Abstract
Objective:
It is unclear whether uterine fibroids are associated with the occurrence of hypertensive disorders of pregnancy (HDP). Thus, this study aimed to evaluate the association between uterine fibroids and HDP in a prospective cohort.
Methods:
Overall, 2404 pregnant women who received antenatal care were enrolled in a prospective cohort in China between 2014 and 2016; 2277 women met the inclusion criteria of this study. The clinical characteristics of participants were assessed via questionnaires and physical examinations at baseline (before the 20th week of gestation), 21st–27th, 28th–34th, and 35th–39th gestational weeks. Ultrasound examination was performed before the 20th week of pregnancy to determine the presence of uterine fibroids. Linear mixed-effect and Cox proportional hazard regression models were used to analyze the association of uterine fibroids with blood pressure and HDP.
Results:
Of 2277 pregnant women, 242 (10.6%) had uterine fibroids, and 45 (2.0%) subsequently developed HDP. The incidence of HDP in women with and without uterine fibroids was 5% (n = 12) and 1.6% (n = 33), respectively. The longitudinal SBPs and DBPs were significantly higher in women with uterine fibroids than in those without. The multivariable Cox model showed that the presence of uterine fibroids was associated with increased HDP risk (adjusted hazard radio: 2.95, 95% confidence interval: 1.35–6.44).
Conclusion:
Uterine fibroids in early pregnancy were associated with an increased HDP risk. Blood pressure of women with uterine fibroids should be closely monitored, and HDP preventive measures are crucial.
Keywords: hypertensive disorders of pregnancy, leiomyoma, preeclampsia, prospective studies
INTRODUCTION
Hypertensive disorder of pregnancy (HDP) affects 6–8% of pregnant women, and remains a major cause of high maternal and perinatal morbidity and mortality worldwide [1–4]. Compared with pregnant women without HDP, women with gestational hypertension have a 1.4-to-2.2-fold increased risk of obstetric complications during pregnancy, and the risk is higher in those with severe preeclampsia [5]. HDP can induce severe maternal complications, such as cardiocerebrovascular diseases, liver and kidney failure, disseminated intravascular coagulation, and pulmonary edema. Because of the placenta dysfunction, there is also increased risk of abortion, preterm birth, fetal growth restriction, fetal distress and stillbirth in HDP [6]. Early identification of high-risk pregnant women with HDP allows intensive monitoring and interventions to reduce the incidence of obstetric complications.
The prevalence of uterine fibroids in women of childbearing age and in pregnant women ranges from 20 to 40% and 1 to 10%, respectively [7–9]. Studies suggest that the presence of uterine fibroids is associated with increased blood pressure (BP) level, and the prevalence of hypertension in women with uterine fibroids has been shown to be as high as 40% [10–12]. Several studies indicated that women with hypertension have a higher risk for fibroids [10,13,14]. In contrast, the risk of hypertension is greater in women with fibroids than in those without fibroids [12,15–17]. Uterine fibroids are usually asymptomatic and are often discovered during an ultrasound examination performed in early pregnancy [18]. Under rapid hormonal changes, notable fibroid growth is observed during the first trimester of pregnancy [19], leading to an increased incidence of obstetric complications [20]. However, it is unclear whether uterine fibroids are associated with the occurrence of HDP. Considering the similar pathogeneses of hypertension and HDP, we hypothesized that uterine fibroids would increase the risk of HDP.
Therefore, we examined the relationship of uterine fibroids diagnosed before the 20th week of pregnancy with a longitudinal BP pattern and the risk of HDP based on a large prospective cohort established in China between 2014 and 2016.
METHODS
This prospective cohort enrolled pregnant women who received antenatal care in the First Affiliated Hospital of Shantou University Medical College in Shantou, China. The study enrolled women aged 20–45 years who were confirmed to be pregnant via ultrasound before the 20th week of gestation. The exclusion criteria were: ectopic pregnancy, history of hypertension or diabetes, secondary hypertension, and severe mental disorders. A total of 2404 pregnant women who met the criteria were enrolled between March 2014 and June 2016. All the pregnant women were followed up at 21st–27th, 28th–34th, and 35th–39th gestational weeks. Baseline and follow-up details were collected using structural questionnaires, which included demographic data, physical and laboratory examination, and individual and family medical histories. In order to examine the causal relationship between uterine fibroids and HDP, we also excluded participants with SBP at least 140 mmHg or DBP at least 90 mmHg before the 20th week of gestation, history of HDP or gestational diabetes mellitus, miscarriage before the 20th week of gestation, and those who had undergone in-vitro fertilization. The design framework of this study is shown in Fig. 1. The study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College and all participants provided written informed consent prior to inclusion in this study.
FIGURE 1.
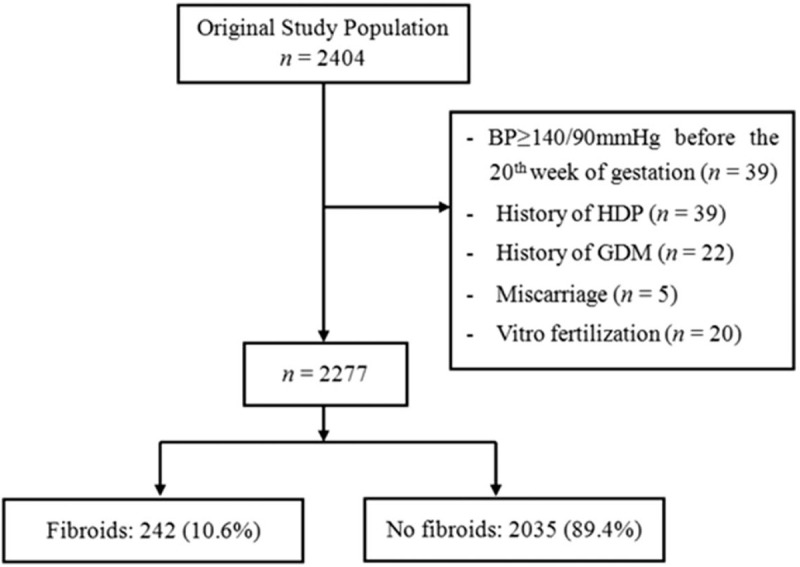
Designed framework of the study. Flow diagram showing selection of study population, sample size, and exclusions. BP, blood pressure; GDM, gestational diabetes mellitus; HDP, hypertensive disorder of pregnancy.
BP was measured thrice by trained nurses at baseline and at each follow-up, using an Omron HEM-7052 automatic BP monitor (Omron Healthcare Ltd., Dalian, China) according to the standard measurement procedure recommended by the American Heart Association [21]. An average of three BP readings was used for analysis.
Other parameters, including maternal age, baseline BP, fasting plasma glucose, monthly per capita income, educational level, nulliparity (yes or no), folic acid supplement intake during pregnancy (yes or no), family history of hypertension (yes or no), family history of diabetes mellitus (yes or no), smoking (yes or no), alcohol consumption (yes or no), weight, and height were obtained by a questionnaire. The weight and height were used to calculate BMI.
Maternal outcomes were obtained from medical records. In this study, HDP included gestational hypertension and preeclampsia. Gestational hypertension was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg without proteinuria, which develops after 20 weeks of gestation. Preeclampsia was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg after 20 weeks of gestation and proteinuria (defined as ≥300 mg of protein in a 24-h urine specimen or ≥1+ in two random urine samples collected at least 4 h apart) [22].
All ultrasound measurements were performed by an experienced operator before the 20th week of gestation. Women with uterine fibroids were defined as those for whom ultrasonography examination revealed at least one fibroid.
Continuous variables are expressed as mean ± standard deviation, and categorical variables as absolute numbers with percentage. Chi-square tests and t tests were used for group comparisons. Linear mixed-effect regression models were used to evaluate the effect of uterine fibroids on the changing profile of SBP and DBP during pregnancy. This regression technique accommodates time-dependent and time-independent covariates. The variables, gestational age and gestational age squared, were included in linear mixed-effects regression models to fit the quadratic function of the association of BP with time [23]. Gestational age was treated in the models both as a random and fixed effect, whereas other covariates including maternal age, BMI, nulliparity, income, education, folic acid supplement, smoking, alcohol consumption, and family history of hypertension and diabetes were treated as fixed effects. The results are expressed as regression coefficients [95% confidence interval (CI)]. Three models adjusted for potential confounders were used to examine the association between uterine fibroids and BP pattern: model 1 was adjusted for gestational age (linear and quadratic terms). Model 2 was adjusted for gestational age, maternal age, baseline BMI, and educational level. Model 3 was further adjusted for monthly per capita income, nulliparity, folic acid supplement intake during pregnancy, smoking, alcohol consumption, family history of hypertension, and family history of diabetes. This analysis strategy provides verifications of whether the association between uterine fibroids and BP change during pregnancy is independent of other covariates. The curves of longitudinal BP change with gestational age were estimated using quadratic regression function as the pattern of variation of SBP and DBP during pregnancy approaches a parabola [24].
The cumulative incidence of HDP was estimated using the Kaplan–Meier method and compared using the log-rank test. Three Cox proportional hazards regression models were constructed to determine the independent predictive value of uterine fibroids for HDP. Model 1 was unadjusted, whereas model 2 was adjusted for maternal age, baseline BMI and educational level, family history of hypertension, family history of diabetes, and baseline SBP and DBP. Model 3 was further adjusted for folic acid supplement intake during pregnancy, nulliparity, monthly per capita income, smoking, alcohol consumption, and fasting plasma glucose. Furthermore, to assess the relationship of uterine fibroids with the severity of HDP, we classified women with HDP into gestational hypertension or preeclampsia group, and performed ordinal logistic regression using the fully adjusted model. Statistical analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA). Two-tailed P values less than 0.05 were considered statistically significant.
RESULTS
After exclusion, 2277 participants were analyzed. The baseline characteristics of this study are shown in Table 1. The mean gestational week of enrollment and ultrasound examination was 13.4 ± 3.4 and 10.4 ± 3.6, respectively. The mean maternal age and baseline BMI were 30.5 ± 4.5 years and 20.5 ± 3.0 kg/m2, respectively. Of these 2277 pregnant women, 242 (10.6%) were identified with uterine fibroids before the 20th week of gestation. Forty-five (2.0%) pregnant women subsequently developed HDP during follow-up. The incidence of HDP in women with uterine fibroids and without uterine fibroids was 5.0% (n = 12) and 1.6% (n = 33), respectively.
TABLE 1.
Characteristics of participants according to fibroid status, from 2014 to 2016, China
| Characteristics | Total (n = 2277) | No fibroid (n = 2035) | Fibroid (n = 242) | P value |
| Maternal age (years) | 30.5 ± 4.5 | 30.3 ± 4.4 | 32.3 ± 5.0 | <0.001 |
| BMI (kg/m2) | 20.5 ± 3.0 | 20.4 ± 3.0 | 21.5 ± 3.1 | <0.001 |
| Monthly per capita income (yuan) | ||||
| <3000 | 934 (41.0) | 840 (41.3) | 94 (38.8) | 0.628 |
| 3000–5000 | 722 (31.7) | 646 (31.7) | 76 (31.4) | |
| >5000 | 621 (27.3) | 549 (27.0) | 72 (29.8) | |
| Educational level | ||||
| Below high school | 488 (21.4) | 451 (22.2) | 37 (15.3) | 0.031 |
| High school | 520 (22.8) | 466 (22.9) | 54 (22.3) | |
| Beyond high school | 1269 (55.7) | 1118 (54.9) | 151 (62.4) | |
| Nulliparity | 1022 (44.9) | 927 (45.6) | 95 (39.3) | 0.063 |
| Folic acid supplement | 1592 (69.9) | 1419 (69.7) | 173 (71.5) | 0.573 |
| Family history of hypertension | 424 (18.6) | 371 (18.2) | 53 (21.9) | 0.166 |
| Family history of diabetes mellitus | 300 (13.2) | 259 (12.7) | 41 (16.9) | 0.067 |
| SH-smoking | 1169 (51.3) | 1032 (50.7) | 137 (56.6) | 0.083 |
| Smoking | 47 (2.1) | 42 (2.1) | 5 (2.1) | 0.998 |
| Alcohol | 609 (26.7) | 549 (27.0) | 60 (24.8) | 0.468 |
| SBP | 109.5 ± 10.2 | 109.3 ± 10.0 | 111.3 ± 11.0 | 0.004 |
| DBP | 68.8 ± 8.1 | 68.7 ± 8.1 | 69.7 ± 8.3 | 0.061 |
| FPG | 4.3 ± 1.3 | 4.3 ± 1.3 | 4.3 ± 1.3 | 0.625 |
Data are presented as means ± standard deviation or number (percentage). FPG, fasting plasma glucose; HDP, hypertensive disorders of pregnancy; SH-smoking, second hand smoking during pregnancy.
Figure 2 shows the longitudinal patterns of SBP and DBP change during pregnancy according to the status of uterine fibroids. During pregnancy, both SBP and DBP began to decline in early pregnancy; a trough occurred in mid-pregnancy and then increased until delivery. Women with fibroids had higher SBP and DBP than those without fibroids. The distribution of maternal BP throughout pregnancy is shown in Figure S1. Table 2 shows the longitudinal association of fibroids with SBP and DBP change during pregnancy, before and after controlling for potential confounding factors. Fully adjusted models suggested that there was a significantly higher longitudinal SBP (regression coefficient: 1.89; 95% CI: 0.66–3.12; P = 0.003) and DBP (regression coefficient: 0.98; 95% CI: 0.02–1.94; P = 0.047) in uterine fibroids group.
FIGURE 2.
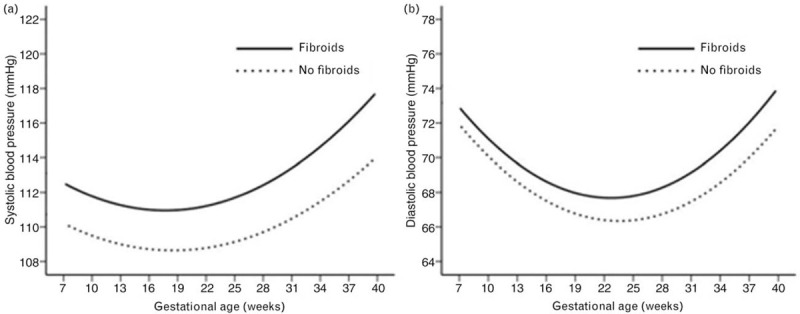
Longitudinal trend of blood pressure changes during pregnancy. Longitudinal trend of SBP (a) and DBP (b) change with gestational age versus uterine fibroids (n = 242 in fibroid group and n = 2035 in non fibroid group).
TABLE 2.
Longitudinal Associations of SBP and DBP levels during pregnancy with uterine fibroids
| SBP | DBP | ||||||
| Model | Covariates in model | β for fibroid | 95% CI | P value | β for fibroid | 95% CI | P value |
| 1 | Gestational age | 2.66 | 1.42–3.90 | <0.001 | 1.51 | 0.55–2.48 | 0.002 |
| 2 | Gestational age, age, BMI, education | 1.97 | 0.73–3.21 | 0.002 | 1.03 | 0.07–2.00 | 0.036 |
| 3 | Gestational age, age, BMI, income, education, Fa, Nullip, Smoking, Alcohol, fHP, fDM | 1.89 | 0.66–3.12 | 0.003 | 0.98 | 0.02–1.94 | 0.047 |
Values were based on linear mixed-effects regression models and reflect the difference in maternal BP between the uterine fibroids group and the reference group. Results were regression coefficients (95% confidence interval), and it indicates that its of statistical significant when 95% CI does not contain 0. β, regression coefficient; CI, confidence interval; education, educational level; Fa, folic acid supplement intake during pregnancy; fDM, family history of diabetes mellitus; fHP, family history of hypertension; income, monthly per capita income; Nullip, nulliparous; alcohol, alcohol consumption.
Kaplan–Meier curve analysis showed that the cumulative incidence of HDP was significantly higher in women with uterine fibroids (Fig. 3). To clarify the association between uterine fibroids and the risk of HDP, Cox proportional hazards models were constructed (Table 3). An unadjusted model revealed that women with uterine fibroids had a 2.57-fold increased risk of HDP compared with women without uterine fibroids (hazard ratio: 3.57; 95% CI: 1.83–6.94; P < 0.001) (model 1). After adjustment for maternal age, baseline BMI, educational level, family history of hypertension and diabetes, and baseline SBP and DBP, the presence of uterine fibroids was still associated with high risk of HDP (hazard radio: 3.12; 95% CI: 1.55–6.27; P = 0.001) (model 2). Finally, a fully adjusted model including all variables yielded similar results (hazard radio: 2.95; 95% CI: 1.35–6.44; P = 0.007) (model 3). Additionally, ordinal logistic regression model showed that compared with no fibroids, women with fibroids were 2.4 times more likely to have at least one grade increase in HDP severity (Table 4).
FIGURE 3.
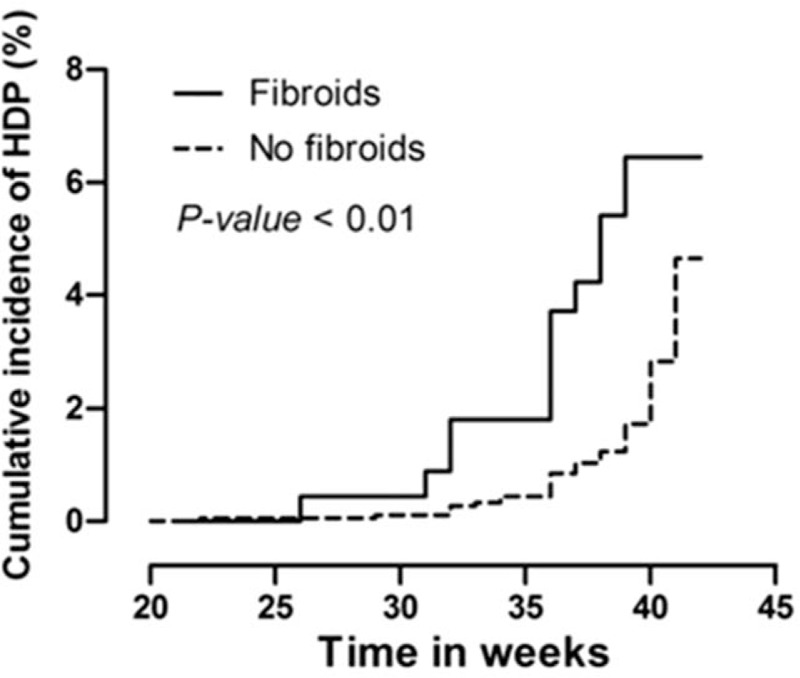
Kaplan–Meier curve of cumulative incidence of hypertensive disorder of pregnancy. Kaplan–Meier curve for hypertensive disorders of pregnancy (HDP) women by the status of uterine fibroids. P value was generated based on the log-rank test (n = 242 in fibroid group and n = 2035 in non fibroid group).
TABLE 3.
Associations of the risk of hypertensive disorders of pregnancy with uterine fibroids
| Model | Covariates in model | HR for fibroid | 95% CI | P-value |
| 1 | Null | 3.57 | 1.83–6.94 | <0.001 |
| 2 | Age, BMI, Education, fHP, fDM, SBP, DBP | 3.12 | 1.55–6.27 | 0.001 |
| 3 | Model 2 + Fa, Nullip, Income, Smoking, Alcohol, FPG | 2.95 | 1.35–6.44 | 0.007 |
Results were showed as hazards ratio (95% confidence interval) derived from Cox proportional-hazards model. alcohol, alcohol consumption; HR, hazards radio; CI, confidence interval; Fa, folic acid supplement intake during pregnancy; fHP, family history of hypertension; fDM, family history of diabetes mellitus; FPG, fasting plasma glucose; income, monthly per capita income; Nullip, nulliparous.
TABLE 4.
The relationship of uterine fibroid with the severity of hypertensive disorders of pregnancy
| Fibroid | OR | 95% CI | P value |
| No fibroid | Reference | Reference | Reference |
| Fibroid | 2.43 | 1.09, 5.42 | 0.029 |
Results were showed as odds ratio (95% confidence interval) derived from Ordinal logistic regression (fully adjusted model).
DISCUSSION
HDP is a risk factor for severe pregnancy outcomes, and it is beneficial to identify pregnant women with a high risk of HDP. In this large prospective cohort study, we found that the presence of uterine fibroids was associated with increased longitudinal SBP and DBP in pregnant women. Compared with those without fibroids, women with uterine fibroids diagnosed before the 20th week of gestation had a higher risk for HDP during pregnancy. These results may contribute to early detection of women who are at high risk for HDP.
Previous studies showed that the prevalence of uterine fibroids increased with increasing premenopausal age [25]. One possible mechanism is related to the hormonal changes associated with increasing age [26]. Increased BMI may increase the risk of uterine fibroids through inflammatory mechanisms [27,28]. Additionally, central obesity, insulin resistance and hyperlipidemia, which were related to BMI, were all associated with a higher risk of uterine fibroids [29]. As expected, participants in the fibroids group were older and present a statistically higher BMI in our study. However, the association between uterine fibroids and the increased risk of HDP remained significant after adjusting for age, BMI, and other confounders.
Consistent with the study by Farias et al.[24] that included 199 healthy pregnant women, our large sample analysis shows that during pregnancy, both SBP and DBP notably decrease from early pregnancy to mid-pregnancy but subsequently increase until delivery. A case–control study showed that the level of BP was higher in the uterine fibroid group than in the control group [17], and we demonstrated a similar result in pregnant women. Previous studies have suggested a relationship between uterine fibroids and hypertension [12–17]. Findings from cross-sectional and case–control studies indicate that women with hypertension are at higher risk of developing fibroids [10,13,14]. Moreover, a prospective study by Boynton–Jarrett et al. found that for every 10 mmHg increase in DBP, the fibroid risk would be increased by approximately 10% [11]. In contrast, several studies have indicated that the risk of hypertension is greater in women with fibroids than in those without fibroids [15–17]. In a recent case–control study, Haan et al.[12] reported a notably high risk of hypertension in women with fibroids after adjusting for several cardiovascular disease risk factors. Regarding the association of uterine fibroids with HDP in pregnant women, our research could make a significant contribution to this field. Our study was based on a large prospective cohort and suggested a causal relationship between uterine fibroids and the risk of HDP. According to our study, pregnant women with uterine fibroids should be classified as high-risk for HDP. Thus, closer BP monitoring and HDP preventive measures, such as those recommended by LeFevre et al.[30], become necessary.
The incidence of HDP was lower in our cohort than in cohorts of other studies [3,6]. The reason for this could be the exclusion of some risk factors for HDP in our cohort, such as a history of hypertension, history of diabetes, and an elevated BP level before the 20th week of pregnancy. This strategy, combined with adjusting for several known risk factors, aimed to better explain the causal relationship between uterine fibroids and HDP. It is still unclear how uterine fibroids induce HDP. Poorly perfused placenta in early pregnancy is an important stage in the development of HDP [31]. Therefore, it is reasonable to speculate that the expansion of fibroids in early pregnancy may cause poor placental perfusion by compressing uterine blood vessels, leading to an increased risk of HDP. Combined with these effect, several molecules secreted from fibroids, such as thromboxane A2 and endothelin-1 [32,33], which are associated with endothelial dysfunction by inducing inflammation and oxidative stress response, may be other risk factors for HDP [34]. These suggest that the mechanism of HDP induction by uterine fibroids requires further study.
This study had some limitations that warrant discussion. First, in this pregnancy cohort, we did not have prepregnancy measurements, such as BMI and BP, and the history of hypertension or diabetes was based on self-reporting. However, the participants included in this study were aged 20–45 years without severe mental disorders, and had normal communication, expression, and cognition abilities, so the recall bias for the history of hypertension and diabetes should be relatively small. Second, a dose–response analysis based on the size, type, and number of uterine fibroids was not performed owing to the relatively small sample of HDP cases available. Third, since the design was observational, we can only speculate on the possible pathogeneses of HDP caused by uterine fibroids where the precise molecular mechanisms still remain unclear. However, our study contributes to the literature as it provides new evidence on the causal relationship between uterine fibroids and the occurrence of HDP. To our knowledge, this is the first study to evaluate the association of uterine fibroids with HDP in a large prospective cohort.
In conclusion, this prospective study of pregnant women suggests that women with uterine fibroids identified during early pregnancy have a high risk of HDP, even after adjusting for multiple confounding factors. Considering the adverse effects of HDP on pregnancy, pregnant women with uterine fibroids should have their BP closely monitored, and the preventive measures for HDP are necessary.
ACKNOWLEDGEMENTS
Participants involved in this study for their critical contributions.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding sources: The study was funded in part by grants from Key Disciplinary Project of Clinical Medicine under the Guangdong High-level University Development Program; Guangdong University Innovation Team Project (Nature) (2019KCXTD003); 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG19B); ‘Dengfeng Project’ for the construction of high-level hospitals in Guangdong Province–the First Affiliated Hospital of Shantou University Medical College Supporting Funding; Funding for Guangdong Medical Leading Talent, the First Affiliated Hospital, SUMC.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Yequn Chen, Mengyue Lin, Pi Guo contributed equally to this work.
Abbreviations: BP, blood pressure; CI, confidence interval; Fa, folic acid supplement intake during pregnancy; fDM, family history of diabetes mellitus; fHP, family history of hypertension; FPG, fasting plasma glucose; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy
Supplemental digital content is available for this article.
REFERENCES
- 1.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens 2008; 21:521–526. [DOI] [PubMed] [Google Scholar]
- 2.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Preeclampsia. The Lancet 2016; 387:999–1011. [DOI] [PubMed] [Google Scholar]
- 3.Naderi S, Tsai SA, Khandelwal A. Hypertensive disorders of pregnancy. Curr Atheroscler Rep 2017; 19:15. [DOI] [PubMed] [Google Scholar]
- 4.Souza JP, Gulmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013; 381:1747–1755. [DOI] [PubMed] [Google Scholar]
- 5.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009; 113:1299–1306. [DOI] [PubMed] [Google Scholar]
- 6.Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One 2014; 9:e100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy an ultrasound-screening study. Obstet Gynecol 2009; 113:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drayer SM, Catherino WH. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int J Gynaecol Obstet 2015; 131:117–122. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, Wang X, Zou L, Li G, Chen Y, Li C, et al. Adverse obstetric outcomes in pregnant women with uterine fibroids in China: a multicenter survey involving 112,403 deliveries. PLoS One 2017; 12:e0187821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol 2001; 153:11–19. [DOI] [PubMed] [Google Scholar]
- 11.Boynton-Jarrett R, Rich-Edwards J, Malspeis S, Missmer SA, Wright R. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol 2005; 161:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haan YC, Diemer FS, Van Der Woude L, Van Montfrans GA, Oehlers GP, Brewster LM. The risk of hypertension and cardiovascular disease in women with uterine fibroids. J Clin Hypertens (Greenwich) 2018; 20:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parazzini F, Chiaffarino F, Polverino G, Chiantera V, Surace M, La Vecchia C. Uterine fibroids risk and history of selected medical conditions linked with female hormones. Eur J Epidemiol 2004; 19:249–253. [DOI] [PubMed] [Google Scholar]
- 14.Radin RG, Rosenberg L, Palmer JR, Cozier YC, Kumanyika SK, Wise LA. Hypertension and risk of uterine leiomyomata in US black women. Hum Reprod 2012; 27:1504–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luoto R, Rutanen EM, Auvinen A, Fibroids hypertension. A cross-sectional study of women undergoing hysterectomy. J Reprod Med 2001; 46:359–364. [PubMed] [Google Scholar]
- 16.Sivri N, Yalta T, Sayin C, Yalta K, Ozpuyan F, Tastekin E, Yetkin E. Evaluation of cardiovascular risk factors in women with uterine leimyoma: is there a link with atherosclerosis? Balkan Med J 2012; 29:320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haan YC, Oudman I, de Lange ME, Timmermans A, Ankum WM, van Montfrans GA, et al. Hypertension risk in Dutch women with symptomatic uterine fibroids. Am J Hypertens 2015; 28:487–492. [DOI] [PubMed] [Google Scholar]
- 18.De Vivo A, Mancuso A, Giacobbe A, Savasta LM, De Dominici R, Dugo N, et al. Uterine myomas during pregnancy: a longitudinal sonographic study. Ultrasound Obstet Gynecol 2011; 37:361–365. [DOI] [PubMed] [Google Scholar]
- 19.Chill HH, Karavani G, Rachmani T, Dior U, Tadmor O, Shushan A. Growth pattern of uterine leiomyoma along pregnancy. BMC Womens Health 2019; 19:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz VL, Dotters DJ, Droegemeuller W. Complications of uterine leiomyomas in pregnancy. Obstet Gynecol 1989; 73:593–596. [PubMed] [Google Scholar]
- 21.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, et al. Council on High Blood Pressure Research Professional and Public Education Subcommittee, American Heart Association. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005; 7:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts JM, Pearson G, Cutler J, Lindheimer M. NHLBI Working Group on Research on Hypertension During Pregnancy. Pregnancy, summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 2003; 41:437–445. [DOI] [PubMed] [Google Scholar]
- 23.Kac G, Mendes RH, Farias DR, Eshriqui I, Rebelo F, Benaim C, et al. Hepatic, renal and inflammatory biomarkers are positively associated with blood pressure changes in healthy pregnant women: a prospective cohort. Medicine (Baltimore) 2015; 94:e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farias DR, Franco-Sena AB, Rebelo F, Schlussel MM, Salles GF, Kac G. Total cholesterol and leptin concentrations are associated with prospective changes in systemic blood pressure in healthy pregnant women. J Hypertens 2014; 32:127–134. [DOI] [PubMed] [Google Scholar]
- 25.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997; 90:967–973. [DOI] [PubMed] [Google Scholar]
- 26.Rice KE, Secrist JR, Woodrow EL, Hallock LM, Neal JL. Etiology, diagnosis, and management of uterine leiomyomas. J Midwifery Womens Health 2012; 57:241–247. [DOI] [PubMed] [Google Scholar]
- 27.Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol 2016; 59:2–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Protic O, Toti P, Islam MS, Occhini R, Giannubilo SR, Catherino WH, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res 2016; 364:415–427. [DOI] [PubMed] [Google Scholar]
- 29.Tak YJ, Lee SY, Park SK, Kim YJ, Lee JG, Jeong DW, et al. Association between uterine leiomyoma and metabolic syndrome in parous premenopausal women: a case-control study. Medicine (Baltimore) 2016; 95:e5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeFevre ML. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 161:819–826. [DOI] [PubMed] [Google Scholar]
- 31.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009; 30: Suppl A: S32–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace K, Chatman K, Porter J, Scott J, Johnson V, Moseley J, LaMarca B. Enodthelin 1 is elevated in plasma and explants from patients having uterine leiomyomas. Reprod Sci 2014; 21:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vainio M, Riutta A, Koivisto AM, Mäenpää J. Prostacyclin, thromboxane A and the effect of low-dose ASA in pregnancies at high risk for hypertensive disorders. Acta Obstet Gynecol Scand 2004; 83:1119–1123. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2008; 294:H541–H550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


