Abstract
Purpose of review
Schizophrenia is a heterogeneous psychiatric disorder with a different, but not necessarily milder clinical presentation in women as compared to men. These sex differences have largely been attributed to the protective role of estrogens. This article reviews the current state of estrogen research in schizophrenia.
Recent findings
Estrogens regulate important pathophysiological pathways in schizophrenia, including dopamine activity, mitochondrial function, and the stress system. Estrogen deficiency is common in both sexes and is associated with increases in psychotic symptoms. Hyperprolactinemia causes secondary estrogen deficiency and can be a reaction to stress, or secondary to prolactin-raising antipsychotics. Therefore, prolactin-sparing antipsychotics should be preferred especially in premenopausal women, who are more prone to hyperprolactinemia. Premenopausal women furthermore require lower doses of antipsychotics than men, since estrogens raise the availability and efficacy of antipsychotics.
Summary
The past years have established the importance of estrogens in the pathophysiology of schizophrenia and have shown its relevance to clinical practice through its influence on antipsychotic drug efficacy. Future research should focus on the neurobiological and clinical effect of contraceptives in premenopausal women with schizophrenia. Furthermore, the potential of estrogen-like augmentation with raloxifene and phytoestrogens in schizophrenia should be established in the coming years.
Keywords: estrogen, psychosis, schizophrenia, sex differences, sex steroids
INTRODUCTION
Robust sex disparities are recognized in schizophrenia incidence, age of onset, risk factors, symptomatology, and disease course [1▪▪]. Men experience more severe negative and cognitive symptoms and perform worse on social functioning, whereas affective symptoms, self-harm, and suicide attempts are more frequent among women [1▪▪,2,3]. This different presentation can cause diagnostic delay in women [1▪▪]. Despite earlier claims of a more benign clinical course, recent studies showed that women need just as many re-hospitalizations as men [1▪▪] and a meta-analysis found similar recovery rates for women (12.9%) and men (12.1%) [4]. Although women initially achieve better recovery, advantages on the long term are less evident [5]. Several factors may be associated with these sex disparities in schizophrenia, such as the faster brain development in women, different gender roles, differences in personality and different risk factors [1▪▪,6,7]. For example, both childhood trauma and substance abuse are associated with the development of schizophrenia, but the occurrence of these triggering factors differs largely between the sexes [1▪▪,8].
The past years provided mounting evidence that the clinical differences between men and women with schizophrenia can partly be attributed to estrogens [9–12,13▪]. Evidence for this estrogen hypothesis is at least twofold. On the one hand, women are increasingly vulnerable to psychosis onset and experience more severe symptoms during low estrogenic phases (e.g. after menopause) [6,11,12]. On the other hand, estrogens have direct neuroprotective actions, for example by decreasing neuro-inflammation, promoting synaptic plasticity, and by influencing major neurotransmitter systems relevant for schizophrenia, such as dopamine signaling [12,14–16]. Through their influence on dopaminergic signaling, estrogens reduce impulsivity and risk for substance abuse in women [9–12,16]. In the current review, we summarize recent work regarding the role of estrogens in the pathology and treatment of schizophrenia. Specifically, we discuss new findings regarding the major neurobiological mechanisms underlying the estrogen hypothesis, as well as developments in the role of estrogens in treatment strategies. We further summarize recent implications of estrogens for clinical practice.
Box 1.

no caption available
Neurobiological action of estrogens in schizophrenia
There are three types of estrogen, of which estradiol (estradiol-17β) has the highest concentration in the brain [17]. Estrogens can exert direct neuroprotective effects via genomic and nongenomic mechanisms (Fig. 1) [18]. Genomic actions involve activation of the estrogen receptor alpha (ERα) or beta (ERβ). Upon binding, these receptors promote gene expression of antiapoptotic genes and neuroprotective growth factors, whereas the expression of pro-inflammatory molecules is repressed [17,19]. Previous studies have shown that genetic variants of the ERα coding gene (ESR1) are associated with schizophrenia development and symptomatology [10,20,21]. For example, the CC-homozygote of single nucleotide polymorphism (SNP) rs2234693 is more common in schizophrenia and has been associated with more severe general symptoms, whereas T-allele carriers have an earlier age of onset [10,20]. A genetic variant of the same gene, SNP rs2144025, is associated with specific symptoms, such as grandiose delusions and alogia [21]. Estrogens can engage in rapid nongenomic signaling events through activation of ERα/β, but specifically through binding to the G protein-coupled estrogen receptor (GPER) (Fig. 1) [22▪]. GPERs are for example highly expressed in hippocampal neurons where they activate pathways involved in cognitive functioning [23].
FIGURE 1.
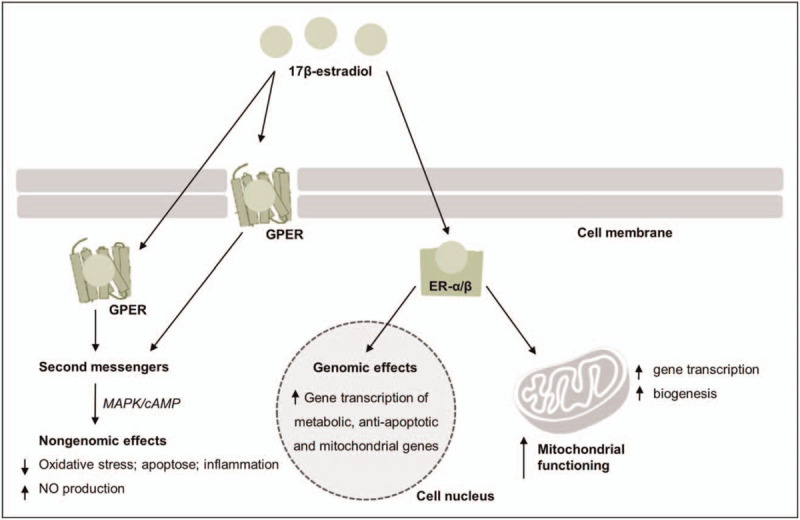
A simplified overview of the putative mechanisms involved in estrogens genomic and nongenomic actions. Genomic mechanisms involve activation of the estrogen receptors (ERα/β) by estrogen, which then translocate either to the cell nucleus or to the mitochondria resulting in transcription activation in the cell nucleus or in the mitochondria. Nongenomic mechanisms involve binding of estrogens to ERs or to a G protein coupled receptor (GPER), either at the plasma membrane or intracellularly to activate second messenger systems, such as those involving mitogen-activated protein kinase (MAPK) or cyclic adenosine 3’,5’-monophosphate (cAMP) pathways. ERα/β activation can ameliorate mitochondrial activity directly by enhancing mitochondrial functioning through promoting gene transcription of mitochondrial DNA and indirectly, by promoting gene transcription of mitochondrial and metabolic genes in the cell nucleus. NO, nitric oxide.
Estrogens and dopamine
Although hyperactive dopamine signaling is known as a central mechanism affected in schizophrenia [24,25], its association with estrogens is less known. Dopaminergic function is regulated by estrogens in several ways (Fig. 2) [16,26–29]. Estrogens increase dopamine sensitivity of dopamine D2/D3 receptors in the ventral tegmental area (VTA) [30], which is part of the mesolimbic (associated with positive symptoms) and the mesocortical pathway (associated with negative and cognitive symptoms) [24,31]. By increasing dopamine sensitivity in the VTA, estrogens can thus reduce psychotic symptoms.
FIGURE 2.
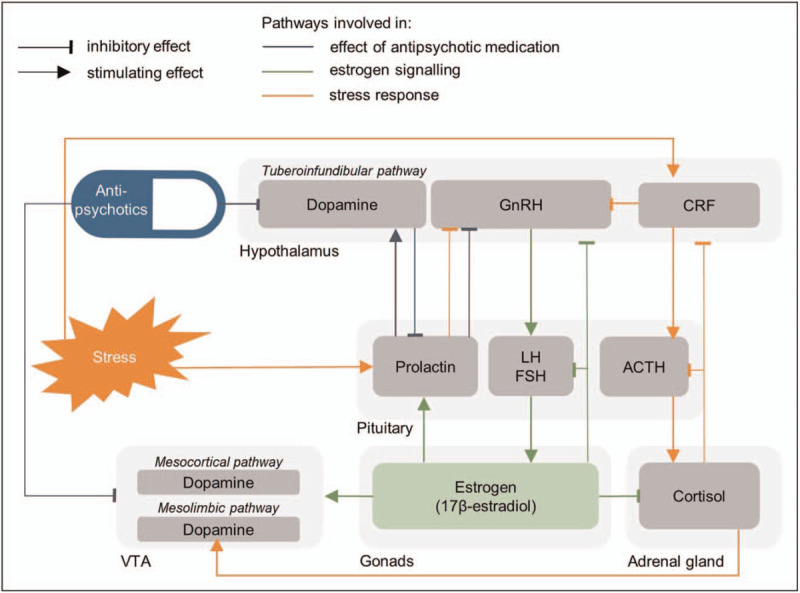
A simplified schematic overview of the interaction between estrogen signaling in orange, the stress response in red and antipsychotic effects in blue. ACTH, Adrenocorticotropic hormone; CRF, cortisol releasing factor; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
Estrogens also regulate dopamine activity through Catechol-O-methyltransferase (COMT), an enzyme that degrades dopamine (Fig. 3) [32,33]. Since estrogens inhibit COMT gene transcription [34▪,35], estrogen deficiency can lead to increased COMT activity and decreased dopaminergic functioning. Moreover, recent studies show that COMT inhibits estrogen activity [34▪,36,37].
FIGURE 3.
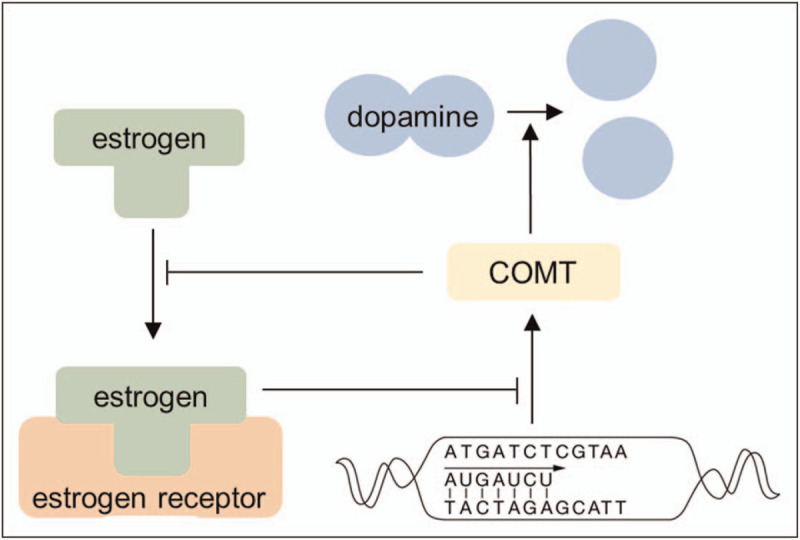
A simplified overview of the putative interaction between estrogens and Catechol-o-methyltransferase (COMT). Estrogens decrease COMT expression, inhibiting effect COMT gene transcription through activation of the estrogen receptor. COMT enzyme inhibits estrogen activity, through binding to estrogen.
Estrogens and the mitochondria
Mitochondrial deficits have been implicated as an important schizophrenia risk factor [38,39]. Mitochondria are the main providers of energy for cellular activities and mitochondrial deficits impair synaptic signaling, neurotransmission, and neurodevelopment [22▪,39]. Recent research revealed that estrogens ameliorate mitochondrial activity directly by promoting gene transcription of mitochondrial DNA and indirectly by promoting gene transcription of mitochondrial genes in the cell nucleus (Fig. 1) [22▪]. Animal studies show that females have better mitochondrial function than males, reflected in increased mitochondrial biogenesis, oxidative capacity, and antioxidant defense as well as less release of mitochondrial apoptotic factors) [22▪,40]. Preclinical and clinical studies indicate that augmentation with plant-derived estrogens (i.e. phytoestrogens) improves mitochondrial functionality and decreases oxidative stress [22▪]. However, additional research is required to extend these findings to schizophrenia patients.
Estrogens and response to stress
Estrogens influence the activity of the hypothalamic-pituitary-adrenal axis (HPA-axis) and thereby contribute to the intrinsic differences in stress responses that are found between healthy men and women [41,42▪]. Higher estrogen levels constitute a protective effect that modulates the stress response, whereas estrogen deficiency leads to increased activity (i.e. dysregulation) of the HPA-axis [43▪▪,44–46]. In schizophrenia, both sexes show a lower response to acute elevations in cortisol that may result from higher baseline cortisol levels [46]. This blunted stress response is more evident in male patients [47], whereas in female patients, low estrogen phases are associated with decreased stress resilience and increased HPA-axis dysregulation [48,49].
The role of estrogens in stress vulnerability is also reflected in the sex-dependent reaction to childhood trauma [50]. In schizophrenia, a history of childhood trauma results in sex-specific illness trajectories. Although both sexes have an earlier age of onset in case of trauma, female patients show more depressive symptoms, whereas male patients show more negative symptoms [50]. Moreover, a more pronounced impact on longer-term outcome was observed in men. Childhood trauma thus seems to amplify the typical sex-specific expression of symptoms, possibly mediated by estrogens effects on the HPA-axis [45,50]. To make things more complicated, the type of trauma also differs between girls and boys, with girls more often experiencing traumatic situations involving caregivers, which constitutes trauma that is especially difficult to cope with [50].
Estrogen deficiency and hyperprolactinemia
Estrogen deficiency is common in both women and men with schizophrenia over the course of their disease; in prodromal and untreated phases of psychosis as well as in chronic schizophrenia [6,9,11,51–54]. On a clinical level, psychotic vulnerability is increased in women during low estrogenic periods (e.g. in the premenstrual cycle phase or after menopause) [55,56▪]. Simultaneously with the menopausal drop of estrogen levels, both prevalence and relapse rates of psychosis rise, whereas psychotic symptoms decrease when estrogen levels are high, for example during pregnancy [57–60]. A recent study furthermore showed that later menarche in women at clinical high risk (CHR) for psychosis (i.e. later rise of estrogen levels) was associated with abnormalities in hippocampal connectivity [61▪]. Similarly, earlier studies indicate that later menarche is associated with an earlier onset of schizophrenia [62,63]. However, the larger literature reports contradicting evidence, including two cohort studies [64,65]. Although men naturally have lower estrogen levels than women, several studies found that men with schizophrenia have even lower levels of estrogens compared to healthy men [51,52]. In addition, male patients have lower levels of testosterone (which is converted to estrogens in the brain) [66,67].
Estrogen defiency is often the result of hyperprolactinemia, which in itself can have different causes [63,64]. Antipsychotic medication can cause hyperprolactinemia (see Table 1 for an overview based on Peuskens et al.[68] and Gonzalez-Rodriguez et al.[69▪]), since dopamine inhibits prolactin production via the tuberoinfundibular pathway (Fig. 2) [69▪]. However, hyperprolactinemia is also observed in antipsychotic-naïve patients of both sexes with (prodromal) psychosis [70–75,76▪▪]. It is hypothesized that this primary hyperprolactinemia is a result of the stress response system, since stress can induce the secretion of prolactin [9]. Prolactin in turn stimulates dopamine secretion, which can induce psychotic symptoms (Fig. 2) [63]. Within this framework, primary estrogen deficiency results from stress-induced increased prolactin secretion. This hypothesis is in line with recent studies showing that higher prolactin levels are associated with more severe psychotic [76▪▪] and negative [73] symptoms in these treatment naïve patients. Other studies showed, conversely, that prolactin levels are inversely associated with treatment response [77–79]. Whether and how prolactin is involved as a pathophysiologic factor in schizophrenia thus remains not fully understood.
Table 1.
Estimated effects of antipsychotics on prolactin levels
| Antipsychotic | Estimated prolactin elevation |
| Aripiprazole | 0 |
| Quetiapine | 0/+ |
| Asenapine | + |
| Clozapine | + |
| Sertindole | + |
| Ziprasidone | + |
| Lurasidone | +/++ |
| Olanzapine | ++ |
| Amisulpride | +++ |
| Haloperidol | +++ |
| Paliperidone | +++ |
| Risperidone | +++ |
0, minimal to no risk; +, low risk; ++, moderate risk; +++, high risk.
Although hyperprolactinemia is well known for its side-effects (e.g. Galactorrhea, cessation of normal cyclic ovarian function and hirsutism) its effect on estrogen levels is less well known, but at least as severe. The resulting secondary estrogen deficiency can lead to serious side-effects such as sexual dysfunction, decreased libido and infertility, but also to increased risk for osteoporosis and cardiovascular morbidity [80]. It has been suggested that antipsychotic-induced hyperprolactinemia is a potential explanation for the decreased biological fertility that is observed in women and men with schizophrenia [81]. In women, prolactin inhibits follicle stimulating hormone (FSH) via inhibition of gonadotropin-releasing hormone (GnRH), thereby preventing ovulation. In men, prolactin inhibits the release of gonadotropins in a similar way, which has a direct effect on spermatogenesis [82,83]. Interestingly, a recent review showed that exposure to prolactin-raising medication during pregnancy was associated with increased risks of congenital malformations, whereas prolactin-sparing medication was not [84]. Yet, more research is needed to establish the effect of antipsychotic use during pregnancy.
Taken together, hyperprolactinemia should be minimized as much as possible. This is especially true for women with intrinsicillay higher estrogen levels that should be maintained (i.e. premenopausal women), especially since they are most vulnerable to hyperprolactinemia compared to other women and men [69▪]. Prolactin-sparing medication should therefore be preferred over prolactin-raising medication (see Table 1). Interestingly, aripiprazole treatment even showed to have a reducing effect on prolactin levels [85,86].
Estrogens and antipsychotic treatment
Estrogens directly raise the plasma concentration of antipsychotic drugs by regulating enzymes that metabolize antipsychotics, which is most evident for clozapine and olanzapine [87▪]. These antipsychotic drugs are both metabolized in the liver by the same isozyme (CYP1A2), of which the activity is inhibited by estrogens. In the CNS, estrogens increase the sensitivity of dopamine D2 receptors and thus they augment the efficacy of (D2-receptor binding) antipsychotics [88,89]. Eugene and Misiak [89] showed that women require only half of the olanzapine dosage as compared to men to achieve equal occupacy of the dopamine D2 receptor. The authors attribute much of this effect to the modulation of D2 receptors by estrogen. Moreover, a recent review even argued that estrogen may act as an antipsychotic agent itself, considering that it targets dopamine signaling in a similar way as antipsychotics do [90▪].
Since estrogens raise the availability of antipsychotics, premenopausal women require lower antipsychotic dosages [91]. However, considering that psychotic symptoms increase during low estrogenic phases of the menstrual cycle [32,87▪,92], younger women can benefit from slight increases in antipsychotic doses during these phases, to prevent monthly exacerbations in their illness [56▪]. For women who are sensitive to these monthly symptom increases, oral estrogenic contraceptives may be helpful. Similarly, higher doses of antipsychotics are required after menopause, when symptoms increase and estrogen levels decline [32,86]. A recent meta-analysis showed that during pregnancy, when estrogen levels increase, decreased plasma levels are observed for quetiapine and aripiprazole, but not for olanzapine [93]. Pregnant women and their unborn child may therefore benefit from adjustments in the dosing of certain antipsychotics, to minimize the burden of side-effects of (overdosed) antipsychotics.
Despite some disagreement among clinical data, women are more vulnerable to side-effects of antipsychotics, including sexual dysfunction, cardiovascular effects and specific metabolic symptoms (i.e. weight gain and low HDL-cholesterol) as compared to men [94–97]. A large cross-sectional study [98] furthermore reported that women with schizophrenia are 134% more likely to develop metabolic syndrome, whereas male patients are 85% more likely to develop metabolic syndrome, when compared to healthy men and women, respectively. Importantly, antipsychotic-induced obesity adds to the risk of future metabolic dysfunction and cardiovascular problems. Another large trial [99] concluded that especially female patients taking olanzapine and clozapine represent a group at high risk for metabolic cardiovascular complications. This increased risk for adverse reactions may reflect the relative overdosage of antipsychotics female patients frequently experience [87▪,89]. In addition, side effects increase when antipsychotics are combined with other psychotropic drugs such as antidepressants, which is more common among women [1▪▪,94]. Clinicians should therefore be wary of prescribing antipsychotics that induce weight gain to both pre and postmenopausal women.
Estrogenic treatment
Estrogenic contraceptives are commonly prescribed to premenopausal women, including women with schizophrenia, yet little is known about their clinical effects. Estrogenic contraceptives stabilize but also lower endogeneous estrogen levels as they are counterbalanced with higher exogeneous estrogen levels [100,101]. Instead, progestogen-only contraception, such as medroxyprogesterone acetate, can lower both endogeneous and exogeneous estrogen levels [101,102]. Although so far the effect of contraceptives in schizophrenia has not been studied, two epidemiological studies demonstrated that especially progestogen-only contraceptives increase the risk of depressive symptoms and suicide in the general population [103–105]. Given the prevalent affective symptoms in women with schizophrenia [1▪▪], estrogenic contraceptives (e.g. ethinylestradiol) or combined contraceptives (e.g. ethinylestradiol/levonorgestel) should be preferred over progestogen-only contraception until further research has been done regarding the potential risks and benefits of contraceptive use in schizophrenia [13▪].
Double-blind, placebo-controlled, randomized trials provide evidence that estrogen augmentation is beneficial for women with schizophrenia [106–108], although not all clinical studies have reported significant effects (e.g. [109]). Estrogen treatment is less suitable for long-term treatment due to its considerable side effects on the sex organs of both sexes [13▪]. Estrogen augmentation has been mostly investigated during and after menopause, a period in which estrogenic therapies can target both psychotic and menopausal complaints such as sleep disturbances and mood swings [106,108,110]; complaints which in themselves increase the risk of psychotic relapse [9].
Selective estrogen receptor modulators (SERMs), such as raloxifene, are an alternative for estrogens that seems more suitable for long term use [19] since SERMs have estrogenic effects on the brain and bone tissue, whereas having antiestrogenic effects on other tissues (such as breast and uterus) [111]. A recent meta-analysis [112] revealed that raloxifene improves symptom severity in schizophrenia, although the results on cognition are less consistent [113,114]. To date, studies have been predominantly performed in postmenopausal women, although some studies also included men and younger women and showed the latter two groups also to benefit from raloxifene augmentation [115,116]. Yet, further studies are needed to confirm its efficacy in men and premenopausal women.
Several preliminary studies have identified genetic and hormonal biomarkers that may aid to predict the response to raloxifene. For example, raloxifene is more effective in improving cognitive symptoms when endogenous estrogen levels are low [117▪]. Additionally, Kindler et al.[118] showed that raloxifene increases activity in the prefrontal cortex during emotional response inhibition in both genders. Increased activity was greater in AA-homozygotes for rs9340799 of the ESR1 gene, relative to G carriers. These results were however not reflected by Labad et al., who performed a pharmacogenetic study on the effects of raloxifene in postmenopausal women [119]. Yet, the latter study did show that CC-homozygotes of the rs1042597 of the UGT1A8 gene showed more improvements in negative symptoms in response to raloxifene, when compared to G carriers. ESR1 rs2234693 genotype was furthermore associated with a distinct response in general psychopathology [119]. In addition, protein levels of specificity protein 4 that interacts with ERα are indicated as potential biomarker for raloxifene efficacy [120▪]. Although these initial results require replication, they suggest potential for future personalized pharmacotherapy.
Preclinical and translational studies indicate that phytoestrogens can also be a natural alternative to SERMs [43▪▪,121–123]. For example, the larvae of the mealworm Tenebrio Molitor contain high concentrations of phytoestrogens and have high antioxidant and anti-inflammatory effects [124]. Administration of these larvae in female ovariectomized mice restored estrogen deficiency in a similar way as artificial SERMs [43▪▪]. Moreover, HPA-axis deregulation was restored after treatment. Phytoestrogens, present in certain food products like tofu, can have both estrogenic and antiestrogenic effects. Therefore, they can have a more beneficial side-effect profile when compared to conventional estrogen augmentation. However, the transformation of phytoestrogens by intestinal microbiota is essential for reaping their beneficial effects, since the metabolites of phytoestrogens (e.g. equol) have more estrogenic/antiestrogenic and antioxidant activity than their precursors [125]. In this way, the health effects of phytoestrogens are strongly determined by the gut bacteria of each individual. More studies on the bioavailability and bioactivity of different types of phytoestrogens are essential to establish their function in schizophrenia, either as SERM augmentation therapy or as dietary product.
CONCLUSION
In summary, recent findings support the decisive role of estrogens in schizophrenia. Estrogens regulate clinical symptoms through their influence on with dopamine pathways, as well as by regulating mitochondrial functioning and the stress response system. Estrogen deficiency is common in schizophrenia and is often related to hyperprolactinemia in both medication-naïve and chronic patients. In order to minimize the risk of estrogen deficiency, prolactin-sparing antipsychotics (e.g. aripiprazole) should be preferred, especially in premenopausal women as they are more susceptible to estrogen deficiency following hyperprolactinemia. Since estrogens raise the availability of antipsychotic drugs, which needs to be taken into account for the establishment of optimal starting doses in women. Furthermore, premenopausal women generally require lower drug dosages than men and postmenopausal women whereas women may require slight increases of their dose to prevent relapse of symptoms during low estrogenic phases. Furthermore, progestogen-only contraceptives are known to constitute permanent low estrogen levels in premenopausal women, which causes depressive symptoms in the general population. In order to preserve and protect natural estrogen levels, estrogenic contraceptives should be preferred over progestogen-only contraceptives. Although the past decade has firmly established the efficacy and safety of estrogen-like augmentation with raloxifene in postmenopausal women, forthcoming clinical trials should assess whether these findings extend to men and premenopausal women. With this approach, we expect that the protective role of estrogen will become increasingly important for schizophrenia treatment in the coming years.
Acknowledgements
We would like to thank Shiral Gangadin for his critical reading of the article. In addition, we acknowledge the valuable contribution of authors we have been unable to cite due to space constraints, as selections had to be made on the basis of relevancy and novelty.
Financial support and sponsorship
This study was sponsored by the Dutch Medical Research Organisation ZonMW, project number 686125.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Sommer IE, Tiihonen J, van Mourik A, et al. The clinical course of schizophrenia in women and men—a nation-wide cohort study. NPJ Schizophr 2020; 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]; A large cohort study that shows that schizophrenia has a different, but not necessarily milder course in women as compared to men.
- 2.Jongsma HE, Turner C, Kirkbride JB, Jones PB. International incidence of psychotic disorders, 2012-17: a systematic review and meta-analysis. Lancet Public Health 2019; 4:e229–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dama M, Veru F, Schmitz NS, et al. Sex differences in clinical and functional outcomes among patients treated in an early intervention service for psychotic disorders: an observational study. Can J Psychiatry 2019; 64:Article number 070674371985406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull 2013; 6:1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeman MV. Does gender influence outcome in schizophrenia? Psychiatr Q 2019; 90:173–184. [DOI] [PubMed] [Google Scholar]
- 6.Häfner H. Gender differences in schizophrenia. Psychoneuroendocrinology 2003; 28:17–54. [DOI] [PubMed] [Google Scholar]
- 7.Rössler W, Hengartner MP, Ajdacic-Gross V, et al. Sex differences in sub-clinical psychosis-results from a community study over 30years. Schizophr Res 2012; 139:176–182. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari A, Gonzalez A. Biological alterations affecting risk of adult psychopathology following childhood trauma: a review of sex differences. Clin Psychol Rev 2018; 66:69–79. [DOI] [PubMed] [Google Scholar]
- 9.Riecher-Rössler A. Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry 2017; 4:63–72. [DOI] [PubMed] [Google Scholar]
- 10.Weickert CS, Miranda-angulo AL, Wong J, et al. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet 2008; 17:2293–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markham JA. Sex steroids and schizophrenia. Rev Endocr Metab Disord 2012; 13:187–207. [DOI] [PubMed] [Google Scholar]
- 12.Gogos A, Sbisa AM, Sun J, et al. A role for estrogen in schizophrenia: clinical and preclinical findings. Int J Endocrinol 2015; 2015:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol 2019; 53:Article number 100743. [DOI] [PubMed] [Google Scholar]; This paper is the most recent one to extensively review current evidence of estrogen-like treatments in women with schizophrenia.
- 14.Gonçalves VF, Cuperfain AB, Kennedy JL. Sex differences in schizophrenia: estrogen and mitochondria. Neuropsychopharmacology 2019; 44:216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 2017; 95:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014; 231:1581–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespo-Castrillo A, Arevalo M-A. Microglial and astrocytic function in physiological and pathological conditions: estrogenic modulation. Int J Mol Sci 2020; 21:Article number 3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gogos A, van den Buuse M. Comparing the effects of 17β-oestradiol and the selective oestrogen receptor modulators, raloxifene and tamoxifen, on prepulse inhibition in female rats. Schizophr Res 2015; 168:634–639. [DOI] [PubMed] [Google Scholar]
- 19.Arevalo M-A, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 2015; 16:17–29. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Li W, Zhao J, et al. Association of estrogen receptor alpha gene polymorphism with age at onset, general psychopathology symptoms, and therapeutic effect of schizophrenia. Behav Brain Funct 2013; 9:Article number 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinsonneault JK, Frater JT, Kompa B, et al. Intronic SNP in ESR1 encoding human estrogen receptor alpha is associated with brain ESR1 mRNA isoform expression and behavioral traits. PLoS One 2017; 12:e0179020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪.Torrens-Mas M, Pons D-G, Sastre-Serra J, et al. Sexual hormones regulate the redox status and mitochondrial function in the brain. Pathological implications. Redox Biol 2020; 31:Article number 101505. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review article evalutates the important role of estrogens in mitochondrial function, and assessess the potential role of phytoestrogens in the treatment of brain pathologies by reviewing clinical and preclinical trials on this subject.
- 23.Kim J, Schalk JC, Koss WA, et al. Dorsal hippocampal actin polymerization is necessary for activation of G-protein-coupled estrogen receptor (GPER) to increase CA1 dendritic spine density and enhance memory consolidation. J Neurosci 2019; 39:9598–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstein JJ, Chohan MO, Slifstein MK, et al. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry 2017; 81:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards AC, Bacanu SA, Bigdeli TB, et al. Evaluating the dopamine hypothesis of schizophrenia in a large-scale genome-wide association study. Schizophr Res 2016; 176:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dluzen DE, McDermott JL, Liu B. Estrogen alters MPTP-induced neurotoxicity in female mice: effects on striatal dopamine concentrations and release. J Neurochem 2002; 66:658–666. [DOI] [PubMed] [Google Scholar]
- 27.Miller DB, Ali SF, O’Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci 1998; 844:153–165. [PubMed] [Google Scholar]
- 28.Kishi Y, Takahashi J, Koyanagi M, et al. Estrogen promotes differentiation and survival of dopaminergic neurons derived from human neural stem cells. J Neurosci Res 2005; 79:279–286. [DOI] [PubMed] [Google Scholar]
- 29.Küppers E, Ivanova T, Karolczak M, Beyer C. Estrogen: a multifunctional messenger to nigrostriatal dopaminergic neurons. J Neurocytol 2000; 29:375–3825. [DOI] [PubMed] [Google Scholar]
- 30.Vandegrift BJ, You C, Satta R, et al. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS One 2017; 12:e0187698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28:325–334. [DOI] [PubMed] [Google Scholar]
- 32.González-Castro TB, Hernández-Díaz Y, Juárez-Rojop IE, et al. The Role of a Catechol-O-Methyltransferase (COMT) Val158Met genetic polymorphism in schizophrenia: a systematic review and updated meta-analysis on 32,816 subjects. NeuroMol Med 2016; 18:216–231. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Song J, Yuan P, et al. Orientation and cellular distribution of membrane-bound catechol-O-methyltransferase in cortical neurons: implications for drug development. J Biol Chem 2011; 286:34752–34760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Dean B, Parkin GM, Gibbons AS. Associations between catechol-O-methyltransferase (COMT) genotypes at rs4818 and rs4680 and gene expression in human dorsolateral prefrontal cortex. Exp Brain Res 2020; 238:477–486. [DOI] [PubMed] [Google Scholar]; This study demonstrated that the levels of COMT were dependent on specific COMT genotypes that possess estrogen receptor elements and that their expression could thus be regulated by (catechol)estrogens, which are substrates for COMT that occupy and activate oestrogen receptors.
- 35.Dumas JA, Makarewicz JA, Bunn J, et al. Dopamine-dependent cognitive processes after menopause: the relationship between COMT genotype, estradiol, and working memory. Neurobiol Aging 2018; 72:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schütze N, Vollmer G, Tiemann I, et al. Catecholestrogens are MCF-7 cell estrogen receptor agonists. J Steroid Biochem Mol Biol 1993; 46:781–789. [DOI] [PubMed] [Google Scholar]
- 37.Parkin GM, Udawela M, Gibbons A, et al. Catechol-O-methyltransferase (COMT) genotypes are associated with varying soluble, but not membrane-bound COMT protein in the human prefrontal cortex. J Hum Genet 2018; 63:1251–1258. [DOI] [PubMed] [Google Scholar]
- 38.Cuperfain AB, Zhang ZL, Kennedy JL, Gonçalves VF. The complex interaction of mitochondrial genetics and mitochondrial pathways in psychiatric disease. Mol Neuropsychiatry 2018; 4:52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni P, Chung S. Mitochondrial dysfunction in schizophrenia. BioEssays 2020; 42:Article number 1900202. [DOI] [PubMed] [Google Scholar]
- 40.Ventura-Clapier R, Moulin M, Piquereau J, et al. Mitochondria: a central target for sex differences in pathologies. Clin Sci 2017; 131:803–822. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein JM, Jerram M, Abbs B, et al. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 2010; 30:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Zuloaga DG, Heck AL, De Guzman RM, Handa RJ. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol Sex Differ 2020; 11:Article number 44. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study comprehensively reviews the pivotal role of estrogens in regulating the HPA-axis and the behavioral responses to stress.
- 43▪▪.Kim G-H, Baek HK, Lee JS, et al. Chronic oral administration of tenebrio molitor extract exhibits inhibitory effect on glucocorticoid receptor overexpression in the hippocampus of ovariectomy-induced estrogen deficient mice. J Food Sci 2019; 84:687–694. [DOI] [PubMed] [Google Scholar]; A pivotal preclinical study that was the first to demonstrate that Tenebrio molitor extract of the yellow mealworm possesses antiosteoporotic and neuroprotective effects in ovariectomized mice.
- 44.Goldstein JM, Lancaster K, Longenecker JM, et al. Sex differences, hormones, and fMRI stress response circuitry deficits in psychoses. Psychiatry Res Neuroimaging 2015; 232:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol 2014; 35:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pruessner M, Cullen AE, Aas M, Walker EF. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci Biobehav Rev 2017; 73:191–218. [DOI] [PubMed] [Google Scholar]
- 47.Zorn JV, Schür RR, Boks MP, et al. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 2017; 77:25–36. [DOI] [PubMed] [Google Scholar]
- 48.Riecher-Rössler A, Butler S, Kulkarni J. Sex and gender differences in schizophrenic psychoses—a critical review. Arch Womens Ment Health 2018; 21:627–648. [DOI] [PubMed] [Google Scholar]
- 49.Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015; 59:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pruessner M, King S, Vracotas N, Abadi S, et al. Gender differences in childhood trauma in first episode psychosis: association with symptom severity over two years. Schizophr Res 2019; 205:30–37. [DOI] [PubMed] [Google Scholar]
- 51.Halari R, Kumari V, Mehrotra R, et al. The relationship of sex hormones and cortisol with cognitive functioning in schizophrenia. J Psychopharmacol 2004; 18:366–374. [DOI] [PubMed] [Google Scholar]
- 52.Doğan Bulut S, Bulut S. The relationship between sex hormone profiles and symptoms of schizophrenia in men. Compr Psychiatry 2016; 69:186–192. [DOI] [PubMed] [Google Scholar]
- 53.Teresa Pons-Cabrera M, Sagué-Vilavella M, et al. Hypothalamic-pituitary-gonadal axis hormones in antipsychotic naïve first-episode of psychosis and healthy controls. Abstract Schizophrenia International Research Society (SIRS) 2020 Congress. Schizophr Bull 2020; 46: Suppl 1: S236. [Google Scholar]
- 54.Maric N, Popovic V, Jasovic-Gasic M, et al. Cumulative exposure to estrogen and psychosis: a peak bone mass, case-control study in first-episode psychosis. Schizophr Res 2005; 73:351–355. [DOI] [PubMed] [Google Scholar]
- 55.Gurvich C, Gavrilidis E, Worsley R, et al. Psychoneuroendocrinology menstrual cycle irregularity and menopause status in fluence cognition in women with schizophrenia. Psychoneuroendocrinology 2018; 96:173–178. [DOI] [PubMed] [Google Scholar]
- 56▪.Reilly TJ, Sagnay de la Bastida VC, Joyce DW, et al. Exacerbation of psychosis during the perimenstrual phase of the menstrual cycle: systematic review and meta-analysis. Schizophr Bull 2020; 46:78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent meta-analysis that showed an increased rate of admissions of psychosis during the perimenstrual phase of the menstrual cycle.
- 57.Searles S, Makarewicz JA, Dumas JA. The role of estradiol in schizophrenia diagnosis and symptoms in postmenopausal women. Schizophr Res 2018; 196:35–38. [DOI] [PubMed] [Google Scholar]
- 58.da Silva TL, Ravindran AV. Contribution of sex hormones to gender differences in schizophrenia: a review. Asian J Psychiatr 2015; 18:2–14. [DOI] [PubMed] [Google Scholar]
- 59.González-Rodríguez A, Guàrdia A, Álvarez Pedrero A, et al. Women with schizophrenia over the life span: health promotion, treatment and outcomes. Int J Environ Res Public Health 2020; 17:Article number 5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grigoriadis S, Seeman MV. The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Can J Psychiatry 2002; 47:437–442. [DOI] [PubMed] [Google Scholar]
- 61▪.Damme KSF, Ristanovic I, Vargas T, Mittal VA. Timing of menarche and abnormal hippocampal connectivity in youth at clinical-high risk for psychosis. Psychoneuroendocrinology 2020; 117:Article number 104672. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important study showing that later menarche in women at clinical high risk (CHR) for psychosis (i.e. later rise of estrogen levels) is associated with abnormalities in hippocampal connectivity.
- 62.Cohen RZ, Seeman MV, Gotowiec A, Kopala L. Earlier puberty as a predictor of later onset of schizophrenia in women. Am J Psychiatry 1999; 156:1059–1064. [DOI] [PubMed] [Google Scholar]
- 63.Galdos PM, van Os JJ, Murray RM. Puberty and the onset of psychosis. Schizophr Res 1993; 10:7–14. [DOI] [PubMed] [Google Scholar]
- 64.Boden JM, Fergusson DM, Horwood LJ. Age of menarche and psychosocial outcomes in a New Zealand birth cohort. J Am Acad Child Adolesc Psychiatry 2011; 50:132–140.e5. [DOI] [PubMed] [Google Scholar]
- 65.Welham J, Scott J, Williams G, et al. Emotional and behavioural antecedents of young adults who screen positive for nonaffective psychosis: a 21-year birth cohort study. Psychol Med 2009; 39:625–634. [DOI] [PubMed] [Google Scholar]
- 66.Lodha P, Karia S. Testosterone and schizophrenia: a clinical review. Ann Indian Psychiatry 2019; 3:92. [Google Scholar]
- 67.Misiak B, Frydecka D, Loska O, et al. Testosterone, DHEA and DHEA-S in patients with schizophrenia: a systematic review and meta-analysis. Psychoneuroendocrinology 2018; 89:92–102. [DOI] [PubMed] [Google Scholar]
- 68.Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014; 28:421–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69▪.González-Rodríguez A, Labad J, Seeman MV. Antipsychotic-induced Hyperprolactinemia in aging populations: prevalence, implications, prevention and management. Prog Neuro-Psychopharmacol Biol Psychiatry 2020; 101:109941. [DOI] [PubMed] [Google Scholar]; An important narrative review that emphasizes the importance of avoiding anitpsychotic-induced hyperprolactinemia.
- 70.Delgado-Alvarado M, Tordesillas-Gutierrez D, Ayesa-Arriola R, et al. Plasma prolactin levels are associated with the severity of illness in drug-naive first-episode psychosis female patients. Arch Womens Ment Health 2019; 22:367–373. [DOI] [PubMed] [Google Scholar]
- 71.Ittig S, Studerus E, Heitz U, et al. Estradiol production suppressed by prolactin in at-risk mental state and first episode psychosis female patients? Preliminary results. Eur Psychiatry 2017; 41:S267–S1267. [Google Scholar]
- 72.González-Blanco L, Greenhalgh AMD, Garcia-Rizo C, et al. Prolactin concentrations in antipsychotic-naïve patients with schizophrenia and related disorders: a meta-analysis. Schizophr Res 2016; 174:156–160. [DOI] [PubMed] [Google Scholar]
- 73.El Sayed El Taweel M, Abdalla AM. Evaluation of prolactin levels in male patients with first-episode schizophrenia and its correlation with psychopathology. Middle East Curr Psychiatry 2017; 24:49–54. [Google Scholar]
- 74.Petrikis P, Tigas S, Tzallas AT, et al. Prolactin levels in drug-naïve patients with schizophrenia and other psychotic disorders. Int J Psychiatry Clin Pract 2016; 20:165–169. [DOI] [PubMed] [Google Scholar]
- 75.Petruzzelli MG, Margari M, Peschechera A, et al. Hyperprolactinemia and insulin resistance in drug naive patients with early onset first episode psychosis. BMC Psychiatry 2018; 18:Article number 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76▪▪.Tharoor H, Mohan G, Gopal S. Title of the article: sex hormones and psychopathology in drug naïve Schizophrenia. Asian J Psychiatr 2020; 52:Article number 102042. [DOI] [PubMed] [Google Scholar]; This study detected a positive relationship between prolactin levels and psychotic symptoms in untreated first episode schizophrenia, which indicates that prolactin levels could be a potential marker for symptom severity.
- 77.Kleinman JE, Weinberger DR, Rogol AD, et al. Plasma prolactin concentrations and psychopathology in chronic schizophrenia. Arch Gen Psychiatry 1982; 39:Article number 655. [DOI] [PubMed] [Google Scholar]
- 78.Segal M, Avital A, Berstein S, et al. Prolactin and estradiol serum levels in unmedicated male paranoid schizophrenia patients. Prog Neuro-Psychopharmacol Biol Psychiatry 2007; 31:378–382. [DOI] [PubMed] [Google Scholar]
- 79.Segal M, Avital A, Rojas M, et al. Serum prolactin levels in unmedicated first-episode and recurrent schizophrenia patients: a possible marker for the disease's subtypes. Psychiatry Res 2004; 127:227–235. [DOI] [PubMed] [Google Scholar]
- 80.De Hert M, Detraux J, Peuskens J. Second-generation and newly approved antipsychotics, serum prolactin levels and sexual dysfunctions: a critical literature review. Expert Opin Drug Saf 2014; 13:605–624. [DOI] [PubMed] [Google Scholar]
- 81.MacCabe JH, Koupil I, Leon DA. Lifetime reproductive output over two generations in patients with psychosis and their unaffected siblings: the Uppsala 19151929 birth cohort multigenerational study. Psychol Med 2009; 39:Article number 1667. [DOI] [PubMed] [Google Scholar]
- 82.Dabbous Z, Atkin SL. Hyperprolactinaemia in male infertility: clinical case scenarios. Arab J Urol 2018; 16:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buvat J. Hyperprolactinemia and sexual function in men: a short review. Int J Impot Res 2003; 15:373–377. [DOI] [PubMed] [Google Scholar]
- 84.Damkier P, Videbech P. The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs 2018; 32:351–366. [DOI] [PubMed] [Google Scholar]
- 85.Jiang X-J, Wu F-X, Zhang J-P, et al. Effects of risperidone and aripiprazole on serum levels of prolactin, testosterone and estradiol in female patients with schizophrenia. Drug Res 2018; 68:410–414. [DOI] [PubMed] [Google Scholar]
- 86.Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One 2013; 8:e70179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87▪.Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology 2020; 163:Article number 107631. [DOI] [PubMed] [Google Scholar]; Impotant review article on de gender and sex differences in the effects of antipsychotic drugs.
- 88.González-Rodríguez A, Seeman MV. Pharmacotherapy for schizophrenia in postmenopausal women. Expert Opin Pharmacother 2018; 19:809–821. [DOI] [PubMed] [Google Scholar]
- 89.Eugene AR, Masiak J. A pharmacodynamic modelling and simulation study identifying gender differences of daily olanzapine dose and dopamine D2-receptor occupancy. Nord J Psychiatry 2017; 71:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90▪.Hodgetts S, Hausmann M. Antipsychotic effects of sex hormones and atypical hemispheric asymmetries. Cortex 2020; 127:313–332. [DOI] [PubMed] [Google Scholar]; This paper describes why estrogens could be seen as antipsychotic agents, considering their neuromodulatory effects.
- 91.González-Rodríguez A, Seeman MV. The association between hormones and antipsychotic use: a focus on postpartum and menopausal women. Ther Adv Psychopharmacol 2019; 9:Article number 204512531985997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lange B, Mueller JK, Leweke FM, Bumb JM. How gender affects the pharmacotherapeutic approach to treating psychosis - a systematic review. Expert Opin Pharmacother 2017; 18:351–362. [DOI] [PubMed] [Google Scholar]
- 93.Westin AA, Brekke M, Molden E, et al. Treatment with antipsychotics in pregnancy: changes in drug disposition. Clin Pharmacol Ther 2018; 103:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iversen TSJ, Steen NE, Dieset I, et al. Side effect burden of antipsychotic drugs in real life - impact of gender and polypharmacy. Prog Neuro-Psychopharmacol Biol Psychiatry 2018; 82:263–271. [DOI] [PubMed] [Google Scholar]
- 95.Lucca J, Ramesh M, Ram D. Gender differences in the occurrences and pattern of adverse drug reactions in psychiatric patients: a prospective observational study. Trop J Med Res 2017; 20:Article number 84. [Google Scholar]
- 96.Meyer JM, Nasrallah HA, McEvoy JP, et al. The clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res 2005; 80:9–18. [DOI] [PubMed] [Google Scholar]
- 97.Castellani LN, Costa-Dookhan KA, McIntyre WB, et al. Preclinical and clinical sex differences in antipsychotic-induced metabolic disturbances: a narrative review of adiposity and glucose metabolism. J Psychiatry Brain Sci 2019; 4:e190013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005; 80:19–32. [DOI] [PubMed] [Google Scholar]
- 99.Kraal AZ, Ward KM, Ellingrod VL. Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacol Bull 2017; 47:8–21. [PMC free article] [PubMed] [Google Scholar]
- 100.Raeder F, Heidemann F, Schedlowski M, et al. No pills, more skills: the adverse effect of hormonal contraceptive use on exposure therapy benefit. J Psychiatr Res 2019; 119:95–101. [DOI] [PubMed] [Google Scholar]
- 101.Lewis CA, Kimmig ACS, Zsido RG, et al. Effects of hormonal contraceptives on mood: a focus on emotion recognition and reactivity, reward processing, and stress response. Curr Psychiatry Rep 2019; 21:Article number 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporos Int 2019; 30:2391–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skovlund CW, Mørch LS, Kessing LV, et al. Association of hormonal contraception with suicide attempts and suicides. Am J Psychiatry 2018; 175:336–342. [DOI] [PubMed] [Google Scholar]
- 104.Skovlund CW, Mørch LS, Kessing LV, Lidegaard O. Association of hormonal contraception with depression. JAMA Psychiatry 2016; 97:478–489. [DOI] [PubMed] [Google Scholar]
- 105.Zettermark S, Vicente RP, Merlo J. Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: a pharmacoepidemiological study on 800 000 Swedish women. PLoS One 2018; 13:e0194773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heringa SM, Begemann MJH, Goverde AJ, Sommer IEC. Sex hormones and oxytocin augmentation strategies in schizophrenia: a quantitative review. Schizophr Res 2015; 168:603–613. [DOI] [PubMed] [Google Scholar]
- 107.Weiser M, Levi L, Zamora D, et al. Effect of adjunctive estradiol on schizophrenia among women of childbearing age. JAMA Psychiatry 2019; 76:Article number 1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kulkarni J, Gavrilidis E, Wang W, et al. Estradiol for treatment-resistant schizophrenia: a large-scale randomized-controlled trial in women of child-bearing age. Mol Psychiatry 2015; 20:695–702. [DOI] [PubMed] [Google Scholar]
- 109.Louzã MR, Marques AP, Elkis H, et al. Conjugated estrogens as adjuvant therapy in the treatment of acute schizophrenia: a double-blind study. Schizophr Res 2004; 66:97–100. [DOI] [PubMed] [Google Scholar]
- 110.Lascurain MB, Camuñas-Palacín A, Thomas N, et al. Improvement in depression with oestrogen treatment in women with schizophrenia. Arch Womens Ment Health 2020; 23:149-154. [DOI] [PubMed] [Google Scholar]
- 111.Ellis AJ, Hendrick VM, Williams R, Komm BS. Selective estrogen receptor modulators in clinical practice: a safety overview. Expert Opin Drug Saf 2015; 14:921–934. [DOI] [PubMed] [Google Scholar]
- 112.de Boer J, Prikken M, Lei WU, et al. The effect of raloxifene augmentation in men and women with a schizophrenia spectrum disorder: a systematic review and meta-analysis. NPJ Schizophr 2018; 4:Article number 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weickert TW, Weickert CS. Raloxifeneimproves cognition in schizophrenia: spurious result or valid effect? Front Psychiatry 2017; 8:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huerta-Ramos E, Labad J, Cobo J, et al. Effects of raloxifene on cognition in postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Eur Arch Psychiatry Clin Neurosci 2020; 270:729–737. [DOI] [PubMed] [Google Scholar]
- 115.Weickert TW, Weinberg D, Lenroot R, et al. Adjunctive raloxifene treatment improves attention and memory in men and women with schizophrenia. Mol Psychiatry 2015; 20:685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khodaie-Ardakani MR, Khosravi M, Zarinfard R, et al. A placebo-controlled study of raloxifene added to risperidone in men with chronic schizophrenia. Acta Med Iran 2015; 53:337–345. [PubMed] [Google Scholar]
- 117▪.Gurvich C, Hudaib A, Gavrilidis E, et al. Raloxifene as a treatment for cognition in women with schizophrenia: the influence of menopause status. Psychoneuroendocrinology 2019; 100:113–119. [DOI] [PubMed] [Google Scholar]; An important clinical trial that showed that SERM raloxifene is more effective when endogenous estrogen levels are low, indicating that its efficacy may be predictable by hormonal biomarkers.
- 118.Kindler J, Weickert CS, Schofield PR, et al. Raloxifene increases prefrontal activity during emotional inhibition in schizophrenia based on estrogen receptor genotype. Eur Neuropsychopharmacol 2016; 26:1930–1940. [DOI] [PubMed] [Google Scholar]
- 119.Labad J, Martorell L, Huerta-Ramos E, et al. Pharmacogenetic study of the effects of raloxifene on negative symptoms of postmenopausal women with schizophrenia: A double-blind, randomized, placebo-controlled trial. Eur Neuropsychopharmacol 2016; 26:1683–1689. [DOI] [PubMed] [Google Scholar]
- 120▪.Vila È, Huerta-Ramos E, Núñez C, et al. Specificity proteins 1 and 4 in peripheral blood mononuclear cells in postmenopausal women with schizophrenia: a 24-week double-blind, randomized, parallel, placebo-controlled trial. Eur Arch Psychiatry Clin Neurosci 2019; 269:941–948. [DOI] [PubMed] [Google Scholar]; This study revealed that protein levels of specificity protein 4 may be a potential biomarker for raloxifene efficacy.
- 121.Puranik NV, Srivastava P, Bhatt G, et al. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various parameters and molecular modelling approach. Sci Rep 2019; 9:Article number 7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tungmunnithum D, Thongboonyou A, Pholboon A, et al. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines 2018; 5:Article number 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rodríguez-Landa JF, Cueto-Escobedo J, Puga-Olguín A, et al. The phytoestrogen genistein produces similar effects as 17 β -estradiol on anxiety-like behavior in rats at 12 weeks after ovariectomy. Biomed Res Int 2017; 2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Son YJ, Choi SY, Hwang IK, et al. Could defatted mealworm (Tenebrio molitor) and mealworm Oil be used as food ingredients? Foods 2020; 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Landete JM, Arqués J, Medina M, et al. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr 2016; 56:1826–1843. [DOI] [PubMed] [Google Scholar]


