Summary
With a nervous system that has about a billion times fewer neurons than the human brain, C. elegans was initially not regarded as a model for studies on learning. However, the collective effort of the C. elegans field in the past several decades has shown that the worm displays plasticity in its behavioral response to a wide range of sensory cues in the environment. As a bacteria-feeding worm, C. elegans is highly adaptive to the bacteria enriched in its habitat, especially those that are pathogenic and pose a threat to survival. It uses several common forms of behavioral plasticity that last for different amounts of time, including imprinting and adult-stage associative learning, to modulate its interactions with pathogenic bacteria. Probing the molecular, cellular and circuit mechanisms underlying these forms of experience-dependent plasticity has identified signaling pathways and regulatory insights that are conserved in more complex animals.
C. elegans senses and responds to diverse environmental cues
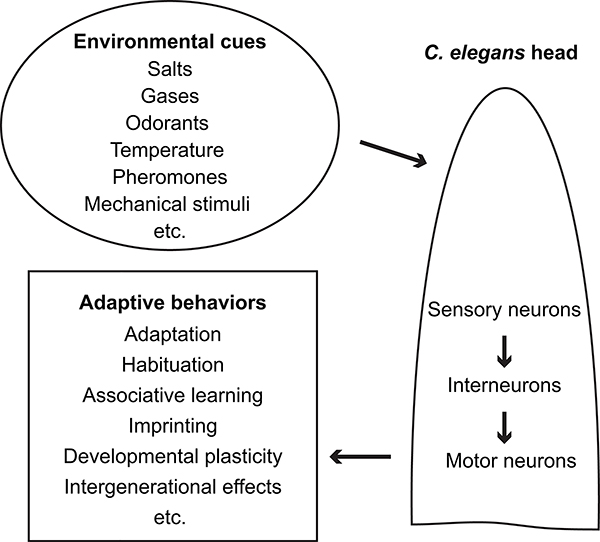
Animals live in different ecological niches that are characteristic of different chemical, physical and biological cues and have likely evolved sensorimotor systems that are able to detect and respond to the environmental conditions of their habitats. C. elegans feeds on bacteria and is often found in decaying fruits or other organic matters that are rich in bacteria (Felix and Duveau, 2012; Frezal and Felix, 2015; Samuel et al., 2016). It navigates its environment by detecting and responding to various chemical cues, including odorants and salts, temperature, pheromones, gases, as well as mechanical stimuli [(Figure 1) and (Aoki and Mori, 2015; Bargmann, 2006; Brandt et al., 2012; Bretscher et al., 2008; Butcher et al., 2007; Chalfie, 2009; Cheung et al., 2005; de Bono and Maricq, 2005; Goodman et al., 2014; Goodman and Sengupta, 2019; Gray et al., 2004; Hallem et al., 2011; Hao et al., 2018; Jeong et al., 2005; Kaplan and Horvitz, 1993; Kim et al., 2009; Macosko et al., 2009; Pierce-Shimomura et al., 2001; Reddy et al., 2011; Schafer, 2015; Srinivasan et al., 2008; Srinivasan et al., 2012; White and Jorgensen, 2012; White et al., 2007)]. Some of the odorants that are attractive to C. elegans can be produced by plants and may serve as cues representing an environment that is abundant in bacteria. In addition, C. elegans is known to navigate within a thermal gradient and the ambient temperature significantly regulates the development and life span of a worm. The sensorimotor response to chemical cues and temperature have been extensively studied in C. elegans. For example, a few ciliated sensory neurons use G proteins, cyclic nucleotide-gated channels (CNGs), and G-protein coupled seven-transmembrane receptors to detect odorants. The calcium-permeable CNGs transform odorant information into intracellular signals, which produce intercellular signals to engage postsynaptic interneurons and downstream motor neurons to generate movement towards or away from the odorants (Bargmann, 2006; de Bono and Maricq, 2005). Similarly, the major sensory neurons, as well as their intracellular signaling pathways, that perceive and respond to external salt concentration, ambient temperature gradient, pheromones, gases and mechanical cues have been identified and characterized (Aoki and Mori, 2015; Bargmann, 2006; Brandt et al., 2012; Bretscher et al., 2008; Butcher et al., 2007; Chalfie, 2009; Cheung et al., 2005; de Bono and Maricq, 2005; Goodman et al., 2014; Goodman and Sengupta, 2019; Gray et al., 2004; Hallem et al., 2011; Hao et al., 2018; Jeong et al., 2005; Kaplan and Horvitz, 1993; Kim et al., 2009; Macosko et al., 2009; Pierce-Shimomura et al., 2001; Reddy et al., 2011; Schafer, 2015; Srinivasan et al., 2008; Srinivasan et al., 2012; White and Jorgensen, 2012; White et al., 2007). The behavioral strategies and the underlying neural circuits through which C. elegans navigates in a sensory environment are also intensively investigated, such as those described in (Aprison and Ruvinsky, 2019; Bargmann, 2006; Chalasani et al., 2007; de Bono and Maricq, 2005; Donnelly et al., 2013; Goodman and Sengupta, 2019; Gordus et al., 2015; Gray et al., 2005; Iino and Yoshida, 2009; Ikeda et al., 2020; Jang et al., 2012; Kaplan et al., 2020; Kato et al., 2015; Kunitomo et al., 2013; Li et al., 2014; Liu et al., 2018; Luo et al., 2014; Macosko et al., 2009; Pierce-Shimomura et al., 1999; Schafer, 2015; Tsalik and Hobert, 2003; Venkatachalam et al., 2016; Wen et al., 2018; White et al., 2007). The molecular, cellular and circuit bases for these sensorimotor responses provide the substrates for experience-dependent regulation to generate learning. The studies that investigate various forms of learning in C. elegans have been reviewed elsewhere (Alcedo and Zhang, 2013; de Bono and Maricq, 2005; McDiarmid et al., 2019; Sasakura and Mori, 2013). Here, we will focus on several learning paradigms that regulate the interactions between C. elegans and pathogenic bacteria.
Figure 1.
Diverse adaptive behaviors in response to environmental cues in C. elegans.
Environmental cues induce plasticity across different timescales
C. elegans displays both adaptation and habituation, two common forms of non-associative learning (Figure 1). C. elegans is attracted to several chemical odorants, such as Isoamyl alcohol and benzaldehyde; however, prolonged exposure to these volatile chemicals reduces the sensory response to the odorants and generates adaptation that lasts for a couple hours (Colbert and Bargmann, 1995; Inoue et al., 2013; Kaye et al., 2009). It is shown that during adaptation the endogenous small RNA (endo-siRNA)-mediated gene expression regulation in the sensory neuron that detects isoamyl alcohol and benzaldehyde downregulates a guanylyl cyclase that is critical for the G-protein coupled signaling pathway underlying the sensing of the odorants (Juang et al., 2013). These results reveal a novel function of endo-siRNA pathways in regulating gene expression in response to olfactory experience. In addition, the worm reverses from a mild mechanical stimulus that is delivered to its body or nose and senses the stimulus using receptor neurons, several of which contain distinct morphological features (Chalfie, 2009; Kaplan and Horvitz, 1993; Schafer, 2015). Tapping the cultivating plate also generates mechanical stimuli that trigger reversals. However, tapping for multiple times reduces the amplitude of the reversals (Rankin et al., 1990). This type of behavioral changes is analogous to habituation previously characterized in Alysia and cats, where multiple stimulation with a benign mechanical stimulus leads to a reduction in response (Bailey and Chen, 1983; Spencer et al., 1966). Repeated habituation training under certain conditions can generate memory that lasts for 24 hours (Rose et al., 2002).
In addition to non-associative learning, previous studies have shown that olfactory responses can be respectively enhanced or weakened by paring odorant exposure with the presence or absence of food, which presumably represents an appetitive or aversive environment (Figure 1). Various neuronal circuits and molecular pathways have been characterized in regulating these associative learning behaviors [(Alcedo and Zhang, 2013; de Bono and Maricq, 2005) and the references therein]. C. elegans also remembers the salt concentration under its cultivation condition and seeks this concentration when tested in a salt gradient after the training. However, if the worm is kept at a salt concentration in the absence of food, it avoids the concentration during the post-training rest (Kunitomo et al., 2013; Luo et al., 2014; Saeki et al., 2001; Tomioka et al., 2006). As a critical condition, the cultivation temperature significantly modulates the navigation of the worm in a temperature gradient during post-training tests (Aoki and Mori, 2015; Biron et al., 2006; Goodman et al., 2014; Goodman and Sengupta, 2019; Hedgecock and Russell, 1975; Mori and Ohshima, 1995). Some of these forms of behavioral plasticity resemble associative learning identified in vertebrate animals and in fruit flies. While a one-time massed training in these paradigms often generates memories for a couple hours, spaced training can generate long-term memories that last for 16 hours (Kauffman et al., 2010).
Experimental power of C. elegans facilitates dissection of plasticity mechanisms
The ease of applying forward and reverse genetic approaches to characterize gene function in C. elegans and the knowledge of genetic identities and synaptic connectivities of the worm neurons facilitates studies on learning and behavioral plasticity in C. elegans in several important ways:
Because the worm neurons are defined in their genetic making and synaptic connectivity, we are able to identify the neurons where the gene products implicated in learning are generated and act, as well as their presynaptic and post-synaptic neurons. These analyses provide us with knowledge on neuronal circuits underlying various forms of learning behaviors.
By applying in vivo imaging and genetic manipulations, we can identify experience-dependent changes in the activity and the connectivity of the learning circuit that are correlated with behavioral changes and characterize the causality of these changes in generating learned behavior.
Once the key neurons underlying learning are identified, we can also analyze gene expression in these neurons in naive and trained animals in order to identify genes that display training-correlated changes in their expression and address the function of these molecules in learning.
Meanwhile, the ease of performing genetic analyses in the C. elegans nervous system also makes it feasible to conduct genetic screens in order to identify new functions of characterized genes and pathways in learning, as well as identify new genes with previously unknown functions.
Interactions with pathogenic bacteria that modulate behavior
C. elegans feeds on bacteria in the wild and in the laboratory setting. A wide range of different bacteria strains, including many in the Pseudomonas genus, are found to be associated with C. elegans isolated from its natural habitats (Felix and Duveau, 2012; Frezal and Felix, 2015; Samuel et al., 2016; Schulenburg and Felix, 2017). While some of these bacteria serve as food sources, others are pathogenic and kill C. elegans through infections or with secreted toxins [(Hoffman and Aballay, 2019; Irazoqui et al., 2010; Kim and Ewbank, 2018) and the references therein]. Because bacteria play a vital role in the development and survival of C. elegans, it is conceivable that C. elegans has evolved diverse strategies to mediate its interactions with the environmental bacteria.
Bacteria produce multiple types of sensory cues that can be used by the worm to detect and respond to the microbes. In addition to odorants, bacteria also produce water-soluble metabolites, generate or alter concentration of gases. The border and the texture of a bacterial lawn may also generate mechanical stimulation to moving worms. These bacteria-derived sensory cues act in a combinatorial manner to elicit behavioral responses in C. elegans [(Bargmann et al., 1993; Brandt and Ringstad, 2015; Bretscher et al., 2008; Calhoun et al., 2015; Cheung et al., 2005; Flavell et al., 2013; Gramstrup Petersen et al., 2013; Gray et al., 2004; Ha et al., 2010; Hallem et al., 2011; Hao et al., 2018; Harris et al., 2019; Meisel et al., 2014; Ooi and Prahlad, 2017a; Pradel et al., 2007; Reddy et al., 2011; Rhoades et al., 2019; Sawin et al., 2000; Tran et al., 2017), Flavell and Kim this issue and the references therein]. The diversity of the sensory cues is consistent with multiple signaling pathways that are identified to mediate bacteria-worm interactions.
Adult-stage learning of pathogenic bacteria
Some pathogenic bacteria, such as the Pseudomonas aeruginosa strain PA14, infect C. elegans after being ingested, which leads to a slow death of the worm during a course of several days (Tan et al., 1999). Thus, pathogenic bacteria likely signal both food and danger to the worms. The odorants of several pathogenic bacteria, including PA14, are attractive to the worms that are cultivated under the standard condition by feeding on E. coli OP50 at 20 – 22 °C (Ha et al., 2010; Jin et al., 2016; Zhang et al., 2005). When newly transferred to a lawn of PA14, worms feed on the lawn. However, in the next few hours worms start to leave the lawn [Figure 2 and (Chang et al., 2011)]. The virulence of the bacteria, as well as several bacteria-derived chemicals act together to repel the worms from the lawn. The mechanisms underlying the changes in behavior and physiology of the worm over this process are separately reviewed (Hoffman and Aballay, 2019; Kim and Ewbank, 2018; Meisel and Kim, 2014) and (Flavell and Kim, this issue)].
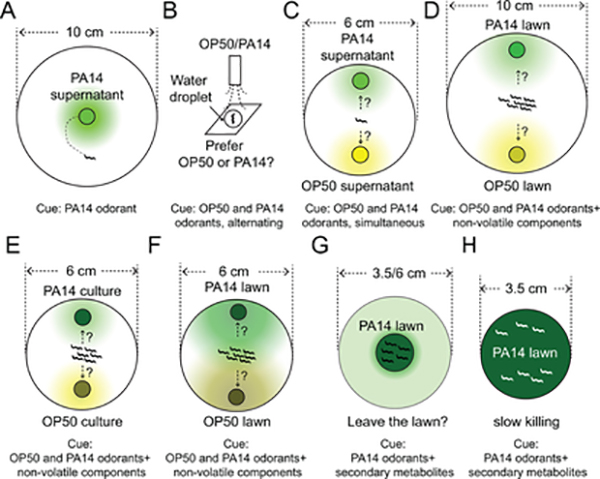
Figure 2. Schematic diagrams showing assays for behavioral responses to PA14.
(A) A small drop of supernatant of PA14 culture is used as the source of odorants to examine attractive steering movements, when a worm starts from a position relatively close to the odorant source.
(B) To test the relative preference between the odorants of PA14 and the odorants of E. coli OP50, two airstreams saturated with the odorants of OP50 or the odorants of PA14 are used to deliver alternating stimuli to individual worms swimming in a small drop of buffer in an airtight chamber.
(C, D) Two small drops of supernatant of bacterial culture are put on a plate immediately before the assay (C) or two small drops of bacterial culture are quickly air-dried before the assay (D) to measure the preference between the two odorant mixtures in a single worm (C) or a population of worms (D). In D, the plate does not contain peptone and therefore does not support growth of the bacteria.
(E, F) A small plate with two bacteria lawns grown on the plate for a few hours (E) or for 24 hours (F) to be used as odorant sources. During cultivation, the bacterial lawns may produce cues diffused into the medium, produce or alter the concentration of gases in the lawn areas.
(G, H) A bacterial lawn centered on a small plate (G) or completely covering a small plate (H) prepared by fully growing first at 37°C and then at 25°C to examine the lawn avoidance/occupancy or survival of the worms over time.
Do worms learn to associate the aversiveness of PA14 with sensory cues produced by the pathogenic bacteria to generate retrievable memory of the bacterium? This question can be addressed by testing the response to PA14-derived sensory cues in the naive, i.e. E. coli-raised, worms and the trained, i.e. PA14-fed, worms [(Ha et al., 2010; Jin et al., 2016; Liu et al., 2018; Zhang et al., 2005) and Figure 2A – 2F]. Previously, by probing the worms with an assay that resembles the standard chemotaxis assay on olfactory responses or with an assay that uses airstreams saturated with the odorants of tested bacteria, it is shown that after feeding on PA14 for 4 – 6 hours, the adult worms learn to reduce their preference for the odorants of the bacterium (Figure 2A) (Ha et al., 2010; Jin et al., 2016; Liu et al., 2018; Zhang et al., 2005). This type of learning in the adult C. elegans is contingent on the pathogenicity of the training bacteria and a serotonin signal. The training-dependent change in the olfactory response is specific for the odorants of the training bacteria (Figure 2A – 2C) (Choi et al., 2020; Ha et al., 2010; Jin et al., 2016; Liu et al., 2018; Zhang et al., 2005). Together, these results indicate a learned association between pathogenicity and the odorants of the training bacterium. Since pathogenic bacteria represent a critical constraint to the survival of C. elegans, it is conceivable that C. elegans has evolved the ability to associate the olfactory cues of some pathogenic bacteria with the virulence, which regulates subsequent interactions with the pathogen. The learned behavioral response is reversible, which suggests a temporary modulation of the nervous system (Ha et al., 2010; Jin et al., 2016; Liu et al., 2018; Zhang et al., 2005).
Several different methods have been used to analyze the changes in behavioral responses to pathogenic bacterium PA14 after feeding on PA14 [Figure 2 and (Ha et al., 2010; Hao et al., 2018; Horspool and Chang, 2017; Jin et al., 2016; Lee and Mylonakis, 2017; Liu et al., 2018; Ma et al., 2017; Meisel et al., 2014; Miller et al., 2015; Moore et al., 2019; Ooi and Prahlad, 2017; Singh and Aballay, 2019; Wolfe et al., 2019; Zhang et al., 2005). Some of these methods differ in the types of the sensory cues that they examine, the spatial and temporal patterns of the cues, and the behavioral strategies that they measure (Figure 2). For example, the behavioral strategies used to distinguish between two simultaneously present bacterial odorants (Figure 2C – 2F) are likely different from those used to respond to two alternating bacterial odorants (Figure 2B). The odorant gradient established by a point source is not linear (Tanimoto et al., 2017). Therefore, the size of the testing plate is important for the intensity and the spatial pattern of the bacterial odorants sensed by the worms (Figure 2C and 2D). The duration for which the testing bacteria were placed or grown on the testing plate or the temperature used to cultivate the testing bacteria generates different mixtures of the sensory cues (Figure 2E and 2F). In addition, separating the training process from the testing assay makes it possible to examine whether a retrievable memory is formed. Furthermore, the assay that measures the occupancy of a bacterial lawn grown on an assay plate likely measures sensory responses elicited by direct contacts of the worms with the stimuli, including metabolites that are not volatile and mechanical cues, in addition to olfactory responses (Figure 2). While worms feeding on a lawn of pathogenic bacterium PA14 gradually leave the lawn over time (Figure 2G and 2H), it usually takes longer for the worms to significantly leave the lawn than to learn to reduce the preference for the odorants of PA14. Because different molecular and neuronal apparatus are employed to detect, process and generate behavioral responses to these various types of sensory information, studies using these different assay conditions enriched our understanding of different pathways through which the worm interacts with pathogenic bacteria.
Imprinting
Interestingly, training the worms by feeding on pathogenic bacteria during the first larval stage (L1) for 12 hours forms the aversive memory of the odorants of the pathogens that can be retrieved during the adult stage [Figure 1 and (Jin et al., 2016)]. This form of memory is comparable to the imprinted memory characterized in various vertebrate animals (Lorenz, 1935; Nevitt et al., 1994; Wilson and Sullivan, 1994). The worms imprint not only the odorants associated with the aversive experience, but also the odorants associated with food sources to form a long-lasting appetitive memory [Figure 1 and (Remy and Hobert, 2005)]. Mapping the neural circuits for the imprinting of pathogen odorants and the retrieval of the aversive memory, which take place two days apart, show that different circuits subserve learning and retrieval (Jin et al., 2016). In addition to the odorants that often represent food, pheromones also signal significant environmental conditions, such as the density of the conspecifics, to the worm (Butcher et al., 2007; Jeong et al., 2005; Macosko et al., 2009; Srinivasan et al., 2008; Srinivasan et al., 2012; White et al., 2007). Exposing the worms during the L1 stage to a repulsive pheromone enhances the avoidance of the pheromone during the adult stage by strengthening the synaptic connectivity between a pheromone-sensing neuron and its downstream motor neurons [Figure 1 and (Hong et al., 2017)]. Starvation during the L1 stage also profoundly alters the wiring of the nervous system by regulating neurotransmitters that respond to food availability (Bayer and Hobert, 2018). It is conceivable that during the early larval development when the nervous system is being formed, strong neuronal activities in response to environmental conditions reprogram the developmental process to generate persistent changes. A couple of studies show that harsh conditions during development systematically regulate gene expression and modulate the anatomy and activity of the nervous system, which produce behavioral changes that last into the adult stage. For example, when the worm density is high and food is relatively sparse, the larval worms can enter a diapause state, dauer, that halts the development for days until the conditions improve (Golden and Riddle, 1984). Adult animals that have experienced the dauer stage exhibit distinct behavioral traits, including those important for food seeking. The molecules that regulate chromatin structures and endogenous RNAi pathways mediate dauer formation, which potentially modulate the expression of genes underlying dauer-inducing changes in anatomy and behavior (Bharadwaj and Hall, 2017; Hall et al., 2010; Ow et al., 2018; Pradhan et al., 2019).
Intergenerational effects
Because pathogenic bacteria serve as food sources and critical survival constraints to the worm, it is plausible that exposure to the pathogens modulates the nervous system and the behavior of the progenies. A recent study shows that training adult hermaphrodites by feeding on PA14 for 4 hours, which is known to produce a robust aversive memory that associates PA14 odorants with virulence in the adult mothers, increases the progeny’s preference for the PA14 odorants (Pereira et al., 2020). Many animals prefer the food that they are exposed to in utero (Liu and Urban, 2017; Nehring et al., 2015; Todrank et al., 2011). These results suggest that with 4-hour exposure the food response elicited by PA14 is significant in the hermaphrodite mothers and that the resulting signals modulate the progeny developing in the uterus. Increasing the duration of PA14 exposure to 8 hours enhances the infection to the mothers (Troemel et al., 2006) and reduces the preference for PA14 in the progeny (Pereira et al., 2020). These findings suggest that longer exposure to PA14 induces a stronger response to the pathogenicity of PA14, which changes the response of the progeny to PA14 from attraction to avoidance. While 4 to 8-hour parental training with PA14 produces robust aversive memory in the hermaphrodite mothers, their modulatory effects on the progeny are limited to the first generation of the offspring (Pereira et al., 2020).
Further increasing the duration of PA14 exposure to 24 hours that starts at the L4 larval stage not only generates avoidance of PA14 in the exposed mothers, but also produces PA14 avoidance in the progeny for 4 generations (Moore et al., 2019). Similar to the effect of 4-hour training on the progeny, the multi-generational effect requires the endo-RNAi pathway and the piRNA pathway (Moore et al., 2019; Pereira et al., 2020). Small RNA pathways also mediate olfactory adaptation by regulating the expression of genes critical for odorant sensation (Juang et al., 2013). However, these behavioral changes differ in their durations and modulatory effects, which suggest distinct mechanisms through which the underlying small RNA pathways alter the nervous system and behavior.
In addition to food-seeking related behavior, exposing the worms to pathogenic bacteria generates intergenerational effects on other physiological traits critical for survival. For example, exposure to Pseudomonas vranovensis, another bacterium pathogenic to C. elegans, generates multigenerational effects and enhances immune resistance to the pathogen in the offspring (Burton et al., 2019). Meanwhile, exposure to certain pathogenic bacteria for two consecutive generations induces formation of dauers, a dormant development stage that is highly resistant to environmental stresses (Palominos et al., 2017). These studies reveal multiple ways that the worm has evolved to adapt its development and function to the bacteria in its environment. Interestingly, pathogenic bacteria are not the only environmental conditions that impact the worm for multiple generations. It has been shown that parental experiences, including dietary restriction, osmotic stress, temperature changes, olfactory imprinting, and prolonged starvation, can regulate the physiology of the offspring, some of which last for several generations and are mediated by small RNA pathways (Burton et al., 2017; Burton et al., 2019; Das et al., 2020; Demoinet et al., 2017; Greer et al., 2011; Hibshman et al., 2016; Jobson et al., 2015; Klosin et al., 2017; Ni et al., 2016; Palominos et al., 2017; Posner et al., 2019; Rechavi et al., 2014; Remy, 2010; Schott et al., 2014; ). These studies together show that C. elegans has evolved diverse adaptive strategies to generate long-term plasticity that lasts for multiple generations. The short lifespan of C. elegans and the ease to conduct genetic experiments on it makes this line of research productive. It will be informative to compare the mechanistic insights identified in studies on intergenerational effects in different animals to understand the difference and similarity in the logic of these regulations.
Outlook
C. elegans lives in a bacteria-rich environment that represents a vast amount of opportunities and constraints to its survival, reproduction and evolution. We are at the beginning stage to understand its impressive adaptive responses encoded in a compact genome. In addition to its responses to pathogenic bacteria, several recent studies also reveal interesting interactions between C. elegans and its commensal bacteria species. These studies show that C. elegans utilizes neurotransmitters or vitamins produced by the environmental bacteria (O’Donnell et al., 2020; Urrutia et al., 2020; Wei and Ruvkun, 2020) to maintain or modulate various physiological events and neural functions. These findings together with those investigating the interactions of C. elegans and pathogenic bacteria have established C. elegans as a promising system to probe mechanisms underlying gut-brain interactions. Together, these studies allow us to leverage the experimental powers provided by a model organism to investigate the function of the nervous system in an ethological and evolutionary context.
Acknowledgements
The work in the Zhang laboratory is supported by the National Institutes of Health (DC009852, R21MH117386, NS115484).
References
- Alcedo J, and Zhang Y (2013). Molecular and cellular circuits underlying Caenorhabditis elegans olfactory plasticity. In Invertebrate learning and memory, Menzel R, and Benjamine P, eds. (San Diego, CA, USA: Elsevier Academic Press Inc.), pp. 112–123. [Google Scholar]
- Aoki I, and Mori I (2015). Molecular biology of thermosensory transduction in C. elegans. Curr Opin Neurobiol 34, 117–124. [DOI] [PubMed] [Google Scholar]
- Aprison EZ, and Ruvinsky I (2019). Coordinated Behavioral and Physiological Responses to a Social Signal Are Regulated by a Shared Neuronal Circuit. Curr Biol 29, 4108–4115 e4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, and Chen M (1983). Morphological basis of long-term habituation and sensitization in Aplysia. Science 220, 91–93. [DOI] [PubMed] [Google Scholar]
- Bargmann CI (2006). Chemosensation in C. elegans. WormBook, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, and Horvitz HR (1993). Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515–527. [DOI] [PubMed] [Google Scholar]
- Bayer EA, and Hobert O (2018). Past experience shapes sexually dimorphic neuronal wiring through monoaminergic signalling. Nature 561, 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj PS, and Hall SE (2017). Endogenous RNAi Pathways Are Required in Neurons for Dauer Formation in Caenorhabditis elegans. Genetics 205, 1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron D, Shibuya M, Gabel C, Wasserman SM, Clark DA, Brown A, Sengupta P, and Samuel AD (2006). A diacylglycerol kinase modulates long-term thermotactic behavioral plasticity in C. elegans. Nat Neurosci 9, 1499–1505. [DOI] [PubMed] [Google Scholar]
- Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, Pocock R, and Ringstad N (2012). A single gene target of an ETS-family transcription factor determines neuronal CO2-chemosensitivity. PLoS One 7, e34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt JP, and Ringstad N (2015). Toll-like Receptor Signaling Promotes Development and Function of Sensory Neurons Required for a C. elegans Pathogen-Avoidance Behavior. Curr Biol 25, 2228–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher AJ, Busch KE, and de Bono M (2008). A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105, 8044–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Furuta T, Webster AK, Kaplan RE, Baugh LR, Arur S, and Horvitz HR (2017). Insulin-like signalling to the maternal germline controls progeny response to osmotic stress. Nat Cell Biol 19, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton NO, Riccio C, Dallaire A, Price J, Jenkins B, Koulman A, and Miska EA (2019). C. elegans heritably adapts to P. vranovensis infection via a mechanism that requires the cysteine synthases cysl-1 and cysl-2. Nat Commu doi: 101038/s41467-020-15555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RA, Fujita M, Schroeder FC, and Clardy J (2007). Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol 3, 420–422. [DOI] [PubMed] [Google Scholar]
- Calhoun AJ, Tong A, Pokala N, Fitzpatrick JA, Sharpee TO, and Chalasani SH (2015). Neural Mechanisms for Evaluating Environmental Variability in Caenorhabditis elegans. Neuron 86, 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, and Bargmann CI (2007). Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450, 63–70. [DOI] [PubMed] [Google Scholar]
- Chalfie M (2009). Neurosensory mechanotransduction. Nat Rev Mol Cell Biol 10, 44–52. [DOI] [PubMed] [Google Scholar]
- Chang HC, Paek J, and Kim DH (2011). Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature 480, 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BH, Cohen M, Rogers C, Albayram O, and de Bono M (2005). Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol 15, 905–917. [DOI] [PubMed] [Google Scholar]
- Choi M-K, Liu H, Wu T, Yang W, and Zhang Y (2020). NMDAR-mediated modulation of gap junction circuit regulates olfactory learning in C. elegans. Nature Communications https://doiorg/101038/s41467-020-17218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, and Bargmann CI (1995). Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14, 803–812. [DOI] [PubMed] [Google Scholar]
- Das S, Ooi FK, Cruz Corchado J, Fuller LC, Weiner JA, and Prahlad V (2020). Serotonin signaling by maternal neurons upon stress ensures progeny survival. Elife 9:e55246. doi: 10.7554/eLife.55246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono M, and Maricq AV (2005). Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci 28, 451–501. [DOI] [PubMed] [Google Scholar]
- Demoinet E, Li S, and Roy R (2017). AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc Natl Acad Sci USA 114, E2689–E2698. doi: 10.1073/pnas.1616171114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, Samuel AD, and Alkema MJ (2013). Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol 11, e1001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MA, and Duveau F (2012). Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biol 10, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, and Bargmann CI (2013). Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frezal L, and Felix MA (2015). C. elegans outside the Petri dish. Elife 4:e05849. doi: 10.7554/eLife.05849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JW, and Riddle DL (1984). The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev Biol 102, 368–378. [DOI] [PubMed] [Google Scholar]
- Goodman MB, Klein M, Lasse S, Luo L, Mori I, Samuel A, Sengupta P, and Wang D (2014). Thermotaxis navigation behavior. WormBook, 1–10. doi: 10.1895/wormbook.1891.1168.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, and Sengupta P (2019). How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli. Genetics 212, 25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordus A, Pokala N, Levy S, Flavell SW, and Bargmann CI (2015). Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 161, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramstrup Petersen J, Rojo Romanos T, Juozaityte V, Redo Riveiro A, Hums I, Traunmuller L, Zimmer M, and Pocock R (2013). EGL-13/SoxD specifies distinct O2 and CO2 sensory neuron fates in Caenorhabditis elegans. PLoS Genet 9, e1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, and Bargmann CI (2005). A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A 102, 3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, Ellis RE, Marletta MA, and Bargmann CI (2004). Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430, 317–322. [DOI] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, and Brunet A (2011). Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 479, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, and Zhang Y (2010). Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68, 1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SE, Beverly M, Russ C, Nusbaum C, and Sengupta P (2010). A cellular memory of developmental history generates phenotypic diversity in C. elegans. Curr Biol 20, 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, Ratsch G, Miller DM 3rd, Horvitz HR, Sternberg PW, and Ringstad N (2011). Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci U S A 108, 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Yang W, Ren J, Hall Q, Zhang Y, and Kaplan JM (2018). Thioredoxin shapes the C. elegans sensory response to Pseudomonas produced nitric oxide. Elife 7:e36833. doi: 10.7554/eLife.36833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Wu T, Linfield G, Choi MK, Liu H, and Zhang Y (2019). Molecular and cellular modulators for multisensory integration in C. elegans. PLoS Genet 15, e1007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock EM, and Russell RL (1975). Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 72, 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibshman JD, Hung A, and Baugh LR (2016). Maternal Diet and Insulin-Like Signaling Control Intergenerational Plasticity of Progeny Size and Starvation Resistance. PLoS Genet 12, e1006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C, and Aballay A (2019). Role of neurons in the control of immune defense. Curr Opin Immunol 60, 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Ryu L, Ow MC, Kim J, Je AR, Chinta S, Huh YH, Lee KJ, Butcher RA, Choi H, et al. (2017). Early Pheromone Experience Modifies a Synaptic Activity to Influence Adult Pheromone Responses of C. elegans. Curr Biol 27, 3168–3177 e3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horspool AM, and Chang HC (2017). Superoxide dismutase SOD-1 modulates C. elegans pathogen avoidance behavior. Sci Rep 7, 45128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y, and Yoshida K (2009). Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci 29, 5370–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Nakano S, Giles AC, Xu L, Costa WS, Gottschalk A, and Mori I (2020). Context-dependent operation of neural circuits underlies a navigation behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A 117, 6178–6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Sawatari E, Hisamoto N, Kitazono T, Teramoto T, Fujiwara M, Matsumoto K, and Ishihara T (2013). Forgetting in C. elegans is accelerated by neuronal communication via the TIR-1/JNK-1 pathway. Cell Rep 3, 808–819. [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Urbach JM, and Ausubel FM (2010). Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Kim K, Neal SJ, Macosko E, Kim D, Butcher RA, Zeiger DM, Bargmann CI, and Sengupta P (2012). Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron 75, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong E, Lee W, Kim YH, Kim K, and Paik YK (2005). Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433, 541–545. [DOI] [PubMed] [Google Scholar]
- Jin X, Pokala N, and Bargmann CI (2016). Distinct Circuits for the Formation and Retrieval of an Imprinted Olfactory Memory. Cell 164, 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juang BT, Gu C, Starnes L, Palladino F, Goga A, Kennedy S, and L’Etoile ND (2013). Endogenous nuclear RNAi mediates behavioral adaptation to odor. Cell 154, 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobson MA, Jordan JM, Sandrof MA, Hibshman JD, Lennox AL, and Baugh LR (2015). Transgenerational Effects of Early Life Starvation on Growth, Reproduction, and Stress Resistance in Caenorhabditis elegans. Genetics 201, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Salazar Thula O, Khoss N, and Zimmer M (2020). Nested Neuronal Dynamics Orchestrate a Behavioral Hierarchy across Timescales. Neuron 105, 562–576 e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JM, and Horvitz HR (1993). A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci U S A 90, 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kaplan HS, Schrodel T, Skora S, Lindsay TH, Yemini E, Lockery S, and Zimmer M (2015). Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669. [DOI] [PubMed] [Google Scholar]
- Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, and Murphy CT (2010). Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol 8, e1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JA, Rose NC, Goldsworthy B, Goga A, and L’Etoile ND (2009). A 3’UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron 61, 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, and Lehner B (2017). Transgenerational transmission of environmental information in C. elegans. Science 356, 320–323. [DOI] [PubMed] [Google Scholar]
- Kim DH, and Ewbank JJ (2018). Signaling in the innate immune response. WormBook 2018, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, and Sengupta P (2009). Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326, 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, and Iino Y (2013). Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nat Commun 4, 2210. [DOI] [PubMed] [Google Scholar]
- Lee K, and Mylonakis E (2017). An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Rep 20, 2501–2512. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Zheng M, and Xu XZ (2014). Encoding of both analog- and digital-like behavioral outputs by one C. elegans interneuron. Cell 159, 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, and Urban NN (2017). Prenatal and Early Postnatal Odorant Exposure Heightens Odor-Evoked Mitral Cell Responses in the Mouse Olfactory Bulb. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang W, Wu T, Duan F, Soucy E, Jin X, and Zhang Y (2018). Cholinergic Sensorimotor Integration Regulates Olfactory Steering. Neuron 97, 390–405 e393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K (1935). Der kumpan in der umwelt des vogels. J Ornithol 83, 137–213. [Google Scholar]
- Luo L, Wen Q, Ren J, Hendricks M, Gershow M, Qin Y, Greenwood J, Soucy ER, Klein M, Smith-Parker HK, et al. (2014). Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 82, 1115–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Zhang L, Dai LL, Khan RU, and Zou CG (2017). mir-67 regulates P. aeruginosa avoidance behavior in C. elegans. Biochem Biophys Res Commun 494, 120–125. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, and Bargmann CI (2009). A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiarmid TA, Yu AJ, and Rankin CH (2019). Habituation Is More Than Learning to Ignore: Multiple Mechanisms Serve to Facilitate Shifts in Behavioral Strategy. Bioessays 41, e1900077. [DOI] [PubMed] [Google Scholar]
- Meisel JD, and Kim DH (2014). Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol 35, 465–470. [DOI] [PubMed] [Google Scholar]
- Meisel JD, Panda O, Mahanti P, Schroeder FC, and Kim DH (2014). Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 159, 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EV, Grandi LN, Giannini JA, Robinson JD, and Powell JR (2015). The Conserved G-Protein Coupled Receptor FSHR-1 Regulates Protective Host Responses to Infection and Oxidative Stress. PLoS One 10, e0137403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RS, Kaletsky R, and Murphy CT (2019). Piwi/PRG-1 Argonaute and TGF-beta Mediate Transgenerational Learned Pathogenic Avoidance. Cell 177, 1827–1841 e1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, and Ohshima Y (1995). Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376, 344–348. [DOI] [PubMed] [Google Scholar]
- Nehring I, Kostka T, von Kries R, and Rehfuess EA (2015). Impacts of in utero and early infant taste experiences on later taste acceptance: a systematic review. J Nutr 145, 1271–1279. [DOI] [PubMed] [Google Scholar]
- Nevitt GA, Dittman AH, Quinn TP, and Moody WJ Jr. (1994). Evidence for a peripheral olfactory memory in imprinted salmon. Proc Natl Acad Sci U S A 91, 4288–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JZ, Kalinava N, Chen E, Huang A, Trinh T, & Gu SG (2016). A transgenerational role of the germline nuclear RNAi pathway in repressing heat stress-induced transcriptional activation in C. elegans. Epigenetics and Chromatin, 9(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MP, Fox BW, Chao PH, Schroeder FC, and Sengupta P (2020). A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 583(7816): 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi FK, and Prahlad V (2017b). Olfactory experience primes the heat shock transcription factor HSF-1 to enhance the expression of molecular chaperones in C. elegans. Sci Signal 10(501): eaan4893. doi: 10.1126/scisignal.aan4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow MC, Borziak K, Nichitean AM, Dorus S, and Hall SE (2018). Early experiences mediate distinct adult gene expression and reproductive programs in Caenorhabditis elegans. PLoS Genet 14, e1007219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palominos MF, Verdugo L, Gabaldon C, Pollak B, Ortiz-Severin J, Varas MA, Chavez FP, and Calixto A (2017). Transgenerational Diapause as an Avoidance Strategy against Bacterial Pathogens in Caenorhabditis elegans. mBio 8(5):e01234–17. doi: 10.1128/mBio.01234-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AG, Gracida X, Kagias K, and Zhang Y (2020). C. elegans aversive olfactory learning generates diverse intergenerational effects. BioRxiv, bioRxiv 2020.2002.2007.939017; doi: https://doi.org/939010.931101/932020.939002.939007.939017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Faumont S, Gaston MR, Pearson BJ, and Lockery SR (2001). The homeobox gene lim-6 is required for distinct chemosensory representations in C. elegans. Nature 410, 694–698. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Morse TM, and Lockery SR (1999). The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci 19, 9557–9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner R, Toker IA, Antonova O, Star E, Anava S, Azmon E, Hendricks M, Bracha S, Gingold H, and Rechavi O (2019). Neuronal Small RNAs Control Behavior Transgenerationally. Cell 177, 1814–1826 e1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E, Zhang Y, Pujol N, Matsuyama T, Bargmann CI, and Ewbank JJ (2007). Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc Natl Acad Sci U S A 104, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S, Quilez S, Homer K, and Hendricks M (2019). Environmental Programming of Adult Foraging Behavior in C. elegans. Curr Biol 29, 2867–2879 e2864. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Beck CD, and Chiba CM (1990). Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res 37, 89–92. [DOI] [PubMed] [Google Scholar]
- Rechavi O, Houri-Ze’evi L, Anava S, Goh WSS, Kerk SY, Hannon GJ, and Hobert O (2014). Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KC, Hunter RC, Bhatla N, Newman DK, and Kim DH (2011). Caenorhabditis elegans NPR-1-mediated behaviors are suppressed in the presence of mucoid bacteria. Proc Natl Acad Sci U S A 108, 12887–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy JJ (2010). Stable inheritance of an acquired behavior in Caenorhabditis elegans. Curr Biol 20, R877–878. [DOI] [PubMed] [Google Scholar]
- Remy JJ, and Hobert O (2005). An interneuronal chemoreceptor required for olfactory imprinting in C. elegans. Science 309, 787–790. [DOI] [PubMed] [Google Scholar]
- Rhoades JL, Nelson JC, Nwabudike I, Yu SK, McLachlan IG, Madan GK, Abebe E, Powers JR, Colon-Ramos DA, and Flavell SW (2019). ASICs Mediate Food Responses in an Enteric Serotonergic Neuron that Controls Foraging Behaviors. Cell 176, 85–97 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Kaun KR, and Rankin CH (2002). A new group-training procedure for habituation demonstrates that presynaptic glutamate release contributes to long-term memory in Caenorhabditis elegans. Learn Mem 9, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, and Iino Y (2001). Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204, 1757–1764. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Rowedder H, Braendle C, Felix MA, and Ruvkun G (2016). Caenorhabditis elegans responses to bacteria from its natural habitats. Proc Natl Acad Sci U S A 113, E3941–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H, and Mori I (2013). Behavioral plasticity, learning, and memory in C. elegans. Curr Opin Neurobiol 23, 92–99. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, and Horvitz HR (2000). C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26, 619–631. [DOI] [PubMed] [Google Scholar]
- Schafer WR (2015). Mechanosensory molecules and circuits in C. elegans. Pflugers Arch 467, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D, Yanai I, & Hunter CP (2014). Natural RNA interference directs a heritable response to the environment. Scientific Reports, 4. 10.1038/srep07387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H, and Felix MA (2017). The Natural Biotic Environment of Caenorhabditis elegans. Genetics 206, 55–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, and Aballay A (2019). Intestinal infection regulates behavior and learning via neuroendocrine signaling. Elife 88:e50033. doi: 10.7554/eLife.50033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WA, Thompson RF, and Neilson DR Jr. (1966). Response decrement of the flexion reflex in the acute spinal cat and transient restoration by strong stimuli. J Neurophysiol 29, 221–239. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, and Schroeder FC (2008). A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 454, 1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, and Schroeder FC (2012). A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol 10, e1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Mahajan-Miklos S, and Ausubel FM (1999). Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci U S A 96, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto Y, Yamazoe-Umemoto A, Fujita K, Kawazoe Y, Miyanishi Y, Yamazaki SJ, Fei X, Busch KE, Gengyo-Ando K, Nakai J, et al. (2017). Calcium dynamics regulating the timing of decision-making in C. elegans. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todrank J, Heth G, and Restrepo D (2011). Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc Biol Sci 278, 1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, and Iino Y (2006). The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51, 613–625. [DOI] [PubMed] [Google Scholar]
- Tran A, Tang A, O’Loughlin CT, Balistreri A, Chang E, Coto Villa D, Li J, Varshney A, Jimenez V, Pyle J, et al. (2017). C. elegans avoids toxin-producing Streptomyces using a seven transmembrane domain chemosensory receptor. Elife 6:e23770. doi: 10.7554/eLife.23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, and Kim DH (2006). p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2, e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, and Hobert O (2003). Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol 56, 178–197. [DOI] [PubMed] [Google Scholar]
- Urrutia A, Garcia-Angulo VA, Fuentes A, Caneo M, Legue M, Urquiza S, Delgado SE, Ugalde J, Burdisso P, and Calixto A (2020). Bacterially produced metabolites protect C. elegans neurons from degeneration. PLoS Biol 18, e3000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam V, Ji N, Wang X, Clark C, Mitchell JK, Klein M, Tabone CJ, Florman J, Ji H, Greenwood J, et al. (2016). Pan-neuronal imaging in roaming Caenorhabditis elegans. Proc Natl Acad Sci U S A 113, E1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, and Ruvkun G (2020). Lysosomal activity regulates Caenorhabditis elegans mitochondrial dynamics through vitamin B12 metabolism. BioRxiv bioRxiv 2020.04.20.049502; doi: 10.1101/2020.04.20.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q, Gao S, and Zhen M (2018). Caenorhabditis elegans excitatory ventral cord motor neurons derive rhythm for body undulation. Philos Trans R Soc Lond B Biol Sci 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JQ, and Jorgensen EM (2012). Sensation in a single neuron pair represses male behavior in hermaphrodites. Neuron 75, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JQ, Nicholas TJ, Gritton J, Truong L, Davidson ER, and Jorgensen EM (2007). The sensory circuitry for sexual attraction in C. elegans males. Curr Biol 17, 1847–1857. [DOI] [PubMed] [Google Scholar]
- Wilson DA, and Sullivan RM (1994). Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol 61, 1–18. [DOI] [PubMed] [Google Scholar]
- Wolfe GS, Tong VW, Povse E, Merritt DM, Stegeman GW, Flibotte S, and van der Kooy D (2019). A Receptor Tyrosine Kinase Plays Separate Roles in Sensory Integration and Associative Learning in C. elegans. eNeuro 6(4):ENEURO.0244–18.2019. doi: 10.1523/ENEURO.0244-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, and Bargmann CI (2005). Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438, 179–184. [DOI] [PubMed] [Google Scholar]