Abstract
Purpose of review
Excessive synaptic pruning has first been suggested by Irwin Feinberg (1982) as an important pillar in the pathophysiology in schizophrenia (SCZ). This article reviews recent developments highlighting factors implicated in aberrant synaptic pruning and its contribution to disease onset and emergence of cognitive symptoms in SCZ. Unraveling these factors provides new insights for potential prevention and treatment strategies for psychotic disorders.
Recent findings
Increased pruning in SCZ was recently confirmed by a positron emission tomography-study employing the novel tracer [11C]UCB-J, demonstrating the consequential loss of synaptic density. Recent evidence supports the contributing role of astrocytes and increased complement-mediated microglial pruning in disease onset and cognitive symptoms in SCZ. Increased microglial pruning is mediated specifically by C4. Furthermore, environmental factors (e.g., infections and stress) can lead to dysbiosis which was recently linked to microglial activation and pruning in SCZ.
Summary
Recent findings render the pruning machinery a potential target for early treatment and prevention in individuals at high risk for SCZ. Minocycline can improve cognition in SCZ, probably by reducing excessive pruning. Probiotics might also have beneficial effects on cognition, although recent findings are not encouraging. N-acetyl-cysteine recovers functional connectivity in SCZ both in vitro and in vivo, making it an interesting candidate.
Keywords: astrocytes, cognitive symptoms, microglia, schizophrenia, synaptic pruning
INTRODUCTION
Schizophrenia (SCZ) and related psychotic disorders are heterogeneous psychiatric disorders that are estimated to affect ∼20 million people worldwide, placing a significant burden on individual well-being and global health [1]. In addition to positive and negative symptoms, disabling cognitive symptoms are present in a subpopulation of SCZ patients. Cognitive clustering shows three groups: one that is cognitively intact, one with moderate cognitive deficits in only one or a few domains, and one with severe cognitive dysfunction [2]. The latter group shows global deficits, affecting numerous cognitive domains ranging from executive functioning to working memory, planning, verbal fluency, and problem solving [3]. Whereas positive symptoms can be effectively mitigated by antipsychotic medication in the majority of patients, treatments alleviating cognitive symptoms are scarce [4,5]. Given the, as of yet, largely elusive pathophysiology underlying cognitive impairments in SCZ, a better understanding of the mechanisms behind this symptom is essential to aid the development of effective treatments and preventive strategies.
One of the most prominent hypotheses unraveling the pathophysiology of SCZ stems from the eighties. In 1982, Feinberg postulated that a crucial step in the development of SCZ is a faulty programmed synaptic pruning process [6]. Synaptic pruning is a healthy neurodevelopmental process important for the proper establishment and maturation of functional neural networks by eliminating infrequently used synapses whereas maintaining frequently used connections. Whereas pruning occurs throughout life, certain critical developmental periods are characterized by a peak in pruning. Particularly during adolescence, brain regions involved in higher cognitive functions have been found to be subject to extensive pruning [7▪]. According to Feinberg, SCZ may arise due to a faulty programmed pruning process, resulting in excessive synaptic pruning during adolescence [6].
Recently, Feinberg's hypothesis was supported in a PET-study employing the novel tracer [11C]UCB-J to image synaptic vesicle glycoprotein 2A (SV2A). This study demonstrated a significant and large decrease (Cohen's d = 0.8) in synaptic density in vivo in 18 SCZ patients compared to 18 healthy controls (HC) [8▪▪]. Such a reduction in synaptic density has a profound impact on cortical connectivity [7▪,9]. In line with this, a recent meta-analysis across 14 studies (743 individuals with ultra-high risk [UHR] for psychosis, 588 HC) found abnormalities in gray matter volumes (an indirect measure of synaptic density) in UHR individuals. Compared to HC, UHR individuals showed increased gray matter volumes in the median cingulate, right fusiform gyrus, left superior temporal gyrus, and right thalamus (P < 0.001 in all studies) and decreased volumes in the superior frontal gyrus bilaterally (P < 0.001 in all studies) [10]. The loss of synaptic density may impair cognitive functioning by affecting certain neuronal circuitry, which would be reflected in an abnormal functional connectivity (FC). Indeed, Xian-Bin et al. (2019) recently assessed resting-state FC in 24 individuals with clinical high risk for psychosis (CHR), 19 patients with first-episode psychosis (FEP), and 47 HC. Results of this investigation revealed hypoconnectivities in CHR and FEP patients compared to HC between posterior insula and somatosensory areas (Effect sizes HC vs. FEP: 0.96–1.26; Effect sizes HC vs. CHR: 0.82–1.06) and between dorsal anterior insula and putamen (Effect sizes HC vs. FEP: 1.07–1.25; Effect sizes HC vs. CHR: 0.87–0.95) [11].
Although these recent studies support Feinberg's theory in showing decreased synaptic density and aberrant structural and FC in SCZ, the question remains which factors contribute to the emergence of aberrant synaptic pruning in SCZ. To advance our understanding of the pathophysiological machinery, the current review focuses on the latest scientific developments with respect to factors contributing to excessive synaptic pruning in SCZ. An overarching concept providing a well-established connection between genetic and environmental factors implicated in pruning is given by the notion of an immune system dysregulation in SCZ, with a crucial role assigned to abnormal microglial activation linked to increased complement system activation.
Box 1.
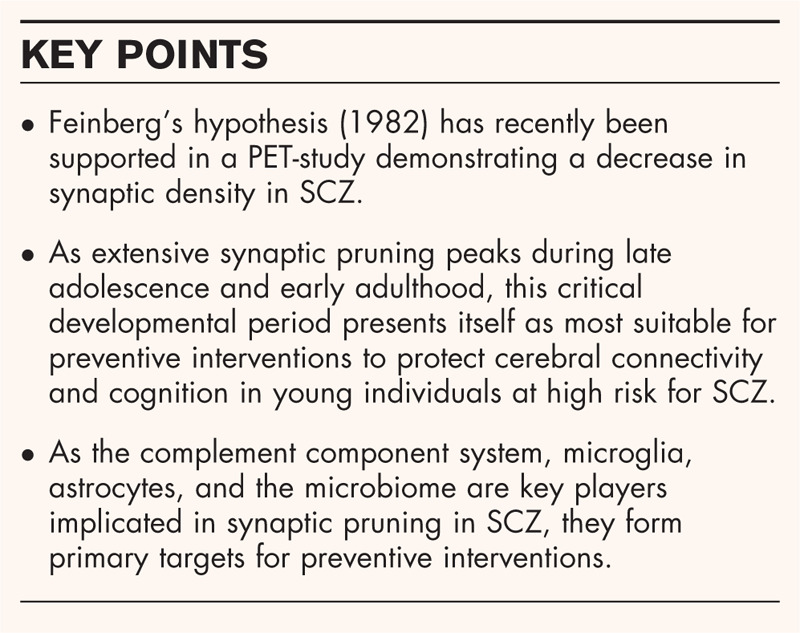
no caption available
MICROGLIAL SYNAPTIC PRUNING
Microglia are resident macrophages of the CNS and play a crucial role in its immunity. Main functions of microglia entail phagocytosis of pathogens and disrupted or apoptotic neuronal parts such as infrequently used synapses [12]. The traditionally demarcated term ‘microglial activation’ nowadays comprises the highly diverse functional and morphological microglial reactions to triggers such as stress, trauma, neuroinflammation, or neurodegeneration [13]. Persistent overactivation of microglia can be induced by increased abundance of activated complement system proteins like C3 and C4 [14] and is associated with increased pro-inflammatory cytokine release (e.g., TNF-a, IL-1b, and IL-33) [15,16]. Moreover, prolonged microglial overactivation mediated by increased complement activation may cause excessive synaptic pruning [14,15]. Support for this comes from a recent meta-analysis reporting significantly higher microglial density and increased levels of pro-inflammatory cytokine proteins in postmortem brain samples of SCZ patients compared to HC [17].
In several lines of research, Stevens et al. impressively demonstrated the crucial role of microglial and the complement system in synaptic pruning during neurodevelopment. In one of their recent studies, the group revealed microglial pruning in hippocampal neurons to be phosphatidylserine (PS-) and TREM2-dependent in mice. PS has been found to be a potential molecular component that tags certain neuronal elements, thereby signaling subsequent microglial engulfment. Interestingly, the involvement of PS in microglia-mediated pruning has been demonstrated to be highest during critical developmental periods characterized by a peak in microglial pruning. Finally, with their finding that C1q peaks during developmental pruning periods and the observation of decreased microglial engulfment of PS-marked elements in C1q knock-out mice, the group further highlights the role of complement during developmental microglial pruning [18]. Recent findings implicate dysfunctional complement system as an important driver of abnormal microglial activation [19▪▪].
The complement system as mediator of microglial synaptic pruning
The classical complement cascade is an integral part of the innate immune system. By opsonizing pathogens or abnormal cell parts and releasing inflammatory mediators, complement proteins direct microglial phagocytosis to engulf and destroy their target [20▪]. In particular, C3 and C4 have most often been reported to trigger opsonization of synapses, thereby inducing synapse phagocytosis by microglia [21]. Whereas C4 is mainly involved in the classical pathway, its pathway successor C3 represents the complement protein of fundamental importance for proper opsonization common to all complement pathways [22]. A recent meta-analysis compared plasma complement protein levels and activity between SCZ patients and HC. Pooled estimated means did not reveal differences between the two groups in 11 studies of C3 and 10 studies of C4, even though individual studies reported significant increases in C3 and C4 in SCZ compared to HC [23]. Two more recent studies (not included in the meta-analysis) added to these inconsistencies. Kopczynska et al. suggested that dysregulations in C3 and C4 may be used as biomarkers to distinguish FEP patients from HC, but found a (nonsignificant) decrease of C3 in FEP compared to HC, rather than in increase [22]. In contrast, Cardozo et al. reported a significant increase in C3 as well as C4 blood levels in SCZ patients compared to HC [7▪]. So far, reports on complement levels in SCZ remain highly inconsistent.
Recently, Sellgren et al.[19▪▪] investigated microglial synaptic pruning in vitro in SCZ patient-derived neuronal cultures. Results of this study not only confirmed the key role of abnormal microglia in excessive pruning in SCZ, but also highlighted the crucial involvement of complement in this process. Their findings show that increased microglial pruning is mediated by C4 [19▪▪]. In accordance, SCZ's strongest genetic association in the major histocompatibility complex has been found to result partly from structurally altered alleles from the complement C4 gene. Such variations have been further revealed to result in an enhanced C4a expression [24]. Importantly, Sellgren et al. also demonstrated the antibiotic minocycline to decrease the aberrant synapse elimination induced by complement-mediated microglial pruning [19▪▪]. These findings support the notion of dysfunctional complement-mediated microglial pruning and present highly significant implications for a potential treatment target. In that regard, genetically overexpressed active C4 may induce increased activation of its pathway successor C3, leading to excessive opsonization of synapses, and ultimately resulting in increased synapse phagocytosis by microglia.
THE EMERGING ROLE OF ASTROCYTES IN SYNAPTIC PRUNING
In addition to complement-mediated microglial pruning, astrocytes have recently been implicated in pruning [20▪,25]. Astrocytes can produce cytokine interleukin (IL)-33, which directly enhances the phagocytic action of microglial cells. Interestingly, synaptic maturation has been reported to be accompanied by an increase in IL-33. This may reflect a homeostatic loop in which increased numbers of synapses constitute a signal for astrocytes to produce IL-33 for the induction of microglial pruning [25]. Astrocytes can also release transforming growth factor b (TGF-b) which enhances the expression of the complement cascade initiating C1q protein, ultimately resulting in the release of C3. Subsequent microglial activation leads to the release of C1q, IL-1a, and TNF-a, which results in reactive astrocytes with a diminished ability to promote synapse formation [20▪]. Astrocytes may also use another phagocytic pathway, via receptors Multiple EGF-like domains 10 (MEG10) and MER Tyrosine Kinase (MERTK), which is independent of C1q. Both pathways mediate synaptic pruning during development [26]. Support comes from findings of excess synapses in MEGF10 and MERTK deficient mice, reflecting missing circuit refinement [26].
ENVIRONMENTAL FACTORS IMPLICATED IN SYNAPTIC PRUNING
Although the above-mentioned description focuses on the role of astrocytes and complement-mediated microglial pruning, certain environmental factors are known to affect synaptic pruning as well. These factors can be interpreted in light of the two-hit model of SCZ. This theory posits that an initial perinatal-immune activation in response to genetic and/or environmental alterations induces a first hit, and subsequent stressors or psychological trauma in childhood or adolescence poses a second hit [14,27]. Perinatal-immune activation can be influenced by a genetic liability that predisposes the individual for an overexpression of complement component proteins and pro-inflammatory cytokines [15,21,28,29]. Together, these factors can prime microglia which, in response to later stressful events, become overactivated, inducing excessive opsonization of synapses, leading to pathological pruning.
Maternal immune activation (MIA), perinatal insults, and early-life stress form environmental factors that can prime microglia [15,30]. MIA produces an increase in pro-inflammatory cytokines and MMP-9 release in the fetal body, both of which are inducers of microglial activity [28]. Prenatal infections and MIA are furthermore associated with microglial overactivation in children [31]. Interestingly, prenatal infections have recently been reported to cause astrocytes to react in a hypersensitive manner to stimuli in the future [32]. Furthermore, MIA and perinatal stress can lead to elevated microglial density and activity [15]. Although these perinatal immune activation factors prime microglia, a shift toward prolonged microglial overaction phenotype takes place only after exposure to stress or traumatic stimuli during adolescence, a time which coincidence with the onset of first clinical symptoms in SCZ [15]. Indeed, evidence points toward subtle, yet significant increases in pro-inflammatory cytokines in adults with childhood trauma, which itself has been associated with increased risk to develop SCZ [14]. Finally, cannabis usage during early adolescence may disturb neurodevelopmental processes as it affects microglial function by causing an upregulation of CB2 receptors, thereby modifying synaptic pruning [33]. The effect of cannabis usage on brain connectivity has recently been demonstrated in a sample of 54 psychotic patients (29 patients who use cannabis, 25 patients who did not use cannabis) and 38 HC (16 HC who use cannabis, 22 HC who did not use cannabis). Results revealed increased connectivity in the dorsal attention network (P = 0.019) and visual dorsal attention internetwork (P = 0.036) in cannabis consuming patients compared to nonusing controls. Interestingly, however, there was no significant difference between patients with cannabis usage and patients without cannabis usage in terms of connectivity [34]. Given that the current state of literature on the effect of cannabis consumption on connectivity in SCZ patients points toward mixed results, further research is warranted.
The gut–brain axis allows for bidirectional communication between the gut and brain via pathways involving the immune system, autonomic nervous system, endocrine, and enteric nervous system [35]. The brain can influence the microbiota of the gut via these pathways. Conversely, the intestinal microbiota can affect cognition by influencing levels of short-chain fatty acids (SCFA), serotonin, GABA and other neurotransmitters [36]. Interestingly, the critical developmental period for cortical maturation characterized by a peak in synaptic pruning coincidences with the time of maturation of the intestinal microbiota [37▪], pointing toward a role of the microbiome in microglial activation [38]. Early life environmental factors such as cesarian section, antibiotic treatment, stress or infections can lead to dysbiosis, which can affect early brain maturation [35,39]. In germ-free mice, absence of gut microbiota negatively affects cognition, stress tolerance and social behavior by altering the regulation [39] and expression of genes implicated in synapse organization in microglia [40]. In humans, there is also evidence that microglial function and activation can be modified by the intestinal microbiota, especially during the critical developmental time [37▪].
CLINICAL IMPLICATIONS
Converging lines of evidence point toward an overactive pruning machinery in SCZ as a potent target for preventive and therapeutic actions.
Minocycline is a tetracyclic antibiotic with anti-inflammatory properties and has been proven to be safe, well-tolerated and effective in SCZ patients [41▪,42], as a recent randomized double-blind, placebo-controlled study demonstrated that the administration of minocycline to 75 SCZ patients led to significantly reduced cognitive deficits and levels of IL-6 and IL-1b compared to placebo [43]. Further support comes from a meta-analysis on randomized placebo-controlled trials in SCZ patients investigating the effect of adjuvant anti-inflammatory agents on distinct clinical outcome variables. Based on two trials (133 SCZ patients), they concluded that minocycline significantly improved cognitive functioning in SCZ patients compared to placebo, specifically with regard to executive functioning (Hedges’ g: first trial = 1.35; second trial = 0.17) and visual learning/memory (effect size 0.94). Furthermore, no significant differences in side effects between minocycline and placebo have been found [44]. A potential explanation for this positive effect of minocycline is that it reduces extensive pruning, as shown in cell lines by Sellgren [19▪▪]. Alternatively, minocycline may primarily affect the microbiome, which could lead to alterations in SCFA and GABA production, also known to affect cognition [45].
Manipulation of the microbial environment by the administration of probiotics (beneficiary microbiota bacteria) or prebiotics (nutritional fibers that serve as food for these bacteria) may affect brain function and improve cognition [35,36]. A recent meta-analysis investigated the effect of probiotics (11 studies, 724 participants), prebiotics (5 studies, 355 participants), and fermented food (6 studies, 472 participants) in a mixed group of HC and patients with a wide range of brain and somatic disorders on cognition. Pooled results did not demonstrate beneficial effects of probiotics, prebiotics or fermented food on cognitive outcome measures. Authors of this meta-analysis suggested that the absence of significant findings for pooled estimates may be due to high clinical heterogeneity, which ranged from fibromyalgia, to cirrhosis, to Alzheimer, depression and more [46]. Findings of another meta-analysis assessing 7 controlled clinical trials in a mixed sample of patients with Alzheimer, fibromyalgia, depression, minimal hepatic encephalopathy, and HC, and 11 animal studies did support the beneficial effect of probiotics on cognitive functioning in both human and animals. Interestingly, the beneficial effect of probiotics was more pronounced in cognitively impaired individuals compared to HC [47]. Overall, mixed results of the effects of probiotics and the absence of investigations of the effect of pre or probiotics on cognition in SCZ patients warrant further research.
Finally, to prevent the loss of brain connectivity by increased pruning, the administration of N-acetyl-cysteine (NAC) supplementation may be beneficial. Mullier et al.[48] compared the effect of 6 months NAC supplementation to placebo on FC between cingulate cortex areas in 20 early psychotic patients and 74 HC. This pilot study demonstrated that, compared to placebo, supplementation of NAC in early psychotic individuals increased the FC between designated areas implicated in positive symptoms and processing speed [48]. However, replication studies are warranted.
Based on the peak of excessive pruning in late adolescence and early adulthood, it may prove most beneficial to administer minocycline, NAC, or possibly pro/prebiotics to prevent over-pruning, early on in adolescent individuals who are at high risk to develop SCZ. Even though the peak in synaptic pruning occurs during adolescence, it is a process part of brain homeostasis taking place throughout life. Therefore, interventions with minocycline may still be useful after adolescence. It is encouraging that these three interventions are all well tolerated with few if any side-effects [44]. People who could potentially benefit from such interventions are those with first degree family with SCZ and polygenic risk scores involving the complement system, as has been elegantly demonstrated by Sellgren et al. in vitro [19▪▪].
CONCLUSION
The present review outlines recent developments in the elucidation of the pathophysiological mechanism underlying aberrant synaptic pruning in SCZ, and alterations succeeding it. Genetically overexpressed complement proteins C3 and C4 contribute to a generally enhanced pro-inflammatory state of both periphery and CNS. Together, this results in microglial overactivation for a prolonged period of time, thereby inducing excessive synaptic pruning. The resulting loss of synaptic density has recently been confirmed by a PET-study employing a novel tracer [11C]UCB-J. Astrocytes contribute to excessive pruning through their ability to directly enhance microglial phagocytic actions. The ability of the intestinal microbiota to modify microglial function and activation implicate the human microbiome as a modifiable factor in pruning as well. Minocycline has been demonstrated to be safe and beneficial in improving cognitive functioning in SCZ. Although individual studies reported minocycline and NAC to improve cognitive functioning, further research is warranted to replicate their unambiguous beneficial effects on cognition.
Acknowledgements
The authors would like to thank Shiral Gangadin for the critical reading of the review. We would also like to thank Fynn Elvers for the valuable assistance in writing the review.
Financial support and sponsorship
This work is supported by a grant to I.S. from ZonMw mental health (GGZ) (ZonMw, project code: 63631 001 0).
Conflicts of interest
I.S. is a consultant to Gabather, received research support from Janssen Pharmaceuticals Inc. and Sunovion Pharmaceuticals Inc. The rest of the authors do not have any conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.World Health Organization. Mental Disorders 2019, Available from: https://www.who.int/news- room/fact-sheets/detail/schizophrenia. [Accessed 11 November 2020] [Google Scholar]
- 2.Carruthers SP, Gurvich CT, Meyer D, et al. Exploring heterogeneity on the wisconsin card sorting test in schizophrenia spectrum disorders: a cluster analytical investigation. J Int Neuropsychol Soc 2019; 25:750–760. [DOI] [PubMed] [Google Scholar]
- 3.Chen P, Ye E, Jin X, et al. Association between thalamocortical functional connectivity abnormalities and cognitive deficits in schizophrenia. Sci Rep 2019; 9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinkeviciute I, Begemann M, Prikken M, et al. Efficacy of different types of cognitive enhancers for patients with schizophrenia: a meta-analysis. NPJ Schizophr 2018; 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn RS, Winter van Rossum I, Leucht S, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry 2018; 5:797–807. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982; 17:319–334. [DOI] [PubMed] [Google Scholar]
- 7▪.Cardozo PL, de Lima IBQ, Maciel EMA, et al. Synaptic elimination in neurological disorders. Curr Neuropharmacol 2019; 17:1071–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review discussing the role of the complement cascade in abnormal synaptic pruning and its effect on brain connectivity in schizophrenia, among others.
- 8▪▪.Onwordi EC, Halff EF, Whitehurst T, et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat Commun 2020; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first in vivo PET-study demonstrating the decrease of synaptic density in schizophrenic patients compared to healthy controls by employing the novel tracer [11C]UCB-J.
- 9.Van Berlekom AB, Muflihah CH, Snijders GJLJ, et al. Synapse pathology in schizophrenia: a meta-analysis of postsynaptic elements in postmortem brain studies. Schizophr Bull 2020; 46:374–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Y, Ou Y, Pan P, et al. Brain structural abnormalities as potential markers for detecting individuals with ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res 2019; 209:22–31. [DOI] [PubMed] [Google Scholar]
- 11.Li XB, Wang LB, Xiong YB, et al. Altered resting-state functional connectivity of the insula in individuals with clinical high-risk and patients with first-episode schizophrenia. Psychiatry Res 2019; 282:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Marques TR, Ashok AH, Pillinger T, et al. Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med 2019; 49:2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubbelaar ML, Kracht L, Eggen BJL, Boddeke EWGM. The kaleidoscope of microglial phenotypes. Front Immunol 2018; 9:1753.doi: 10.3389/fimmu.2018.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongan D, Ramesar M, Föcking M, et al. Role of inflammation in the pathogenesis of schizophrenia: a review of the evidence, proposed mechanisms and implications for treatment. Early Interv Psychiatry 2020; 14:385–397. [DOI] [PubMed] [Google Scholar]
- 15.Howes OD, McCutcheon R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 2017; 7:e1024–e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conti P, Lauritano D, Caraffa A, et al. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: suppressor effect of IL-37. Eur J Pharmacol 2020; 875:173035.doi: 10.1016/j.ejphar.2020.173035. [DOI] [PubMed] [Google Scholar]
- 17.Van Kesteren CFMG, Gremmels H, De Witte LD, et al. Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 2017; 7:e1075–e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott-Hewitt N, Perrucci F, Morini R, et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J 2020; 39:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Sellgren CM, Gracias J, Watmuff B, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 2019; 22:374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is of particular importance given their elegant demonstration of the role of abnormal microglia and C4 in excessive pruning in schizophrenia. Provides also evidence that minocycline decreases aberrant synaptic pruning induced by complement-mediated microglial pruning.
- 20▪.Druart M, Le Magueresse C. Emerging roles of complement in psychiatric Disorders. Front Psychiatry 2019; 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review highlighting the involvement of complement in microglial and astrocytic synaptic pruning in schizophrenia, among others.
- 21.Lee JD, Coulthard LG, Woodruff TM. Complement dysregulation in the central nervous system during development and disease. Semin Immunol 2019; 45:101340. [DOI] [PubMed] [Google Scholar]
- 22.Kopczynska M, Zelek W, Touchard S, et al. Complement system biomarkers in first episode psychosis. Schizophr Res 2019; 204:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mongan D, Sabherwal S, Susai SR, et al. Peripheral complement proteins in schizophrenia: a systematic review and meta-analysis of serological studies. Schizophr Res 2020; 222:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekar A, Bialas AR, De Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016; 530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vainchtein ID, Molofsky AV. Astrocytes and microglia. Sickness Health Trends Neurosci 2020; 43:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman C, Egert L, Di Benedetto B. Astrocytic-neuronal crosstalk gets jammed: alternative perspectives on the onset of neuropsychiatric disorders. Eur J Neurosci 2020; 9:e14900. [DOI] [PubMed] [Google Scholar]
- 27.Feigenson KA, Kusnecov AW, Silverstein SM. Inflammation and the two-hit hypothesis of schizophrenia. Neurosci Biobehav Rev 2014; 38:72–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitanihirwe BKY, Woo TUW. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr Res 2020; 218:28–35. [DOI] [PubMed] [Google Scholar]
- 29.Momtazmanesh S, Zare-Shahabadi A, Rezaei N. Cytokine alterations in schizophrenia: an updated review. Front Psychiatry 2019; 10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Comer AL, Carrier M, Tremblay MÈ, Cruz-Martín A. The inflamed brain in schizophrenia: the convergence of genetic and environmental risk factors that lead to uncontrolled neuroinflammation. Front Cell Neurosci 2020; 14:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Zhang Z, Lin W, et al. Modulating microglia activation prevents maternal immune activation induced schizophrenia-relevant behavior phenotypes via arginase 1 in the dentate gyrus. Neuropsychopharmacology 2020; 45:1896–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasov VV, Svistunov AA, Chubarev VN, et al. Alterations of astrocytes in the context of schizophrenic dementia. Front Pharmacol 2020; 10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel PK, Leathem LD, Currin DL, Karlsgodt KH. Adolescent neurodevelopment and vulnerability to psyCHOSIS. Biol Psychiatry 2020; 89:184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sami MB, McCutcheon RA, Ettinger U, et al. Cannabis use linked to altered functional connectivity of the visual attentional connectivity in patients with psychosis and controls. Schizophr Bull Open 2020; 1:1–11. [Google Scholar]
- 35.Golofast B, Vales K. The connection between microbiome and schizophrenia. Neurosci Biobehav Rev 2020; 108:712–731. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt C. Thinking from the gut the microbiome may. Nat Publ Gr 2015; 312:s12–s15. [DOI] [PubMed] [Google Scholar]
- 37▪.Eltokhi A, Janmaat IE, Genedi M, et al. Dysregulation of synaptic pruning as a possible link between intestinal microbiota dysbiosis and neuropsychiatric disorders. J Neurosci Res 2020; 98:1335–1369. [DOI] [PubMed] [Google Scholar]; Review discussing the role of the intestinal microbiota in microglial synaptic pruning in neurodevelopmental disorders such as schizophrenia.
- 38.Erny D, Prinz M. How microbiota shape microglial phenotypes and epigenetics. Glia 2020; 68:1655–1672. [DOI] [PubMed] [Google Scholar]
- 39.Springer, Khandaker GM, Meyer U, Jones Editors PB. Neuroinflammation and schizophrenia. 2020. [Google Scholar]
- 40.Chu C, Murdock MH, Jing D, et al. The microbiota regulate neuronal function and fear extinction learning. Nature 2019; 574:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Çakici N, Van Beveren NJM, Judge-Hundal G, et al. An update on the efficacy of anti-inflammatory agents for patients with schizophrenia: a meta-analysis. Psychol Med 2019; 49:2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meta-analysis suggesting the beneficial effect of minocycline and N-acetylcysteine on cognitive functioning in schizophrenia.
- 42.Zheng W, Zhu XM, Zhang QE, et al. Adjunctive minocycline for major mental disorders: a systematic review. J Psychopharmacol 2019; 33:1215–1226. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Zheng H, Wu R, et al. The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr Res 2019; 212:92–98. [DOI] [PubMed] [Google Scholar]
- 44.Cho M, Lee TY, Kwak YB, et al. Adjunctive use of anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Aust N Z J Psychiatry 2019; 53:742–759. [DOI] [PubMed] [Google Scholar]
- 45.Hasebe K, Rivera LR, Smith CM, et al. Modulation of high fat diet-induced microbiome changes, but not behaviour, by minocycline. Brain Behav Immunity 2019; 82:309–318. [DOI] [PubMed] [Google Scholar]
- 46.Marx W, Scholey A, Firth J, et al. Prebiotics, probiotics, fermented foods and cognitive outcomes: a meta-analysis of randomized controlled trials. Neurosci Biobehav Rev 2020; 118:472–484. [DOI] [PubMed] [Google Scholar]
- 47.Lv T, Ye M, Luo F, et al. Probiotics treatment improves cognitive impairment in patients and animals: a systematic review and meta-analysis. Neurosci Biobehav Rev 2020; 120:159–172. [DOI] [PubMed] [Google Scholar]
- 48.Mullier E, Roine T, Griffa A, et al. N-acetyl-cysteine supplementation improves functional connectivity within the cingulate cortex in early psychosis: a pilot study. Int J Neuropsychopharmacol 2019; 22:478–487. [DOI] [PMC free article] [PubMed] [Google Scholar]


