Supplemental digital content is available in the text.
Key Words: CARDIOVASCULAR DISEASE, CIGARETTE SMOKING, EXERCISE, INFLAMMATION, INSULIN, POSTPRANDIAL LIPEMIA
ABSTRACT
Purpose
Cigarette smoking is an independent risk factor for coronary heart disease and is associated with impaired postprandial metabolism. Acute exercise reduces postprandial lipemia and improves other coronary heart disease risk markers in nonsmokers. Less is known about responses in cigarette smokers.
Methods
Twelve male cigarette smokers (mean ± SD; age = 23 ± 4 yr, body mass index = 24.9 ± 3.0 kg·m−2) and 12 male nonsmokers (age = 24 ± 4 yr, body mass index = 24.1 ± 2.0 kg·m−2) completed two, 2-d conditions (control and exercise) in a randomized crossover design. On day 1, participants rested for 9 h (0800–1700) in both conditions except a 60-min treadmill run (65% ± 7% peak oxygen uptake, 2.87 ± 0.54 MJ) was completed between 6.5 and 7.5 h (1430–1530) in the exercise condition. On day 2 of both conditions, participants rested and consumed two high-fat meals over 8 h (0900–1700) during which 13 venous blood samples and nine resting arterial blood pressure measurements were collected.
Results
Smokers exhibited higher postprandial triacylglycerol and C-reactive protein than nonsmokers (main effect group effect size [Cohen’s d] ≥ 0.94, P ≤ 0.034). Previous day running reduced postprandial triacylglycerol, insulin, and systolic and diastolic blood pressure (main effect condition d ≥ 0.28, P ≤ 0.044) and elevated postprandial nonesterified fatty acid and C-reactive protein (main effect condition d ≥ 0.41, P ≤ 0.044). Group–condition interactions were not apparent for any outcome across the total postprandial period (0–8 h; all P ≥ 0.089), but the exercise-induced reduction in postprandial triacylglycerol in the early postprandial period (0–4 h) was greater in nonsmokers than smokers (−21%, d = 0.43, vs −5%, d = 0.16, respectively; group–condition interaction P = 0.061).
Conclusions
Acute moderate-intensity running reduced postprandial triacylglycerol, insulin, and resting arterial blood pressure the day after exercise in male cigarette smokers and nonsmokers. These findings highlight the ability of acute exercise to augment the postprandial metabolic health of cigarette smokers and nonsmokers.
Cigarette smoking is a major cause of premature mortality and constitutes a strong independent risk factor for cardiovascular disease and type 2 diabetes (1,2). The propensity for chronic disease development in cigarette smokers has largely been attributed to the atherogenic, inflammatory, and oxidative stress consequences of chronic cigarette smoke exposure (3). Central to these pathways, cigarette smoking is related to adverse alterations in the fasted lipid and lipoprotein profile (4), which manifest synergistically with perturbations in other physiological systems, including impaired insulin sensitivity (5), greater systemic inflammation (6), and elevated production of reactive oxygen species triggering markers of oxidative stress (7,8). Emerging evidence suggests that the antioxidant enzymes, peroxiredoxin 4 (PRDX-4) and superoxide dismutase 3 (SOD3), are associated positively and negatively with coronary heart disease (CHD) risk, respectively (9,10). Extracellular PRDX-4 concentrations are indicative of endogenous oxidative stress (11), and SOD3 is the only known antioxidant enzyme to scavenge reactive oxygen species in the extracellular milieu. Whether SOD3 and PRDX-4 differ according to smoking status is not known and requires investigation.
Elevated postprandial triacylglycerol (TAG) concentrations are an independent risk factor for cardiovascular disease (12), and the retention of TAG-rich lipoprotein remnants in the arterial intima can contribute to vascular inflammation and atherosclerosis (13). Contemporary evidence from Mendelian randomization studies also support a causal role for TAG-mediated pathways in CHD (14); however, the clinical cardiovascular benefit of reducing TAG and TAG-rich lipoprotein remnants in the postprandial state has yet to be established. Previous data demonstrate that cigarette smokers exhibit an exaggerated postprandial lipemic response and are more insulin resistant than nonsmokers (15–18). Given the profound health detriments of long-term cigarette smoking and the overall poor success rate of smoking cessation (19), therapeutic lifestyle strategies targeting postprandial metabolism may confer important cardiovascular and metabolic health benefits in this at-risk population.
Compelling evidence indicates that single bouts of moderate- to high-intensity exercise transiently reduce circulating postprandial TAG concentrations in healthy nonsmokers (20). The exercise-induced reduction in postprandial lipemia has also been shown to coincide with transient changes in other risk markers for CHD, including favorable reductions in postprandial insulin, interleukin 6 (IL-6), and resting blood pressure (21–23). Although epidemiological evidence suggests that regular physical activity may lower the risk of cardiovascular mortality in smokers (24), the effect of previous acute exercise on postprandial CHD risk markers in cigarette smokers is not known and merits investigation.
The aim of this study was to compare the effects of acute moderate-intensity running on fasting and postprandial risk markers for CHD in male cigarette smokers and nonsmokers. The primary outcome was postprandial TAG concentrations, but several other CHD risk markers were also assessed, including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glucose, insulin, C-reactive protein (CRP), IL-6, PRDX-4, SOD3, and blood pressure. It was hypothesized that cigarette smokers would exhibit impaired postprandial CHD risk markers compared with nonsmokers, but a single bout of exercise would reduce postprandial lipemia to a similar, if not greater, extent in smokers.
METHODS
Participants
The study was conducted in accordance with the principles set out in the Declaration of Helsinki and was approved by the institutional Ethics Approvals Sub-Committee. Twelve male cigarette smokers (20 to 30 yr) and 12 male nonsmokers (20 to 34 yr) provided written informed consent and completed the study between August 2017 and October 2018 (see Figure, Supplemental Digital Content 1, for participant flow chart, http://links.lww.com/MSS/C201). All participants were free from cardiovascular and metabolic disease, were not taking any medications, and were body mass stable in the previous 3 months (≤ ±3 kg). Participants had a body mass index ≤29.9 kg·m−2 and a resting arterial blood pressure <160/90 mm Hg. Cigarette smokers were recruited if they currently smoked at least one cigarette per day and had done so for at least 12 months, whereas nonsmokers had never smoked cigarettes. Groups were matched for age and body mass index (Table 1). The study was registered at ClinicalTrials.gov (trial identifier: NCT03735186).
TABLE 1.
Physical and physiological characteristics at baseline.
| Cigarette Smokers (n = 12) | Nonsmokers (n = 12) | Nonsmokers vs Cigarette Smokers | ||
|---|---|---|---|---|
| Mean Difference (95% CIa) | d | |||
| Age (yr) | 23 ± 4 | 24 ± 4 | −1 (−4 to 2) | 0.28 |
| Stature (cm) | 176.5 ± 8.6 | 178.0 ± 7.6 | −1.5 (−8.3 to 5.3) | 0.19 |
| Body mass (kg) | 77.8 ± 12.0 | 76.6 ± 9.1 | 1.2 (−7.8 to 10.2) | 0.11 |
| Body mass index (kg·m−2) | 24.9 ± 3.0 | 24.1 ± 2.0 | 0.8 (−1.4 to 2.9) | 0.30 |
| Waist circumference (cm) | 84.7 ± 11.0 | 80.8 ± 5.1 | 3.9 (−3.4 to 11.2) | 0.46 |
| Body fat (%) | 18.0 ± 5.7 | 16.8 ± 5.2 | 1.2 (−3.4 to 5.8) | 0.22 |
| Lean body mass (kg) | 63.4 ± 8.3 | 63.8 ± 8.9 | −0.4 (−7.6 to 6.9) | 0.04 |
| V˙O2peak (L·min−1) | 3.38 ± 0.48 | 3.60 ± 0.74 | −0.22 (−0.75 to 0.31) | 0.35 |
| V˙O2peak (mL·kg−1⋅min−1) | 43.7 ± 4.6 | 46.7 ± 5.3 | −2.9 (−7.1 to 1.3) | 0.59 |
| Resting SBP (mm Hg) | 129 ± 13 | 124 ± 11 | 4 (−6 to 14) | 0.37 |
| Resting DBP (mm Hg) | 77 ± 9 | 69 ± 6 | 8 (1 to 14)b | 1.04 |
| Fasting TAG (mmol·L−1) | 1.22 (0.99 to 1.49) | 0.72 (0.54 to 0.96) | 68% (21% to 134%)b | 1.32 |
| Fasting TC (mmol·L−1) | 4.40 (3.90 to 4.98) | 3.97 (3.52 to 4.49) | 11% (−6% to 30%) | 0.53 |
| Fasting HDL-C (mmol·L−1) | 1.20 (1.08 to 1.33) | 1.45 (1.28 to 1.65) | −17% (−29% to −4%)b | 1.04 |
| Fasting LDL-C (mmol·L−1) | 2.43 (2.04 to 2.90) | 1.83 (1.57 to 2.13) | 33% (7% to 65%)b | 1.09 |
| Fasting NEFA (mmol·L−1) | 0.28 ± 0.06 | 0.41 ± 0.22 | −0.13 (−0.26 to 0.01) | 0.77 |
| Fasting glucose (mmol·L−1) | 4.70 ± 0.33 | 4.69 ± 0.19 | 0.00 (−0.23 to 0.23) | 0.01 |
| Fasting insulin (pmol·L−1) | 23.4 (15.7 to 34.8) | 24.7 (20.9 to 29.2) | −5% (−37% to 42%) | 0.11 |
| HOMA-IR | 0.81 (0.53 to 1.25) | 0.86 (0.72 to 1.02) | −5% (−39% to 46%) | 0.11 |
| Adipo-IR | 6.58 (4.06 to 10.66) | 9.01 (6.14 to 13.21) | −27% (−59% to 30%) | 0.46 |
| Fasting CRP (mg·L−1) | 1.25 (0.69 to 2.25) | 0.51 (0.25 to 1.04) | 145% (3% to 483%)b | 0.87 |
| Fasting IL-6 (pg·mL−1) | 1.39 (1.02 to 1.89) | 1.07 (0.80 to 1.42) | 30% (−12% to 93%) | 0.56 |
| Fasting TNF-α (pg·mL−1) | 1.24 (0.99 to 1.54) | 1.01 (0.87 to 1.16) | 23% (−4% to 58%) | 0.71 |
| Fasting SOD3 (ng·mL−1) | 1.00 (0.77 to 1.31) | 0.83 (0.60 to 1.16) | 20% (−19% to 80%) | 0.39 |
| Fasting PRDX-4 (ng·mL−1) | 12.2 (10.4 to 14.2) | 9.3 (7.3 to 12.0) | 30% (−1% to 71%) | 0.81 |
Values for TAG, TC, HDL-C, LDL-C, insulin, HOMA-IR, Adipo-IR, CRP, IL-6, TNF-α, SOD3, and PRDX-4 are geometric mean (95% CI), and statistical analyses are based on log-transformed data. All other values are mean ± SD. Data are analyzed using linear mixed models with group (cigarette smokers or nonsmokers) included as a fixed factor.
aFor normally distributed data, values represent the mean absolute difference (95% CI of the mean absolute difference between the groups). For log-transformed data, values represent the ratio of geometric means (95% CI for the ratio of geometric means between the groups).
bMain effect of group P ≤ 0.044.
Preliminary Measures
Participants attended the laboratory for a preliminary visit to undergo screening, familiarization, anthropometric measurements, and exercise testing. Specifically, participants completed questionnaires to assess health status, habitual physical activity levels, and smoking habits (cigarette smokers only). Stature was determined using a stadiometer (Avery Industrial Ltd., Leicester, UK), and body mass and body fat percentage were measured using a body composition analyzer (Seca GmbH & Co. KG, Hamburg, Germany). Waist circumference was determined at the narrowest point between the iliac crest and the xiphoid process using a measuring tape. Resting arterial blood pressure was measured in the semisupine position after 10 to 15 min rest using a digital monitor (Omron M5-1; Matsusaka Co., Yokosuka, Japan), and the mean of three measurements was calculated.
After familiarization with the treadmill (ExciteMed; Technogym, Cesena, Italy), two preliminary exercise tests were performed: 1) submaximal incremental treadmill test to determine the relationship between running speed, oxygen consumption, and heart rate; and 2) incremental uphill treadmill test to determine peak oxygen uptake (V˙O2peak). The submaximal incremental test composed of 4 × 4-min stages starting at a treadmill speed between 5 and 9 km·h−1 and increasing by 1 to 1.5 km·h−1 at the start of each subsequent stage. After 30 min of recovery, participants completed a V˙O2peak test at a fixed individual running speed (7.0 to 9.5 km·h−1). The treadmill gradient was set to 0% at the start of the test and increased by 1% every minute until volitional exhaustion.
During both exercise tests, expired air samples were measured continuously using a breath-by-breath gas analysis system (MetaMax® 3B; Cortex Biophysik, Leipzig, Germany). Heart rate was monitored continuously (Polar T31; Polar Electro, Kempele, Finland), and RPE (25) was measured at predetermined intervals throughout the tests (submaximal: final 30 s of each 4-min stage; V˙O2peak: final 10 s of the stage at 2-min intervals and at test termination). To determine V˙O2peak, a 30-s rolling average was calculated from the breath-by-breath V˙O2 data, and an average of these data was subsequently calculated in 30-s blocks; the highest value was recorded as V˙O2peak. Data from the 4 × 4 min submaximal incremental and V˙O2peak tests were used to determine the exercise intensity in the main experimental exercise condition.
Experimental Design
Using a randomized mixed-measures crossover design, participants completed two, 2-d experimental conditions separated by at least 1 wk: 1) exercise and 2) rest control. The block randomization sequence stratified by smoking status was generated by the lead investigator (TFA), who also enrolled participants and assigned participants to the condition order. The study design is presented in Figure 1. Participants refrained from strenuous physical activity and weighed, recorded, and replicated their dietary intake in the 48 h before day 1 of each condition. Participants were also asked not to consume caffeine or alcohol in the 24 h before day 1 of each condition.
FIGURE 1.
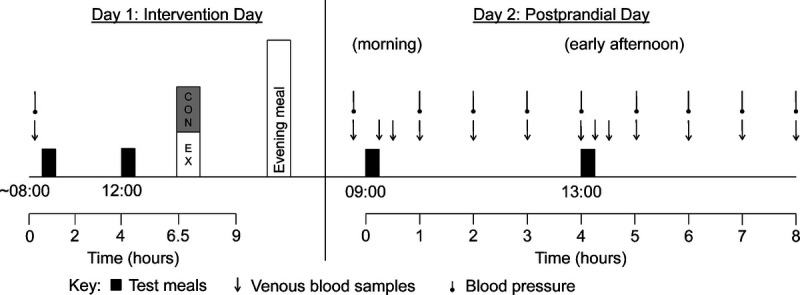
Schematic of the study design. CON, control; EX, exercise.
Day 1
Participants arrived at the laboratory at 0800 after a 10-h overnight fast. A fasting blood sample was collected via venipuncture of an antecubital vein, and resting arterial blood pressure was measured. Standardized breakfast and lunch were provided at 0830 and 1200, respectively. Participants rested in the laboratory for the duration of the control condition until 1700. Identical procedures were completed in the exercise condition, except participants completed 1 h of moderate-intensity treadmill exercise at 60% of their V˙O2peak between 1430 and 1530. Expired air samples were measured using an online breath-by-breath gas analysis system to maintain the target exercise intensity and to estimate gross energy expenditure and substrate utilization (26). Heart rate was monitored continuously, and RPE was recorded at 10-min intervals during the exercise session. Participants were provided with a standardized evening meal consisting of a pizza to consume before 2100 (2511 kJ, 32% fat, 52% carbohydrate, 16% protein). Additional food or drink items apart from plain water were not permitted until arriving at the laboratory the next day.
Day 2
Participants arrived at the laboratory at 0800 after fasting overnight for at least 10 h and rested in the laboratory throughout both conditions until 1700. After 15 min seated rest, resting arterial blood pressure was measured, and an 18 G cannula (BD Venflon; Becton–Dickinson, Helsingborg, Sweden) was inserted into an antecubital vein before a fasting blood sample was drawn. Participants consumed a standardized breakfast and lunch at 0 h (0900) and 4 h (1300), respectively. Subsequent venous blood samples were collected at 0.25, 0.5, 1, 2, 3, 4, 4.25, 4.5, 5, 6, 7, and 8 h after the start of the breakfast meal, and resting arterial blood pressure was measured at hourly intervals until 8 h (1700).
Test Meals
On days 1 and 2, breakfast consisted of plain croissants, milk chocolate spread, double cream, and chocolate milkshake, which provided 60 kJ energy per kilogram body mass (57% fat, 35% carbohydrate, and 8% protein). Lunch consisted of white bread, cheddar cheese, butter, and chocolate milkshake, which provided 60 kJ energy per kilogram body mass (57% fat, 32% carbohydrate, and 11% protein). Breakfast and lunch meals were each consumed within 10 min, and water was provided ad libitum throughout each condition.
Blood Sampling
Plasma TAG and glucose concentrations were determined in all samples. Plasma concentrations of TC, HDL-C, LDL-C, and tumor necrosis factor α (TNF-α) were quantified from fasted samples on day 1 and day 2. Plasma insulin and nonesterified fatty acid (NEFA) concentrations were determined on day 1 in the fasted state and on day 2 at 0, 0.5, 1, 3, 4, 4.5, 6, and 8 h. Concentrations of CRP and IL-6 were measured on day 1 in the fasted state and on day 2 at 0, 2, 5, and 8 h. Plasma SOD3 and PRDX-4 concentrations were measured on day 1 in the fasted state and on day 2 at 0, 2, 4, and 8 h.
Participants rested in a semisupine position during blood sampling. Venous blood samples were drawn into precooled 9 mL EDTA Monovette tubes (Sarstedt, Leicester, UK) and immediately centrifuged at 1165g for 10 min at 4°C (Labofuge 400R; Thermo Scientific, Langenselbold, Germany). The plasma supernatant was dispensed into Cryovials and stored at −80°C for later analysis.
Biochemical Analysis
Concentrations of plasma TAG, TC, HDL-C, LDL-C, glucose, CRP (HORIBA Medical, Montpellier, France), and NEFA (Randox Laboratories Ltd., County Antrim, UK) were determined spectrophotometrically using commercially available kits (high-sensitivity detection was used for CRP) and a benchtop analyzer (Pentra 400, HORIBA Medical). Concentrations of plasma insulin (Mercodia, Uppsala, Sweden), IL-6, and TNF-α (high-sensitivity kits; R&D Systems, Abingdon, UK) were determined using commercially available enzyme-linked immunosorbent assays. Plasma SOD3 and PRDX-4 concentrations were quantified using in-house enzyme-linked immunosorbent assays (27) developed using commercially available antigens and antibodies (Abcam, Cambridge, UK; Sigma Aldrich, Dorset, UK). The human SOD3 antigen and rabbit antiserum directed against human SOD3 were developed as previously described (28,29).
To eliminate interassay variation, samples from each participant were analyzed in the same run. The within-batch coefficient of variation for each assay was as follows: 1.6% for TAG, 0.8% for TC, 0.7% for HDL-C, 0.8% for LDL-C, 1.6% for NEFA, 0.6% for glucose, 3.2% for insulin, 0.9% for CRP, 5.0% for IL-6, 3.2% for TNF-α, 6.8% for SOD3, and 5.1% for PRDX-4.
Statistical Analyses
Based on previous data from our laboratory (21), it was estimated that a sample size of 24 participants would have >90% power to detect a 0.407 mmol·L−1·h−1 reduction in time-averaged total area under the curve (AUC) for TAG after acute exercise using a two-tailed t-test, assuming an SDdiff of 0.494 mmol·L−1·h−1 and adopting an α value of 0.05. The primary outcome measured in this study was time-averaged total AUC for TAG, and the secondary end points were TC, HDL-C, LDL-C, NEFA, glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR), adipose tissue insulin resistance index (Adipo-IR), CRP, IL-6, TNF-α, SOD3, PRDX-4, and arterial blood pressure. Standard equations were used to calculate HOMA-IR (30) and Adipo-IR (31). Time-averaged total AUC was calculated for blood pressure and concentrations of plasma TAG, NEFA, glucose, insulin, CRP, IL-6, SOD3, and PRDX-4 using the trapezoidal method for the total postprandial period (0–8 h). Time-averaged total AUC was also calculated for plasma TAG, NEFA, glucose, and insulin in the morning (0–4 h) and afternoon (4–8 h) periods. Time-average incremental AUC relative to the fasted or nadir value was calculated for biochemical outcomes (32).
Data were analyzed using the software package SPSS (SPSS version 23; SPSS Inc., Chicago, IL). The model residuals of the outcome variables were explored using histograms. Normally distributed data are presented as mean ± SD. Fasting concentrations and time-averaged total AUC values for TAG, TC, HDL-C, LDL-C, insulin, CRP, IL-6, TNF-α, SOD3, and PRDX-4 demonstrated a positively skewed distribution. These variables were natural log-transformed before analysis and are presented descriptively as geometric mean (95% confidence interval [95% CI]).
Fasting plasma constituents and resting arterial blood pressure at baseline on day 1 were similar between the exercise and the control conditions (all P ≥ 0.102); therefore, the average was calculated and used for all subsequent analysis. Physical characteristics and exercise responses were compared between cigarette smokers and nonsmokers using linear mixed models with group (12 cigarette smokers vs 12 nonsmokers) included as a fixed factor. Linear mixed models were used to examine differences in HOMA-IR, Adipo-IR, and fasting and AUC values for plasma constituents and blood pressure with condition (exercise vs control) and group (12 cigarette smokers vs 12 nonsmokers) included as fixed factors.
Absolute standardized effect sizes (Cohen’s d) were calculated by dividing the difference between the mean values (exercise vs control or cigarette smokers vs nonsmokers) with the pooled SD. An effect size of 0.2 was considered the minimum important difference, 0.5 moderate, and 0.8 large (33). For normally distributed variables, pairwise comparisons are based on mean differences and the respective 95% CI of the mean absolute difference. For log-transformed variables, pairwise comparisons are based on the ratio of geometric means and 95% CI for the ratio of geometric means. Statistical significance was accepted as P < 0.05. All P values are presented as exact values (to three decimal places) except very low values, which are displayed as P < 0.001. Interpretation of the data is based on the 95% CI and effect sizes rather than more conventional dichotomous hypothesis testing (34).
RESULTS
Participant Characteristics
Cigarette smokers smoked a mean ± SD (range) of 9 ± 5 (4–20) cigarettes per day, had smoked cigarettes for 6 ± 4 (1 to 15) yr, and had 3.1 ± 3.1 (0.3 to 9.8) pack-years of smoking. Cigarette smokers exhibited higher fasting plasma concentrations of TAG (d = 1.32, P = 0.004), LDL-C (d = 1.09, P = 0.014), CRP (d = 0.87, P = 0.044), and PRDX-4 (d = 0.81, P = 0.059); lower concentrations of HDL-C (d = 1.04, P = 0.018); and higher resting DBP (d = 1.04, P = 0.018) than nonsmokers at baseline (Table 1). No other between-group differences were observed in baseline physical and physiological characteristics (P ≥ 0.096) (Table 1).
Exercise Responses
The relative exercise intensity (percent of V˙O2peak) was matched between groups (d = 0.31, P = 0.449), but the treadmill running speed was lower in cigarette smokers than that in nonsmokers (d = 0.89, P = 0.040) (Table 2). No other differences were observed in treadmill running responses between cigarette smokers and nonsmokers (P ≥ 0.123) (Table 2).
TABLE 2.
Responses to treadmill exercise in cigarette smokers and nonsmokers.
| Cigarette Smokers (n = 12) | Nonsmokers (n = 12) | Nonsmokers vs Cigarette Smokers | ||
|---|---|---|---|---|
| Mean Difference (95% CIa) | d | |||
| Treadmill speed (km·h−1) | 7.7 ± 1.0 | 8.7 ± 1.2 | −1.0 (−2.0 to −0.1)b | 0.89 |
| Heart rate (bpm) | 159 ± 13 | 159 ± 11 | 0 (−11 to 10) | 0.03 |
| Rating of perceived exertion | 11 ± 2 | 12 ± 2 | −1 (−3 to 1) | 0.52 |
| Oxygen uptake (L·min−1) | 2.14 ± 0.40 | 2.36 ± 0.48 | −0.21 (−0.58 to 0.16) | 0.48 |
| Percent V˙O2peak (%) | 63.4 ± 7.7 | 65.8 ± 7.2 | −2.3 (−8.7 to 4.0) | 0.31 |
| Respiratory exchange ratio | 0.95 ± 0.05 | 0.92 ± 0.04 | 0.03 (−0.01 to 0.07) | 0.62 |
| Fat oxidation (g) | 12.6 ± 8.5 | 18.5 ± 9.4 | −5.9 (−13.5 to 1.7) | 0.65 |
| Carbohydrate oxidation (g) | 144 ± 36 | 140 ± 33 | 4 (−25 to 33) | 0.12 |
| Gross energy expenditure (kJ) | 2788 ± 509 | 2954 ± 584 | −166 (−630 to 298) | 0.30 |
All values are mean ± SD. Data were analyzed using linear mixed models with group (cigarette smokers or nonsmokers) included as a fixed factor.
a95% CI of the mean absolute difference between the groups.
bMain effect of group P = 0.040.
Fasting Plasma Concentrations
Fasting plasma metabolite concentrations on day 2 are presented in Table 3. Main effects of group revealed higher fasting plasma concentrations of TAG (d = 1.44, P < 0.001), LDL-C (d = 0.93, P = 0.024), CRP (d = 0.91, P = 0.034), and IL-6 (d = 0.82, P = 0.042) and lower fasting plasma HDL-C concentrations (d = 0.96, P = 0.019) in cigarette smokers compared with nonsmokers. Fasting plasma TC, NEFA, glucose, insulin, TNF-α, SOD3, and PRDX-4 concentrations; HOMA-IR; and Adipo-IR were similar between cigarette smokers and nonsmokers (P ≥ 0.098).
TABLE 3.
Fasting plasma concentrations and resting arterial blood pressure on day 2 of the control and exercise conditions in cigarette smokers and nonsmokers.
| Cigarette Smokers (n = 12) | Nonsmokers (n = 12) | Nonsmokers vs Cigarette Smokers | Control vs Exercise | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Mean Difference (95% CIa) | d | Mean Difference (95% CIa) | d | |
| TAG (mmol·L−1) | 1.22 (0.97 to 1.53) | 1.01 (0.81 to 1.26) | 0.67 (0.51 to 0.87) | 0.60 (0.50 to 0.73) | 75% (32% to 132%)b | 1.44 | −13% (−23% to −3%)c | 0.37 |
| TC (mmol·L−1) | 4.45 (3.90 to 5.07) | 4.33 (3.84 to 4.87) | 4.01 (3.57 to 4.51) | 3.93 (3.50 to 4.40) | 11% (−6% to 29%) | 0.51 | −2% (−6% to 1%) | 0.12 |
| HDL-C (mmol·L−1) | 1.25 (1.09 to 1.42) | 1.17 (1.04 to 1.32) | 1.49 (1.32 to 1.69) | 1.44 (1.28 to 1.63) | −18% (−30% to −3%)b | 0.96 | −5% (−9% to −0.2%)c | 0.23 |
| LDL-C (mmol·L−1) | 2.46 (2.03 to 2.98) | 2.44 (2.06 to 2.88) | 1.90 (1.62 to 2.23) | 1.89 (1.62 to 2.20) | 29% (4% to 62%)b | 0.93 | −1% (−5% to 3%) | 0.03 |
| NEFA (mmol·L−1) | 0.35 ± 0.16 | 0.46 ± 0.20 | 0.37 ± 0.14 | 0.53 ± 0.22 | −0.04 (−0.16 to 0.07) | 0.29 | 0.13 (0.03 to 0.23)c | 0.88 |
| Glucose (mmol·L−1) | 4.67 ± 0.25 | 4.60 ± 0.36 | 4.62 ± 0.18 | 4.48 ± 0.25 | 0.09 (−0.12 to 0.29) | 0.40 | −0.10 (−0.20 to −0.01)c | 0.48 |
| Insulin (pmol·L−1) | 24.9 (17.7 to 34.9) | 22.9 (16.6 to 31.8) | 18.4 (14.5 to 23.4) | 18.1 (13.8 to 23.6) | 31% (−6% to 83%) | 0.58 | −5% (−23% to 17%) | 0.11 |
| HOMA-IR | 0.86 (0.61 to 1.22) | 0.78 (0.56 to 1.09) | 0.63 (0.49 to 0.81) | 0.60 (0.45 to 0.79) | 33% (−6% to 88%) | 0.61 | −7% (−25% to 15%) | 0.16 |
| Adipo-IR | 7.78 (4.79 to 12.63) | 9.21 (5.39 to 15.71) | 6.39 (4.38 to 9.33) | 7.82 (5.09 to 11.99) | 20% (−26% to 93%) | 0.26 | 20% (−18% to 77%) | 0.27 |
| CRP (mg·L−1) | 1.09 (0.63 to 1.87) | 1.47 (0.80 to 2.70) | 0.40 (0.21 to 0.79) | 0.70 (0.35 to 1.41) | 138% (7% to 429%)b | 0.91 | 53% (17% to 101%)c | 0.45 |
| IL-6 (pg·mL−1) | 1.40 (1.04 to 1.88) | 1.59 (1.11 to 2.29) | 0.96 (0.70 to 1.32) | 1.05 (0.74 to 1.48) | 48% (2% to 117%)b | 0.82 | 11% (−11% to 40%) | 0.22 |
| TNF-α (pg·mL−1) | 1.16 (0.92 to 1.45) | 1.14 (0.94 to 1.39) | 1.00 (0.86 to 1.16) | 1.01 (0.88 to 1.16) | 14% (−9% to 45%) | 0.45 | 0% (−7% to 7%) | 0.00 |
| SOD3 (ng·mL−1) | 1.03 (0.78 to 1.36) | 1.07 (0.86 to 1.34) | 0.78 (0.54 to 1.12) | 0.73 (0.50 to 1.05) | 40% (−7% to 111%) | 0.66 | −1% (−9% to 7%) | 0.02 |
| PRDX-4 (ng·mL−1) | 11.1 (9.1 to 13.6) | 11.8 (9.5 to 14.7) | 11.0 (9.1 to 13.2) | 9.8 (8.3 to 11.7) | 10% (−11% to 38%) | 0.32 | −2% (−15% to 12%) | 0.07 |
| SBP (mm Hg) | 134 ± 19 | 130 ± 10 | 126 ± 10 | 129 ± 10 | 5 (−5 to 14) | 0.31 | 0 (−5 to 5) | 0.01 |
| DBP (mm Hg) | 76 ± 11 | 72 ± 8 | 68 ± 9 | 72 ± 5 | 4 (−2 to 10) | 0.36 | 0 (−4 to 4) | 0.01 |
Values for TAG, TC, HDL-C, LDL-C, insulin, HOMA-IR, Adipo-IR, CRP, IL-6, TNF-α, SOD3, and PRDX-4 are geometric mean (95% CI) and statistical analyses are based on log-transformed data. All other values are mean ± SD. Data were analyzed using linear mixed models with group (cigarette smokers or nonsmokers) and condition (exercise or control) included as fixed factors.
Linear mixed models revealed no group–condition interactions P ≥ 0.070.
aFor normally distributed data, values represent the mean absolute difference (95% CI of the mean absolute difference between groups or conditions). For log-transformed data, values represent the ratio of geometric means (95% CI for the ratio of geometric means between groups or conditions).
bMain effect of group P ≤ 0.042.
cMain effect of condition ≤0.043.
Main effects of condition revealed lower fasting concentrations of TAG (d = 0.37, P = 0.014), HDL-C (d = 0.23, P = 0.043) and glucose (d = 0.48, P = 0.038) and higher fasting concentrations of NEFA (d = 0.88, P = 0.012) and CRP (d = 0.45, P = 0.003) in the exercise condition compared with the control condition. No group–condition interactions were observed in fasting metabolite concentrations or indicators of insulin resistance (P ≥ 0.175).
Postprandial Plasma Concentrations
Total postprandial period (0–8 h)
The time-averaged total AUC values for the entire postprandial period (0–8 h) are displayed in Supplemental Table 1 (Supplemental Digital Content 2, Time-averaged total area under the curve values (0–8 h) for postprandial plasma concentrations and resting arterial blood pressure on day 2 of the control and exercise conditions, http://links.lww.com/MSS/C202) and are presented graphically in Figure 2 (TAG, glucose, insulin, and NEFA) and Figure 3 (IL-6, CRP, SOD3, and PRDX-4).
FIGURE 2.
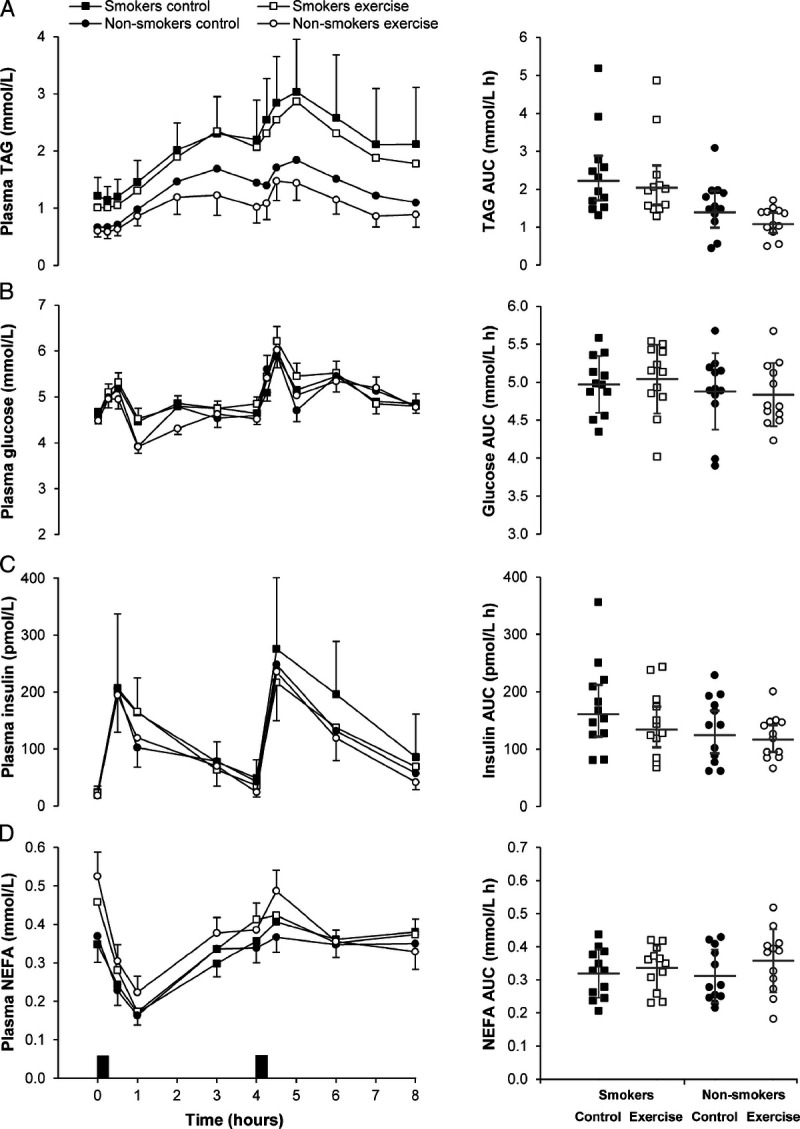
Plasma concentrations of TAG (A), glucose (B), insulin (C), and NEFA (D) in the exercise and control conditions for male cigarette smokers (n = 12) and male nonsmokers (n = 12). Data points on left-hand panels represent geometric mean (95% CI) for TAG and insulin, and mean ± SEM for glucose and NEFA. Black rectangles indicate consumption of breakfast and lunch meals. Data points on right-hand panels represent individual data values for time-averaged total area under the curve (AUC) (0–8 h). The gray solid line (—) indicates the geometric mean (95% CI) for TAG and insulin, and mean ± SD for glucose and NEFA. Linear mixed models for time-averaged total AUC (0–8 h) identified a main effect of group for TAG (P = 0.004), and a main effect of condition for TAG (P = 0.002), insulin (P = 0.038), and NEFA (P = 0.044). No group–condition interactions were identified (all P ≥ 0.089).
FIGURE 3.
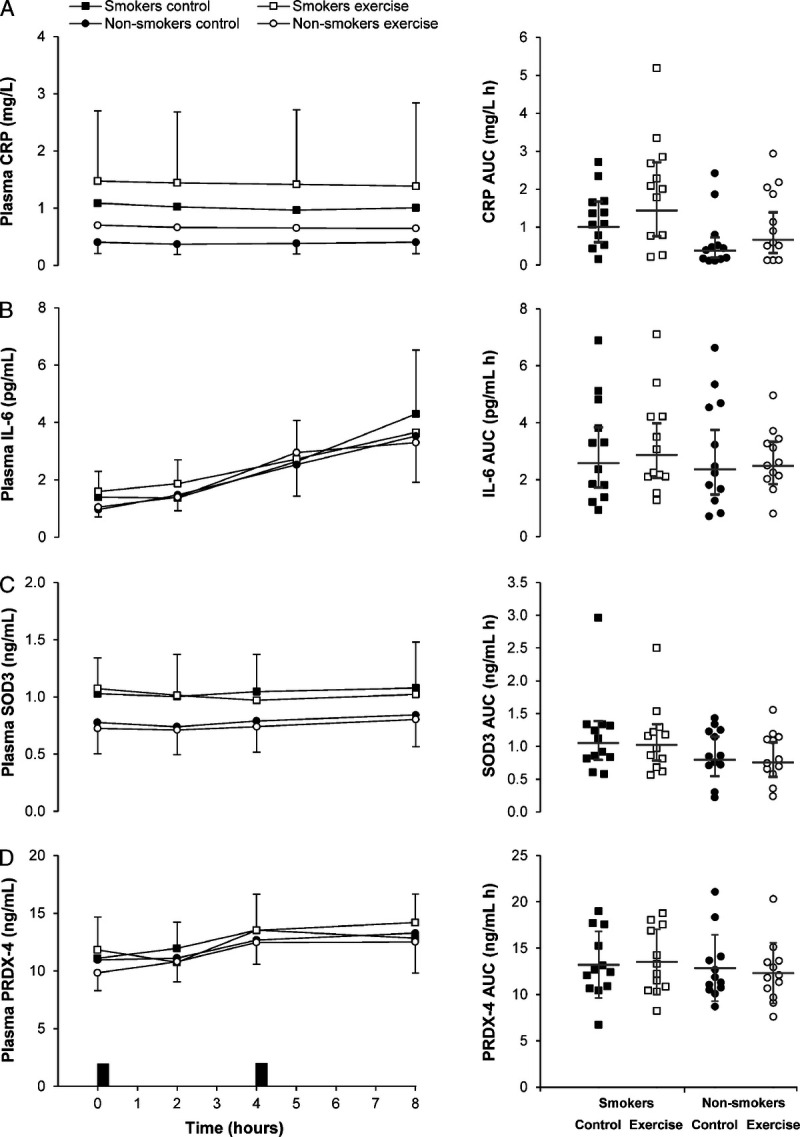
Plasma concentrations of CRP (A), IL-6 (B), SOD3 (C), and PRDX-4 (D) in the exercise and control conditions for male cigarette smokers (n = 12) and male nonsmokers (n = 12). Data points on left-hand panels represent geometric mean (95% CI). Black rectangles indicate consumption of breakfast and lunch meals. Data points on right-hand panels represent individual data values for time-averaged total area under the curve (AUC) (0–8 h), and the gray solid line (―) indicates the geometric mean (95% CI). Linear mixed models for time-averaged total AUC (0–8 h) identified a main effect of group for CRP (P = 0.034), and a main effect of condition for CRP (P = 0.004). No group–condition interactions were identified (all P ≥ 0.386).
A main effect of group revealed higher time-averaged total AUC (0–8 h) for TAG (d = 1.15, P = 0.004) and CRP (d = 0.94, P = 0.034) in cigarette smokers than that in nonsmokers. The time-averaged total AUC (0–8 h) for NEFA, glucose, insulin, IL-6, SOD3, and PRDX-4 were similar between groups (P ≥ 0.162).
A main effect of condition revealed lower time-averaged total AUC (0–8 h) for TAG (d = 0.35, P = 0.002) and insulin (d = 0.28, P = 0.038) and higher time-averaged total AUC for NEFA (d = 0.41, P = 0.044) and CRP (d = 0.50, P = 0.004) in the exercise condition compared with the control condition. No between-condition differences were identified in the time-averaged total AUC (0–8 h) for glucose, IL-6, SOD3, and PRDX-4 (all P ≥ 0.163). The magnitude of change in postprandial metabolite concentrations (total AUC, 0–8 h) the day after exercise was similar in cigarette smokers and nonsmokers (all group–condition interactions P ≥ 0.089).
Analysis of the time-averaged incremental AUC values (0–8 h) revealed broadly comparable findings to the total AUC analysis (see Supplemental Table 2, Supplemental Digital Content 3, Time-averaged incremental positive area under the curve values (0–8 h) for postprandial plasma concentrations on day 2 of the control and exercise conditions, http://links.lww.com/MSS/C203). The time-averaged incremental AUC was higher in cigarette smokers compared with nonsmokers for CRP (d = 1.34, P = 0.004) and lower in the exercise condition compared with the control condition for insulin (d = 0.35, P = 0.029). The 95% CI for the mean difference in time-averaged incremental AUC for TAG overlapped zero (main effect group P = 0.074; main effect condition P = 0.127), but the response was higher in cigarette smokers than nonsmokers (mean difference 0.47 mmol·L−1·h−1, d = 0.65) and lower in the exercise condition than that in the control condition (mean difference 0.16 mmol·L−1·h−1, d = 0.22). Group–condition interactions were not apparent for any time-averaged incremental AUC outcome (all P ≥ 0.148).
Morning period (0–4 h) and afternoon period (4–8 h)
The time-averaged total AUC for TAG, NEFA, glucose, and insulin in the morning period (0–4 h) and the afternoon period (4–8 h) are displayed in Table 4.
TABLE 4.
Time-averaged total area under the curve values for postprandial plasma concentrations on day 2 of the control and exercise conditions in cigarette smokers and nonsmokers in the morning period (0–4 h) and afternoon period (4–8 h).
| Cigarette Smokers (n = 12) | Nonsmokers (n = 12) | Nonsmokers vs Cigarette Smokers | Control vs Exercise | |||||
|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Mean Difference (95% CIa) | d | Mean Difference (95% CIa) | d | |
| TAG (mmol·L−1·h−1) | ||||||||
| Morning period | 1.88 (1.53 to 2.30) | 1.78 (1.43 to 2.21) | 1.30 (0.91 to 1.84) | 1.02 (0.77 to 1.34) | 59% (13% to 124%)b | 1.02 | −14% (−22% to −5%)c | 0.32 |
| Afternoon period | 2.53 (1.84 to 3.46) | 2.29 (1.72 to 3.04) | 1.48 (1.05 to 2.09) | 1.13 (0.89 to 1.44) | 86% (27% to 171%)b | 1.19 | −17% (−26% to −6%)c | 0.35 |
| NEFA (mmol·L−1·h−1) | ||||||||
| Morning period | 0.26 ± 0.08 | 0.29 ± 0.08 | 0.27 ± 0.08 | 0.33 ± 0.12 | −0.02 (−0.09 to 0.05) | 0.27 | 0.05 (0.01 to 0.08)c | 0.54 |
| Afternoon period | 0.38 ± 0.08 | 0.38 ± 0.07 | 0.35 ± 0.10 | 0.38 ± 0.10 | 0.01 (−0.06 to 0.07) | 0.11 | 0.02 (−0.02 to 0.05) | 0.18 |
| Glucose (mmol·L−1·h−1) | ||||||||
| Morning period | 4.77 ± 0.43 | 4.80 ± 0.49 | 4.58 ± 0.50 | 4.45 ± 0.30 | 0.27 (−0.05 to 0.59) | 0.58 | −0.05 (−0.24 to 0.14) | 0.11 |
| Afternoon period | 5.18 ± 0.44 | 5.28 ± 0.51 | 5.17 ± 0.56 | 5.22 ± 0.60 | 0.03 (−0.36 to 0.42) | 0.06 | 0.08 (−0.14 to 0.30) | 0.15 |
| Insulin (pmol·L−1·h−1) | ||||||||
| Morning period | 123 (92 to 165) | 119 (88 to 161) | 100 (77 to 130) | 100 (79 to 126) | 22% (−13% to 70%) | 0.45 | −2% (−15% to 13%) | 0.04 |
| Afternoon period | 195 (146 to 261) | 146 (111 to 193) | 146 (103 to 207) | 131 (107 to 161) | 22% (−14% to 74%) | 0.40 | −18% (−29% to −6%)c | 0.39 |
Values for TAG and insulin are geometric mean (95% CI) and statistical analyses are based on log-transformed data. All other values are mean ± SD. Data were analyzed using linear mixed models with group (cigarette smokers or nonsmokers) and condition (exercise or control) included as fixed factors.
Linear mixed models revealed no group–condition interactions P ≥ 0.061.
aFor normally distributed data, values represent the mean absolute difference (95% CI of the mean absolute difference between groups or conditions). For log-transformed data, values represent the ratio of geometric means (95% CI for the ratio of geometric means between groups or conditions).
bMain effect of group P ≤ 0.011.
cMain effect of condition P ≤ 0.014.
The time-averaged total AUC for TAG was higher in cigarette smokers than that in nonsmokers in the morning period (0–4 h) (d = 1.02, P = 0.011) and afternoon period (4–8 h) (d = 1.19, P = 0.003). No between-group differences were identified in time-averaged total AUC for NEFA, glucose, and insulin in the morning period (0–4 h) (P ≥ 0.092) or afternoon period (4–8 h) (P ≥ 0.255).
A main effect of condition revealed lower time-averaged total AUC for TAG in the exercise condition than the control condition in the morning period (0–4 h) (d = 0.32, P = 0.005) and afternoon period (4–8 h) (d = 0.35, P = 0.005). The time-averaged total AUC for NEFA was higher in the exercise condition than that in the control condition in the morning period (0–4 h) (d = 0.54, P = 0.014), and the time-averaged total AUC for insulin was lower in the exercise condition than that in the control condition in the afternoon period (d = 0.39, P = 0.008). No other between-condition differences were identified in postprandial metabolite concentrations in the morning (0–4 h) (P ≥ 0.581) or afternoon (4–8 h) (P ≥ 0.353) periods.
The magnitude of reduction in time-averaged total AUC for TAG the day after exercise was greater in nonsmokers than cigarette smokers in the morning period (0–4 h) (−21%, 95% CI = −31% to −10%, d = 0.43, vs −5%, 95% CI = −17% to 9%, d = 0.16, respectively; group–condition interaction P = 0.061). No other group–condition interactions were identified in the morning period (0–4 h) (all P ≥ 0.361) or afternoon period (4–8 h) (all P ≥ 0.162).
Systolic and Diastolic Blood Pressure in the Fasted and Postprandial State
Resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the fasted state were similar between groups and conditions (main effect group, P ≥ 0.211; main effect condition, P ≥ 0.870; group–condition interaction, P ≥ 0.070) (Table 3). The time-averaged total AUC values (0–8 h) for SBP and DBP are displayed in Supplemental Table 1 (Supplemental Digital Content 2, http://links.lww.com/MSS/C202) and are presented graphically in Figure 4. A main effect of condition revealed lower time-averaged total AUC for SBP (d = 0.28, P = 0.038) and DBP (d = 0.32, P = 0.044) in the exercise condition compared with the control condition. The time-averaged total AUC values for SBP and DBP were similar between cigarette smokers and nonsmokers (P ≥ 0.765), and the magnitude of change after exercise was similar in both groups (group–condition interaction P ≥ 0.089).
FIGURE 4.
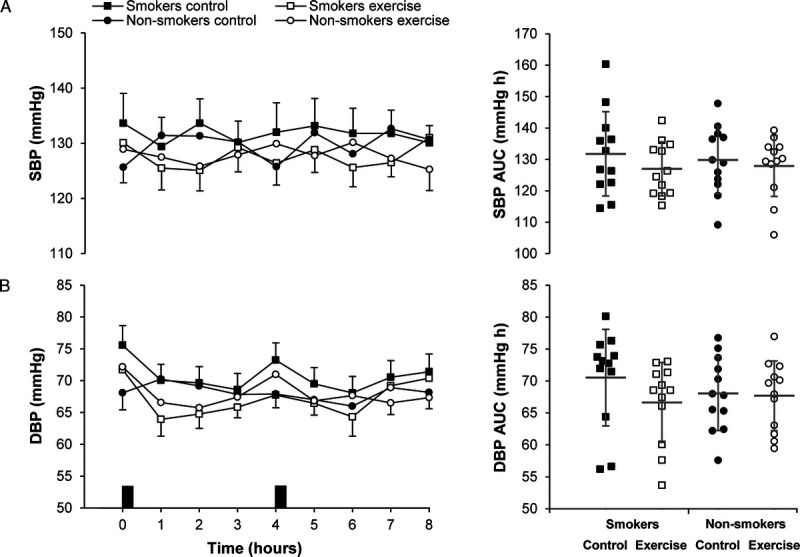
Resting arterial SBP (A) and DBP (B) in the exercise and control conditions for male cigarette smokers (n = 12) and male nonsmokers (n = 12). Data points on left-hand panels represent mean ± SEM. Black rectangles indicate consumption of breakfast and lunch meals. Data points on right-hand panels represent individual data values for time-averaged total area under the curve (AUC) (0–8 h), and the gray solid line (―) indicates the mean ± SD. Linear mixed models for time-averaged total AUC (0–8 h) identified a main effect of condition for SBP (P = 0.038) and DBP (P = 0.044). No main effect of group (all P ≥ 0.765) or group–condition interactions (all P ≥ 0.089) were identified.
DISCUSSION
The primary finding of the present study was that a single bout of moderate-intensity running elicited similar reductions in postprandial TAG, insulin, and resting arterial blood pressure the next day in response to high-fat meals in male cigarette smokers and nonsmokers. The small-to-moderate reduction in postprandial TAG the day after exercise was greater in nonsmokers than cigarette smokers in the early postprandial period. Fasting and postprandial concentrations of TAG and CRP were markedly higher in cigarette smokers than nonsmokers, and postprandial CRP and NEFA were elevated the day after exercise irrespective of smoking status.
In line with previous findings, cigarette smokers exhibited higher fasting TAG, higher fasting LDL-C, and lower fasting HDL-C than nonsmokers (4,17). The impaired fasting lipid and lipoprotein profile in cigarette smokers coincided with a markedly higher postprandial TAG response to high-fat meals compared with nonsmokers. This is consistent with previous studies in individuals with similar smoking behavior to our participants (average 9 cigarettes per day, 5 yr smoking) (16) and in more established heavier cigarette smokers (average 18–23 cigarettes per day, 25–33 yr smoking) (15,17,18) even after 48 h of smoking abstinence (15). The exaggerated postprandial lipemic profile in cigarette smokers has been linked to the accumulation of chylomicrons and its remnants arising through the down-regulation of lipoprotein lipase (LPL)–mediated lipolysis of TAG at target tissues and/or defects in the hepatic removal of lipoprotein remnants (18,35). TAG-rich lipoprotein remnants have been identified in atherosclerotic plaques (13), and cigarette smoking provokes oxidative modification of LDL-C (36). Speculatively, it is possible that increased arterial exposure to chylomicron remnants in addition to (oxidized) LDL-C over prolonged periods may contribute to vascular inflammation and atherosclerotic plaque development in cigarette smokers.
Insulin resistance is a further potential factor implicated in the exaggerated postprandial TAG response in cigarette smokers, with convincing evidence that smokers are more insulin resistant than nonsmokers (5,17). This link is plausible given that hyperinsulinemia induced by insulin resistance is associated with lower skeletal muscle LPL activity (37), and impaired insulin-evoked suppression of hepatic VLDL release is evident with insulin resistance (38). Moreover, nicotine-induced lipolysis in adipose tissue may increase free fatty acid supply for hepatic reesterification to TAG and secretion in VLDL (39). However, we observed no between-group differences in the postprandial insulin, glucose, and NEFA profiles or in the crude indicators of insulin resistance (HOMA-IR and Adipo-IR). Other studies have also not reported the divergent insulin, glucose, or NEFA responses that would support a role for insulin resistance and/or nicotine-induced lipolysis in contributing to the greater postprandial lipemia in cigarette smokers (15,40). Further work is required to elucidate the complex mechanisms that perturb the metabolic milieu in cigarette smokers, which are undoubtedly complicated by the plethora of toxic stimuli delivered by cigarette smoke (41).
It is well documented that acute exercise performed up to 18 h before a high-fat meal reduces postprandial TAG concentrations in nonsmokers (20). The present study corroborates and advances this finding by demonstrating that the transient exercise-induced reduction in postprandial lipemia extends to cigarette smokers. The clinical significance of this finding cannot be determined, but recent data reporting that TAG-lowering omega 3 therapy (using icosapent ethyl) reduced the risk of cardiovascular end points in statin-treated patients with elevated fasting TAG are encouraging (42). Although the mechanisms responsible for the TAG-lowering effect of exercise were not assessed in this study, both the enhanced clearance of TAG by the upregulation of LPL (43) and/or the secretion of fewer TAG-richer VLDL particles from the liver, which have a higher affinity for LPL (44), have been implicated. Despite the encouraging effect of previous day exercise in cigarette smokers, their postprandial TAG profile after exercise remained higher than the control values of the nonsmokers, suggesting that at least acutely exercise does not completely eradicate the greater postprandial TAG response in cigarette smokers.
Interestingly, the benefits of previous day exercise on postprandial lipemia appeared delayed until after the high-fat lunch in cigarette smokers. This prompted a greater exercise-induced reduction in postprandial TAG in nonsmokers during the morning period (0–4 h) despite the cigarette smokers exhibiting higher TAG values. Although the underlying reason is unclear, it is possible that not controlling smoking between the laboratory visits on day 1 and day 2 may interfere with the mechanistic pathways stimulating the TAG-lowering effect of exercise. This represents a limitation of the current study as smoking behavior was not measured during this period so any differences between the conditions could potentially influence the ensuing postprandial responses. Further correlation analysis did not identify any relationship between smoking pack-years and postprandial responses, and subgroup analyses comparing postprandial responses directly between “lighter” (0.3–1.8 pack-years) and “heavier” (2.6–9.8 pack-years) cigarette smokers stratified by the median split of smoking pack-years did not provide any evidence of a smoking intensity effect (data not shown). However, these findings should be interpreted with caution given that the sample comprised a small group of young and relatively novice cigarette smokers, which limits the ability to clearly differentiate smoking intensity. Although further work is required to confirm how cigarette smoking and exercise interact to influence postprandial metabolic health, including in heavier and/or in more established smokers, our findings support the promotion of exercise as a strategy to transiently alleviate the exaggerated postprandial lipemic response in cigarette smokers.
Another important finding from this study was the reduction in postprandial insulin the day after exercise in both groups, which became prominent in the afternoon period (4–8 h). Although favorable reductions in postprandial insulin have been observed the day after exercise in nonsmokers (22), exercise-induced reductions in postprandial insulin, and commensurate changes in postprandial glycaemia, are not reported consistently (21,23,45). The reduction in postprandial TAG and insulin the day after exercise coincided with a lowering of resting SBP and DBP in the postprandial period. This supports previous research in nonsmokers (23) and provides further evidence that the hypotensive effect of acute exercise may persist up to 25.5 h after exercise (46). Collectively, these findings emphasize an important role for exercise in promoting acute postprandial metabolic health benefits in cigarette smokers and nonsmokers.
Chronic low-grade inflammation plays a pivotal role in the etiology of CHD and type 2 diabetes in cigarette smokers (3). This notion is supported by the aberrations in systemic and vascular inflammatory markers accompanying chronic cigarette smoke exposure (6). The elevated fasting CRP and IL-6 and the higher postprandial CRP in the cigarette smokers of this study mirror previous findings and are further indicative of the profound adverse consequences of cigarette smoking (6,47). Our findings also revealed increased fasting and postprandial CRP the day after exercise regardless of smoking status. Acute exercise causes a marked but transient increase in IL-6 (up to ~1 h after exercise), which triggers a cascade of anti-inflammatory responses and is thought to stimulate hepatic CRP synthesis within 24 h of exercise (48,49). Although CRP is mainly proinflammatory, the postexercise increase in CRP may evoke counteracting anti-inflammatory effects through the upregulation of IL-1 receptor antagonists from circulating monocytes (50). However, the importance of this response is uncertain, and acute exercise-induced increases in CRP are not reported universally (22,51).
The antioxidant enzyme PRDX-4 has been associated with disease outcomes in CHD (9,52), and its extracellular concentrations are reflective of endogenous oxidative stress (11) and inflammation (53). Smoking-related oxidative stress can provoke cellular damage to lipids, proteins, and nucleic acids, and it is thought to play a pivotal role in promoting a proatherogenic environment (3). Cigarette smokers exhibit higher concentrations of oxidative stress biomarkers, including F2-isoprostanes (7) and protein carbonyls (8), and the marginally higher fasted PRDX-4 at baseline in the cigarette smokers of this study further supports these findings; however, no other differences in PRDX-4 or SOD3 were evident between the groups or in response to exercise. Given that fasting PRDX-4 and SOD3 have been shown to increase immediately after high- but not moderate-intensity exercise (27), the absence of an exercise effect in this study may reflect the time delay between the exercise bout and blood sampling and/or the intensity of the exercise stimulus.
In conclusion, male cigarette smokers exhibited higher concentrations of postprandial TAG and CRP than male nonsmokers. Acute moderate-intensity exercise reduced postprandial TAG, insulin, and resting arterial blood pressure the next day in cigarette smokers and nonsmokers, albeit the small-to-moderate reduction in postprandial lipemia in the early postprandial period appeared blunted in cigarette smokers. These findings emphasize the ability of acute exercise to augment the postprandial metabolic health of cigarette smokers and nonsmokers.
Supplementary Material
Acknowledgments
The research team would like to thank all the participants who took part in our study for the time and help given throughout. Without their participation, this research would not have been possible. This research was supported by the NIHR Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care.
The authors declare that they have no conflicts of interest. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation and do not constitute endorsement by the American College of Sport Medicine.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
TAREQ F. ALOTAIBI, Email: t.alotaibi@lboro.ac.uk.
ALICE E. THACKRAY, Email: A.E.Thackray@lboro.ac.uk.
MATTHEW J. ROBERTS, Email: M.Roberts3@lboro.ac.uk.
TURKI M. ALANAZI, Email: T.Alanazi@lboro.ac.uk.
NICOLETTE C. BISHOP, Email: N.C.Bishop@lboro.ac.uk.
ALEX J. WADLEY, Email: A.J.Wadley@bham.ac.uk.
JAMES A. KING, Email: J.A.King@lboro.ac.uk.
EMMA O’DONNELL, Email: E.ODonnell@lboro.ac.uk.
MICHAEL C. STEINER, Email: ms346@leicester.ac.uk.
SALLY J. SINGH, Email: sally.singh@uhl-tr.nhs.uk.
REFERENCES
- 1.Mons U Müezzinler A Gellert C, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. Br Med J. 2015;350:h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–64. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–7. [DOI] [PubMed] [Google Scholar]
- 4.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. Br Med J. 1989;298:784–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339(8802):1128–30. [DOI] [PubMed] [Google Scholar]
- 6.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89(9):1117–9. [DOI] [PubMed] [Google Scholar]
- 7.Morrow JD Frei B Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. [DOI] [PubMed] [Google Scholar]
- 8.Pignatelli B Li CQ Boffetta P, et al. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer Res. 2001;61(2):778–84. [PubMed] [Google Scholar]
- 9.Abbasi A Corpeleijn E Postmus D, et al. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J Am Heart Assoc. 2012;1(5):e002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Z, Spaulding HR. Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol. 2020;32:101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte J, Struck J, Köhrle J, Müller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35:460–5. [DOI] [PubMed] [Google Scholar]
- 12.Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 13.Chapman MJ Ginsberg HN Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes MV Asselbergs FW Palmer TM, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Axelsen M, Eliasson B, Joheim E, Lenner RA, Taskinen MR, Smith U. Lipid intolerance in smokers. J Intern Med. 1995;237:449–55. [DOI] [PubMed] [Google Scholar]
- 16.Bloomer RJ, Solis AD, Fisher-Wellman KH, Smith WA. Postprandial oxidative stress is exacerbated in cigarette smokers. Br J Nutr. 2008;99:1055–60. [DOI] [PubMed] [Google Scholar]
- 17.Eliasson B, Mero N, Taskinen MR, Smith U. The insulin resistance syndrome and postprandial lipid intolerance in smokers. Atherosclerosis. 1997;129:79–88. [DOI] [PubMed] [Google Scholar]
- 18.Mero N, Syvänne M, Eliasson B, Smith U, Taskinen MR. Postprandial elevation of ApoB-48-containing triglyceride-rich particles and retinyl esters in normolipemic males who smoke. Arterioscler Thromb Vasc Biol. 1997;17(10):2096–102. [DOI] [PubMed] [Google Scholar]
- 19.Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health. 2010;32:71–82. [DOI] [PubMed] [Google Scholar]
- 20.Freese EC, Gist NH, Cureton KJ. Effect of prior exercise on postprandial lipemia: an updated quantitative review. J Appl Physiol. 2014;116:67–75. [DOI] [PubMed] [Google Scholar]
- 21.Arjunan SP, Bishop NC, Reischak-Oliveira A, Stensel DJ. Exercise and coronary heart disease risk markers in South Asian and European men. Med Sci Sports Exerc. 2013;45:1261–8. [DOI] [PubMed] [Google Scholar]
- 22.Arjunan SP Deighton K Bishop NC, et al. The effect of prior walking on coronary heart disease risk markers in South Asian and European men. Eur J Appl Physiol. 2015;115:2641–51. [DOI] [PubMed] [Google Scholar]
- 23.Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of brisk walking reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr. 2008;88:1225–31. [DOI] [PubMed] [Google Scholar]
- 24.Hedblad B, Ogren M, Isacsson SO, Janzon L. Reduced cardiovascular mortality risk in male smokers who are physically active. Results from a 25-year follow-up of the prospective population study men born in 1914. Arch Intern Med. 1997;157:893–9. [PubMed] [Google Scholar]
- 25.Borg GA. Perceived exertion: a note on “history” and methods. Med Sci Sports. 1973;5(2):90–3. [PubMed] [Google Scholar]
- 26.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34. [DOI] [PubMed] [Google Scholar]
- 27.Wadley AJ Keane G Cullen T, et al. Characterization of extracellular redox enzyme concentrations in response to exercise in humans. J Appl Physiol (1985). 2019;127(3):858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottfredsen RH Tran SM-H Larsen UG, et al. The C-terminal proteolytic processing of extracellular superoxide dismutase is redox regulated. Free Radic Biol Med. 2012;52(1):191–7. [DOI] [PubMed] [Google Scholar]
- 29.Iversen MB Gottfredsen RH Larsen UG, et al. Extracellular superoxide dismutase is present in secretory vesicles of human neutrophils and released upon stimulation. Free Radic Biol Med. 2016;97:478–88. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 31.Gastaldelli A Cusi K Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. [DOI] [PubMed] [Google Scholar]
- 32.Narang BJ, Atkinson G, Gonzalez JT, Betts JA. A tool to explore discrete-time data: the time series response analyser. Int J Sports Nutr Ex Metab. 2020;1–8. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 34.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “P < 0.05.” Am Stat. 2019;73:1–19. [Google Scholar]
- 35.Sharrett AR Ballantyne CM Coady SA, Atherosclerosis Risk in Communities Study Group, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2001;104:1108–13. [DOI] [PubMed] [Google Scholar]
- 36.Harats D, Ben-Naim M, Dabach Y, Hollander G, Stein O, Stein Y. Cigarette smoking renders LDL susceptible to peroxidative modification and enhanced metabolism by macrophages. Atherosclerosis. 1989;79(2–3):245–52. [DOI] [PubMed] [Google Scholar]
- 37.Pollare T, Vessby B, Lithell H. Lipoprotein lipase activity in skeletal muscle is related to insulin sensitivity. Arterioscler Thromb. 1991;11:1192–203. [DOI] [PubMed] [Google Scholar]
- 38.Malmström R Packard CJ Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40(4):454–62. [DOI] [PubMed] [Google Scholar]
- 39.Hellerstein MK Benowitz NL Neese RA, et al. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J Clin Invest. 1994;93:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grøndahl MF Bagger JI Lund A, et al. Effects of smoking versus nonsmoking on postprandial glucose metabolism in heavy smokers compared with nonsmokers. Diabetes Care. 2018;41:1260–7. [DOI] [PubMed] [Google Scholar]
- 41.Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. 2nd ed. Boca Raton (FL): CRC Press; 2013. [Google Scholar]
- 42.Bhatt DL Steg PG Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 43.Gill JM, Herd SL, Vora V, Hardman AE. Effects of a brisk walk on lipoprotein lipase activity and plasma triglyceride concentrations in the fasted and postprandial states. Eur J Appl Physiol. 2003;89(2):184–90. [DOI] [PubMed] [Google Scholar]
- 44.Ghafouri K, Cooney J, Bedford DK, Wilson J, Caslake MJ, Gill JMR. Moderate exercise increases affinity of large very low-density lipoproteins for hydrolysis by lipoprotein lipase. J Clin Endocrinol Metab. 2015;100(6):2205–13. [DOI] [PubMed] [Google Scholar]
- 45.Trombold JR, Christmas KM, Machin DR, Kim IY, Coyle EF. Acute high-intensity endurance exercise is more effective than moderate-intensity exercise for attenuation of postprandial triglyceride elevation. J Appl Physiol. 2013;114:792–800. [DOI] [PubMed] [Google Scholar]
- 46.Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. J Appl Physiol. 2017;122:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEvoy JW Nasir K DeFilippis AP, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–15. [DOI] [PubMed] [Google Scholar]
- 49.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–62. [DOI] [PubMed] [Google Scholar]
- 50.Pue CA, Mortensen RF, Marsh CB, Pope HA, Wewers MD. Acute phase levels of C-reactive protein enhance IL-1 beta and IL-1ra production by human blood monocytes but inhibit IL-1 beta and IL-1ra production by alveolar macrophages. J Immunol. 1996;156(4):1594–600. [PubMed] [Google Scholar]
- 51.Markovitch D, Tyrrell RM, Thompson D. Acute moderate-intensity exercise in middle-age men has neither an anti- nor proinflammatory effect. J Appl Physiol. 2008;105:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knoops B, Argyropoulou V, Becker S, Ferté L, Kuznetsova O. Multiple roles of peroxiredoxins in inflammation. Mol Cells. 2016;39:60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipinski S Pfeuffer S Arnold P, et al. Prdx4 limits caspase-1 activation and restricts inflammasome-mediated signaling by extracellular vesicles. EMBO J. 2019;38(20):e101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


