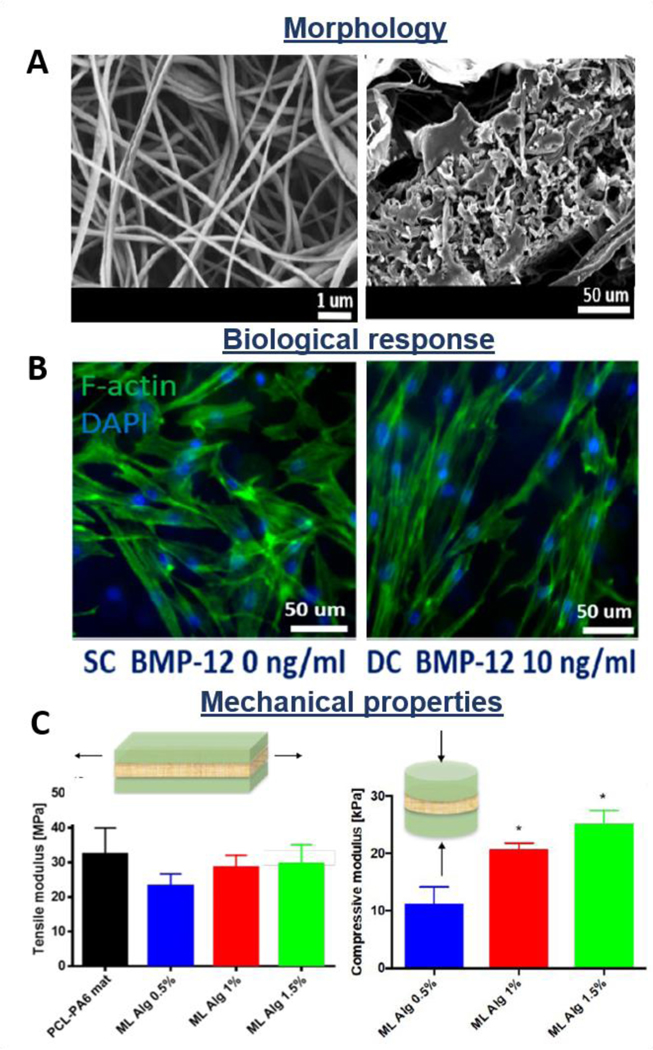
Additionally, layer-by-layer scaffolds, where electrospun, knitted or braided structures are layered with hydrogels, are also considered promising alternatives for reproducing the native characteristic of tendon/ligament tissues [90,102,139,199,200]. Zhao et al. fabricated a multi-layered system made of fibrin gel loaded with bFGF and MSCs sheet sandwiched between two PLGA knitted structures. The scaffold implanted in a rat model revealed sufficient biomechanical properties and expression of tenogenic markers by incorporated MSCs, promoting tissue repair and regeneration [201]. Jayasree et al. proposed a braided scaffold made of PCL/collagen/bFBF layered with alginate hydrogel and subjected to dynamic mechanical stretching into a bioreactor system. Results revealed tenocyte viability, growth and expression of tenogenic markers in vitro, while oriented collagen formation was observed in a rabbit model in vivo [202]. On the other hand, Chainani et al. fabricated a multi-layered electrospun PCL scaffold coated by tendon-derived matrix (TDM), and evaluated the biological response of human adipose-derived stem cells (hASCs). They investigated the effect of the TDM layer, demonstrating high cell infiltration and collagen production by histological and immunofluorescence analysis, respectively. Moreover, the maintenance of mechanical properties during the culture time was also reported, showing the stability of this kind of construct [195]. The results are supported by a study of Yan et al., who showed homogeneous cell distribution and penetration on multi-layered PCL/methacrylated gelatin (Figure 12A,B) [90]. Moreover, the maintenance of mechanical properties during the culture time was also demonstrated (Figure 12C) [90]. The novel multi-layered scaffolds also have the ability to simultaneously load and deliver cells and growth factors, preserving their viability and bioactivity, respectively. Manning et al. designed a multi-layered scaffold consisting of interchanging layers of heparin/fibrin-based hydrogels and PLGA electrospun nanofibers. Additionally, authors encapsulated platelet-derived growth factor BB (PDGF-BB) and ASCs into the hydrogel delivery system. In vitro and in vivo studies showed the homogeneous distribution of cells into the hydrogel structure, their viability, and the continuous release of growth factors [203]. In another study, authors proposed a twisted PLLA electrospun fibrous bundle surrounded by thin PLLA electrospun layer coated with chitosan hydrogel to recapitulate the native tendon ECM. Results showed good biocompatibility in terms of attachment and growth of tenocytes seeded onto the scaffold. Moreover, a low protein absorption was detected, revealing the scaffold potential for preventing adhesion of surrounded tissues in vivo [74]. Rinoldi et al. proposed 3D multi-layered composite scaffolds composed of synthetic electrospun nanofibers coated with layers of hydrogel loaded with MSCs (Figure 13A) [204]. The electrospun matrix was made of PCL and polyamide 6 (PA6) and it was proven to provide the mechanical properties and bear the whole construct. On the other hand, the hydrogel layers were composed of gelatin methacryloyl (GelMA) and alginate and mimic a micro-environment suitable for cell encapsulation and growth (Figure 13B,C). Constructs were mounted and cultured into a custom-built bioreactor, where mechanical and biochemical stimulation was applied. The addition of BMP-12 was optimized in terms of concentration, to promote the tenogenic differentiation of MSCs. In vitro results showed the positive effect of the combined stimuli in terms of proliferation, alignment (Figure 13B), as well as tenogenic differentiation of MSCs [204].
Figure 12.
Morphological, biological, and mechanical properties of multi-layered scaffolds: PCL electrospun aligned mat layered with methacrylated gelatin. A) Scaffold morphology: Fluorescent and SEM images; B) Cell distribution onto the scaffold: actin staining image; C) Anisotropic mechanical properties of the scaffold (on the left-hand side, cross vs longitudinal direction); modulus of crosslinked (CC), not crosslinked (NC) and alternative layers (PC) constructs during culture time (on the right-hand side). Reproduced with permission [90]. Copyright 2016, Elsevier Ltd.
Figure 13.
Morphological, biological, and mechanical properties of multi-layered scaffolds: PCL-PA6 electrospun matrix coated by hydrogel layers formed from 10% GelMA and different concentrations of alginate. A) SEM images of electrospun core (left) and edge of the scaffold (right); B) Fluorescence images of cell cytoskeleton (non-stimulated vs stimulated samples); C) Tensile and compressive moduli of the scaffolds. Reproduced with permission [204]. Copyright 2019, American Chemical Society.
Multi-layered scaffolds appear the most promising candidates for tendon TE due to the great advantage of combining the beneficial properties of each layer in the final engineered system. This approach synergizes the properties of different source-materials, morphologies, and fabrication technologies resulting in composite structures which can potentially make the most in terms of biological response as well as mechanical and structural properties. Moreover, the possibility of loading and releasing bioactive molecules is also enabled.
4. Fiber-based scaffolds for tissue interfaces
Engineering of tissue interfaces has always been considered a great challenge. The main difficulty in this field is the design of gradient scaffolds with zonal structure, architectures, compositions, and mechanical properties. However, this aspect is crucial to develop systems which can guarantee the physiological biofunctionality of the tissue [205].
Herein, fiber-based scaffolding approaches for engineering tendon/ligament-bone and tendon-muscle interfaces are described and discussed.
4.1. Constructs for tendon/ligament-to-bone engineering
The main requirement for engineering tendon/ligament-bone junction is developing a single scaffold with gradient mineralized and non-mineralized regions, various collagen interlacing and hard-to-soft mechanical characteristics. More specifically, three different tissue regions should be designed: tendon/ligament, fibrocartilage interface and bone. [206]
In case of injuries, due to the poor regeneration properties of the tissue, a lack of gradient characteristics occurs at the interface, where mainly unorganized scar tissue is formed. This phenomenon can create a considerable properties mismatch between tendon/ligament and bone regions, often leading to re-failure. [206]
In literature, scaffolds for tendon/ligament-to-bone engineering are commonly designed combining various phases (e.g. biphasic, triphasic) or continuous gradients. Several fiber-based platforms have been developed for this application, including knitted, braided, electrospun and multi-layered systems [206–208]. In this frame, the knitting technology was applied to fabricate a silk construct, further combined with aligned collagen fibers. The authors presented bone marrow stem cells adhesion and osteogenic differentiation in vitro, while adequate biomechanical properties were detected in vivo [208]. On the other hand, the braiding technique was used to produce triphasic braided scaffolds made of PLA. The different braided regions showed zonal mechanical characteristics, recapitulating the tissue junction properties [206].
Electrospinning of scaffolds composed of synthetic (e.g. PCL, PLGA), naturally-based (e.g collagen, silk) and composite (e.g PLGA/hydroxyapatite) biomaterials have also been reported for engineering of tendon/ligament- bone interface [207,209,210]. The multiple-phased scaffolds have been obtained by varying the orientation, architecture and distribution of electrospun fibers in district regions [211]. Electrospinning of aligned-to-random fibrous constructs was implemented in order to reproduce the collagen fiber distribution and orientation in tendon-to-bone junction [212]. Cells seeded on the biphasic construct reported zonal morphological differences, showing oriented vs unorganized cell distribution in aligned and random fibrous regions, respectively [212,213]. Electrospun constructs loaded with growth factors have also been proposed by Reifenrath et al. [214]. Gradient profiles in terms of growth factor and mineral distribution were additionally achieved on aligned-to-random scaffolds by Chen and co-workers. The development of a BMP-2 and nanohydroxyapatite (nanoHA) graded electrospun matrix resulted in a gradient differentiation of bone marrow stem cells cultured onto the constructs. Consistently, higher expression of osteogenic markers was detected in areas with higher content of BMP-2/nanoHA [215]. Similarly, Jiang et al. fabricated mineral graded silk scaffolds, showing gradient characteristics in terms of mechanical properties and stem cells differentiation [216].
Additionally, multi-layered systems have been proposed for tendon/ligament-to-bone applications, including electrospun meshes combined with woven structures and films [217,218]. Zhang et al. produced a PCL electrospun mat loaded with BMP-7 and rolled on a PET woven construct, resulting in a degradable-nondegradable hybrid structures. The scaffolds showed good biocompatibility, sufficient mechanical properties in vivo and induced osteogenesis [217]. Constructs formed from different electrospun layers have also been developed for tendon-bone interface [219]. Li et al. designed a dual-layered electrospun matrix made of PLLA and PLLA/nanoHA composite [220]; while Cai et al. obtained a dual-layered silk/PLCL fibrous scaffold by depositing random fibers on previously spun aligned fibers [219]. Results showed oriented collagen formation and tissue regeneration.
Distinctive signs of progress have been recently reported on the development of fiber-based scaffolds for tendon/ligament-bone interface. However, issues related to loading and delivery of cells in vivo as well as mismatching of mineral gradient distribution remain still unsolved.
4.2. Systems for tendon-muscle junction
The design of the tendon-muscle interface requires great efforts due to the significant difference in properties between the two tissues. Indeed, muscles are mainly composed of cells, while ECM is the primary tendon component. This results in a mismatch of tissue vascularization and consequent metabolic demand. Mechanical properties are also distinct: tendons have higher mechanical characteristics in terms of stiffness, while muscles are compliant and more elastic [221]. The tissue interface involves three components: tendon, myotendinous junction (MTJ) and muscle [222].
When the interface is damaged or injured, restoring the gradient architecture of the myotendinous junction is crucial to guarantee the transferring of loads from muscle to tendon and to ensure the proper tissue functionalities.
Zonal changes related to extracellular matrix, cell component and mechanical features should be considered while engineering MTJs [223]. Fiber-based scaffolding systems for this application have not been widely explored, and just a few studies are published on this topic [224]. Ladd et al. fabricate synthetic electrospun matrices made of PCL and PLLA-collagen for MTJ applications. The authors simultaneously spun the two electrospinning solutions obtaining a three-region platform. The biocompatibility along with adhesion of myoblasts and fibroblasts seeded on the construct was demonstrated, while the formation of myotubes was observed [224]. Additionally, different mechanical profiles were detected, successfully mimicking the MTJ trend characteristics.
Up to date, not many fiber-based studies are focused on MTJ applications; however, it is expected that the field of fibrous systems for this application will exponentially grow in the near future.
5. Concluding remarks and future directions
The highly organized hierarchical fibrous structure of tendons and ligaments is designed to carry loads and to supply the biomechanical functions, such as sustaining, bearing, and reinforcing the joints. The production of fiber-based scaffolds composed of biomaterials derived from biological or synthetic sources is considered a great approach to reproduce the collagen fibrous orientation and to promote the repair and regeneration of tendon and ligament tissues. In this review strand fiber, woven, knitted, braided, and braid-twisted fibrous structures, as well as wet- and electro-spun fibrous scaffolds were described and discussed. Although strand, woven, and braided fibrous scaffolds provided immediate stability after the implantation as well as collagen deposition, they caused inflammatory reaction, synovitis, and long-term fatigue phenomena, limiting their clinical use. In order to overcome these disadvantages, a new generation of braid-twisted scaffolds was developed. Their good abrasion resistance and adequate mechanical properties as well as their great porosity which promotes cell infiltration, proliferation, and tissue infiltration, make the braid-twisted structures a promising candidate for tendon and ligament regeneration purposes. Nevertheless, the electrospinning method allows the control of fiber diameter, porosity, and mechanical properties to reproduce the natural physiology of the tissues. However, the small pore size of the nanofibrous constructs might not permit an optimal cell infiltration and tissue ingrowth. On the other hand, the wet-spinning approach allowed the fabrication of 3D cell-laden structures, providing fibrous systems which can mimic the ECM features. Even though wet-spun aligned fibrous systems might guide cell orientation, the mechanical properties of those natural hydrogel-based scaffolds often do not match the mechanical properties of tendons/ligaments. For these reasons, the combination of different techniques is considered crucial to produce scaffolds which can recapitulate the native tissue properties and promote tissue regeneration. Thus, multi-layered systems, where each compartment can be independently produced and tailored, have emerged as the most effective alternatives for tendon and ligament TE. Potentially, these constructs might combine the advantages of different platforms produced with distinct techniques, combining synthetic and natural polymers, and simultaneously providing mechanical support and biocompatible microenvironments (Table 2).
Table 2.
Summary of advantages and disadvantages of fiber-based scaffolds.
| Fiber-based scaffold | Degradability | Mechanical properties | Possibility of cell encapsulation | Collagen deposition | Fatigue phenomena and synovitis |
|---|---|---|---|---|---|
| Strand fibers | +/− | + | − | + | + |
| Woven fibers | − | + | − | + | + |
| Knitted fibers | + | + | − | + | + |
| Braided fibers | +/− | + | + | + | + |
| Braid-twisted fibers | + | + | − | + | − |
| Electrospun fibers | + | + | − | + | − |
| Wet-spun fibers | + | − | + | + | − |
| Multi-layered fibers | + | + | + | + | − |
3D multi-layered scaffolds may soon be used for the treatment of tendon injuries. This approach can create gradient multi-layered scaffolds that can potentially regenerate tissue interfaces and eventually favor the integration of the developed systems as well as functional recovery. Most probably, fabrication methods and strategies for obtaining more compact and stable multi-layered scaffolds will be developed in the near future, to minimize delamination phenomena which may occur between layers, resulting in the failure of the implants.
Acknowledgements
Authors would like to thank the funding from the National Center for Research and Development (STRATEGMED1/233224/10/NCBR/2014, project START). AT acknowledges financial support from National Institutes of Health (GM126831, AR073822).
Biography

Wojciech Święszkowski obtained his Ph.D. in Mechanical Engineering at Warsaw University of Technology (WUT), Poland and he completed a 4-year postdoctoral fellowship at TU Delft, the Netherlands. He obtained his habilitation at the Faculty of Materials Science and Engineering at WUT, where he is Professor and leader of the Biomaterials group. He was a visiting professor at several universities abroad, including Harvard University and TU Wien. His research interests involve design of advanced biomaterials for engineering cartilage, bone, tendon and nerve tissues, development of 3D-printing and bioprinting technologies, computer modeling and characterization of biomaterials, and development of drug delivery systems.
Contributor Information
Chiara Rinoldi, Materials Design Division, Faculty of Materials Science and Engineering, Warsaw University of Technology. Warsaw 02-507, Poland.
Ewa Kijeńska-Gawrońska, Materials Design Division, Faculty of Materials Science and Engineering, Warsaw University of Technology. Warsaw 02-507, Poland; Centre for Advanced Materials and Technologies CEZAMAT, Warsaw University of Technology. Warsaw 02-822, Poland.
Ali Khademhosseini, Department of Bioengineering, Department of Chemical and Biomolecular Engineering, Department of Radiology, California NanoSystems Institute (CNSI), University of California. Los Angeles, CA 90095, USA; Terasaki Institute for Biomedical Innovation (TIBI), Los Angeles, CA 90024, USA.
Ali Tamayol, Department of Biomedical Engineering, University of Connecticut, Farmington, CT 06030, USA.
Wojciech Swieszkowski, Materials Design Division, Faculty of Materials Science and Engineering, Warsaw University of Technology. Warsaw 02-507, Poland.
References
- [1].Laurencin CT, Freeman JW, Biomaterials 2005, 26, 7530. [DOI] [PubMed] [Google Scholar]
- [2].Ratcliffe A, Butler DL, Dyment NA, Cagle PJ, Proctor CS, Ratcliffe SS, Flatow EL, Ann. Biomed. Eng 2015, 43, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andarawis-Puri N, Flatow EL, Soslowsky LJ, J. Orthop. Res 2015, 33, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rathbone S, Cartmell S, in Tissue Eng. Tissue Organ Regen (Ed: Eberli D), InTech, 2011, pp. 131–162. [Google Scholar]
- [5].Thorpe CT, Birch HL, Clegg PD, Screen HRC, Tendon Regen. 2015, 3. [Google Scholar]
- [6].Reese SP, Maas SA, Weiss JA, J. Biomech 2010, 43, 1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].James R, Kesturu G, Balian G, Chhabra AB, J. Hand Surg. Am 2008, 33, 102. [DOI] [PubMed] [Google Scholar]
- [8].Wang JHC, J. Biomech 2006, 39, 1563. [DOI] [PubMed] [Google Scholar]
- [9].Butler DL, Grood ES, Noyes FR, Zernicke RF, Exerc. Sport Sci. Rev 1978, 6. [PubMed] [Google Scholar]
- [10].Beynnon BD, Fleming BC, J Biomech 1998, 31, 519. [DOI] [PubMed] [Google Scholar]
- [11].Nau T, World J. Orthop 2015, 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Murray MM, Fleming BC, J. Orthop. Res 2013, 31, 1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang T, Gardiner BS, Lin Z, Rubenson J, Kirk TB, Wang A, Xu J, Smith DW, Lloyd DG, Zheng MH, Tissue Eng. Part B Rev 2013, 19, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weiss ND, Yale J Biol. Med 1991, 64, 194. [Google Scholar]
- [15].Saghazadeh S, Rinoldi C, Schot M, Kashaf SS, Sharifi F, Jalilian E, Nuutila K, Giatsidis G, Mostafalu P, Derakhshandeh H, et al. , Adv. Drug Deliv. Rev 2018, 127, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dyment NA, Galloway JL, Curr. Mol. Biol. reports 2015, 1, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Viidik A, in Biomech. Diarthrodial Joints (Ed: Ratcliffe A), Springer-Verlag, New York, 1990, pp. 4–38. [Google Scholar]
- [18].Chaput C, Duval N, 1997, Ligaments, 143. [Google Scholar]
- [19].Longo UG, Lamberti A, Maffulli N, Denaro V, Br. Med. Bull 2010, 94, 165. [DOI] [PubMed] [Google Scholar]
- [20].Negahi Shirazi A, Chrzanowski W, Khademhosseini A, Dehghani F, in Eng. Miner. Load Bear. Tissues (Eds: Bertassoni LE, Coelho PG), Springer International Publishing, Cham, 2015, pp. 161–186. [Google Scholar]
- [21].Leong NL, Petrigliano FA, McAllister DR, J. Biomed. Mater. Res. - Part A 2014, 102, 1614. [DOI] [PubMed] [Google Scholar]
- [22].Rodrigues MT, Reis RL, Gomes ME, Tissue Eng J. Regen. Med 2013, 7, 673. [DOI] [PubMed] [Google Scholar]
- [23].Yilgor C, Yilgor Huri P, Huri G, Stem Cells Int. 2011, 2012, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vunjak-Novakovic G, Altman G, Horan R, Kaplan DL, Annu. Rev. Biomed. Eng 2004, 6, 131. [DOI] [PubMed] [Google Scholar]
- [25].Langer R, Vacanti JP, Science (80-. ). 1993, 260, 920. [DOI] [PubMed] [Google Scholar]
- [26].Nerem RM, in Med. Sci, 2009, pp. 27–42. [Google Scholar]
- [27].Celikkin N, Rinoldi C, Costantini M, Trombetta M, Rainer A, Święszkowski W, Mater. Sci. Eng. C 2017, 78, 1277. [DOI] [PubMed] [Google Scholar]
- [28].Costantini M, Testa S, Rinoldi C, Celikkin N, Idaszek J, Colosi C, Barbetta A, Gargioli C, Święszkowski W, in Biofabrication 3D Tissue Model., The Royal Society Of Chemistry, 2019, pp. 184–215. [Google Scholar]
- [29].Idaszek J, Kijeńska E, Łojkowski M, Swieszkowski W, Appl. Surf. Sci 2016, 388, 762. [Google Scholar]
- [30].No YJ, Castilho M, Ramaswamy Y, Zreiqat H, Adv. Mater 2020, 32, 1904511. [DOI] [PubMed] [Google Scholar]
- [31].Lee J, Guarino V, Gloria A, Ambrosio L, Tae G, Kim YH, Jung Y, Kim S-H, Kim SH, J. Biomater. Sci. Polym. Ed 2010, 21, 1173. [DOI] [PubMed] [Google Scholar]
- [32].Davarinos N, O’Neill BJ, Curtin W, Davarinos CW, O’Neill N, Neill BJ,BJO, Curtin W, Adv. Orthop. Surg 2014, 2014, 6. [Google Scholar]
- [33].Skutek M, Griensven M, Zeichen J, Brauer N, Bosch U, Eur. J. Appl. Physiol 2001, 86, 48. [DOI] [PubMed] [Google Scholar]
- [34].Galloway MT, Lalley AL, Shearn JT, J. Bone Joint Surg. Am 2013, 95, 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang T, Lin Z, Day RE, Gardiner B, Landao-Bassonga E, Rubenson J, Kirk TB, Smith DW, Lloyd DG, Hardisty G, et al. , Biotechnol. Bioeng 2013, 110, 1495. [DOI] [PubMed] [Google Scholar]
- [36].Il Lee K, Lee JS, Kim JG, Kang KT, Jang JW, Shim YB, Moon SH, J. Biomed. Mater. Res. - Part A 2013, 101, 3152. [DOI] [PubMed] [Google Scholar]
- [37].Youngstrom DW, Barrett JG, Stem Cells Int. 2015, 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sawadkar P, Player D, Bozec L, Mudera V, J. Tissue Eng. Regen. Med 2020, 14, 135. [DOI] [PubMed] [Google Scholar]
- [39].Sensini A, Cristofolini L, Zucchelli A, Focarete ML, Gualandi C, DE Mori A, Kao AP, Roldo M, Blunn G, Tozzi G, J. Microsc 2019, 00, 1. [DOI] [PubMed] [Google Scholar]
- [40].Tetsunaga T, Furumatsu T, Abe N, Nishida K, Naruse K, Ozaki T, J. Biomech 2009, 42, 2097. [DOI] [PubMed] [Google Scholar]
- [41].Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW, Biomaterials 2005, 26, 1261. [DOI] [PubMed] [Google Scholar]
- [42].Qin T-W, Sun Y-L, Thoreson AR, Steinmann SP, Amadio PC, An K-N, Zhao C, Biomaterials 2015, 51, 43. [DOI] [PubMed] [Google Scholar]
- [43].Tomás AR, Goncąlves AI, Paz E, Freitas P, Domingues RMA, Gomes ME, Nanoscale 2019, 11, 18255. [DOI] [PubMed] [Google Scholar]
- [44].Yates EW, Rupani A, Foley GT, Khan WS, Cartmell S, Anand SJ, Stem Cells Int. 2012, 2012, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pauly HM, Kelly DJ, Popat KC, Trujillo NA, Dunne NJ, McCarthy HO, Haut Donahue TL, J. Mech. Behav. Biomed. Mater 2016, 61, 258. [DOI] [PubMed] [Google Scholar]
- [46].Full SM, Delman C, Gluck JM, Abdmaulen R, Shemin RJ, Heydarkhan-Hagvall S, J. Biomed. Mater. Res. Part B Appl. Biomater 2015, 103, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bosworth LA, Alam N, Wong JK, Downes S, J. Mater. Sci. Mater. Med 2013, 24, 1605. [DOI] [PubMed] [Google Scholar]
- [48].Cooper JA, Bailey LO, Carter JN, Castiglioni CE, Kofron MD, Ko FK, Laurencin CT, Biomaterials 2006, 27, 2747. [DOI] [PubMed] [Google Scholar]
- [49].Chen J, Moreau J, Horan R, Collette A, Bramano D, Volloch V, Richmond J, Vunjak-Novakovic G, Kaplan DL, Altman GH, Cult. Cells Tissue Eng 2005, 191. [Google Scholar]
- [50].Li F, Li B, Wang QM, Wang JHC, Cell Motil. Cytoskeleton 2008, 65, 332. [DOI] [PubMed] [Google Scholar]
- [51].Wang JH-C, Yang G, Li Z, Shen W, J. Biomech 2004, 37, 573. [DOI] [PubMed] [Google Scholar]
- [52].Cristino S, Grassi F, Toneguzzi a Piacentini S, Grigolo B, Santi S, Riccio M, Tognana E, Facchini a, Lisignoli G, J. Biomed. Mater. Res. A 2005, 73, 275. [DOI] [PubMed] [Google Scholar]
- [53].Yee Lui PP, Stem Cells Cloning Adv. Appl 2015, 8, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Costa-Almeida R, Calejo I, Gomes ME, Int. J. Mol. Sci 2019, 20, 3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gonçalves AI, Costa-Almeida R, Gershovich P, Rodrigues MT, Reis RL, Gomes ME, in Tendon Regen. - Underst. Tissue Physiol. Dev. to Eng. Funct Substitutes (Eds: Gomes ME, Reis RL, Rodrigues M), Academic Press, Boston, 2015, pp. 187–203. [Google Scholar]
- [56].Liu S, Qin M, Hu C, Wu F, Cui W, Jin T, Fan C, Biomaterials 2013, 34, 4690. [DOI] [PubMed] [Google Scholar]
- [57].Thomopoulos S, Das R, Sakiyama-Elbert S, Silva MJ, Charlton N, Gelberman RH, Ann. Biomed. Eng 2010, 38, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sakiyama-Elbert SE, Das R, Gelberman RH, Harwood F, Amiel D, Thomopoulos S, J Hand Surg Am 2009, 33, 1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Evrova O, Burgisser GM, Ebnother C, Adathala A, Calcagni M, Bachmann E, Snedeker JG, Scalera C, Giovanoli P, Vogel V, et al. , Biomaterials 2019, 232, 119722. [DOI] [PubMed] [Google Scholar]
- [60].Thomopoulos S, Das R, Silva MJ, Sakiyama-Elbert S, Harwood FL, Zampiakis E, Kim HM, Amiel D, Gelberman RH, J. Orthop. Res 2009, 27, 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, Chan K-M, Life Sci. 2003, 72, 2965. [DOI] [PubMed] [Google Scholar]
- [62].Zhao S, Zhao J, Dong S, Huangfu X, Li B, Yang H, Zhao J, Cui W, Int. J. Nanomedicine 2014, 9, 2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Roth SP, Brehm W, Gross C, Scheibe P, Schubert S, Burk J, Int. J. Mol. Sci 2019, 20, 5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB, Biomed. Mater 2011, 6, 25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Moreau JE, Chen J, Horan RL, Kaplan DL, Altman GH, Tissue Eng. 2005, 11, 1887. [DOI] [PubMed] [Google Scholar]
- [66].Alimohammadi M, Aghli Y, Fakhraei O, Moradi A, Passandideh-Fard M, Ebrahimzadeh MH, Khademhosseini A, Tamayol A, Mousavi Shaegh SA, ACS Biomater. Sci. Eng 2020, 6, 4356. [DOI] [PubMed] [Google Scholar]
- [67].Ramos DM, Abdulmalik S, Arul MR, Rudraiah S, Laurencin CT, Mazzocca AD, Kumbar SG, Polym. Adv. Technol 2019, 30, 1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sensini A, Gualandi C, Zucchelli A, Boyle LA, Kao AP, Reilly GC, Tozzi G, Cristofolini L, Focarete ML, Sci. Rep 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Olvera D, Schipani R, Sathy BN, Kelly DJ, Biomed. Mater 2019, 14, 35016. [DOI] [PubMed] [Google Scholar]
- [70].Gabler C, Saß JO, Gierschner S, Lindner T, Bader R, Tischer T, Biomed Res. Int 2018, 2018, DOI 10.1155/2018/6432742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liao JCY, He M, Gan AWT, Wen F, Tan LP, Chong AKS, J. Biomed. Mater. Res. - Part B Appl. Biomater 2018, 106, 2605. [DOI] [PubMed] [Google Scholar]
- [72].Sheng D, Li J, Ai C, Feng S, Ying T, Liu X, Cai J, Ding X, Jin W, Xu H, et al. , J. Mater. Chem. B 2019, 7, 4801. [DOI] [PubMed] [Google Scholar]
- [73].Almeida H, Domingues RMA, Mithieux SM, Pires RA, Gonçalves AI, Gómez-Florit M, Reis RL, Weiss AS, Gomes ME, ACS Appl. Mater. Interfaces 2019, 11, 19830. [DOI] [PubMed] [Google Scholar]
- [74].Nivedhitha Sundaram M, Deepthi S, Mony U, Shalumon KT, Chen JP, Jayakumar R, Int. J. Biol. Macromol 2019, 122, 37. [DOI] [PubMed] [Google Scholar]
- [75].Dong Q, Cai J, Wang H, Chen S, Liu Y, Yao J, Shao Z, Chen X, Acta Biomater. 2020, 106, 102. [DOI] [PubMed] [Google Scholar]
- [76].Touré ABR, Mele E, Christie JK, Nanomaterials 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hortensius R, Mozdzen L, Harley B, in (Eds: Gomes ME, Reis RL, Rodrigues MTBT-TR), Academic Press, Boston, 2015, pp. 349–380.
- [78].Vaquette C, Kahn C, Frochot C, Nouvel C, Six JL, De Isla N, Luo LH, Cooper-White J, Rahouadj R, Wang X, J. Biomed. Mater. Res. - Part A 2010, 94, 1270. [DOI] [PubMed] [Google Scholar]
- [79].Erisken C, Zhang X, Moffat KL, Levine WN, Lu HH, Tissue Eng. Part A 2012, 19, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Surrao DC, Waldman SD, Amsden BG, Acta Biomater. 2012, 8, 3997. [DOI] [PubMed] [Google Scholar]
- [81].Hakimi O, Mouthuy P-A, Carr A, Int. J. Exp. Pathol 2013, 94, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Deniz P, Guler S, Çelik E, Hosseinian P, Aydin HM, Mater. Sci. Eng. C 2020, 106, 110293. [DOI] [PubMed] [Google Scholar]
- [83].Zeugolis DI, Khew ST, Yew ESY, Ekaputra AK, Tong YW, Yung L-YL, Hutmacher DW, Sheppard C, Raghunath M, Biomaterials 2008, 29, 2293. [DOI] [PubMed] [Google Scholar]
- [84].Gigante A, Cesari E, Busilacchi A, Manzotti S, Kyriakidou K, Greco F, Di Primio R, Mattioli-Belmonte M, J. Orthop. Res 2009, 27, 826. [DOI] [PubMed] [Google Scholar]
- [85].Majima T, Funakosi T, Iwasaki N, Yamane ST, Harada K, Nonaka S, Minami A, Nishimura SI, J. Orthop. Sci 2005, 10, 302. [DOI] [PubMed] [Google Scholar]
- [86].Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL, Biomaterials 2002, 23, 4131. [DOI] [PubMed] [Google Scholar]
- [87].Contessi Negrini N, Tarsini P, Tanzi MC, Farè S, J. Appl. Polym. Sci 2019, 136, 1. [Google Scholar]
- [88].Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL, J. Biomed. Mater. Res. A 2003, 67, 559. [DOI] [PubMed] [Google Scholar]
- [89].Chaudhury S, Holland C, Thompson MS, Vollrath F, Carr AJ, J. Shoulder Elb. Surg 2012, 21, 1168. [DOI] [PubMed] [Google Scholar]
- [90].Yang G, Lin H, Rothrauff BB, Yu S, Tuan RS, Acta Biomater. 2016, 35, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kim MS, Kim G, Carbohydr. Polym 2014, 114, 213. [DOI] [PubMed] [Google Scholar]
- [92].Shao H-J, Chen CS, Lee Y-T, Wang J-H, Young T-H, J. Biomed. Mater. Res. A 2010, 93, 1297. [DOI] [PubMed] [Google Scholar]
- [93].Pacheco DP, Zorzetto L, Petrini P, Biomed. Compos 2017, 59. [Google Scholar]
- [94].Fallahi A, Khademhosseini A, Tamayol A, Trends Biotechnol. 2016, 34, 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Fallahi A, Yazdi IK, Serex L, Lesha E, Faramarzi N, Tarlan F, Avci H, Costa-Almeida R, Sharifi F, Rinoldi C, et al. , ACS Biomater. Sci. Eng 2019, 6, 1112. [DOI] [PubMed] [Google Scholar]
- [96].Wu Y, Han Y, Wong YS, Fuh JYH, Tissue Eng J. Regen. Med 2018, 12, 1798. [DOI] [PubMed] [Google Scholar]
- [97].Akbari M, Tamayol A, Bagherifard S, Serex L, Mostafalu P, Faramarzi N, Mohammadi MH, Khademhosseini A, Adv. Healthc. Mater 2016, 5, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Guner MB, Dalgic AD, Tezcaner A, Yilanci A, Keskin D, Biomed. Mater 2020, 15, 065014. [DOI] [PubMed] [Google Scholar]
- [99].Yang S, Shi X, Li X, Wang J, Wang Y, Luo Y, Biomaterials 2019, 207, 61. [DOI] [PubMed] [Google Scholar]
- [100].Akbari M, Tamayol A, Laforte V, Annabi N, Najafabadi AH, Khademhosseini A, Juncker D, Adv Funct Mater 2015, 24, 4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tamayol A, Najafabadi AH, Aliakbarian B, Arab-Tehrany E, Akbari M, Annabi N, Juncker D, Khademhosseini A, Adv. Healthc. Mater 2015, 4, 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Costa-Almeida R, Domingues RMA, Fallahi A, Avci H, Yazdi IK, Akbari M, Reis RL, Tamayol A, Gomes ME, Khademhosseini A, J. Tissue Eng. Regen. Med 2017, 12, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Saveh-Shemshaki N, Nair LS, Laurencin CT, Acta Biomater. 2019, 94, 64. [DOI] [PubMed] [Google Scholar]
- [104].Kumar N, Sharma a K., Singh GR, Gupta P, J. Vet. Med. A. Physiol. Pathol. Clin. Med 2002, 49, 161. [DOI] [PubMed] [Google Scholar]
- [105].Jenkins DH, McKibbin B, J. Bone Joint Surg. Br 1980, 62-B, 497. [DOI] [PubMed] [Google Scholar]
- [106].Blazewicz M, Eur. Cell. Mater 2001, 2, 21. [DOI] [PubMed] [Google Scholar]
- [107].Albright JA, Keating EM, Marino AA, Ph D, 1984, 16. [Google Scholar]
- [108].Jenkins DH, J. Bone Joint Surg. Br 1978, 60-B, 520. [DOI] [PubMed] [Google Scholar]
- [109].Dasar U, Gursoy S, Akkaya M, Algin O, Isik C, Bozkurt M, J. Orthop. Surg 2016, 24, 188. [DOI] [PubMed] [Google Scholar]
- [110].Debnath UK, Fairclough JA, Williams RL, Knee 2004, 11, 259. [DOI] [PubMed] [Google Scholar]
- [111].García-Ruíz JP, Lantada AD, Materials (Basel). 2017, 11, DOI 10.3390/ma11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Silver FH, Freeman JW, Seehra GP, J. Biomech 2003, 36, 1529. [DOI] [PubMed] [Google Scholar]
- [113].Panas-Perez E, Gatt CJ, Dunn MG, J. Mater. Sci. Mater. Med 2013, 24, 257. [DOI] [PubMed] [Google Scholar]
- [114].No YJ, Tarafder S, Reischl B, Ramaswamy Y, Dunstan C, Friedrich O, Lee CH, Zreiqat H, ACS Biomater. Sci. Eng. 2020, 6, 1887. [DOI] [PubMed] [Google Scholar]
- [115].Roolker W, Patt TW, van Dijk CN, Vegter M, Marti RK, Knee Surg. Sports Traumatol. Arthrosc 2000, 8, 20. [DOI] [PubMed] [Google Scholar]
- [116].Dahlstedt L, Dalén N, Jonsson U, Acta Orthop. Scand 1990, 61, 217. [DOI] [PubMed] [Google Scholar]
- [117].Murray AW, Macnicol MF, Knee, The 2004, 11, 9. [DOI] [PubMed] [Google Scholar]
- [118].Davarinos N, Neill BJO, Curtin W, Adv. Orthop. Surg 2014, 2014, 1. [Google Scholar]
- [119].Gao K, Chen S, Wang L, Zhang W, Kang Y, Dong Q, Zhou H, Li L, Arthroscopy 2010, 26, 515. [DOI] [PubMed] [Google Scholar]
- [120].Leciejewski M, Królikowska A, Reichert P, Polym. Med 2019, 48, 53. [DOI] [PubMed] [Google Scholar]
- [121].Legnani C, Ventura A, Terzaghi C, Borgo E, Albisetti W, Int. Orthop 2010, 34, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Macnicol MF, Penny D, Leeds-keio T, 1991, 73. [DOI] [PubMed]
- [123].Zaffagnini S, Marcheggiani Muccioli GM, Chatrath V, Bondi A, De Pasquale V, Martini D, Bacchelli B, Marcacci M, Knee Surgery, Sport. Traumatol. Arthrosc 2008, 16, 1026. [DOI] [PubMed] [Google Scholar]
- [124].Dandy DJ, Gray AJR, J. Bone Jt. Surg. - Ser. B 1994, 76, 193. [PubMed] [Google Scholar]
- [125].Denti M, Bigoni M, Dodaro G, Monteleone M, Arosio A, Knee Surg. Sports Traumatol. Arthrosc 1995, 3, 75. [DOI] [PubMed] [Google Scholar]
- [126].Schroven ITJ, Geens S, Beckers L, Lagrange W, Fabry G, Knee Surgery, Sport. Traumatol. Arthrosc 1994, 2, 214. [DOI] [PubMed] [Google Scholar]
- [127].Newman SDS, Atkinson HDE, Willis-Owen CA, Int. Orthop 2013, 37, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Nau T, Lavoie P, Duval N, J. Bone Joint Surg. Br 2002, 84, 356. [DOI] [PubMed] [Google Scholar]
- [129].Yu S-B, Yang R-H, Zuo Z-N, Dong Q-R, Int. J. Clin. Exp. Med 2014, 7, 2511. [PMC free article] [PubMed] [Google Scholar]
- [130].Younesi M, Islam A, Kishore V, Anderson JM, Akkus O, Adv. Funct. Mater 2014, 24, 5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Islam A, Bohl MS, Tsai AG, Younesi M, Gillespie R, Akkus O, 2016, 30, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Learn GD, McClellan PE, Knapik DM, Cumsky JL, Webster-Wood V, Anderson JM, Gillespie RJ, Akkus O, J. Biomed. Mater. Res. - Part B Appl. Biomater 2019, 107, 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wu S, Wang Y, Streubel PN, Duan B, Acta Biomater. 2017, 62, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Laranjeira M, Domingues RMA, Costa-Almeida R, Reis RL, Gomes ME, Small 2017, 13, 1. [DOI] [PubMed] [Google Scholar]
- [135].Ruan D, Zhu T, Huang J, Le H, Hu Y, Zheng Z, Tang C, Chen Y, Ran J, Chen X, et al. , ACS Biomater. Sci. Eng 2019, 5, 5412. [DOI] [PubMed] [Google Scholar]
- [136].Zhang W, Yang Y, Zhang K, Luo T, Tang L, Li Y, J. Biomater. Tissue Eng 2017, 7, 571. [Google Scholar]
- [137].Cai J, Xie X, Li D, Wang L, Jiang J, Mo X, Zhao J, Biomater. Sci 2020, 8, 4413. [DOI] [PubMed] [Google Scholar]
- [138].Sahoo S, Ouyang H, Goh JC-H, Tay TE, Toh SL, Tissue Eng. 2006, 12, 91. [DOI] [PubMed] [Google Scholar]
- [139].Freeman JW, Woods MD, Cromer D. a., Ekwueme EC, Andric T, Atiemo E. a., Bijoux CH, Laurencin CT, J. Biomech 2011, 44, 694. [DOI] [PubMed] [Google Scholar]
- [140].Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, Vanderby R, Li WJ, Biomaterials 2014, 35, 6907. [DOI] [PubMed] [Google Scholar]
- [141].Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT, Biomaterials 2005, 26, 1523. [DOI] [PubMed] [Google Scholar]
- [142].Noyes FR, Barber-Westin SD, Arthroscopy 1994, 10, 371. [DOI] [PubMed] [Google Scholar]
- [143].Kumar K, Maffulli N, Arthroscopy 1999, 15, 422. [DOI] [PubMed] [Google Scholar]
- [144].McPherson GK, V Mendenhall H, Gibbons DF, Plenk H, Rottmann W, Sanford JB, Kennedy JC, Roth JH, Clin. Orthop. Relat. Res 1985, 186. [PubMed] [Google Scholar]
- [145].Kdolsky R, Kwasny O, Schabus R, Clin. Orthop. Relat. Res 1993, 183. [PubMed] [Google Scholar]
- [146].Johnson D, Oper. Tech. Sports Med 1995, 3, 173. [Google Scholar]
- [147].Bowyer GW, Matthews SJ, J R Army Med Corps 1991, 137, 69. [DOI] [PubMed] [Google Scholar]
- [148].Ahlfeld SK, Larson RL, Collins HR, Am. J. Sports Med 1987, 15, 326. [DOI] [PubMed] [Google Scholar]
- [149].Lu HH, Cooper JA, Manuel S, Freeman JW, Attawia MA, Ko FK, Laurencin CT, Biomaterials 2005, 26, 4805. [DOI] [PubMed] [Google Scholar]
- [150].Cooper JAJ, Sahota JS, Gorum WJ 2nd, Carter J, Doty SB, Laurencin CT, Proc. Natl. Acad. Sci. U. S. A 2007, 104, 3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Gwiazda M, Kumar S, Świeszkowski W, Ivanovski S, Vaquette C, J. Mech. Behav. Biomed. Mater 2020, 104, 103631. [DOI] [PubMed] [Google Scholar]
- [152].Yu X, Mengsteab PY, Narayanan G, Nair LS, Laurencin CT, Engineering 2020, DOI 10.1016/j.eng.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Pagán A, Aznar-Cervantes SD, Pérez-Rigueiro J, Meseguer-Olmo L, Cenis JL, J. Biomed. Mater. Res. - Part B Appl. Biomater 2019, 107, 2209. [DOI] [PubMed] [Google Scholar]
- [154].Freeman JW, Woods MD, Laurencin CT, J. Biomech 2007, 40, 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Freeman JW, Woods MD, Cromer DA, Wright LD, Laurencin CT, J. Biomater. Sci. Polym. Ed 2009, 20, 1709. [DOI] [PubMed] [Google Scholar]
- [156].Madhavarapu S, Rao R, Libring S, Fleisher E, Yankannah Y, Freeman JW, TECHNOLOGY 2017, 05, 98. [Google Scholar]
- [157].Mengsteab PY, Conroy P, Badon M, Otsuka T, Kan HM, Vella AT, Nair LS, Laurencin CT, PLoS One 2020, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Nasajpour A, Mandla S, Shree S, Mostafavi E, Sharifi R, Khalilpour A, Saghazadeh S, Hassan S, Mitchell MJ, Leijten J, et al. , Nano Lett. 2017, 17, 6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chlanda A, Kijeńska E, Rinoldi C, Tarnowski M, Wierzchoń T, Swieszkowski W, Micron 2018, 107, 79. [DOI] [PubMed] [Google Scholar]
- [160].Hejazi F, Mirzadeh H, Contessi N, Tanzi MC, Faré S, J. Biomed. Mater. Res. Part A 2017, 105, 1535. [DOI] [PubMed] [Google Scholar]
- [161].Kijeńska E, Swieszkowski W, in Electrospun Mater. Tissue Eng. Biomed. Appl (Ed: E. B. T.-E. M. for T. E. and Kny BA), Woodhead Publishing, 2017, pp. 43–56. [Google Scholar]
- [162].Nasajpour A, Ansari S, Rinoldi C, Rad AS, Aghaloo T, Shin SR, Mishra YK, Adelung R, Swieszkowski W, Annabi N, et al. , Adv. Funct. Mater 2018, 28, 1703437. [Google Scholar]
- [163].Kijeńska-Gawrońska E, Bolek T, Bil M, Swieszkowski W, J. Mater. Chem. B 2019, 4509. [Google Scholar]
- [164].Campiglio CE, Negrini NC, Farè S, Draghi L, Materials (Basel). 2019, 12, 2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rinoldi C, Kijeńska E, Chlanda A, Choinska E, Khenoussi N, Tamayol A, Khademhosseini A, Swieszkowski W, J. Mater. Chem. B 2018, 6, 3116. [DOI] [PubMed] [Google Scholar]
- [166].James R, Toti US, Laurencin CT, Kumbar SG, in Biomed. Nanotechnol. Methods Protoc (Ed: Hurst SJ), Humana Press, Totowa, NJ, 2011, pp. 243–258. [Google Scholar]
- [167].Agarwal S, Wendorff JH, Greiner A, Polymer (Guildf). 2008, 49, 5603. [Google Scholar]
- [168].Mauck RL, Baker BM, Nerurkar NL, a Burdick J, Li W-J, Tuan RS, Elliott DM, Tissue Eng. Part B. Rev 2009, 15, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Sensini A, Gualandi C, Focarete ML, Belcari J, Zucchelli A, Boyle L, Reilly GC, Kao AP, Tozzi G, Cristofolini L, Biofabrication 2019, 11, 035026. [DOI] [PubMed] [Google Scholar]
- [170].Peach MS, James R, Toti US, Deng M, Morozowich NL, Allcock HR, Laurencin CT, Kumbar SG, Biomed. Mater 2012, 7, 45016. [DOI] [PubMed] [Google Scholar]
- [171].Li X, Chen H, Xie S, Wang N, Wu S, Duan Y, Zhang M, Shui L, Int. J. Nanomedicine 2020, 15, 6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Beason DP, Connizzo BK, Dourte LM, Mauck RL, Soslowsky LJ, Steinberg DR, Bernstein J, Shoulder Elb J. Surg. 2012, 21, 245. [DOI] [PubMed] [Google Scholar]
- [173].Yang C, Deng G, Chen W, Ye X, Mo X, Colloids Surf. B. Biointerfaces 2014, 122, 270. [DOI] [PubMed] [Google Scholar]
- [174].Moffat KL, Kwei AS-P, Spalazzi JP, Doty SB, Levine WN, Lu HH, Tissue Eng. Part A 2009, 15, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hakimi O, Murphy R, Stachewicz U, Hislop S, Carr a. J., Eur. Cells Mater 2012, 24, 344. [DOI] [PubMed] [Google Scholar]
- [176].Zhang C, Yuan H, Liu H, Chen X, Lu P, Zhu T, Yang L, Yin Z, Heng BC, Zhang Y, et al. , Biomaterials 2015, 53, 716. [DOI] [PubMed] [Google Scholar]
- [177].Domingues RMA, Chiera S, Gershovich P, Motta A, Reis RL, Gomes ME, Adv. Healthc. Mater 2016, 5, 1364. [DOI] [PubMed] [Google Scholar]
- [178].Shang S, Yang F, Cheng X, Frank Walboomers X, Jansen J. a., Eur. Cells Mater 2010, 19, 180. [DOI] [PubMed] [Google Scholar]
- [179].El Khatib M, Mauro A, Di Mattia M, Wyrwa R, Schweder M, Ancora M, Lazzaro F, Berardinelli P, Valbonetti L, Di Giacinto O, et al. , Cells 2020, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Tu T, Shen Y, Wang X, Zhang W, Zhou G, Zhang Y, Wang W, Liu W, Appl. Mater. Today 2020, 18, 100495. [Google Scholar]
- [181].Ni T, Senthil-Kumar P, Dubbin K, Aznar-Cervantes SD, Datta N, Randolph MA, Cenis JL, Rutledge GC, Kochevar IE, Redmond RW, Lasers Surg. Med 2012, 44, 645. [DOI] [PubMed] [Google Scholar]
- [182].Jin H-J, Chen J, Karageorgiou V, Altman GH, Kaplan DL, Biomaterials 2004, 25, 1039. [DOI] [PubMed] [Google Scholar]
- [183].Maghdouri-White Y, Petrova S, Sori N, Polk S, Wriggers H, Ogle R, Ogle R, Francis M, Biomed. Phys. Eng. Express 2018, 4, DOI 10.1088/2057-1976/aa9c6f. [DOI] [Google Scholar]
- [184].Domingues RMA, Gonçalves AI, Costa-almeida R, Rodrigues MT, Reis RL, Gomes ME, Fabrication of Hierarchical and Biomimetic Fibrous Structures to Support the Regeneration of Tendon Tissues, Elsevier Inc., 2015. [Google Scholar]
- [185].Yang Y, Sun J, Liu X, Guo Z, He Y, Wei D, Zhong M, Guo L, Fan H, Zhang X, Regen. Biomater 2017, 4, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wu HY, Zhang F, Yue XX, Ming JF, Zuo BQ, Mater. Lett 2014, 135, 63. [Google Scholar]
- [187].Rinoldi C, Costantini M, Kijeńska E, Heljak M, Monika C, Buda R, Baldi J, Cannata S, Guzowski J, Gargioli C, et al. , Adv. Healthc. Mater 2019, 1801218, 1. [DOI] [PubMed] [Google Scholar]
- [188].Nowotny J, Aibibu D, Farack J, Nimtschke U, Hild M, Gelinsky M, Kasten P, Cherif C, Aibibu D, Farack J, et al. , J. Biomater. Sci. Polym. Ed 2016, 27, 917. [DOI] [PubMed] [Google Scholar]
- [189].Funakoshi T, Majima T, Iwasaki N, Yamane S, Masuko T, Minami A, Harada K, Tamura H, Tokura S, Nishimura S, J. Biomed. Mater. Res. Part A 2005, 74A, 338. [DOI] [PubMed] [Google Scholar]
- [190].Majima T, Irie T, Sawaguchi N, Funakoshi T, Iwasaki N, Harada K, Minami A, Nishimura S, Proc. Inst. Mech. Eng. Part H J. Eng. Med 2007, 221, 537. [DOI] [PubMed] [Google Scholar]
- [191].Sardelli L, Pacheco DP, Zorzetto L, Rinoldi C, Święszkowski W, Petrini P, J. Appl. Biomater. Funct. Mater 2019, 17, 1. [DOI] [PubMed] [Google Scholar]
- [192].Liu C, Tian S, Bai J, Yu K, Liu L, Liu G, Dong R, Tian D, Int. J. Nanomedicine 2020, 15, 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Liu X, Laurent C, Du Q, Targa L, Cauchois G, Chen Y, Wang X, de Isla N, J. Biomed. Mater. Res. - Part A 2018, 106, 3042. [DOI] [PubMed] [Google Scholar]
- [194].Liu X, Baldit A, de Brosses E, Velard F, Cauchois G, Chen Y, Wang X, de Isla N, Laurent C, Polym. 2020, 12, DOI 10.3390/polym12092163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Chainani A, Hippensteel KJ, Kishan A, Garrigues NW, Ruch DS, Guilak F, Little D, Tissue Eng. Part A 2013, 19, 2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Rashid M, Dudhia J, Dakin SG, Snelling SJB, De Godoy R, Mouthuy PA, Smith RKW, Morrey M, Carr AJ, Sci. Rep 2020, 10, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hakimi O, Mouthuy PA, Zargar N, Lostis E, Morrey M, Carr A, Acta Biomater. 2015, 26, 124. [DOI] [PubMed] [Google Scholar]
- [198].Sharifi-Aghdam M, Faridi-Majidi R, Derakhshan MA, Chegeni A, Azami M, Proc. Inst. Mech. Eng. Part H J. Eng. Med 2017, 231, 652. [DOI] [PubMed] [Google Scholar]
- [199].Zheng Z, Ran J, Chen W, Hu Y, Zhu T, Chen X, Yin Z, Heng BC, Feng G, Le H, et al. , Acta Biomater. 2017, 51, 317. [DOI] [PubMed] [Google Scholar]
- [200].Jiang S, Yan H, Fan D, Song J, Fan C, Int. J. Mol. Sci 2015, 16, 6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Zhao T, Qi Y, Xiao S, Ran J, Wang J, Ghamor-Amegavi EP, Zhou X, Li H, He T, Gou Z, et al. , J. Mater. Chem. B 2019, 7, 2201. [DOI] [PubMed] [Google Scholar]
- [202].Jayasree A, Kottappally Thankappan S, Ramachandran R, Sundaram MN, Chen CH, Mony U, Chen JP, Jayakumar R, ACS Biomater. Sci. Eng 2019, 5, 1476. [DOI] [PubMed] [Google Scholar]
- [203].Manning CN, Schwartz a. G., Liu W, Xie J, Havlioglu N, Sakiyama-Elbert SE, Silva MJ, Xia Y, Gelberman RH, Thomopoulos S, Acta Biomater. 2013, 9, 6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Rinoldi C, Fallahi A, Yazdi I, Campos Paras J, Kijeńska-Gawrońska E, Trujillo-de Santiago G, Tuoheti A, Demarchi D, Annabi N, Khademhosseini A, et al. , ACS Biomater. Sci. Eng 2019, 5, 2953. [DOI] [PubMed] [Google Scholar]
- [205].Rossetti L, Kuntz LA, Kunold E, Schock J, Müller KW, Grabmayr H, Stolberg-Stolberg J, Pfeiffer F, Sieber SA, Burgkart R, et al. , Nat. Mater 2017, 16, 664. [DOI] [PubMed] [Google Scholar]
- [206].Ramakrishna H, Li T, He T, Temple J, King MW, Spagnoli A, Biomater. Res 2019, 23, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Lin Y, Zhang L, Liu NQ, Yao Q, Van Handel B, Xu Y, Wang C, Evseenko D, Wang L, Int. J. Nanomedicine 2019, 14, 5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Qian S, Wang Z, Zheng Z, Ran J, Zhu J, Chen W, Med. Sci. Monit 2019, 25, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Kolluru PV, Lipner J, Liu W, Xia Y, Thomopoulos S, Genin GM, Chasiotis I, Acta Biomater. 2013, 9, 9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Liu W, Yeh Y-C, Lipner J, Xie J, Sung H-W, Thomopoulos S, Xia Y, Langmuir 2011, 27, 9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Samavedi S, Vaidya P, Gaddam P, Whittington AR, Goldstein AS, Biotechnol. Bioeng 2014, 111, 2549. [DOI] [PubMed] [Google Scholar]
- [212].Xie J, Li X, Lipner J, Manning CN, Schwartz AG, Thomopoulos S, Xia Y, Nanoscale 2010, 2, 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Font Tellado S, Bonani W, Balmayor ER, Foehr P, Motta A, Migliaresi C, Van Griensven M, Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering, 2017. [DOI] [PubMed] [Google Scholar]
- [214].Reifenrath J, Wellmann M, Kempfert M, Angrisani N, Welke B, Gniesmer S, Kampmann A, Menzel H, Willbold E, Int. J. Mol. Sci 2020, 21, DOI 10.3390/ijms21031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Chen P, Li L, Dong L, Wang S, Huang Z, Qian Y, Wang C, Liu W, Yang L, ACS Biomater. Sci. Eng 2020, DOI 10.1021/acsbiomaterials.9b01683. [DOI] [PubMed] [Google Scholar]
- [216].Jiang N, He J, Zhang W, Li D, Lv Y, Mater. Sci. Eng. C 2020, 110, DOI 10.1016/j.msec.2020.110711. [DOI] [PubMed] [Google Scholar]
- [217].Zhang P, Han F, Chen T, Wu Z, Chen S, Biomater. Sci 2020, 8, 871. [DOI] [PubMed] [Google Scholar]
- [218].Han F, Zhang P, Chen T, Chao L, Xuejun W, Zhao P, 2019, 14, 9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Cai J, Wang J, Ye K, Li D, Ai C, Sheng D, Jin W, Liu X, Zhi Y, Jiang J, et al. , Int. J. Nanomedicine 2018, 13, 3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Li X, Cheng R, Sun Z, Su W, Pan G, Zhao S, Zhao J, Cui W, Acta Biomater. 2017, 61, 204. [DOI] [PubMed] [Google Scholar]
- [221].VanDusen KW, Larkin LM, Muscle-Tendon Interface, Elsevier Ltd, 2015. [Google Scholar]
- [222].Arrigoni C, Petta D, Bersini S, Mironov V, Candrian C, Moretti M, Biofabrication 2019, 11, 32004. [DOI] [PubMed] [Google Scholar]
- [223].Barajaa MA, Nair LS, Laurencin CT, Bioinspired Scaffold Designs for Regenerating Musculoskeletal Tissue Interfaces, Regenerative Engineering And Translational Medicine, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Ladd MR, Lee SJ, Stitzel JD, Atala A, Yoo JJ, Biomaterials 2011, 32, 1549. [DOI] [PubMed] [Google Scholar]
- [225].Taylor ED, J. Bone Jt. Surg 2010, 92, 170. [Google Scholar]