To the Editor:
A substantial proportion of patients with coronavirus disease (COVID-19) admitted to the ICU require invasive mechanical ventilation for acute respiratory distress syndrome (ARDS), which is still associated with a high mortality rate (1). Applying the optimal positive end-expiratory pressure (PEEP) to ensure lung recruitment while limiting lung hyperinflation remains challenging in ARDS (2, 3). Yet, there are a few simple tools that might help personalize the level of PEEP in those patients at the bedside. Among them, the lung ultrasound (LUS) aeration score and the recruitment-to-inflation (R/I) ratio have the potential to identify patients who are more likely to benefit from PEEP (2). Previous studies indeed suggested that LUS could assess spatial distribution of PEEP-induced lung recruitment but does not reliably detect hyperinflation (4, 5). The R/I ratio is a tool that has recently been developed to evaluate both the potential for lung recruitment and the risk for hyperinflation, but it does not provide regional information about lung recruitment (6–8). Therefore, these two tools, readily available at the bedside in most ICUs, could provide additive and complementary information on lung recruitment.
In the present study, we aimed to assess lung recruitability simultaneously by the R/I ratio and the LUS in patients with COVID-19–related ARDS.
Methods
We conducted a prospective observational study between March 31 and October 29, 2020, in a 26-bed university-affiliated ICU in Lyon, France, which was approved by our institutional ethics committee.
Consecutive adult patients with COVID-19–associated ARDS in whom PEEP-induced lung recruitment was assessed simultaneously with both the LUS and the R/I ratio within the first 48 hours after intubation were included. COVID-19 was biologically confirmed and ARDS diagnosis was based on the Berlin criteria.
Ventilator settings and respiratory mechanics were recorded in sedated and curarized patients, ventilated in volume control with an Evita XL respirator (Dräger Medical). All measurements were performed in patients in the semirecumbent position. The presence of complete airway closure was assessed by measuring the airway opening pressure (AOP), as previously described (6).
PEEP-induced lung recruitment was assessed by the R/I ratio with high and low PEEP set at 15 cm H2O (for 30 min) and 5 cm H2O, respectively; in the event that AOP was >5 cm H2O, R/I was calculated with AOP instead of 5 cm H2O (6–8). Briefly, the recruited volume from low to high PEEP divided by the effective pressure change gave the compliance of the recruited lung; the ratio of this compliance to the compliance at low PEEP gave the R/I ratio (6–8). In the absence of a universally validated cut-off value, the median R/I ratio of the cohort was used to classify patients as high and low recruiters.
LUS scores were calculated at PEEP 15 cm H2O and PEEP 5 cm H2O, by summing regional scores (0–3 points) obtained in six regions of each lung (i.e., up and down anterior, medial, and posterior chest wall) (4, 5, 9, 10). The delta LUS (ΔLUS) score was defined as the difference obtained between low and high PEEP. The LUS reaeration score was also determined according to the method validated by Bouhemad and colleagues (5). All operators were experimented (>100 LUS procedures) and blinded to the R/I ratio results.
Continuous data are expressed as median (first to third quartile). Comparisons were done using nonparametrical tests. Correlations were assessed with the rho Spearman’s correlation test and its 95% confidence intervals (CIs).
Results
Twenty-four patients (age: 69 [63–73] yr; sex ratio: 5) were included (Table 1). The time from first symptoms to ICU admission was 8 (7–10) days. Twenty-two patients (88%) were treated with high-flow nasal oxygen therapy or noninvasive ventilation before intubation.
Table 1.
Patient Characteristics, Respiratory Mechanics, and Lung Recruitability
| All (n = 24) | High Recruiters (n = 12) | Low Recruiters (n = 12) | P Value* | |
|---|---|---|---|---|
| Age, yr | 69 (62 to 73) | 67 (60 to 73) | 70 (63 to 74) | 0.325 |
| Sex, M, n (%) | 20 (83) | 11 (92) | 9 (75) | 0.590 |
| Body mass index, kg/m2 | 31 (27 to 34) | 31 (29 to 33) | 29 (25 to 34) | 0.347 |
| Preexisting conditions, n (%) | ||||
| COPD | 3 (13) | 2 (17) | 1 (8) | >0.999 |
| Hypertension | 11 (46) | 6 (50) | 5 (42) | >0.999 |
| Diabetes | 12 (46) | 6 (50) | 6 (50) | >0.999 |
| Ischemic heart disease | 2 (8) | 1 (8) | 1 (8) | >0.999 |
| SAPS II score | 40 (31 to 44) | 37 (31 to 43) | 41 (31 to 46) | 0.840 |
| Delay between intubation and inclusion, h | 8 (4 to 16) | 9 (4 to 16) | 8 (5 to 13) | 0.728 |
| PaO2/FiO2 at inclusion, mm Hg | 136 (99 to 166) | 111 (92 to 160) | 144 (117 to 179) | 0.141 |
| Severity of ARDS, n (%) | 0.187 | |||
| Mild | 2 (8) | 0 (0) | 2 (17) | — |
| Moderate | 15 (63) | 7 (58) | 8 (67) | — |
| Severe | 7 (29) | 5 (42) | 2 (17) | — |
| Fluid balance from admission, L | −2.0 (−2.0 to 1.1) | 0.8 (−1.7 to 1.2) | −0.2 (−2.3 to 0.1) | 0.219 |
| Baseline respiratory data | ||||
| Vt, ml/kg (PBW) | 6.0 (5.8 to 6.1) | 5.9 (5.8 to 6.1) | 6.0 (5.8 to 6.1) | 0.620 |
| Respiratory rate, cycles/min | 25 (22 to 28) | 25 (23 to 28) | 25 (22 to 28) | 0.726 |
| PEEP, cm H2O | 12 (10 to 15) | 15 (10 to 15) | 12 (10 to 13) | 0.272 |
| Auto-PEEP, cm H2O | 1.0 (0.6 to 1.0) | 1.0 (0.5 to 1.0) | 1.0 (0.6 to 1.0) | 0.650 |
| Pplat, cm H2O | 24 (22 to 27) | 24 (22 to 27) | 24 (21 to 27) | 0.907 |
| Crs, ml/cm H2O | 34 (29 to 48) | 42 (33 to 47) | 32 (29 to 36) | 0.076 |
| Ventilatory ratio† | 1.47 (1.24 to 1.88) | 1.58 (1.40 to 2.16) | 1.29 (1.17 to 1.53) | 0.198 |
| Respiratory data at PEEP 5 and 15 cm H2O | ||||
| AOP > 5 cm H2O, n (%) | 8 (33) | 4 (33) | 4 (33) | 1.000 |
| ΔSpO2/FiO2‡ | 11 (4 to 16) | 11 (4 to 16) | 10 (2 to 16) | 0.788 |
| Vrec, ml | 226 (117 to 356) | 347 (227 to 546) | 120 (76 to 212) | <0.001 |
| Vrec, ml/kg(PBW) | 3.9 (1.9 to 4.6) | 4.5 (3.5 to 7.3) | 2.1 (1.3 to 3.4) | <0.001 |
| Crs at PEEP 5 cm H2O | 37 (29 to 42) | 39 (31 to 47) | 35 (26 to 40) | 0.311 |
| Crs at PEEP 15 cm H2O | 31 (24 to 40) | 39 (29 to 47) | 26 (23 to 31) | 0.012 |
| Crec, ml/cm H2O | 22 (11 to 32) | 32 (24 to 50) | 12 (8 to 21) | <0.001 |
| R/I ratio | 0.70 (0.49 to 0.81) | 0.80 (0.74 to 1.20) | 0.48 (0.20 to 0.57) | <0.001 |
| LUS score at PEEP 5 cm H2O | 24 (22 to 25) | 24 (23 to 25) | 24 (22 to 26) | 0.594 |
| LUS score at PEEP 15 cm H2O | 21 (20 to 23) | 20 (19 to 22) | 22 (21 to 23) | 0.170 |
| ΔLUS score§ | 3 (1 to 4) | 4 (3 to 6) | 2 (0 to 3) | 0.012 |
| Anterior lung regions | 1 (0 to 2) | 2 (0 to 2) | 1 (0 to 2) | 0.633 |
| Lateral lung regions | 0 (0 to 1) | 1 (0 to 2) | 0 (0 to 1) | 0.077 |
| Posterior lung regions | 1 (0 to 1) | 1 (1 to 2) | 0 (0 to 1) | 0.009 |
| LUS reaeration score | 3 (1 to 5) | 4 (3 to 6) | 2 (0 to 3) | 0.017 |
| Anterior lung regions | 1 (0 to 2) | 2 (0 to 3) | 1 (0 to 2) | 0.593 |
| Lateral lung regions | 0 (0 to 1) | 1 (0 to 3) | 0 (0 to 1) | 0.084 |
| Posterior lung regions | 1 (0 to 1) | 1 (1 to 2) | 0 (0 to 1) | 0.009 |
Definitions of abbreviations: AOP = airway opening pressure; ARDS = acute respiratory distress syndrome; COPD = chronic obstructive pulmonary disease; Crec = compliance of the recruited lung; Crs = compliance of the respiratory system; LUS = lung ultrasound score; PBW = predicted body weight; PEEP = positive end-expiratory pressure; Pplat= plateau pressure; R/I ratio = recruitment-to-inflation ratio; SAPS II = Simplified Acute Physiology Score II; SpO2 = oxygen saturation as measured by pulse oximetry; Vrec = recruited lung volume.
Data are expressed as median (first to third quartile) unless otherwise indicated.
P values refer to the comparison between the high- and low-recruiter groups.
Defined as (V̇e × PaCO2)/(predicted body weight × 100 × 37.5).
ΔSpO2/FiO2 = SpO2/FiO2 at PEEP 15 cm H2O − SpO2/FiO2 at PEEP 5 cm H2O.
ΔLUS = LUS score at PEEP 5 cm H2O − LUS score at PEEP 15 cm H2O.
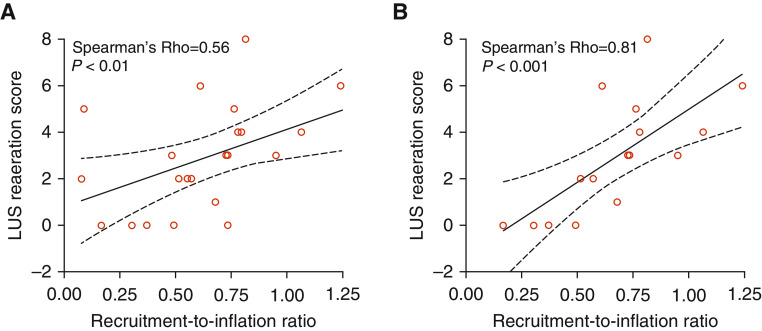
Most patients (n = 22/24, 92%) presented moderate to severe ARDS (Table 1). Complete airway closure was present in one-third of cases; none of them had an AOP above the high PEEP (Table 1). The median R/I ratio was 0.7, thus defining 12 high recruiters (0.8 [0.8–1.1]) and 12 low recruiters (0.5 [0.3–0.6]) (Table 1). The LUS scores measured at low and high PEEP did not significantly differ between the two subgroups, whereas both ΔLUS and LUS reaeration scores were significantly higher in high recruiters (Table 1). The difference in LUS reaeration scores was mainly driven by more reaeration in lateral and posterior lung regions in the recruiters (Table 1). We found significant (P < 0.01) correlations between R/I ratio and both ΔLUS and LUS reaeration scores (rho = 0.55 [95% CI, 0.18–0.78] and 0.56 [95% CI, 0.19–0.79], respectively). These correlations were even stronger (rho = 0.82 [95% CI, 0.54–0.94] and 0.81 [95% CI, 0.52–0.93], respectively; P < 0.001) when considering only patients without complete airway closure at 5 cm H2O (Figure 1).
Figure 1.
Correlation between recruitment-to-inflation (R/I) ratio and lung ultrasound (LUS) reaeration score. (A and B) The continuous line shows the linear regression (with 95% confidence interval in dashed lines) between the R/I ratio and the LUS reaeration score in 24 patients with coronavirus disease (COVID-19)–induced acute respiratory distress syndrome with and without airway opening pressure (AOP) >5 cm H2O (A) and in the 16 patients without AOP >5 cm H2O (B). Of note, the R/I ratio is a continuous variable, whereas the LUS reaeration score is a discrete variable in the linear regression.
Discussion
The main and new finding of the present study is that the R/I ratio at a threshold of 0.7 correlated with the LUS reaeration score, in particular in patients without complete airway closure. To the best of our knowledge, this is the first report combining the R/I ratio and LUS to assess lung recruitment, even considering patients with non–COVID-19 ARDS.
Our result extends the previous validation of the LUS reaeration score against the recruited volume measured by the volume–pressure curve (4), by consistently underscoring the need to consider AOP when assessing lung recruitment by the spirometric methods.
In our study, the LUS reaeration score was significantly higher in the posterior lung regions of recruiters than in nonrecruiters but not in the anterior lung regions. This important finding suggests that lung recruitment occurred in the dependent lung regions, which is the desirable effect of PEEP or of any other method used to elicit this process. The fact that compliance of the respiratory system was greater in the former than in the latter, and that ventilatory ratio, a marker of dead space, was not different between the two groups, argue against a significant hyperinflation.
In the princeps study by Chen and colleagues in non–COVID-19 ARDS, the R/I ratio that differentiated recruiters from nonrecruiters, corresponding to the median value of their cohort, was 0.5. In the present study, the median value of the R/I ratio was 0.7, which was used for the analysis. Nevertheless, we found similar results at a threshold of 0.5 (data not shown).
Our study is limited by the fact that it is single-centered and that the gold standard method for lung recruitment assessment (i.e., quantitative computed tomography scan) was not used. Also, we cannot rule out that PEEP greater than 15 cm H2O might have recruited lung in some low recruiters. Our findings confirm, however, previous reports about the feasibility of the R/I ratio measurement in COVID-19 (6–8). Moreover, the measurements were not repeated over time as it could be that recruitability would change. This should be the purpose of further studies.
Supplementary Material
Footnotes
Author Contributions: N.S., E.C., and C.G. designed the study. N.S., E.C., and A.D. collected the data. N.S., L.A., M.C., and C.G. analyzed the data and drafted the manuscript. E.C. and A.D. critically revised the manuscript. All authors approved the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202012-4447LE on February 9, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turbil E, Terzi N, Cour M, Argaud L, Einav S, Guérin C. Positive end-expiratory pressure-induced recruited lung volume measured by volume-pressure curves in acute respiratory distress syndrome: a physiologic systematic review and meta-analysis. Intensive Care Med. 2020;46:2212–2225. doi: 10.1007/s00134-020-06226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz MJ. High versus low PEEP in non-recruitable collapsed lung tissue: possible implications for patients with COVID-19. Lancet Respir Med. 2020;8:e44. doi: 10.1016/S2213-2600(20)30180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 5.Chiumello D, Mongodi S, Algieri I, Vergani GL, Orlando A, Via G, et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med. 2018;46:1761–1768. doi: 10.1097/CCM.0000000000003340. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Del Sorbo L, Grieco DL, Junhasavasdikul D, Rittayamai N, Soliman I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome: a clinical trial. Am J Respir Crit Care Med. 2020;201:178–187. doi: 10.1164/rccm.201902-0334OC. [DOI] [PubMed] [Google Scholar]
- 7.Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beloncle FM, Pavlovsky B, Desprez C, Fage N, Olivier PY, Asfar P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. 2020;10:55. doi: 10.1186/s13613-020-00675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Zieleskiewicz L, Markarian T, Lopez A, Taguet C, Mohammedi N, Boucekine M, et al. AZUREA Network. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020;46:1707–1713. doi: 10.1007/s00134-020-06186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.