Abstract
Objective:
Angiogenic T cells (Tang cells), a recently discovered T-cell subset, have been reported involved in the repair of endothelial injury. The purpose of this study was to explore the correlation of immunologic senescence and pro-inflammatory capacity of Tang cells with endothelial dysfunction in hypertensive patients.
Methods:
Immunological characteristics of Tang cells (CD3+CD31+CXCR4+) from hypertensive patients with or without endothelial dysfunction were elucidated by surface immunophenotyping and intracellular cytokine staining. Endothelial function was measured by flow-mediated dilation (FMD).
Results:
The frequency of CD28null subset in CD4+ Tang cells was notably elevated in hypertensive patients with endothelial dysfunction, which was negatively associated with FMD. The high frequency of CD28nullCD4+ Tang cells was an independent risk factor of endothelial dysfunction with good diagnostic performance in ROC curve analysis. Immunophenotyping revealed that this specific subset of Tang cells exhibited senescent profile and has low hTERT expression. CD28nullCD4+ Tang cells produced high levels of inflammatory cytokines, IL-6, IFN-γ and TNF-α, and significantly correlated with the systemic inflammation in hypertensive patients with endothelial dysfunction.
Conclusion:
Collectively, our findings demonstrate for the first time that CD28null subset in CD4+ Tang cells with senescent and pro-inflammatory phenotype is dependently correlated with impaired FMD and systemic inflammation, which might contribute to the immunopathologic mechanism of endothelial dysfunction. Identification of a pathogenic CD4+ Tang-cell subset lacking CD28 may offer opportunities for the evaluation and management of endothelial dysfunction in hypertension.
Keywords: angiogenic T cells, endothelial dysfunction, hypertension, immunosenescence, inflammation
INTRODUCTION
Hypertension is a major pathophysiologic contributor to cardiovascular diseases [1]. Dysfunction of vascular endothelium is the hallmark of hypertension, which is characterized as impaired endothelium-dependent vasorelaxation with a pro-inflammatory state and prothrombic properties [2]. Accumulated evidence has widely identified endothelial dysfunction as the first step of the atherosclerotic process and as a fundamental mechanism in the pathophysiology of hypertensive target organ damage and cardiovascular diseases [3].
Chronic inflammation has been considered as a critical trigger of hypertension and contributed to an alteration of endothelial structure and function [4,5]. Aging of the immune system, defined as immunosenescence [6], contributes to the development of chronic inflammation and has been thought to underlie vascular endothelial injury [7,8]. Altered T-cell subsets and dysregulated cytokine profile are marked features of immunosenescence [9]. However, any relationship between the immunosenescence of T cells and impaired endothelial function in hypertension remains to be elucidated.
Senescent T cells exhibit characteristic phenotype, such as lack expression of CD28 and abnormal secretory phenotype [10]. Previous studies found that CD28nullCD8+ T cells increased in patients with hypertension, suggesting a role for senescent T-cell-driven inflammation in hypertension [11]. Recent studies suggest that a specific T-cell subset, termed angiogenic T cells (Tang cells), involve in endothelial repair and promote formation of new blood vessels. Tang cells are characterized by the co-expression of CD3, CD31, and the receptor for the CXC chemokine stromal cell-derived factor-1 (CXCR4) [12,13]. Altered circulating Tang cell frequencies and senescent subsets have been reported to be associated with cardiovascular risk factors in rheumatoid arthritis [13], systemic lupus erythematosus (SLE) [14] and diabetes [15]. The role of senescent Tang cells in hypertension and endothelial dysfunction has yet to be determined.
On the basis of the uncertainties mentioned above, in the present study, we compare the frequencies of Tang cell subsets in hypertensive patients with or without endothelial dysfunction confirmed by flow-mediated dilation (FMD) and investigate the relationship between senescent Tang cells and FMD. We also explore the pro-inflammatory functional phenotype and senescent profile of CD28null Tang and the association between senescent Tang cells and elevated serum pro-inflammatory cytokines in hypertensive patients with endothelial dysfunction. In summary, this study suggests the linkage between senescent Tang cells and endothelial dysfunction and systemic inflammation in hypertension.
METHODS
Study population
The present study included 80 consecutively enrolled patients diagnosed with essential hypertension at the Department of Hypertension and Vascular Disease, the First Affiliated Hospital of Sun Yat-sen University from June 2019 to August 2019. The inclusion criteria for the essential hypertensive patients were as follows: aged 40--65 years; office blood pressure raised with SBP at least 140 mmHg and/or DBP at least 90 mmHg; without any antihypertension medication, herbal supplements, antidepressants or other traditional Chinese medication therapy history. The exclusion criteria were as follows: secondary hypertension; (ii) systemic diseases, such as diabetes, HIV/AIDS, liver disease, chronic renal failure, tuberculosis, and autoimmune diseases; diabetes or prediabetes mellitus; medical history of cardiovascular disease: acute myocardial infarct, stable angina, unstable angina, heart failure, atrial fibrillation, atrioventricular blockade, peripheral vascular disease or cerebrovascular accident. BP measurements were performed according to 2018 ESC/ESH Guidelines for the management of arterial hypertension[16]. All of the participants underwent three BP measurements in two different visits, after 30 min of rest, and the measurements were spaced by 5--10 min intervals, on both the left and right arms, in the sitting and lying positions. Patients underwent a physical examination and laboratory examination at the time of initial enrollment. The baseline characteristics of the study population are summarized in Table 1. Informed consent was provided by all participants, and the study protocol was approved by the Ethics Committees of the First Affiliated Hospital of Sun Yat-sen University.
TABLE 1.
Demographic characteristics and clinical parameters of study population
| HT without ED (n = 32) | HT with ED (n = 48) | P value | |
| Age (years) | 54.1 ± 7.6 | 52.9 ± 4.1 | 0.650 |
| Sex (% male) | 22 (68.8) | 30 (62.5) | 0.566 |
| BMI (kg/m2) | 23.4 ± 1.8 | 23.5 ± 2.3 | 0.787 |
| Heart rate (beat/min) | 65.5 ± 9.0 | 64.5 ± 10.1 | 0.661 |
| Plasma glucose (mmol/l) | 5.42 ± 0.37 | 5.47 ± 0.44 | 0.587 |
| Triglyceride (mmol/l) | 1.26 ± 0.52 | 1.27 ± 0.37 | 0.964 |
| Creatinine (μmol/l) | 80.4 ± 19.8 | 79.5 ± 20.3 | 0.436 |
| eGFR (ml/min per 1.73 m2) | 90.4 ± 17.5 | 89.6 ± 18.2 | 0.615 |
| Total cholesterol (mmol/l) | 5.11 ± 0.45 | 5.24 ± 0.58 | 0.265 |
| LDL cholesterol (mmol/l) | 2.75 ± 0.35 | 2.71 ± 0.40 | 0.622 |
| HDL cholesterol (mmol/l) | 1.44 ± 0.46 | 1.52 ± 0.29 | 0.399 |
| Current smokers (%) | 14 (43.8) | 23 (47.9) | 0.148 |
| Hypertension (%) | 100∗ | 100∗ | – |
| SBP (mmHg) | 161.3 ± 8.1 | 160.9 ± 9.8 | 0.087 |
| DBP (mmHg) | 100.3 ± 4.6 | 101.4 ± 5.7 | 0.353 |
| Flow-mediated Dilation (%) | 7.8 ± 0.6 | 5.8 ± 0.7 | <0.001 |
ED, endothelial dysfunction; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HT, hypertension; LDL, low-density lipoprotein.
P < 0.05 is considered significant.
Measurement of flow-mediated dilation
A high-resolution ultrasonography equipment specialized to measure FMD (UNEXEF18G, UNEX Co, Nagoya, Japan) was used to evaluate FMD according to the guidelines of the American College of Cardiology [17], as previously described in our previous study [18].
FMD was performed in a temperature-controlled room (22 °C) with participants in a resting supine state between 0800 and 1000 h, after at least an 8-h fast. Diameter of brachial artery was automatically imaged by a high-resolution linear artery transducer coupled to computer-assisted analysis software. A blood pressure cuff was placed around the forearm. The brachial artery was visualized longitudinally 5--10 cm above the antecubital crease. Pulsed Doppler flow was evaluated at baseline and during peak hyperemic flow. The baseline longitudinal image of the artery was acquired for 30 s, and then the cuff was inflated 50 mmHg greater than systolic pressure for 5 min. The longitudinal image of the artery was recorded continuously until 1 min after releasing occlusion. FMD was automatically expressed as the percentage change in peak diameter from the baseline diameter. Percentage of FMD [(peak diameter − baseline diameter)/baseline diameter] was used for analysis. Endothelial dysfunction was confirmed by FMD with cutoff at 7% or less [19].
Immunophenotyping analysis of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood using Ficoll-Hypaque (GE Healthcare, Sweden) and analyzed by flow cytometry. PBMCs were stained with fluorochrome-conjugated monoclonal antibodies against surface antigens for 30 min at 4 °C. The antibodies used included anti-CD3-PE-Cy7, anti-CD4-Horizon V500, anti-CD8-APC-Cy7, anti-CD31-FITC, anti-CXCR4-PerCP-Cy5.5, and anti-CD28-APC-H7 (all from BD Biosciences, San Jose, California, USA). Phenotypic characterization of CD28null-Tang and CD28+-Tang subsets was performed by extracellular staining with anti-CD57-APC, anti-CD27-APC, anti-CCR7-APC, or antihTERT-APC or the corresponding isotype controls (all from BD Biosciences). Multicolor flow cytometry was performed using an CytoFlex S (Beckman Coulter), and FlowJo V10 software (Treestar, San Carlos, California, USA) was used to analyze the data.
Intracellular cytokine staining
PBMCs were stimulated with anti-CD3 antibody (0.1 μg/ml) for 6 h. After 1 h of incubation, brefeldin A and monensin (BD Biosciences) were added to stimulate intracellular cytokine protein accumulation. Following surface staining with anti-CD3-PE-Cy7, anti-CD4-Horizon V500, anti-CD8-APC-Cy7, anti-CD31-FITC, anti-CXCR4-PerCP-Cy5.5, and anti-CD28-APC-H7, the cells were fixed and permeabilized using the Fixation/Permeabilization Buffer Kit and further stained for intracellular cytokines with anti-TNF-α-FITC, anti-IFN-γ-FITC, anti-IL-6-PE, and anti-IL-10-APC (all from BD Biosciences).
Cytokine quantification
IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-17, IL-1α, IL-1β, and MCP-1 were analyzed by Cytometric Bead Arrays (BD Biosciences) in accordance with the manufacturer's instructions. ELISA kits were used for TNF-α (PeproTech, Rocky Hill, New Jersey, USA), IFN-γ (BD Biosciences), CRP (Invitrogen, ThermoFisher Scientific) and CX3CL (R&D systems).
Statistical analysis
Continuous variables were reported as the means ± SD. Categorical variables were summarized by the percentage of the group total. All reported probability values were two-sided, and a P less than 0.05 was considered statistically significant. All the parameters were tested for normal distribution using the Kolmogorov--Smirnov test. An independent t tests were used for two group continuous variables. A Mann--Whitney test was performed for two group continuous, nonparametric variables. Discrete variables were compared using the chi-square test. Paired data comparisons were summarized using the paired t-test. Correlation between FMD or cytokines levels and frequency of Tang-cell subsets were tested by Pearson's correlation test. Multivariate logistic regression analysis was performed to identify independent predictors of endothelial dysfunction. Statistical analyzes and graphs were performed with SPSS version 22.0 (SPSS Inc., Chicago, Illinois, USA) and Graphpad Prism version 8 (GraphPad Software Inc., San Diego, California, USA).
RESULTS
Characteristics of participants
The present study included 80 essential hypertensive patients without any treatment for high blood pressure enrolled at the First Affiliated Hospital of Sun Yat-sen University from June 2019 to August 2019. Endothelial dysfunction was confirmed by FMD with cutoff at 7% or less [19] and 32 in 80 hypertensive patients present normal endothelial function. Demographic and clinical characteristics of patients were summarized in Table 1. Baseline characteristics were not significantly different between hypertensive patients without endothelial dysfunction and hypertensive patients with endothelial dysfunction with the exception of FMD.
CD4+ Tang cells from hypertensive patients with endothelial dysfunction exhibit characteristics of senescence
Tang, a specific T-cell subset, have been identified to be involved in the repair of damaged endothelium. To evaluate the prevalence of the circulating Tang cells and subpopulations in hypertensive patients with or without endothelial dysfunction, the percentages of Tang (CD3+CD31+CXCR4+) and CD4+/CD8+ subsets were analyzed by flow cytometry. As shown in Fig. 1, panel a, no significant differences were observed in the frequency of Tang or CD4+/CD8+ subsets between hypertensive patients with endothelial dysfunction and hypertensive patients without endothelial dysfunction.
FIGURE 1.

The frequency of Tang-cell subsets in patients with hypertension. PBMNCs isolated from hypertensive patients (HT) with endothelial dysfunction (ED) (n = 48) or without endothelial dysfunction (N-ED) (n = 32) were stained for CD3, CD4, CD31, CXCR4, and CD28 and analyzed by flow cytometry. (a) Gating strategy of Tang cells. (b) Gating strategy of CD4+ or CD8+ Tang cells. (c--e) The frequency of total Tang cells (c) or CD4+ Tang cells (d) or CD8+ Tang cells was compared between HT patients with ED and HT patients without ED. (f) Representative cytometry plots and cumulative data of CD28 expressing level in CD4+ Tang cells. (g) Representative cytometry plots and cumulative data of CD28 expressing level in CD8+ Tang cells. ∗∗P < 0.01. ED, hypertensive patients with endothelial dysfunction; HT, hypertension; N-ED, hypertensive patients without endothelial dysfunction; PBMNCs, peripheral blood mononuclear cells; Tang, angiogenic T cells.
Loss of CD28 expression is the most consistent biologic indicator of T-cell senescence. We showed significantly decreased level of cellular surface CD28 in CD4+ Tang cells (Fig. 1 panel f) in hypertensive patients with endothelial dysfunction, whereas the level of CD28 in CD8+ Tang-cell subset showed no significant alteration, supporting a disease-associated immunosenescence of CD28nullCD4+ Tang cells.
Therefore, CD4+ Tang cells exhibit characteristic of senescence accumulated in hypertensive patients with endothelial dysfunction and relationship between CD28nullCD4+ Tang cells and endothelial dysfunction deserves further study.
Senescent CD4+ Tang cells from hypertensive patients are correlated with defect endothelial function
Tang cells have been reported participated in repair of endothelial damage and senescence induces dysregulated function of T cells. However, the possible relationship between senescent CD4+ Tang cells and endothelial dysfunction in hypertensive patients has not been studied so far.
Our data revealed that FMD was only associated with the frequency of CD28nullCD4+ Tang cells (Fig. 2, panel e), whereas the frequency of CD28null cells in the CD8+ Tang cell subset showed no significant association with endothelial function (Fig. 2, panel d). Moreover, by means of ROC curve analysis, there was good sensitivity and specificity of CD28nullCD4+ Tang cells (AUC = 0.9118, P < 0.001; cutoff value <9.3%, sensitivity% = 87.5%, specificity% = 81.25%) for endothelial dysfunction (Fig. 2, panel f).
FIGURE 2.
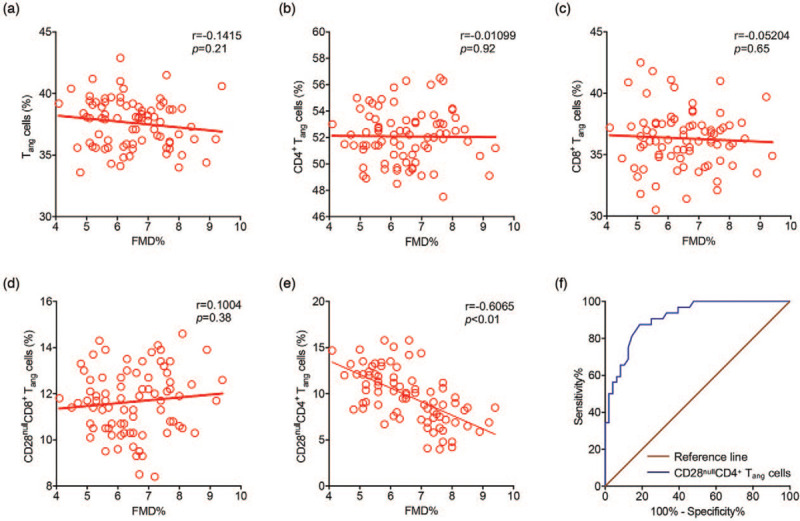
Correlation between flow-mediated dilation and frequency of Tang-cell subsets in patients with hypertension and receiver-operating characteristic curve. (a--e) Correlation between FMD and frequency of Tang-cell subsets were tested using Pearson's correlation test. (f) Receiver-operating characteristic (ROC) Curves of CD28nullCD4+ Tang cells for endothelial dysfunction. FMD, flow-mediated dilation; Tang, angiogenic T cells.
Furthermore, multivariate logistic regression analysis revealed that CD28nullCD4+ Tang-cell subset was an independent risk factor of endothelial dysfunction controlled for age, sex, and SBP (Table 2). Therefore, CD28nullCD4+ Tang cells from hypertensive patients exhibit a potential linkage with endothelial function in hypertensive patients. CD28nullCD4+ Tang cells were deserved a focus of further analyses.
TABLE 2.
Multivariate logistic regression analysis for endothelial dysfunction controlled for age, sex and SBP
| 95% CI | ||||
| Odds ratio | Lower | Upper | P value | |
| Tang cells | ||||
| Age | 0.982 | 0.926 | 1.042 | 0.549 |
| Male sex | 0.654 | 0.236 | 1.811 | 0.414 |
| SBP | 0.944 | 0.895 | 0.995 | 0.032∗ |
| Tang cells | 1.152 | 0.901 | 1.473 | 0.261 |
| CD4+ Tang cells | ||||
| Age | 0.982 | 0.927 | 1.041 | 0.549 |
| Male sex | 0.695 | 0.256 | 1.889 | 0.476 |
| SBP | 0.947 | 0.899 | 0.998 | 0.043∗ |
| CD4+ Tang cells | 0.955 | 0.753 | 1.211 | 0.705 |
| CD8+ Tang cells | ||||
| Age | 0.984 | 0.929 | 1.042 | 0.578 |
| Male sex | 0.687 | 0.252 | 1.872 | 0.463 |
| SBP | 0.947 | 0.898 | 0.998 | 0.043∗ |
| CD8+ Tang cells | 0.994 | 0.811 | 1.217 | 0.951 |
| CD28nullCD4+ Tang cells | ||||
| Age | 1.017 | 0.929 | 1.112 | 0.718 |
| Male sex | 0.924 | 0.219 | 3.897 | 0.914 |
| SBP | 0.966 | 0.902 | 1.035 | 0.326 |
| CD28nullCD4+ Tang cells | 2.551 | 1.677 | 3.880 | <0.001∗ |
| CD28nullCD8+ Tang cells | ||||
| Age | 0.983 | 0.927 | 1.042 | 0.567 |
| Male sex | 0.722 | 0.264 | 1.976 | 0.526 |
| SBP | 0.946 | 0.896 | 0.998 | 0.041∗ |
| CD28nullCD8+ Tang cells | 0.813 | 0.580 | 1.14 | 0.23 |
CI, confidence interval; Tang, angiogenic T cells.
P < 0.05 is considered significant.
CD28nullCD4+ Tang cells from hypertensive patients exhibit senescent profile and pro-inflammatory functional phenotype
Next, we wanted to determine whether the downregulation of CD28 expression in CD4+ Tang cells was accompanied by other senescent features. Thus, we performed a phenotypic characterization of CD28null and CD28+ subsets in CD4+ Tang cells by analyzing the expression of a range of senescent markers usually present in CD28null T cells. Increased expression of CD57 (Fig. 3, panel a) and decreased expression of CCR7 (Fig. 3, panel b) and CD27 (Fig. 3, panel c) supported the prominent senescent profile of CD28nullCD4+ Tang cells. Decreased expression of human telomerase reverse transcriptase (hTERT) is a typical feature of T-cell senescence (Fig. 3, panel d). Thus, we analyzed the expression of hTERT within CD28null and CD28+ subsets in CD4+ Tang cells and found that CD28null subset exhibits marked decline in the expression of hTERT compared with CD28+ subsets (Fig. 3, panel d).
FIGURE 3.
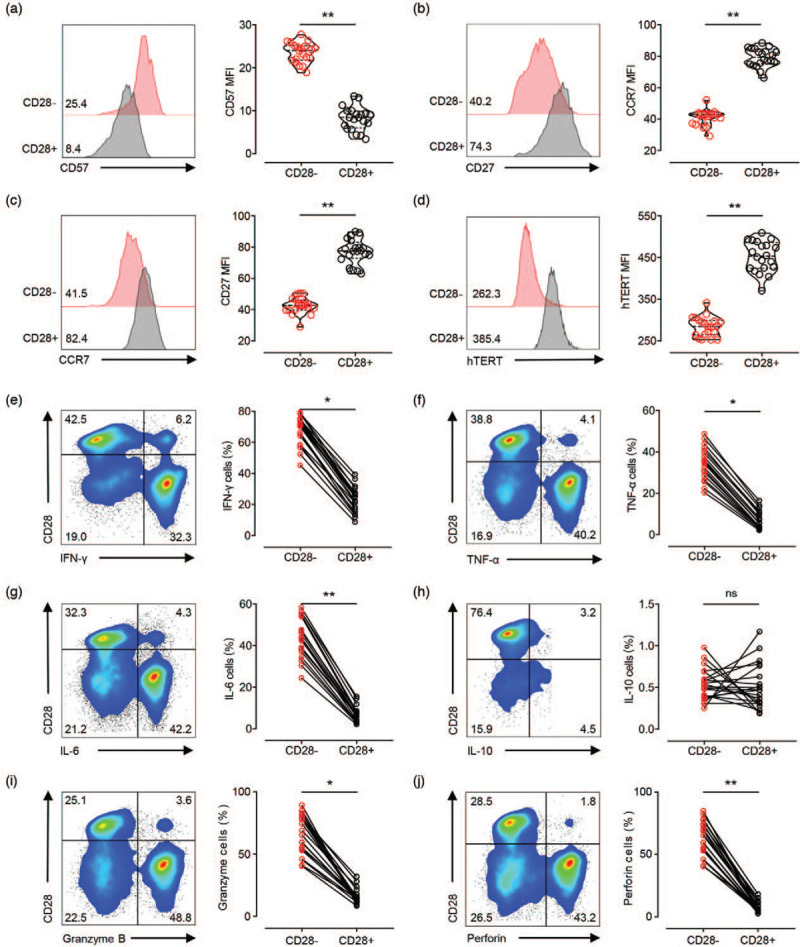
Senescent profile and pro-inflammatory secretory of CD28nullCD4+ Tang cells from hypertensive patients. (a--c) Expression of senescence markers in CD28nullCD4+ Tang cells and CD28+CD4+ Tang cells. Numbers in plots indicate the median fluorescence intensity of each marker staining, with the matched irrelevant control value subtracted. (d) PBMCs were stimulated with 0.5 μg/ml of anti-CD3 antibody for 72 h. Representative cytometry plots and cumulative data of telomerase reverse transcriptase (hTERT) MFI of CD28nullCD4+ Tang cells and CD28+CD4+ Tang cells. (e--h) PBMCs were stimulated with anti-CD3 antibody for 6 h, and intracellular cytokine staining for IFN-γ, TNF-α, IL-6, and IL-10 was performed. The frequency of IFN-γ-, TNF-α- or IL-17A-secreting cells in the CD28nullCD4+ Tang-cell subpopulation was compared with those in the paired CD28+CD4+ Tang-cell subpopulation. (i--j) Intracellular staining for cytotoxic granule proteins was performed. The frequency of granzyme B+ or perforin+ cells in either the CD28nullCD4+ or CD28+CD4+ Tang-cell populations was assessed. ∗P < 0.05; ∗∗P < 0.01. MFI, mean fluorescent intensity; PBMNCs, peripheral blood mononuclear cells; hTERT, human telomerase reverse transcriptase.
The dramatically elevated capacity to secrete pro-inflammatory cytokines is a prominent characteristic of senescent CD4+ T cell. PBMCs were stimulated with anti-CD3 antibody for 6 h, and intracellular staining for IFN-γ, TNF-α, IL-6, and IL-10 was performed. The frequency of IFN-γ, TNF-α, IL-6 or IL-10-secreting cells in the CD28nullCD4+ Tang cell population was compared with those in the paired CD28+CD4+ subpopulation. In this regard, we found that while the frequency of IFN-γ, TNF-α, or IL-6-secreting cells in the CD28nullCD4+ Tang cell population was significantly greater than that in the CD28+ subpopulation, there was no difference in the frequency of IL-10-secreting cells between the two subsets (Fig. 3, panels e--h).
We also evaluated the cytotoxic capacity of CD28nullCD4+ Tang cells vs. CD28+CD4+ Tang cells by intracellular staining of cytotoxic granule proteins, such as granzyme B and perforin, and we found that these cytotoxic granule proteins were highly expressed in the CD28nullCD4+ Tang cells (Fig. 3, panels i and j).
Taken together, these data suggest that CD28nullCD4+ Tang cells, presented typical T-cell senescent profile, might secrete pro-inflammatory cytokines and display higher cytotoxic function. Thus, they might participate in the pathophysiology of endothelial dysfunction through regulation of inflammation.
Increased systemic inflammation correlates with the accumulation of senescent Tang cells
Chronic low-grade inflammation is one of the most important physiopathologic mechanism of endothelial dysfunction. Low-grade inflammation mediated by production of pro-inflammatory cytokines is linked to dysfunctional senescence features of T cells. The senescent profile and pro-inflammatory functional phenotype of CD28nullCD4+ Tang cells from hypertensive patients suggests they might exert a pro-inflammatory, rather than a protective, effect on endothelial cells. However, the linkage between senescent CD4+ Tang cells and systemic inflammation in hypertensive patients remains largely unknown.
Thus, we performed a serological analysis for some common pro-inflammatory cytokines in hypertensive patients. Hypertensive patients with endothelial dysfunction had increased serum concentrations of IL-6, IL-17, IFN-γ, and TNF-α (Table 3 and Fig. 4, panels a--d). Linear regression analyses adjusted by age, sex and SBP revealed positive correlation of these proinflammatory cytokines in hypertensive patients with the accumulation of senescent CD4+ Tang-cell subsets (Table 4 and Fig. 4, panels e--h).
TABLE 3.
Serum levels of cytokines in hypertensive patients
| Cytokine | HT without ED (N = 32) | HT with ED (N = 48) | P value |
| CRP (mg/l) | 0.76 (0.72–0.81) | 0.88 (0.78–1.04) | 0.0108 |
| IL-2 (pg/ml) | 2.67 (2.32–2.97) | 2.72 (2.49–2.98) | 0.5900 |
| IL-4 (pg/ml) | 1.83 (1.59–2.04) | 1.85 (1.58–2.11) | 0.8000 |
| IL-6 (pg/ml) | 0.93 (0.77–1.10) | 1.68 (1.47–1.10) | <0.0001 |
| IL-8 (pg/ml) | 5.39 (4.97–5.60) | 5.53 (4.75–5.86) | 0.6218 |
| IL-10 (pg/ml) | 0.79 (0.60–0.98) | 0.86 (0.69–0.99) | 0.1698 |
| IL-12 (pg/ml) | 1.53 (1.29–1.84) | 1.48 (1.13–1.79) | 0.5746 |
| IL-17 (pg/ml) | 4.22 (3.58–4.80) | 5.46 (4.97–6.18) | <0.0001 |
| IFN-γ (pg/ml) | 0.58 (0.38–0.75) | 1.20 (0.99–1.44) | <0.0001 |
| TNF-α (pg/ml) | 1.72 (1.50–1.96) | 1.99 (1.29–1.93) | <0.0001 |
| IL-1α (pg/ml) | 0.59 (0.45–0.74) | 0.56 (0.39–0.77) | 0.6762 |
| IL-1β (pg/ml) | 0.54 (0.46–0.62) | 0.57 (0.41–0.74) | 0.5257 |
| MCP-1 (pg/ml) | 106.31 (92.83–119.09) | 109.60 (93.07–123.58) | 0.5268 |
Data presented as median (interquartile range). Differences between groups were evaluated by the Mann--Whitney U test. CRP, C-reactive protein; ED, endothelial dysfunction; HT, hypertension.
∗P < 0.05 is considered significant.
FIGURE 4.
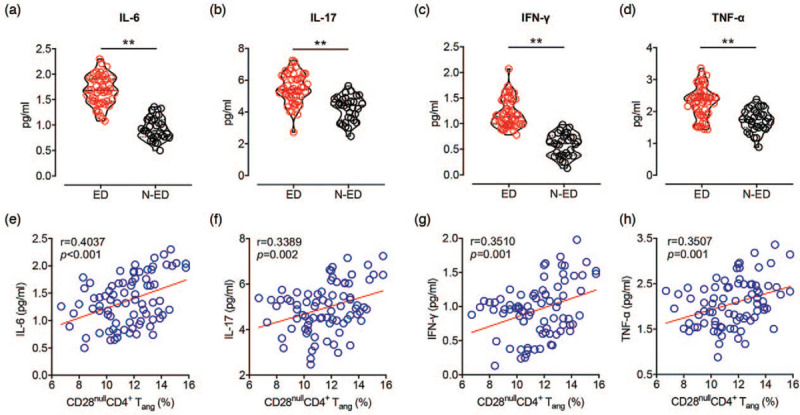
Inflammatory profile is exhibited in the serum of hypertensive patients with endothelial dysfunction and correlates with frequency of CD28nullCD4+ Tang cells. (a--d) Serological analysis of IL-6, IL-17, IFN-γ and TNF-α in hypertensive patients with endothelial dysfunction (ED) (n = 48) and without endothelial dysfunction (N-ED) (n = 32). (e--h) Correlation between serum cytokines s and frequency of CD28nullCD4+ Tang cells were tested using Pearson's correlation test. ∗∗P < 0.01. ED, hypertensive patients with endothelial dysfunction; N-ED, hypertensive patients without endothelial dysfunction; Tang, angiogenic T cells.
TABLE 4.
Association of CD28nullCD4+ Tang cells with serum cytokine serum levels in hypertensive patients
| 95% CI | ||||
| Variable | β coefficient | Lower | Upper | P value |
| CD28nullCD4+ Tang cells | ||||
| IL-6 | 5.110 | 4.147 | 6.072 | 0.036∗ |
| IL-17 | 1.548 | 1.028 | 2.068 | 0.012∗ |
| IFN-γ | 5.543 | 4.451 | 6.636 | <0.01∗ |
| TNF-α | 3.503 | 2.518 | 4.488 | <0.01∗ |
Multiple backward linear regression analyses performed with each cytokine as the dependent variable and age, sex, SBP as covariates.
P < 0.05 is considered significant.
It has been reported that CD4+CD28− T cells does not possess the ability to secrete IL-17, the elevated IL-17 level in hypertensive patients with endothelial dysfunction might be a consequence of memory CD4+ T cells activated by IL-6. The frequency of IL-17+ subpopulation in memory CD4+ T cells (CD4+CD45RO+) were analyzed by flow cytometry. Data showed that frequency of IL17+ subpopulation in CD4+CD45RO+ T cells were elevated in hypertensive patients with endothelial dysfunction (Online Supplemental Figure 2).
Therefore, CD28nullCD4+ Tang cells with notable senescent and pro-inflammatory phenotype were associated with systemic inflammatory environment, which seem to be closely related to endothelial dysfunction in hypertension.
DISCUSSION
The present study provides the first evidence for the presence that the frequency of CD28null cells in the CD4+ Tang-cell population is associated with endothelial dysfunction in patients with hypertension. CD28nullCD4+ Tang cells presents senescent profile and high pro-inflammatory capacity, which might contribute to the immunopathologic mechanism of endothelial dysfunction and be considered as a novel biomarker.
Hypertension appears to have a complicated association with endothelial dysfunction, a phenotypical alteration of the vascular endothelium considered as the initial step in the morbidity of atherosclerosis [2]. In addition, endothelial dysfunction has been shown to be an independent predictor of cardiovascular events [3]. A recently discovered subpopulation of T cells, termed angiogenic T cells (Tang), possesses the ability to facilitate the repair of damaged endothelium and formation of new blood vessels [12]. Reduced Tang population has been previously described in rheumatoid arthritis [13], type 2 diabetes mellitus [15], hypertension-related cerebral small vessel disease [20] and other cardiovascular conditions. By contrast, levels were not reduced in people with SLE, only in the those with rheumatoid arthritis and cardiovascular risk factors [14]. Decreased Tang-cell levels were observed in hypertensive individuals with cerebral small vessel disease [20]. Miao et al.[14] reported increased CD8+ Tang-cell levels in people with SLE, but not CD4+ Tang or total CD3+ Tang cells. However, we did not find any changes in CD4+ or CD8+ Tang or total Tang cells between hypertensive patients with or without endothelial dysfunction. These data highlight the complexity of Tang cells in different pathophysiological states.
Loss of CD28 costimulatory molecule expression is a well known, senescent event of T cells, and CD28null T cells accumulate with age [21]. CD28null phenotype of T cells has been considered a marker of immunosenescence [10,21]. A role for senescent T cells in atherosclerotic diseases has been reported in several studies. Previous studies examining CD28nullCD4+ T cells as a senescent T-cell population suggested a role in promoting vascular inflammation in atherosclerotic diseases [22]. Lopez et al.[23] used CD28 expression to redefine the angiogenic T-cell population and found that CD28nullCD4+ Tang cells were notably increased in the SLE patients. In our study, we revealed that CD28nullCD4+ subset of Tang cells was remarkedly elevated in hypertensive patients with endothelial dysfunction. Furthermore, FMD, as the most widely used technique for the assessment of endothelial function in humans [24], was negatively associated only with the frequency of this subset. ROC curve indicates great diagnostic value of this subset in Tang cells for endothelial dysfunction. Logistic regression showed that level of CD28nullCD4+ Tang cells was an independent risk factor of endothelial dysfunction controlled for age, sex and SBP. As CD4+CD28− T cells have been reported to express C-X3-C Motif Chemokine Receptor 1 (CX3CR1) and endothelial cells are known to express its ligand C-X3-C Motif Chemokine Ligand 1 (CX3CL1), elevated level of plasma CX3CL1 in hypertensive patients with endothelial dysfunction suggested that CXC3CL1/CXC3CR1 axis might involve in regulating the harmful effect of CD4+CD28− Tang cells on endothelium, which is worth to be studied in our further studies (Supplementary Figure 1). Together, these data suggest that CD28nullCD4+ Tang cells might be functionally relevant to the pathogenesis of endothelial dysfunction. For these reasons, we focused on elucidating the functional capacity of CD28nullCD4+ Tang cells in hypertensive patients.
CD4+ subset within the CD28+-Tang in SLE patients presented with a senescent profile has been previously described recently [23]. In line with this, CD28null subset of CD4+ Tang cells in hypertensive patients showed a significant increase in CD57 expression and a remarked decline in CCR7 and CD27 expressions. Moreover, it was shown that CD28nullCD4+ Tang cells have reduced hTERT expression. These data indicate prominent immunosenescent profile.
Elevated production of abundant atypical pro-inflammatory cytokines is a typical feature of senescent T cells [6,25]. Previous publications suggested that they could enhance autoimmunity through the production of pro-inflammatory cytokines that might activate autoreactive T cells [26]. It also has been shown that CD28nullCD4+ T cells, which produced large amounts of inflammatory cytokines, can be isolated from atherosclerotic plaque [27]. Thus, we attempted to investigate the pro-inflammatory cytokines produced by CD28nullCD4+ Tang cells. We hypothesized that the CD28nullCD4+ Tang-cell subset reported in the present study might also exhibit abnormal secretory. Multiparameter flow cytometric analysis revealed that high frequencies of IFN-γ, TNF-α and IL-6-secreting cells were observed in the CD28null subset of CD4+ Tang cells rather than CD28+ subpopulation.
Chronic inflammation is one the most critical mechanisms of endothelial dysfunction [4,8]. Patients with chronic inflammatory diseases, such as rheumatoid arthritis [28], inflammatory bowel diseases [29], lupus [30] and others, manifest endothelial dysfunction, often in the early phase of the disease. Increased inflammatory markers have been reported linked with decreased FMD and linked to a wide variety of major adverse cardiovascular events [8]. According to our experiments, elevated levels of pro-inflammatory mediators, IL-6, IL-17, IFN-γ, and TNF-α are observed systemically in hypertensive patients with endothelial dysfunction. Systemic production of pro-inflammatory mediators is linked to the gathering of phenotypic and dysfunctional senescence features of T cells. Increased systemic levels of pro-inflammatory cytokines in hypertension mentioned above correlated significantly with the accumulation of CD28nullCD4+ Tang cells.
Limitations
Although we demonstrated a relationship between the frequency of senescent CD4+ Tang cells and endothelial dysfunction in hypertension, the current data must be cautiously interpreted. First, the present study was cross-sectional and observational. We could not examine the frequency of CD28nullCD4+ Tang cells prior to occurrence of endothelial dysfunction. The frequencies of CD28nullCD4+ Tang cells before and after occurrence of endothelial dysfunction must be compared in the future. Second, further basic experiments exploring the inflammatory injury effect of CD28nullCD4+ Tang cells on vascular endothelium would provide a more valuable perspective for the role of this Tang cell subset in hypertension and endothelial dysfunction. Third, the small size of the study population restricted a precise analysis on the independent incremental value of CD28nullCD4+ Tang cells as a prognostic factor for endothelial dysfunction over other traditional risk factors in hypertensive patients.
In conclusion, we identified a link between the frequency of CD28nullCD4+ Tang cells and endothelial dysfunction in patients with hypertension. CD28nullCD4+ Tang cells exhibit senescent profile and pro-inflammatory property. This specific subset of CD4+ Tang cells might contribute to endothelial injury via producing high level of pro-inflammatory cytokines. Identification of a pathogenic CD4+ Tang-cell subset lacking CD28 may offer new opportunities for the prevention and treatment of endothelial dysfunction.
ACKNOWLEDGEMENTS
We thank the patients and healthy volunteers involved in this study and also thank the entire Department of Hypertension and vascular disease staff.
We are indebted to Dr Changyou Wu and his laboratory members for their technical assistance with flow cytometry measurement.
Sources of funding: This work was supported by the China Postdoctoral Science Foundation (2019M663309), the National Nature Science Foundation of China (31530023, 81671379), the National Natural Science Foundation of Guangdong (2018A030313807), the Medical Science Foundation of Guangdong (A2020339) and the Project of Guangdong Administration of Traditional Chinese Medicine (20201196).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Gaoxing Zhang, Yuanya Liu, and Yumin Qiu contributed equally to this article.
Abbreviations: CI, confidence interval; CRP, C-reactive protein; ED, endothelial dysfunction; eGFR, estimated glomerular filtration rate; ESC/ESH, European Society of Cardiology/European Society of Hypertension; FMD, flow-mediated dilation; HDL, high-density lipoprotein; hTERT, human telomerase reverse transcriptase; IFN-γ, interferon gamma; LDL, low-density lipoprotein; MFI, mean fluorescent intensity; ROC, receiver-operating characteristic curve; SLE, systemic lupus erythematosus; Tang, angiogenic T cells; TNF-α, tumor necrosis factor-alpha
Supplemental digital content is available for this article.
REFERENCES
- 1.Wang C, Yuan Y, Zheng M, Pan A, Wang M, Zhao M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol 2020; 75:2921–2930. [DOI] [PubMed] [Google Scholar]
- 2.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Endothelial function is impaired in patients receiving antihypertensive drug treatment regardless of blood pressure Level: FMD-J Study (Flow-Mediated Dilation Japan). Hypertension 2017; 70:790–797. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf) 2017; 219:22–96. [DOI] [PubMed] [Google Scholar]
- 4.Zanoli L, Briet M, Empana JP, Cunha PG, Maki-Petaja KM, Protogerou AD, et al. Association for Research into Arterial Structure, Physiology (ARTERY) Society, The European Society of Hypertension (ESH) Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J Hypertens 2020; 38:1682–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017; 70:660–667. [DOI] [PubMed] [Google Scholar]
- 6.Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol 2019; 19:573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantsulaia I, Ciszewski WM, Niewiarowska J. Senescent endothelial cells: potential modulators of immunosenescence and ageing. Ageing Res Rev 2016; 29:13–25. [DOI] [PubMed] [Google Scholar]
- 8.Castellon X, Bogdanova V. Chronic inflammatory diseases and endothelial dysfunction. Aging Dis 2016; 7:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol 2018; 19:10–19. [DOI] [PubMed] [Google Scholar]
- 10.Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol 2016; 9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013; 62:126–133. [DOI] [PubMed] [Google Scholar]
- 12.Berger S, Aronson D, Lavie P, Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med 2013; 187:90–98. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Carrio J, Alperi-Lopez M, Lopez P, Alonso-Castro S, Ballina-Garcia FJ, Suarez A. Angiogenic T cells are decreased in rheumatoid arthritis patients. Ann Rheum Dis 2015; 74:921–927. [DOI] [PubMed] [Google Scholar]
- 14.Miao J, Qiu F, Li T, Zhao P, Zhang K, Lv M, et al. Circulating angiogenic T cells and their subpopulations in patients with systemic lupus erythematosus. Mediators Inflamm 2016; 2016:2842143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Boer SA, Reijrink M, Abdulahad WH, Hoekstra ES, Slart R, Heerspink HJL, et al. Angiogenic T cells are decreased in people with type 2 diabetes mellitus and recruited by the dipeptidyl peptidase-4 inhibitor Linagliptin: a subanalysis from a randomized, placebo-controlled trial (RELEASE study). Diabetes Obes Metab 2020; 22:1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018; 39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Ding ML, Wu F, He W, Li J, Zhang XY, et al. Impaired endothelial repair capacity of early endothelial progenitor cells in hypertensive patients with primary hyperaldosteronemia: role of 5,6,7,8-tetrahydrobiopterin oxidation and endothelial nitric oxide synthase uncoupling. Hypertension 2016; 67:430–439. [DOI] [PubMed] [Google Scholar]
- 19.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation 2005; 111:70–75. [DOI] [PubMed] [Google Scholar]
- 20.Rouhl RP, Mertens AE, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Henskens LH, et al. Angiogenic T-cells and putative endothelial progenitor cells in hypertension-related cerebral small vessel disease. Stroke 2012; 43:256–258. [DOI] [PubMed] [Google Scholar]
- 21.Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009; 30:306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liuzzo G, Goronzy JJ, Yang H, Kopecky SL, Holmes DR, Frye RL, Weyand CM. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation 2000; 101:2883–2888. [DOI] [PubMed] [Google Scholar]
- 23.Lopez P, Rodriguez-Carrio J, Martinez-Zapico A, Caminal-Montero L, Suarez A. Senescent profile of angiogenic T cells from systemic lupus erythematosus patients. J Leukoc Biol 2016; 99:405–412. [DOI] [PubMed] [Google Scholar]
- 24.Maruhashi T, Kihara Y, Higashi Y. Assessment of endothelium-independent vasodilation: from methodology to clinical perspectives. J Hypertens 2018; 36:1460–1467. [DOI] [PubMed] [Google Scholar]
- 25.Vallejo AN. CD28 extinction in human T cells: altered functions and the program of T-cell senescence. Immunol Rev 2005; 205:158–169. [DOI] [PubMed] [Google Scholar]
- 26.Thewissen M, Somers V, Hellings N, Fraussen J, Damoiseaux J, Stinissen P. CD4+CD28null T cells in autoimmune disease: pathogenic features and decreased susceptibility to immunoregulation. J Immunol 2007; 179:6514–6523. [DOI] [PubMed] [Google Scholar]
- 27.Ammirati E, Monaco C, Norata GD. Antigen-dependent and antigen-independent pathways modulate CD4+CD28null T-cells during atherosclerosis. Circ Res 2012; 111:e48–e49. [DOI] [PubMed] [Google Scholar]
- 28.Bordy R, Quirie A, Marie C, Wendling D, Totoson P, Demougeot C. Vascular arginase is a relevant target to improve cerebrovascular endothelial dysfunction in rheumatoid arthritis: evidence from the model of adjuvant-induced arthritis. Transl Stroke Res 2020; 11:4–15. [DOI] [PubMed] [Google Scholar]
- 29.Gravina AG, Dallio M, Masarone M, Rosato V, Aglitti A, Persico M, et al. Vascular endothelial dysfunction in inflammatory bowel diseases: pharmacological and nonpharmacological targets. Oxid Med Cell Longev 2018; 2018:2568569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan HC, Chan HC, Liang CJ, Lee HC, Su H, Lee AS, et al. Role of low-density lipoprotein in early vascular aging associated with systemic lupus erythematosus. Arthritis Rheumatol 2020; 72:972–984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


