Abstract
Rationale: Black adults have worse health outcomes compared with white adults in certain chronic diseases, including chronic obstructive pulmonary disease (COPD).
Objectives: To determine to what degree disadvantage by individual and neighborhood socioeconomic status (SES) may contribute to racial disparities in COPD outcomes.
Methods: Individual and neighborhood-scale sociodemographic characteristics were determined in 2,649 current or former adult smokers with and without COPD at recruitment into SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study). We assessed whether racial differences in symptom, functional, and imaging outcomes (St. George’s Respiratory Questionnaire, COPD Assessment Test score, modified Medical Research Council dyspnea scale, 6-minute-walk test distance, and computed tomography [CT] scan metrics) and severe exacerbation risk were explained by individual or neighborhood SES. Using generalized linear mixed model regression, we compared respiratory outcomes by race, adjusting for confounders and individual-level and neighborhood-level descriptors of SES both separately and sequentially.
Measurements and Main Results: After adjusting for COPD risk factors, Black participants had significantly worse respiratory symptoms and quality of life (modified Medical Research Council scale, COPD Assessment Test, and St. George’s Respiratory Questionnaire), higher risk of severe exacerbations and higher percentage of emphysema, thicker airways (internal perimeter of 10 mm), and more air trapping on CT metrics compared with white participants. In addition, the association between Black race and respiratory outcomes was attenuated but remained statistically significant after adjusting for individual-level SES, which explained up to 12–35% of racial disparities. Further adjustment showed that neighborhood-level SES explained another 26–54% of the racial disparities in respiratory outcomes. Even after accounting for both individual and neighborhood SES factors, Black individuals continued to have increased severe exacerbation risk and persistently worse CT outcomes (emphysema, air trapping, and airway wall thickness).
Conclusions: Disadvantages by individual- and neighborhood-level SES each partly explain disparities in respiratory outcomes between Black individuals and white individuals. Strategies to narrow the gap in SES disadvantages may help to reduce race-related health disparities in COPD; however, further work is needed to identify additional risk factors contributing to persistent disparities.
Keywords: COPD, racial disparities, socioeconomic status, neighborhood disadvantage
At a Glance Commentary
Scientific Knowledge on the Subject
This manuscript expands on knowledge that racial differences occur in respiratory outcomes and disadvantages in individual socioeconomic status (SES) among Black individuals partially explains some of these differences.
What This Study Adds to the Field
We aim to determine whether racial differences in respiratory outcomes are also influenced by neighborhood SES factors, in combination with or independent of individual SES factors.
Black individuals residing in the United States have worse clinical outcomes compared with non-Hispanic white (NHW) individuals in various chronic diseases (1). Though some studies have suggested that U.S. Black individuals have less emphysema compared with NHW individuals for the same amount smoked (2), most studies evaluating racial disparities in chronic obstructive pulmonary disease (COPD) suggest that U.S. Black individuals have worse functional status, more lung function impairment, greater COPD-related exacerbations, and worse quality of life than NHW individuals (2–4). Although Black individuals in the United States are more likely to have lower socioeconomic status (SES) and reside in lower-income neighborhoods compared with NHW individuals (3), the degree to which these racial disparities in respiratory outcomes can be explained by biological differences versus disparities in environmental and social factors remains unclear.
Low individual SES has been associated with higher odds of having COPD as well as greater COPD morbidity and mortality (3). Several studies of COPD, after adjusting for some markers of individual SES, still show Black individual disadvantage persisting for several morbidity measures (3, 5, 6); however, it is not clear the degree to which individual SES may partially explain racial disparities. Furthermore, people of varying individual circumstances may live in neighborhoods of disparate disadvantage for a variety of reasons. Residing in a disadvantaged neighborhood has been associated with an increased risk of other diseases (7, 8), but its role in COPD is less clear, although it has been suggested to impact disease outcomes (9). Independent of an individual’s SES, several factors associated with living in disadvantaged neighborhoods—such as higher stress or depression, poor access to health care, lower quality of care, low access to healthy food, environmental exposures, poor neighborhood walkability, and diminished physical activity—are potential mechanisms by which neighborhood disadvantage may affect COPD outcomes (10–12). Therefore, a potential reason for the persistence of racial disparities in COPD outcomes is that neighborhood-level SES factors also vary by race and are associated with chronic disease health outcomes (7). To our knowledge, the independent and joint effects of individual and neighborhood SES on racial disparities in COPD has not yet been explored.
SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study), a national multicenter cohort study, with its inclusion of participants across many geographical types and regions as well as a relatively large recruitment of Black individuals from diverse backgrounds, offers a valuable opportunity to examine the question of racial disparities in COPD outcomes, disentangling the contribution of individual and neighborhood risk factors on COPD. Using baseline SPIROMICS data and the merged community-level data from U.S. Census Bureau obtained from the ancillary SPIROMICS Air Pollution Study (SPIROMICS AIR) (13), the current investigation aims to determine whether 1) Black participants have worse respiratory outcomes compared with white participants and, if so, 2) whether and to what degree these racial disparities are explained by disparities in individual and/or neighborhood SES.
Methods
Study Design and Participants
The SPIROMICS cohort enrolled 2,982 participants from 2010 to 2015 with the goal of identifying new COPD subgroups and intermediate markers of disease progression. Enrollment criteria for participants with a history of smoking were an age 40–80 years and a smoking history of ≥20 pack-years (14). For the current analyses, we excluded never-smokers (n = 202) and used baseline data on both current and former smokers at risk for COPD but without airway obstruction, as well as those with COPD, which was defined as FEV1/FVC <0.7 (15). We further excluded those reporting mixed race (n = 62) or other race (n = 41) and those not reporting race (n = 16) or missing (n = 12). The analytic cohort consisted of 2,120 participants who self-reported white and 529 who self-reported Black. Few participants (3.8%) were Hispanic (5.5% of Black and 3.4% of white participants) and were included in analyses. SPIROMICS was approved by all participant centers, including Johns Hopkins University, where these analyses were performed.
Exposures
Demographic variables (race, age, sex, income, educational attainment, and marital status), as well as COPD-related risk factors at baseline (smoking status and number of pack-years), body mass index (BMI), and depression were obtained. Using the merged neighborhood-level data from the 2010 U.S. Census Bureau American Community Survey and data from SPIROMICS AIR, individual and neighborhood risk factors were assessed and include tract-level poverty rate, educational attainment, unemployment rate, and median household income (16). The Area Deprivation Index (ADI) National Ranking Score allowed for rankings of neighborhoods by SES disadvantage in a region of interest (17). Variables describing food access were obtained based on data from the U.S. Department of Agriculture for food stores at the census tract level using measures of income and distance to stores (18). All were used as covariates in the adjusted models.
Outcomes
We assessed the Black versus white differences for the following respiratory outcomes: respiratory-specific quality of life (St. George’s Respiratory Questionnaire [SGRQ]) (19), exercise capacity (6-minute-walk test distance [6MWD]) (20), dyspnea scale (modified Medical Research Council [mMRC] scale) (21), COPD health status (COPD Assessment Test [CAT] score) (22), computed tomography (CT) scan metrics (percentage emphysema [defined as percentage of total voxels in the field <−950 Hounsfield units at TLC]) (23), airway wall thickness (for a hypothetical airway with internal perimeter of 10 mm [Pi10]) (24), percentage air trapping (defined as the percentage of total voxels in the field <−856 Hounsfield units at residual volume) (25), and self-reported number of severe exacerbations during follow-up (defined as those leading to a hospitalization or emergency room visit).
Statistical Analysis
Descriptive analyses were used to examine means and SDs or proportions and counts. The unadjusted differences between Black individuals and white individuals regarding demographic risk factors, COPD-related risk factors, neighborhood risk factors, and respiratory outcomes were examined using t tests and χ2 tests. To assess the impact of different sets of covariates on the association between race and outcome, we used linear regression for the continuous outcomes and negative binomial regression for the count outcomes based on generalized linear mixed models with random intercept (for site adjustment). We regressed respiratory outcomes on race with an initial model (model 1) adjusting for individual characteristics (age, sex, smoking history, pack-years, BMI, depression, marital status, and COPD status). We then both separately and sequentially adjusted for individual-level SES (income and education) and neighborhood SES (poverty rate, educational attainment, unemployment rate, median household income, ADI, and food access). In the first sequential adjustment, we first adjusted for individual-level SES (model 2) followed by adjustment for neighborhood-level SES (model 3). In the second sequence, we switched the order, adjusting for neighborhood SES first followed by individual SES (Table E1 in the online supplement). Each model provided a measure of the association between race and the outcome of interest. The difference in the strength of these measures, and the change after adding sequential variables, is then evaluated as the contribution of each set of variables to the Black–white difference in COPD outcomes, using the “difference-method” within the framework of mediation analysis for multiple mediators (26). In this type of analyses, “mediation proportion” is conceptualized as the proportional reduction in the exposure–outcome association from before and after the adjustment with mediators (online supplement).
Sensitivity analyses were conducted adjusting for site as a fixed effect and also adjusting models for individual parameters, including baseline lung function and comorbidity count (27); occupational exposure to hazardous vapor, gas, dust, or fumes (28); and additional neighborhood factors, including urban/rural status, region, and ambient pollution (particulate matter and ozone). Furthermore, we tested for potential interaction between race and COPD status, and stratified models were shown separately for participants with COPD. All parameter estimates in our regression analysis, including mediation proportions and their confidence intervals (CIs), were generated based on a bootstrap approach (26). The threshold for statistical significance was P < 0.05 based on 95% CI. All statistical analyses were performed using STATA version 15.1 (Stata Corp).
Results
A total of 2,649 SPIROMICS participants with available data were included at the time of analysis, of whom 20.0% were Black. Compared with white participants, Black participants were younger, had less college education, had lower income, and had a higher proportion of current smokers but fewer average pack-years smoked. Black participants were also more likely to reside in neighborhoods with worse poverty rate, lower median household income, lower education, higher unemployment, and higher ADI and were less likely to live in areas with limited food access (Table 1).
Table 1.
Participant Characteristics
| Total (N = 2,649) | White (n = 2,120) | Black (n = 529) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr | 63.59 ± 8.91 | 64.95 ± 8.38 | 58.14 ± 8.91 | <0.001 |
| Sex, F, n (%) | 1,219 (46.0) | 953 (45.0) | 266 (50.3) | 0.028 |
| Education, more than HS, n (%) | 1,621 (61.4) | 1,385 (65.5) | 236 (44.7) | <0.001 |
| Income, n (%) | <0.001 | |||
| Under $15,000 | 519 (19.8) | 312 (14.9) | 207 (40.0) | |
| $15,000–$34,999 | 484 (18.5) | 383 (18.2) | 101 (19.5) | |
| $35,000–$49,999 | 326 (12.5) | 283 (13.5) | 43 (8.3) | |
| $50,000–$74,999 | 382 (14.6) | 346 (16.5) | 36 (6.9) | |
| $75,000 or more | 448 (17.1) | 431 (20.5) | 17 (3.3) | |
| Declined to answer | 459 (17.5) | 345 (16.4) | 114 (22.0) | |
| Marital status, married, n (%) | 1,248 (47.2) | 1,129 (53.4) | 119 (22.6) | <0.001 |
| BMI, kg/m2 | 27.87 ± 5.28 | 27.80 ± 5.09 | 28.15 ± 5.99 | 0.180 |
| Smoking status, current smoker, n (%) | 1,038 (39.8) | 703 (33.7) | 335 (64.1) | <0.001 |
| Pack-years | 49.50 ± 27.18 | 51.63 ± 28.71 | 40.96 ± 17.46 | <0.001 |
| Depression scale (HADS-D) | 4.48 ± 3.52 | 4.47 ± 3.55 | 4.53 ± 3.41 | 0.730 |
| Neighborhood | ||||
| Poverty rate (family below poverty level), % | 10.73 ± 10.78 | 8.20 ± 8.19 | 20.89 ± 13.59 | <0.001 |
| Education, 25 yr old or older and less than HS, % | 14.66 ± 11.56 | 12.39 ± 10.07 | 23.78 ± 12.63 | <0.001 |
| Unemployment rate (16 yr old or older and unemployed), % | 5.44 ± 2.97 | 4.87 ± 2.48 | 7.75 ± 3.60 | <0.001 |
| Median household income, ×$1,000 | 58.71 ± 28.07 | 63.68 ± 27.61 | 38.72 ± 19.83 | <0.001 |
| ADI | 41.82 ± 29.62 | 37.64 ± 27.20 | 58.63 ± 32.82 | <0.001 |
| Food access, >0.5 km (if urban) or >16 km (if rural) away from, n (%) | 1,637 (64.2) | 1,387 (67.6) | 250 (50.3) | <0.001 |
| Lung function | ||||
| FEV1% predicted | 72.82 ± 26.43 | 71.80 ± 26.08 | 76.91 ± 27.44 | <0.001 |
| COPD, n (%) | 1,763 (66.6) | 1,484 (70.0) | 279 (52.7) | <0.001 |
| Clinical outcomes | ||||
| CAT | 14.19 ± 8.32 | 13.72 ± 8.11 | 16.04 ± 8.87 | <0.001 |
| mMRC | 1.08 ± 1.00 | 1.06 ± 0.99 | 1.16 ± 1.06 | 0.032 |
| SGRQ total | 33.64 ± 20.65 | 32.62 ± 20.26 | 37.67 ± 21.69 | <0.001 |
| 6MWD, m | 407.6 ± 121.5 | 406.7 ± 118.8 | 411.2 ± 131.9 | 0.461 |
| Imaging characteristics | ||||
| Airway wall thickness (Pi10) | 3.71 ± 0.08 | 3.71 ± 0.08 | 3.72 ± 0.08 | 0.002 |
| Percentage emphysema (−950) | 8.05 ± 10.37 | 8.26 ± 10.30 | 7.20 ± 10.63 | 0.036 |
| Percentage air trapping (−856) | 25.31 ± 21.66 | 26.09 ± 21.27 | 22.16 ± 22.91 | <0.001 |
| Hospitalization | ||||
| Rate of severe exacerbations, n/yr | 0.34 ± 0.98 | 0.30 ± 0.93 | 0.51 ± 1.17 | <0.001 |
Definition of abbreviations: 6MWD = 6-minute-walk test distance; ADI = Area Deprivation Index; BMI = body mass index; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; HADS-D = depression subscale of the Hospital Anxiety and Depression Scale; HS = high school; mMRC = modified Medical Research Council scale; Pi10 = internal perimeter of 10 mm; SGRQ = St. George’s Respiratory Questionnaire.
Data are presented as mean ± SD or n (%). Percentages may not total 100 because of rounding. Scores on the CAT range from 0 to 40, whereas SGRQ scores range from 0 to 100, with lower scores indicating better functioning, with a minimal clinically important difference of 2 points (CAT) and 4 points (SGRQ). Scores for mMRC scale range from 0 to 4, with higher scores indicating more severe breathlessness. Statistical significance was P < 0.05. The ADI ranges from 0 to 100.
Association of Race with Respiratory Outcomes
In unadjusted analysis, Black participants were less likely to have COPD, had a higher FEV1%predicted, less emphysema, and less gas trapping compared with white participants. Despite this, Black participants had worse quality of life and more dyspnea, as evidenced by worse CAT, SGRQ, and mMRC scores and a higher rate of severe exacerbations (0.51 vs. 0.30 per year; P < 0.01) compared with white participants. Black participants also had greater airway wall thickness (Table 1).
Association of Race with Respiratory Outcomes after Adjustment for Individual Characteristics
In models adjusted for individual characteristics (age, sex, smoking status, pack-years, BMI, depression, marital status, and COPD status) and controlling for random site effect (model 1; Table 2), Black participants had all worse measured outcomes compared with white participants. Specifically, in comparison with white participants, Black participants had significantly worse CAT score (β = 1.98; 95% CI, 1.13–2.83), worse SGRQ score (β = 4.33; 95% CI, 2.28–6.27), worse dyspnea (mMRC) (β = 0.16; 95% CI, 0.05–0.26), shorter 6MWD (β = −30.31; 95% CI, −43.08 to −17.93), and higher rate of hospitalization because of severe exacerbation during the study period (incident rate ratio [IRR], 2.35; 95% CI,1.75–3.12) (Figure 1). For CT measures, after adjustment for confounders, Black participants had worse CT metrics, including higher percentage emphysema (β = 1.76; 95% CI, 0.69–2.78), thicker airways (Pi10) (β = 1.57 × 10−2; 95% CI, 0.78 × 10−2 to 2.35 × 10−2), and more air trapping (β = 3.32; 95% CI, 1.28–5.09) compared with white participants (model 1; Table 2).
Table 2.
Association of Race with Respiratory Outcomes and CT Metrics
| Model 1 (Adjusted for Demographics and Individual Clinical Characteristics) | M1a (Mediation from Model 1 to Model 2; Mediation by Individual SES) (%) | Model 2 (Model 1 Also Adjusted for Individual SES) | M2a (Additional Mediation from Model 2 to Model 3; Mediation by Neighborhood SES) (%) | Model 3 (Model 2 Also Adjusted for Neighborhood SES) | M3a (Mediation from Model 1 to Model 3; Total Mediation by Individual and Neighborhood SES) (%) | |
|---|---|---|---|---|---|---|
| COPD health-related outcomes | ||||||
| CAT* | 1.975 (1.127 to 2.832) | 27 | 1.445 (0.590 to 2.292) | 26 | 1.071 (0.135 to 1.983) | 46 |
| mMRC* | 0.159 (0.047 to 0.258) | 30 | 0.112 (−0.003 to 0.209) | 5 | 0.106 (−0.016 to 0.208) | 33 |
| SGRQ overall* | 4.332 (2.278 to 6.265) | 35 | 2.819 (0.765 to 4.764) | 26 | 2.099 (−0.083 to 4.117) | 52 |
| 6MWD* | −30.311 (−43.075 to −17.929) | 33 | −20.196 (−33.156 to −7.145) | 54 | −9.31 (−23.192 to 4.312) | 69 |
| Total severe exacerbation count during follow-up† | 2.351 (1.754 to 3.123) | 7 | 2.209 (1.656 to 2.966) | 32 | 1.709 (1.245 to 2.373) | 37 |
| CT metrics | ||||||
| Pi10* | 0.016 (0.008 to 0.024) | 7 | 0.015 (0.006 to 0.023) | 10 | 0.013 (0.004 to 0.023) | 16 |
| % emphysema* | 1.763 (0.688 to 2.783) | 0 | 1.770 (0.667 to 2.774) | −8 | 1.904 (0.719 to 2.986) | −8 |
| % air trapping* | 3.318 (1.280 to 5.090) | 12 | 2.907 (0.740 to 4.693) | 15 | 2.471 (0.310 to 4.399) | 26 |
Definition of abbreviations: 6MWD = 6-minute-walk test distance; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; CT = computed tomography; mMRC = modified Medical Research Council scale; Pi10 = internal perimeter of 10 mm; SES = socioeconomic status; SGRQ = St. George’s Respiratory Questionnaire.
Model 1 adjusted for participant’s characteristics of age, sex, smoking history, pack-years, body mass index, marital status, and COPD status. Model 2 consisted of model 1 also adjusted for participant SES (education and income). Model 3 consisted of Model 1 also adjusted for participant SES and neighborhood SES (poverty rate, education, unemployment, median household income, Area Deprivation Index, and food access) estimated at the census tract level (block-group level for Area Deprivation Index) using data from the U.S. Census, University of Wisconsin School of Medicine and Public Health, and U.S. Department of Agriculture. Boldface entries indicate a statistically significant association P < 0.05.
Entries represent mean differences (β) and their 95% confidence intervals comparing Black individuals with white individuals.
Entries represent incidence rate ratios and their 95% confidence intervals comparing Black individuals with white individuals.
Figure 1.
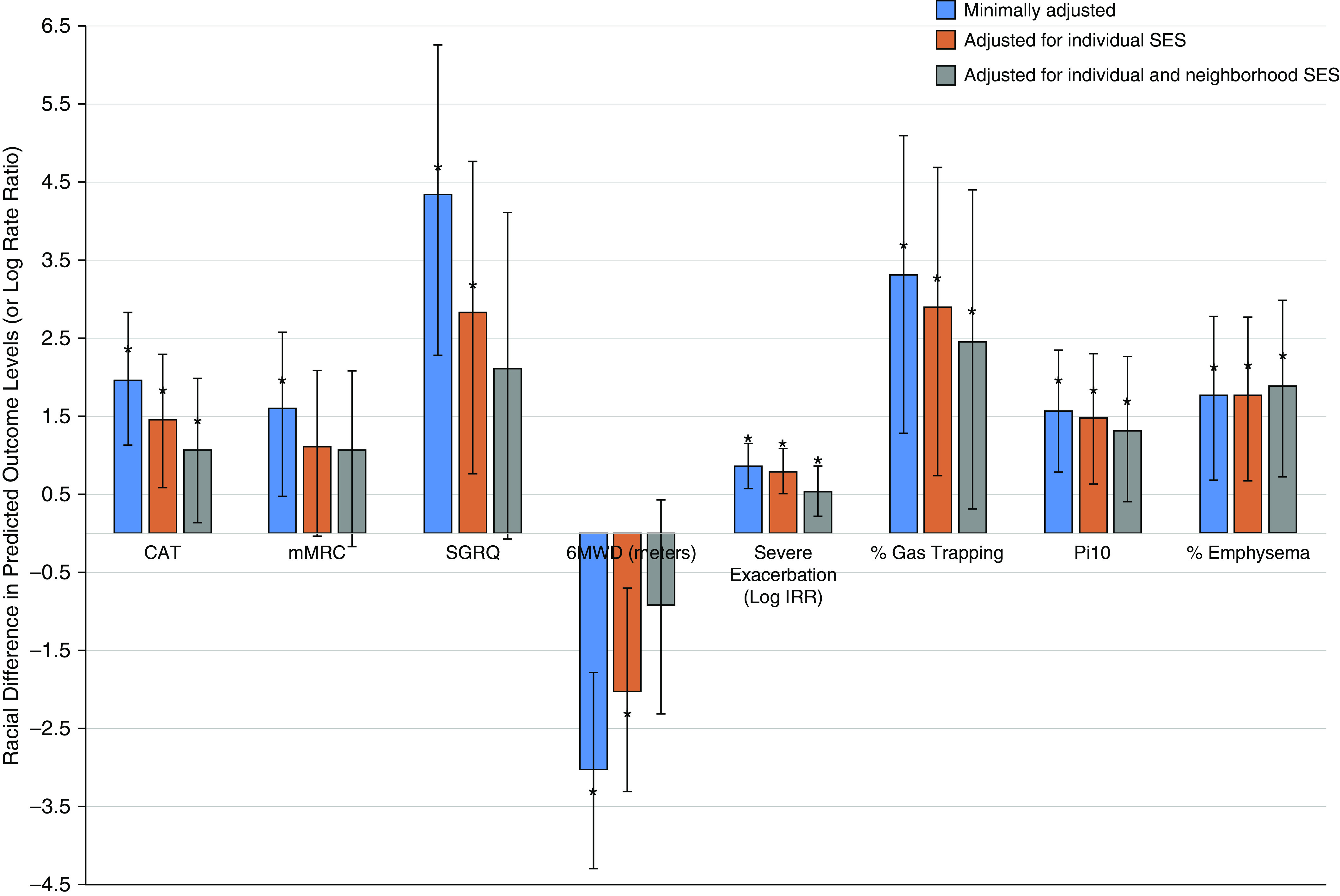
Differences in respiratory outcomes between Black individuals and white individuals are attenuated by individual and neighborhood socioeconomic status (SES). Predicted mean difference in chronic obstructive pulmonary disease outcomes and their 95% confidence intervals are shown sequentially across the following three models: model 1 (blue), which adjusts for demographic and individual characteristics; model 2 (red), which also adjusts model 1 for individual SES; model 3 (gray), which also adjusts model 2 for neighborhood SES. The y-axis represents the differences between Black individuals and white individuals in the levels of the outcome (e.g., index score) except for severe exacerbation, for which the y-axis represents log incident rate ratio. The modified Medical Research Council scale, 6-minute-walk test distance, and airway wall thickness (internal perimeter of 10 mm) were rescaled to fit the chart; the modified Medical Research Council score was multiplied by 10, the 6-minute-walk test distance was divided by 10, and the airway wall thickness (internal perimeter of 10 mm) was multiplied by 100. *Statistical significance of difference in the outcome between Black individuals and white individuals. 6MWD = 6-minute-walk test distance; CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; IRR = incident rate ratio; mMRC = modified Medical Research Council scale; Pi10 = internal perimeter of 10 mm; SGRQ = St. George’s Respiratory Questionnaire.
Association of Race with Respiratory Outcomes after Additional Adjustment for Individual SES
After model 1 was also adjusted for individual-level SES (education and income) (model 2l Table 2), the associations between race and CAT (β = 1.45; 95% CI, 0.59–2.29), SGRQ (β = 2.82; 95% CI, 0.77–4.76), 6MWD (β = −20.2; 95% CI, −33.2 to −7.1), and the rate of severe exacerbation (IRR, 2.21; 95% CI, 1.66–2.97) were attenuated but remained statistically significant, with worse outcomes for Black participants compared with white participants. The association between race and dyspnea was no longer statistically significant (Figure 1).
The analysis shows that individual-level SES contributed up to 35% of the racial disparities in COPD health-related outcomes, with the largest explanatory effect for 6MWD and quality of life (mediation proportion from model 1 to model 2 [M1a]; Table 2). For example, when adjusting for individual-level SES, the mean Black–white difference in total SGRQ declined from 4.3 points (model 1; Table 2) to 2.8 points (model 2; Table 2), representing 35% (95% CI, 21–68%) mediation. Similarly, the racial gap in dyspnea score declined 30% (95% CI, 13%–103%), and race was no longer statistically significantly associated with dyspnea. Individual-level SES also partially explained the race–outcome association in CAT (27%; 95% CI, 16–51%) and 6MWD (33%; 95% CI, 21–60%). For rate of severe exacerbation, the 7% contribution of individual-level SES was not statistically significant (95% CI, −1% to 17%) and the higher rate of severe exacerbations for Black individuals compared with white individuals persisted.
Black participants also continued to have higher percentage emphysema (β = 1.77; 95% CI, 0.68 to 2.78), percentage air trapping (β = 2.91; 95% CI, 0.74 to 4.69), and Pi10 (β = 1.47 × 10−2; 95% CI, 0.65 × 10−2 to 2.30 × 10−2) compared with white participants. The mediation proportion was statistically significant only for air trapping, for which individual-level SES explained a small portion (12%; 95% CI, 2–40%) of the race–outcome association. For percentage emphysema and airway thickness, the racial difference remained largely similar even after accounting for individual-level SES, and neither of the mediation proportions showed statistical significance (M1a; Table 2).
When adjusting by neighborhood-level SES alone (Table E1), the associations between race and outcomes were generally attenuated, as was the case with individual-level SES. In comparison with explanation by individual-level SES alone, the explanation by neighborhood-level SES alone was substantially greater for 6MWD (52% vs. 33%) and for the rate of hospitalization because of severe exacerbation (34% vs. 7%) but lower for dyspnea (15% vs. 30%) (Table E1).
Association of Race with Respiratory Outcomes after Additional Adjustment for Neighborhood SES
When additionally adjusting model 2, which included individual SES, by neighborhood SES (adjusting model 1 by both individual and neighborhood SES) (model 3; Table 2), the associations between race and dyspnea, SGRQ, and 6MWD were no longer statistically significant. The race–outcome associations were attenuated but remained significant for the CAT (β = 1.04; 95% CI, 0.11–1.96) and the rate of severe exacerbations (IRR, 1.67; 95% CI, 1.21–2.33), with worse outcome for Black participants (Table 2, Figure 1).
The analysis shows that neighborhood SES added an additional 26%, 54%, and 32% mediation of racial disparities in CAT score, 6MWD, and the rate of severe exacerbations, respectively (mediation proportion from model 2 to model 3 [M2a]; Table 2); and the combined individual and neighborhood-level SES accounted for up to 69% of the race–outcome association with COPD health-related outcomes (mediation proportion from model 1 to model 3 [M3a]; Table 2). Individual- and neighborhood-level SES explained 46% of the CAT (95% CI, 24–88%), 52% of the SGRQ (95% CI, 27–104%), 69% of the 6MWD (95% CI, 42–123%), 33% of the dyspnea score (95% CI, 2–125%), and 37% of the rate of severe exacerbations (95% CI, 19–65%) (M3a; Table 2).
The race–outcome disparity remained for percentage emphysema, percentage air trapping, and Pi10, with worse outcomes for Black participants (model 3; Table 2). There was some reduction in Black–white difference in airway thickness and air trapping by 16% and 26%, respectively, but none of the mediation proportions for CT phenotypes were statistically significant (M3a; Table 2).
In sensitivity analyses, adjusting for site as a fixed effect (Table E2), results were generally similar in terms of the range of mediation proportion. When models were adjusted for individual parameters (baseline lung function and comorbidity count and occupational exposure) and additional neighborhood factors (urban/rural status, region, and ambient pollution), the conclusions were similar and robust to additional confounding adjustment (Tables E3–E7). We also found no interaction between race and COPD status (all Pinteraction > 0.10), and results for participants with COPD only are shown in Table 3.
Table 3.
Association of Race with Respiratory Outcomes and CT Metrics (COPD-Only)
| Model 1 (Adjusted for Demographics and Individual Clinical Characteristics) | M1a (Mediation from Model 1 to Model 2; Mediation by Individual SES) (%) | Model 2 (Model 1 Also Adjusted for Individual SES) | M2a (Additional Mediation from Model 2 to Model 3; Additional Mediation by Neighborhood SES) (%) | Model 3 (Model 2 Also Adjusted for Neighborhood SES) | M3a (Mediation from Model 1 to Model 3; Total Mediation by Individual and Neighborhood SES) (%) | ||
|---|---|---|---|---|---|---|---|
| COPD health-related outcomes | |||||||
| CAT* | 1.90 (0.77 to 2.96) | 18 | 1.55 (0.44 to 2.65) | 23 | 1.19 (0.05 to 2.35) | 37 | |
| mMRC* | 0.15 (−0.02 to 0.30) | 29 | 0.11 (−0.07 to 0.25) | 23 | 0.08 (−0.09 to 0.25) | 45 | |
| SGRQ overall* | 4.84 (2.46 to 7.11) | 25 | 3.65 (1.27 to 6.04) | 19 | 2.97 (0.25 to 5.58) | 39 | |
| 6MWD* | −36.4 (−54.7 to −16.1) | 23 | −28.0 (−46.1 to −8.5) | 61 | −11.0 (−31.4 to 9.4) | 70 | |
| Total severe exacerbation count during follow-up† | 2.29 (1.69 to 3.06) | 10 | 2.12 (1.57 to 2.87) | 32 | 1.67 (1.19 to 2.37) | 38 | |
| CT metrics | |||||||
| Pi10* | 0.018 (0.008 to 0.028) | 6 | 0.017 (0.007 to 0.028) | 14 | 0.015 (0.003 to 0.026) | 20 | |
| % emphysema* | 1.72 (0.23 to 3.22) | −1 | 1.73 (0.22 to 3.30) | −8 | 1.88 (0.18 to 3.51) | −9 | |
| % air trapping* | 2.86 (−0.01 to 5.51) | 14 | 2.46 (−0.42 to 5.12) | 40 | 1.47 (−1.73 to 4.39) | 49 |
For definition of abbreviations, see Table 2.
The regression analysis was run among patients with COPD only. Model 1 adjusted for participant characteristics of age, sex, smoking history, pack-years, body mass index, marital status, and COPD status. Model 2 consisted of model 1 also adjusted for participant SES (education and income). Model 3 consisted of model 1 also adjusted for participant SES and neighborhood SES (poverty rate, education, unemployment, median household income, Area Deprivation Index, and food access) estimated at the census tract level (block-group level for Area Deprivation Index) using data from the U.S. Census, University of Wisconsin School of Medicine and Public Health, and U.S. Department of Agriculture. Boldface entries indicate a statistically significant association P < 0.05.
Entries represent mean differences (β) and their 95% confidence intervals comparing Black individuals with white individuals.
Entries represent incidence rate ratios and their 95% confidence intervals comparing Black individuals with white individuals.
Discussion
Among participants with heavy smoking history in SPIROMICS, we found that Black participants had worse COPD-related outcomes, including worse respiratory symptoms and quality of life (dyspnea scale, CAT, and SGRQ), worse functional status (6MWD), higher rate of severe exacerbations, and worse CT metrics (percentage emphysema, percentage air trapping, and airway wall thickness [Pi10]) compared with their white counterparts after controlling for participant characteristics, including smoking history and spirometry-confirmed airway obstruction. Individual-level SES and neighborhood-level SES factors explained 12–35% and 26–54% of the racial disparities in respiratory outcomes, respectively. The combined individual- and neighborhood-level SES contributed 33–69% to the race–outcome disparities.
This study adds to the limited body of knowledge that describes the complex relationship between race, SES (individual and neighborhood level), and COPD outcomes. Few studies have reported the presence of racial disparities in COPD outcomes. In a COPDGene study by Han and colleagues (29), after adjusting for educational attainment, Black participants were twice as likely to report an exacerbation that required hospitalization and worse quality of life compared with white participants with COPD. Sarrazin and colleagues (4) similarly showed that Black participants had higher risk for severe exacerbations and were more likely to be admitted to the ICU and receive mechanical ventilation in a study of inpatients with COPD. These studies showed the persistence of racial disparities while adjusting for measures of individual SES; however, other studies were not consistent. Eisner and colleagues (3) showed that Black participants had greater prospective risk of acute COPD exacerbations, which no longer persisted after controlling for individual SES (education and income) and other covariates, including comorbidities, smoking history, BMI, and occupational exposures. Several studies show that low individual SES is linked with poor COPD outcomes; specifically, lower household income or education status have been associated with higher COPD prevalence, COPD mortality, and COPD hospitalization rate (30–35).
In addition, as we have demonstrated in this analysis, Black participants at risk for and with COPD have lower individual SES compared with white participants. Existing studies were limited in their ability to account for SES, and the adjustment for individual SES together with other covariates precluded determining the specific impact that individual SES may have on race–ethnicity associations with COPD health outcomes. The present study adds substantially to the literature by investigating the impact of individual SES on racial disparities across multiple respiratory measures and by considering both individual and neighborhood SES to further add clarity to the contribution of SES to racial disparities. Our results show that individual SES explained up to 35% of racial differences and most notably attenuated differences in patient-reported outcomes and functional status (6MWD); however, except for differences in reported dyspnea, there was persistence of worse respiratory morbidity for Black participants compared with white participants after adjusting for confounders and individual SES alone.
In addition to lower individual SES, we demonstrated that Black participants at risk for and with COPD were more likely to reside in lower-income neighborhoods compared with white participants. Residents of low SES neighborhoods show higher rates of several chronic diseases and overall mortality in comparison with individuals from more affluent neighborhoods, regardless of individual SES (36–40), suggesting an independent influence of neighborhood characteristics on morbidity and mortality (33, 41). Neighborhoods with low SES tend to suffer from limited access to healthy foods, educational and employment opportunities, recreational facilities, health care, and other services and increased exposure to environmental contamination, poor living and working conditions, and violence (42–50). We previously showed that neighborhood deprivation adversely affects COPD outcomes (9), but whether it explains racial disparities had not previously been investigated.
In our study, neighborhood-level SES attenuated the racial disparity in COPD outcomes, above and beyond the contribution of individual SES. Neighborhood SES explained an additional 26–54% of the disparity by race for CAT, 6MWD, and severe exacerbation risk. This is of importance because neighborhood characteristics are rarely taken into consideration in epidemiological studies of COPD. Thus, the combined individual- and neighborhood-level SES showed variable contribution to the Black–white difference in COPD outcomes, with the combined effect of individual and neighborhood SES explaining a significant portion of racial disparity in patient-reported outcomes with no further statistical significant differences in dyspnea, quality of life, or functional status (SGRQ, MRC, and 6MWD) by race. Despite individual and neighborhood SES explaining 37% of racial disparity in severe exacerbation risk, Black subjects continued to have a 1.71 times higher rate of severe exacerbations leading to acute healthcare use compared with white subjects.
Interestingly, neither individual nor neighborhood SES showed a strong contribution to the racial differences in CT metrics. It should be noted that previous studies (2, 51) have suggested that Black participants may have less severe emphysema than white participants when accounting for confounders. In a study by Hansel and colleagues (2), after adjustment for confounders, Black participants with COPD had 2.2% less total percentage emphysema compared with white participants. In the current study, in unadjusted analyses, Black participants also had lower emphysema compared with white participants; however, after adjusting for clinical characteristics and individual- and neighborhood-level SES, Black participants had worse CT metrics (percentage emphysema, percentage air trapping, and airway thickness) compared with white participants. The reversal of race association with CT measures from the unadjusted to the adjusted model was driven by negative confounding by several covariates, including COPD status, BMI, smoking status, and depression. For example, Black participants were less likely to have COPD or be former smokers, whereas those with COPD and who were former smokers had worse emphysema. The reason for differing study results is unclear, but it should be noted that both previous studies included only subjects with COPD, whereas our analyses included former and current smokers with and at risk for COPD. Despite accounting for individual SES, neighborhood SES, and other confounders, the persistent racial disparities seen in severe exacerbation risk and CT phenotypes suggest that there may still be some unmeasured societal factors and personal factors (e.g., stress, poor nutrition, and differences in smoking behaviors) or a biological mechanism, such as differences in susceptibility alleles (52, 53), portending worse prognosis among Black individuals that still need further investigation.
Our results show persistent, though attenuated, disparities in Black participants compared with white participants in COPD outcomes despite adjustment for confounders including individual and neighborhood SES. This has important implications for clinical trials and epidemiologic studies moving forward because of the need to further explore sociocultural, genetic, and biologic risk leading to worse COPD outcomes in Black participants compared with white participants. Our results also emphasize the importance of taking individual and neighborhood SES into account in these investigations. Of note, we looked at a large number of neighborhood markers using easily interpretable, socially relevant markers of SES. Comprehensive neighborhood covariates were obtained from the 2010 U.S. Census data, including neighborhood poverty rate, educational attainment, unemployment rate, median household income, area deprivation, and food accessibility, to comprehensively investigate neighborhood SES disadvantages. Studies have shown that neighborhood SES is an important determinant of healthcare access, and it dictates the sector of the healthcare system in which people receive care (49, 54). Furthermore, individuals with low incomes are more likely to be uninsured and may seek health care less often. We were unable to determine the degree to which healthcare access or use of healthcare resources contributes to the identified health disparities.
A limitation of our study is that the results represent a cross-sectional analysis in which SES and outcomes were ascertained at the same point in time; we are therefore limited in inferring causality or directionality of the observed associations because of the unknown temporal ordering of SES and outcome. Also, to fully recognize racial and socioeconomic health disparities, it is important to understand how ethnic diversity and immigration status impact health outcomes, as these populations may have diverse health beliefs with some language barriers, variation in community healthcare involvement, and differing outlooks toward alternate medicines. Lack of information on country of origin and immigration status is a limitation of this study.
Conclusions
Black individuals have worse health outcomes compared with white individuals across a range of respiratory outcomes, including respiratory symptoms, quality of life, functional status, exacerbation risk, and CT outcomes. These racial disparities in respiratory outcomes are partly explained by SES, and it is important to account for not only individual but also neighborhood disadvantage to fully capture the degree to which SES may explain racial disparity in outcomes. Importantly, differences in patient-reported outcomes and functional status are markedly attenuated once SES factors are taken into consideration. However, it is important to note that even after accounting for both individual and neighborhood SES factors, Black individuals continue to have increased exacerbation risk requiring acute healthcare use even though disparities were significantly attenuated by accounting for SES. Black individuals also had persistently worse CT outcomes (emphysema, air trapping, and airway wall thickness) after adjusting for individual or neighborhood SES factors. Therefore, strategies to narrow the gap in individual SES and neighborhood SES disadvantage may help toward achieving COPD health parity between Black individuals and white individuals; however, further work is needed to identify additional risk factors contributing to worse respiratory morbidity among Black individuals with COPD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS AIR (SPIROMICS [Subpopulations and Intermediate Outcome Measures in COPD] Air Pollution Study) participants and participating physicians, investigators, and staff for making this research possible. More information about the study and how to access SPIROMICS data is available at www.spiromics.org. The authors acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E. Alexis, Ph.D.; Wayne H. Anderson, Ph.D.; Eugene R. Bleecker, M.D.; Richard C. Boucher, M.D.; Elizabeth E. Carretta, M.P.H.; Ronald G. Crystal, M.D.; Jeffrey L. Curtis, M.D.; Claire M. Doerschuk, M.D.; Mark T. Dransfield, M.D.; Christine M. Freeman, Ph.D.; Annette T. Hastie, Ph.D.; Robert J. Kaner, M.D.; Eric C. Kleerup, M.D.; Lisa M. LaVange, Ph.D.; Stephen C. Lazarus, M.D.; Deborah A. Meyers, Ph.D.; John D. Newell, Jr., M.D.; Elizabeth C. Oelsner, M.D., M.P.H.; Wanda K. O’Neal, Ph.D.; Robert Paine III, M.D.; Stephen I. Rennard, M.D.; Donald P. Tashkin, M.D.; Mary Beth Scholand, M.D.; J. Michael Wells, M.D.; Robert A. Wise, M.D.; and Prescott G. Woodruff, M.D., M.P.H. The project officers from the Lung Division of the NHLBI were Lisa Postow, Ph.D., and Thomas Croxton, Ph.D., M.D.
Footnotes
SPIROMICS AIR was supported by the NIH/National Institute of Environmental Health Sciences (R01ES023500). SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca, Bellerophon Therapeutics, Boehringer-Ingelheim Pharmaceuticals, Inc., Chiesi Farmaceutici SpA, Forest Research Institute, Inc., GlaxoSmithKline, Grifols Therapeutics, Inc., Ikaria, Inc., Nycomed GmbH, Takeda Pharmaceutical Company, Novartis Pharmaceuticals Corporation, Regeneron Pharmaceuticals, Inc., and Sanofi. N.N.H. was also supported by National Institute of Minority Health and Health Disparities grant DP50MD010431/Environmental Protection Agency grant 83615001, and C.O.E. and D.C.B. were supported by NHLBI grant T32 HL007534.
Author Contributions: C.O.E., H.W., J.D.K., and N.N.H. were responsible for the concept, design, analysis, and interpretation of data. C.O.E., H.W., and N.N.H. were responsible for drafting of the manuscript. All authors contributed to data analysis, drafting, revision, and final approval of the version submitted for publication, and agree to be accountable for all aspects of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202002-0253OC on October 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kelly RL. Age adjusted to 2000 standard population: all ages. Per 100,000 population. 2015 Kelly Report: health disparities in America. 2015 [accessed 2019 Apr 16]. Available from: https://robinkelly.house.gov/sites/robinkelly.house.gov/files/2015%20Kelly%20Report.pdf.
- 2.Hansel NN, Washko GR, Foreman MG, Han MK, Hoffman EA, DeMeo DL, et al. COPDGene Investigators. Racial differences in CT phenotypes in COPD. COPD. 2013;10:20–27. doi: 10.3109/15412555.2012.727921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisner MD, Blanc PD, Omachi TA, Yelin EH, Sidney S, Katz PP, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65:26–34. doi: 10.1136/jech.2009.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarrazin MV, Cannon KT, Rosenthal GE, Kaldjian LC. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc. 2009;101:656–662. doi: 10.1016/s0027-9684(15)30974-3. [DOI] [PubMed] [Google Scholar]
- 5.Prescott E, Godtfredsen N, Vestbo J, Osler M. Social position and mortality from respiratory diseases in males and females. Eur Respir J. 2003;21:821–826. doi: 10.1183/09031936.03.00047502. [DOI] [PubMed] [Google Scholar]
- 6.Ketelaars CA, Schlösser MA, Mostert R, Huyer Abu-Saad H, Halfens RJ, Wouters EF. Determinants of health-related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 1996;51:39–43. doi: 10.1136/thx.51.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen IH, Yelin EH, Katz P, Eisner MD, Blanc PD. Perceived neighborhood problems and quality of life, physical functioning, and depressive symptoms among adults with asthma. Am J Public Health. 2006;96:873–879. doi: 10.2105/AJPH.2004.059253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kershaw KN, Osypuk TL, Do DP, Chavez PJD, Roux AVD, et al. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. 2015;131:141–148. doi: 10.1161/CIRCULATIONAHA.114.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiatsatos P, Woo H, Paulin LM, Kind A, Putcha N, Gassett AJ, et al. The association between neighborhood socioeconomic disadvantage and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:981–993. doi: 10.2147/COPD.S238933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twardzik E, Judd S, Bennett A, Hooker S, Howard V, Hutto B, et al. Walk Score and objectively measured physical activity within a national cohort. J Epidemiol Community Health. 2019;73:549–556. doi: 10.1136/jech-2017-210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vupputuri S, Rubenstein KB, Derus AJ, Loftus BC, Horberg MA. Factors contributing to racial disparities in influenza vaccinations. PLoS One. 2019;14:e0213972. doi: 10.1371/journal.pone.0213972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peplinski B, McClelland R, Szklo M. Associations between socioeconomic status markers and depressive symptoms by race and gender: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Epidemiol. 2018;28:535–542, e1. doi: 10.1016/j.annepidem.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Hansel NN, Paulin LM, Gassett AJ, Peng RD, Alexis N, Fan VS, et al. Design of the subpopulations and intermediate outcome measures in COPD (SPIROMICS) AIR study. BMJ Open Respir Res. 2017;4:e000186. doi: 10.1136/bmjresp-2017-000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 20013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Census Bureau. Washington, DC: U.S. Census Bureau; 2010. Details about the 2010 release. [accessed 2019 Apr 10]. Available from: https://www.census.gov/programs-surveys/acs/news/data-releases.2010.html. [Google Scholar]
- 17.University of Wisconsin School of Medicine and Public Health. Madison, WI: University of Wisconsin School of Medicine and Public Health; 2013. 2013 area deprivation index v2.0. [accessed 2019 May 23]. Available from: https://www.neighborhoodatlas.medicine.wisc.edu/ [Google Scholar]
- 18.United States Department of Agriculture; Economic research service. Washington, DC: United States Department of Agriculture; 2015. USDA -2015 food access. [accessed 2018 Feb 19]. Available from: [DOI] [Google Scholar]
- 19.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 20.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 21.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 23.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WJ, Silverman EK, Hoffman E, Criner GJ, Mosenifar Z, Sciurba FC, et al. NETT Research Group. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busacker A, Newell JD, Jr, Keefe T, Hoffman EA, Granroth JC, Castro M, et al. A multivariate analysis of risk factors for the air-trapping asthmatic phenotype as measured by quantitative CT analysis. Chest. 2009;135:48–56. doi: 10.1378/chest.08-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 27.Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One. 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulin LM, Smith BM, Koch A, Han M, Hoffman EA, Martinez C, et al. Occupational exposures and computed tomographic imaging characteristics in the SPIROMICS cohort. Ann Am Thorac Soc. 2018;15:1411–1419. doi: 10.1513/AnnalsATS.201802-150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han MK, Curran-Everett D, Dransfield MT, Criner GJ, Zhang L, Murphy JR, et al. COPDGene Investigators. Racial differences in quality of life in patients with COPD. Chest. 2011;140:1169–1176. doi: 10.1378/chest.10-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittemore AS, Perlin SA, DiCiccio Y. Chronic obstructive pulmonary disease in lifelong nonsmokers: results from NHANES. Am J Public Health. 1995;85:702–706. doi: 10.2105/ajph.85.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis DR, Clegg LX, Johnson NJ. Lung disease mortality in the United States: the national longitudinal mortality study. Int J Tuberc Lung Dis. 2009;13:1008–1014. [PMC free article] [PubMed] [Google Scholar]
- 32.Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9:216–226. doi: 10.3109/15412555.2011.648030. [DOI] [PubMed] [Google Scholar]
- 33.Antonelli-Incalzi R, Ancona C, Forastiere F, Belleudi V, Corsonello A, Perucci CA. Socioeconomic status and hospitalization in the very old: a retrospective study. BMC Public Health. 2007;7:227. doi: 10.1186/1471-2458-7-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miravitlles M, Naberan K, Cantoni J, Azpeitia A. Socioeconomic status and health-related quality of life of patients with chronic obstructive pulmonary disease. Respiration. 2011;82:402–408. doi: 10.1159/000328766. [DOI] [PubMed] [Google Scholar]
- 35.Sahni S, Talwar A, Khanijo S, Talwar A. Socioeconomic status and its relationship to chronic respiratory disease. Adv Respir Med. 2017;85:97–108. doi: 10.5603/ARM.2017.0016. [DOI] [PubMed] [Google Scholar]
- 36.Signorello LB, Cohen SS, Williams DR, Munro HM, Hargreaves MK, Blot WJ. Socioeconomic status, race, and mortality: a prospective cohort study. Am J Public Health. 2014;104:e98–e107. doi: 10.2105/AJPH.2014.302156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown AF, Liang LJ, Vassar SD, Stein-Merkin S, Longstreth WT, Jr, Ovbiagele B, et al. Neighborhood disadvantage and ischemic stroke: the cardiovascular health study (CHS) Stroke. 2011;42:3363–3368. doi: 10.1161/STROKEAHA.111.622134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study. Am J Epidemiol. 2010;171:564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 40.Shankardass K, Jerrett M, Milam J, Richardson J, Berhane K, McConnell R. Social environment and asthma: associations with crime and no child left behind programmes. J Epidemiol Community Health. 2011;65:859–865. doi: 10.1136/jech.2009.102806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller GE, Engen PA, Gillevet PM, Shaikh M, Sikaroodi M, Forsyth CB, et al. Lower neighborhood socioeconomic status associated with reduced diversity of the colonic microbiota in healthy adults. PLoS One. 2016;11:e0148952. doi: 10.1371/journal.pone.0148952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bower KM, Thorpe RJ, Jr, Rohde C, Gaskin DJ. The intersection of neighborhood racial segregation, poverty, and urbanicity and its impact on food store availability in the United States. Prev Med. 2014;58:33–39. doi: 10.1016/j.ypmed.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamichhane AP, Warren J, Puett R, Porter DE, Bottai M, Mayer-Davis EJ, et al. Spatial patterning of supermarkets and fast food outlets with respect to neighborhood characteristics. Health Place. 2013;23:157–164. doi: 10.1016/j.healthplace.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hilmers A, Hilmers DC, Dave J. Neighborhood disparities in access to healthy foods and their effects on environmental justice. Am J Public Health. 2012;102:1644–1654. doi: 10.2105/AJPH.2012.300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 46.Walton E. Vital places: facilitators of behavioral and social health mechanisms in low-income neighborhoods. Soc Sci Med. 2014;122:1–12. doi: 10.1016/j.socscimed.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore LV, Diez Roux AV, Evenson KR, McGinn AP, Brines SJ. Availability of recreational resources in minority and low socioeconomic status areas. Am J Prev Med. 2008;34:16–22. doi: 10.1016/j.amepre.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen M, Zhang X, Harris CD, Holt JB, Croft JB. Spatial disparities in the distribution of parks and green spaces in the USA. Ann Behav Med. 2013;45(S1) Suppl 1:S18–S27. doi: 10.1007/s12160-012-9426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. 2005;46:15–31. doi: 10.1177/002214650504600103. [DOI] [PubMed] [Google Scholar]
- 50.Auchincloss AH, Van Nostrand JF, Ronsaville D. Access to health care for older persons in the United States: personal, structural, and neighborhood characteristics. J Aging Health. 2001;13:329–354. doi: 10.1177/089826430101300302. [DOI] [PubMed] [Google Scholar]
- 51.Weinmann GG, Chiang YP, Sheingold S. The National Emphysema Treatment Trial (NETT): a study in agency collaboration. Proc Am Thorac Soc. 2008;5:381–384. doi: 10.1513/pats.200709-154ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Celedón JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, et al. The transforming growth factor-beta1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD) Hum Mol Genet. 2004;13:1649–1656. doi: 10.1093/hmg/ddh171. [DOI] [PubMed] [Google Scholar]
- 53.Demeo DL, Mariani TJ, Lange C, Srisuma S, Litonjua AA, Celedon JC, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet. 2006;78:253–264. doi: 10.1086/499828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Becker G, Newsom E. Socioeconomic status and dissatisfaction with health care among chronically ill African Americans. Am J Public Health. 2003;93:742–748. doi: 10.2105/ajph.93.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


