To the Editor:
E-cigarette or vaping product use–associated lung injury (EVALI) has caused hospitalizations and mortality in the United States since August 2019 (1). The main respiratory clinical symptoms of patients with EVALI include organizing pneumonia and diffuse alveolar damage (2). Lipid-laden macrophages (LLMs) have been seen in the lungs of patients with EVALI (3). LLM typically results from impaired pulmonary surfactant recycling and/or uptake of exogenous lipids (4) and is indicative of impaired innate defense. Moreover, LLM has been proposed as a unique biomarker of patients with EVALI who use vape products that contain tetrahydrocannabinol (THC) and/or vitamin E acetate (5, 6). However, it is currently unproven whether LLM is a unique clinical feature of EVALI, and whether they should be used for diagnosis remains debatable (7). Indeed, in animal studies, exposure to e-cigarette vapor that does not contain THC resulted in accumulation of LLMs, impaired immune responses to viral infection, and decreased pulmonary surfactant expression (8). Some of the results of these studies have been previously reported in the form of abstracts in international conferences (9, 10).
The goal of the current study was to measure Oil Red O (Abcam) staining as a marker of lipid deposition in alveolar macrophages from asymptomatic vapers, and in parallel to assess surfactant levels and antibacterial capacity. All data are presented as mean ± SD. We performed research bronchoscopies on healthy nonsmokers (age, 27.75 ± 7.29 yr; body mass index [BMI], 29.63 ± 5.65 kg/m2; percentage predicted FVC [ppFVC], 104.42 ± 14.06; ppFEV1, 102.25 ± 14.83), smokers (age, 33.63 ± 7.37 yr; BMI, 28.44 ± 6.78 kg/m2; ppFVC, 106.05 ± 11.14; ppFEV1, 101.53 ± 12.70), and vapers (age, 26.40 ± 7.55 yr; BMI, 29.46 ± 6.33 kg/m2; ppFVC, 101.53 ± 8.77; ppFEV1, 101.20 ± 9.34) to obtain macrophages from BAL as previously described (11). Serum cotinine concentrations were measured to verify the tobacco use status of the subjects. Smokers (154.97 ± 109.37 ng/ml) and vapers (122.45 ± 95.36 ng/ml) showed significantly increased cotinine concentrations compared with the nonsmokers (0.02 ± 0.06 ng/ml), with no difference between the two tobacco product user groups. Apart from the female-to-male sex ratio, there were no significant differences in subject demographics. All subjects completed a 2-week smoking or vaping diary before the bronchoscopy. Dual-users (subjects using both combustible tobacco and e-cigarettes) were excluded from the study and all vapers used nicotine in a propylene glycol/vegetable glycerin base. The smokers reported a mean of 10.67 ± 5.54 cigarette use per day and a mean pack-year history of 8.61 ± 5.29. The vapers exhibited a widely variable pattern of e-cigarette use with an average of 13.16 ± 16.72 ml used per day for some subjects and 88.08 ± 82.59 puffs per day for others. The self-reported vaping history indicated the use of second- to fourth-generation e-cigarettes. Five of the vapers were never-smokers and the other vapers had switched to exclusive vaping at least 6 months before bronchoscopy. The BAL macrophages were examined for lipid deposition and BAL fluid was examined for antibacterial capacity and levels of surfactant proteins. One-way ANOVA was used to analyze the statistical difference across the groups. Tukey’s multiple comparisons tests were used for comparing between groups.
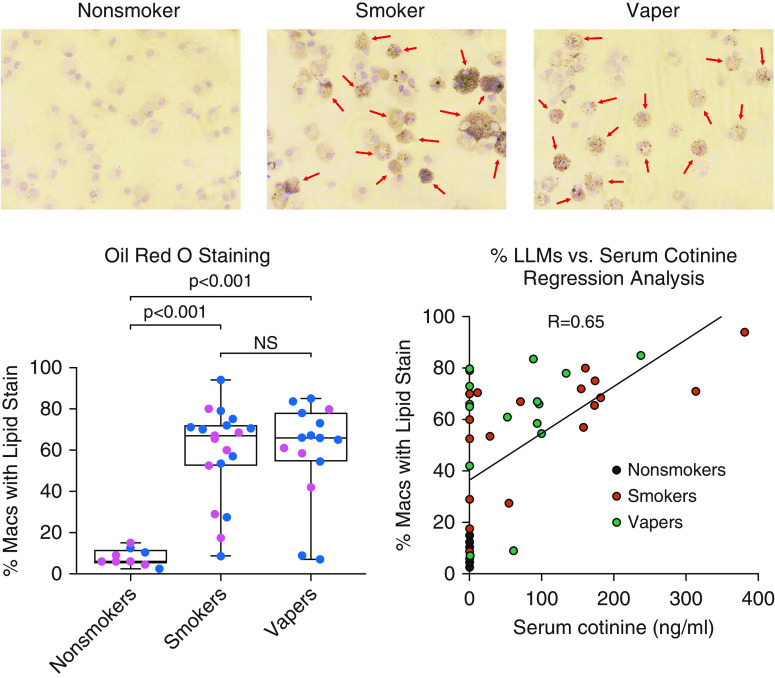
BAL cytospins were stained with Oil Red O stain (Abcam) for the histological visualization of cellular lipid deposition. We observed that otherwise healthy smokers and vapers had significantly increased and comparatively equal numbers of LLM relative to nonsmokers, who showed no evidence of lipid deposition (Figure 1). The extent of macrophage lipid deposition was also not different between asymptomatic smokers and vapers (Figure 1, bottom left), indicating that LLMs may not be a unique feature of patients with EVALI. A significant correlation was observed between the available serum cotinine concentrations and the percentage of LLMs (R = 0.65) (Figure 1, bottom right), suggesting a role for nicotine in LLM formation. Importantly, the evaluation of THC using a drug test kit (Easy@Home catalog no. # EDTH-114) showed no measurable amount of THC in any of our subjects’ BAL samples, suggesting that THC and vitamin E acetate were not the causes of LLM. Furthermore, the presence of LLMs, in both smokers’ and vapers’ lungs, with the correlation to serum cotinine concentrations, indicated that not only are LLMs not specific to patients with EVALI, they are not even specific to vapers.
Figure 1.
Smokers and vapers show increased lipid deposition in alveolar macrophages. BAL samples were collected from healthy nonsmokers, smokers, and vapers to obtain the alveolar macrophages and secreted proteins. Cytospin slides were prepared and Oil Red O stain was used to identify intracellular lipid deposition in alveolar macrophages. The representative images of Oil Red O staining in nonsmokers, smokers, and vapers are shown with positively stained macrophages marked with arrows. The graphs show the percentage of cells with positive staining for lipid deposition per subject. The cytospin slides from smokers (n = 19) and vapers (n = 15) had significantly higher numbers of Oil Red O–positive macrophages than the nonsmokers (n = 9). A regression analysis of available serum cotinine concentrations with the percentage of LLMs showed a strong correlation (R = 0.65). The pink dots are female subjects and the blue dots are male subjects. Tukey’s post hoc test derived the P values that are provided on the graphs. LLMs = lipid-laden macrophages; Macs = macrophages; NS = nonsignificant.
We then measured pulmonary surfactant proteins in concentrated BAL fluid using Western blotting as previously described (11). The proteins were normalized to albumin content in BAL fluid. The most abundant surfactant protein, SP-A (surfactant protein A), was significantly reduced in both smokers and vapers compared with nonsmokers (Figure 2, blot at top left and SP-A graph). Although smokers had a greater reduction in SP-A levels compared with nonsmokers, the difference between smokers’ and vapers’ SP-A levels was not significant (Figure 2, SP-A graph). Other surfactant protein levels remained unaltered across the groups (Figure 2, SP-B to SP-D). To see if antibacterial capacity was functionally affected by product use, we measured Pseudomonas aeruginosa growth in cell-free BAL fluids. We observed significantly increased P. aeruginosa growth (cfu/ml) in both smokers’ and vapers’ BAL fluid, indicating a reduced antibacterial capacity in both smokers and vapers relative to nonsmokers (Figure 2, lower left). Like macrophage lipid deposition, there was no difference in antibacterial capacity between smokers and vapers (Figure 2, lower left). However, only a weak correlation was observed between serum cotinine and cfu/ml values (R = 0.33) (Figure 2, lower right). These data suggested that the impaired antibacterial activity was nicotine independent and that other factors may have been involved in the process.
Figure 2.
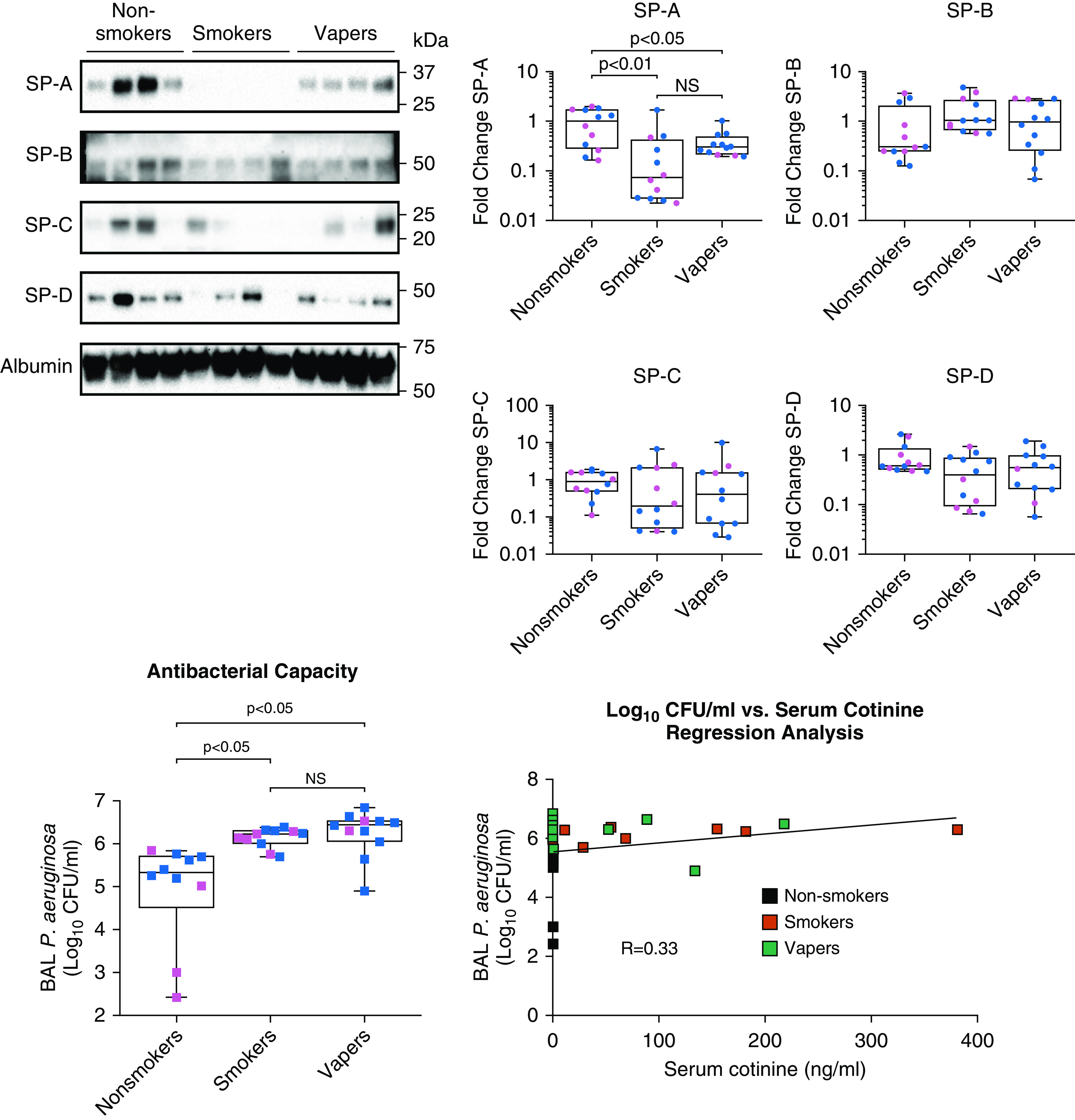
Smokers and vapers show reduced pulmonary surfactant A levels and reduced antibacterial capacity. BAL samples were collected from healthy nonsmokers, smokers, and vapers to obtain the secreted proteins. Concentrated BAL fluid was used for the estimation of SP (surfactant protein) levels by Western blotting. The primary antibodies used were the SP-A antibody (Novus Biologicals), SP-B antibody (ABclonal), SP-C antibody (ABclonal), SP-D antibody (R&D Systems), and human serum albumin antibody (R&D Systems). The densitometric analysis was performed to evaluate the change in expression. The graphs show significantly decreased SP-A expression in both smokers and vapers compared with the nonsmokers, and all other surfactant proteins remained unaltered across the three groups (n = 12 per group). The data are represented as fold change compared with the nonsmokers. For the evaluation of the antibacterial property, BAL fluids were incubated with 103 cfu/ml of P. aeruginosa for 2 hours and plated on Luria-Bertani agar plates. After 24 hours of incubation, colony-forming units were significantly greater in both vapers and smokers (n = 11 each) than in the nonsmokers (n = 10), indicating a reduced pulmonary antimicrobial capacity in users. The regression analysis of available serum cotinine concentrations with cfu/ml showed a weak correlation (R = 0.33). The pink dots and squares are female subjects, and the blue dots and squares are male subjects. Tukey’s post hoc test derived the P values are provided on the graphs. NS = nonsignificant; P. aeruginosa = Pseudomonas aeruginosa.
Our observations demonstrated that asymptomatic vapers had increased LLM accumulation in their lungs. We further observed that SP-A was significantly decreased in both smokers and asymptomatic vapers as compared with nonsmokers, which may compromise alveolar surface tension (12). Furthermore, surfactant proteins have antibacterial activity (13, 14) and the reduced SP-A levels corroborate the observed decreased antibacterial capacity in smokers and vapers. Together, these changes suggest increased susceptibility to infection and, indeed, reduced antibacterial capacity was identified in vapers’ BALs. Interestingly, the available serum cotinine concentrations showed a strong correlation with percentage LLM (R = 0.65) but not cfu/ml values (R = 0.33), indicating more complex mechanisms may be involved in the observed reduced antibacterial capacity in smokers’ and vapers’ lung. These observations are consistent with our previous study, in which we found that vapers’ BAL had increased protease levels to the same extent as smokers’ BAL fluid (11). Interestingly, smokers and asymptomatic vapers had comparatively equally elevated LLM with no evidence of THC and/or vitamin E acetate use, suggesting that LLM may not be a unique feature to patients with EVALI. Our study provides further support to the stipulation that lipid deposition in alveolar macrophages may not be a unique feature of the EVALI diagnosis and expands our current thinking to include the involvement of other lung injuries and altered host defense processes such as antimicrobial activity and surfactant protein levels (7, 15, 16). Furthermore, the strong correlation of LLMs with serum cotinine suggests that lipid deposition within macrophages may be related to tobacco product use irrespective of the pulmonary health status of the subjects. The aberration in antibacterial capacity and surfactant protein levels in otherwise healthy smokers and vapers argues against the perception that e-cigarettes are a safer alternative to conventional combustible tobacco cigarettes. This study has some limitations: our study is cross-sectional with only age-matched groups with limited numbers of subjects. An extended longitudinal study with more age-matched and sex-matched subjects including more vapers without prior smoking history would provide more insight into the deleterious effects of continued e-cigarette use.
Supplementary Material
Footnotes
Supported by NIH/Food and Drug Administration (FDA) grant HL120100, NIH/NHLBI grant HL135642, and NIH/FDA grant HL153698.
Originally Published in Press as DOI: 10.1164/rccm.202009-3507LE on December 17, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Reagan-Steiner S, Gary J, Matkovic E, Ritter JM, Shieh WJ, Martines RB, et al. Lung Injury Response Pathology Working Group. Pathological findings in suspected cases of e-cigarette, or vaping, product use-associated lung injury (EVALI): a case series. Lancet Respir Med. 2020;8:1219–1232. doi: 10.1016/S2213-2600(20)30321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kligerman S, Raptis C, Larsen B, Henry TS, Caporale A, Tazelaar H, et al. Radiologic, pathologic, clinical, and physiologic findings of electronic cigarette or vaping product use-associated lung injury (EVALI): evolving knowledge and remaining questions. Radiology. 2020;294:491–505. doi: 10.1148/radiol.2020192585. [DOI] [PubMed] [Google Scholar]
- 3.Guerrini V, Panettieri RA, Jr, Gennaro ML. Lipid-laden macrophages as biomarkers of vaping-associated lung injury. Lancet Respir Med. 2020;8:e6. doi: 10.1016/S2213-2600(19)30476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 5.Balmes JR. Vaping-induced acute lung injury: an epidemic that could have been prevented. Am J Respir Crit Care Med. 2019;200:1342–1344. doi: 10.1164/rccm.201910-1903ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Lung Injury Response Laboratory Working Group. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy C, Keane MP, Fabre A. Lipid-laden macrophages are not diagnostic of pulmonary alveolar proteinosis syndrome and can indicate lung injury. Am J Respir Crit Care Med. 2020;202:1197–1198. doi: 10.1164/rccm.202005-1880LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madison MC, Landers CT, Gu BH, Chang CY, Tung HY, You R, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129:4290–4304. doi: 10.1172/JCI128531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A, Keating J, Coakley R, Ehrmann B, Alexis N, Tarran R. Vaping associated alterations in the lung lipidome [abstract] Am J Respir Crit Care Med. 2020;201:A7682. [Google Scholar]
- 10.Ghosh A, Keating JE, Coakley RD, Ehrmann BM, Alexis NE, Tarran R. Vaping associated alterations in the lung lipidome [abstract] New Orleans, LA: Society for Research on Nicotine and Tobacco; 2020. [Google Scholar]
- 11.Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR, Jr, Alexis NE, et al. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med. 2019;200:1392–1401. doi: 10.1164/rccm.201903-0615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. FASEB J. 2001;15:59–69. doi: 10.1096/fj.00-0318rev. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awasthi S. Surfactant protein (SP)-A and SP-D as antimicrobial and immunotherapeutic agents. Recent Pat Antiinfect Drug Discov. 2010;5:115–123. doi: 10.2174/157489110791233559. [DOI] [PubMed] [Google Scholar]
- 15.Shields PG, Song MA, Freudenheim JL, Brasky TM, McElroy JP, Reisinger SA, et al. Lipid laden macrophages and electronic cigarettes in healthy adults. EBioMedicine. 2020;60:102982. doi: 10.1016/j.ebiom.2020.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eissenberg T, Maziak W. Are electronic cigarette users at risk for lipid-mediated lung injury? Am J Respir Crit Care Med. 2020;201:1012–1013. doi: 10.1164/rccm.201910-2082LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.