Abstract
A new alternative method for the production of biodiesel from rendered fat, including animal by‐product (ABP) Category 1 tallow, was evaluated. The method consists of a conversion phase, based on esterification and transesterification in a single step (at temperature ≥ 200°C, pressure ≥ 70 bar with a retention time ≥ 15 min), using MgO as a catalyst and in the presence of methanol (10–15%), followed by vacuum distillation (at ≥ 150°C, ≤ 10 mbar) of the end‐product, biodiesel and the co‐product, glycerine. Prions (PrPS c), which are abnormal isoforms of the prion protein, were considered by the applicant to be the most resistant hazard. In accordance with previous EFSA Opinions and current expert evaluation, a reduction in prion infectivity, or detectable PrPS c, of at least 6 log10 should be achieved for the process to be considered equivalent to the processing method laid down in the Regulation (EU) No 142/2011. Published data from an experimental replication of the conversion step of the biodiesel production process under consideration were provided, which showed an at least 6 log10 reduction in detectable PrPS c, by Western blot, in tallow that had been spiked with murine and human prion strains. In addition, it was demonstrated that the presence of methanol does not affect the recovery or detection of PrPS c from a biodiesel substrate. Based on scientific literature, the vacuum distillation step has been shown to be capable of achieving an additional 3 log10 reduction in PrPS c. Therefore, the proposed alternative method is considered to be at least equivalent to the processing method laid down in the legislation for the production of biodiesel from raw materials including Category 1 ABP.
Keywords: animal by‐product, ABP, biodiesel, prion, TSE, methanol, tallow, category 1
Summary
On 10 June 2020, the European Food Safety Authority (EFSA) received from the Austrian Competent Authority (Federal Ministry of Social Affairs, Health, Care and Consumer Protection) an application (mandate and technical dossier: EFSA‐Q‐2020‐00450) under Regulation (EC) No 1069/2009 referring to the evaluation of an alternative method for the production of biodiesel from rendered fat, including animal by‐product (ABP) Category 1 tallow, submitted by BDI‐BioEnergy International GmbH (hereinafter referred to as the applicant).
The proposed new method will be applied using feedstock treated according to method 1 (pressure sterilisation) and after removal of insoluble impurities in excess of 0.15% w/w. The process, called RepCat®, consists of a conversion phase of esterification and transesterification in a single step (at temperature ≥ 200°C, pressure ≥ 70 bar with a retention time ≥ 15 min), using MgO as a catalyst and in the presence of methanol (10–15%), followed by vacuum distillation (at ≥ 150°C, ≤ 10 mbar) of the end‐product, biodiesel.
The material to be treated is rendered fat, including ABP Category 1 tallow. Under Article 20 of Regulation (EC) No 1069/2009, EFSA is required to assess whether the method submitted ensures that any risks to public or animal health are reduced to a degree that is at least equivalent to that achieved by the processing method laid down in the legislation for the same category of ABP. Prions (PrPSc), which are the abnormal isoforms of the prion protein, were considered to be the most resistant hazard by the applicant and the BIOHAZ Panel agreed with the approach of focusing on the capability of the alternative method to reduce prion infectivity or detectable PrPSc. In accordance with previous EFSA Opinions and current expert evaluation, a reduction in prion infectivity, or detectable PrPSc, of at least 6 log10 should be achieved for the process to be considered at least equivalent to the processing method laid down in Regulation (EU) No 142/2011.
The applicant provided data in the form of published studies in which it was shown that methanol does not affect the recovery or detection of prions from a biodiesel substrate, the RepCat® esterification and transesterification process can achieve a reduction in prions of at least 6 log10, as detected by Western blot, and vacuum distillation can provide a further reduction of 3 log10.
The application includes sufficient information on the HACCP plan and the risks of interdependent processes and those associated with the intended end use of biodiesel. The measures proposed in the dossier to deal with these risks are compliant with the relevant legislation.
The application and supporting references have been thoroughly reviewed and the data provided support the conclusion that the RepCat® process can achieve at least a 6 log10 reduction of the hazard. The process can therefore be considered at least equivalent to the processing method laid down in the legislation for the production of biodiesel from raw materials including Category 1 ABP.
1. Introduction
1.1. Background
On 10 June 2020, the European Food Safety Authority (EFSA) received from the Federal Ministry of Social Affairs, Health, Care and Consumer Protection of Austria (Competent Authority, CA) an application (mandate and technical dossier; EFSA‐Q‐2020‐00450) under Regulation (EC) No 1069/20091, for the evaluation of a new alternative process, called RepCat®, ‘for production of biodiesel and distilled glycerine from rendered fat of all categories of animal by‐products’, submitted on behalf of the Austrian‐based company BDI‐BioEnergy International GmbH (hereinafter referred to as the applicant).
The applicant submitted an application as required in the procedure for authorisation of an alternative method of use or disposal of animal by‐products (ABP) or derived products, laid down in Article 20 of the Regulation (EC) No 1069/2009.
During the completeness check, performed according to Regulation (EC) No 1069/2009, it was noticed that some information was missing or incomplete. On 19 August 2020, EFSA sent a letter to the applicant with the following three requests:
According to Section 2.1.2.1 (2.a) of the ‘Statement on technical assistance on the format for applications for new alternative methods for animal by‐products’ from the EFSA BIOHAZ Panel (2010a), the applicant should provide technical data sheets for the equipment used in the relevant process steps.
The applicant has provided a list of references at the end of the technical dossier in which three references cited in the dossier have not been provided. The applicant should complete the reference list accordingly and send all the cited references as separate pdf format files. References should be provided in a dedicated folder ‘References’.
During its review, EFSA has identified no claims for confidentiality. If applicable, and in accordance with Art. 39 of Regulation (EC) No 178/2002, the applicant should kindly indicate the elements of the application dossier for which a request for confidentiality treatment should be stated specifying the applicable section(s) or data sets, and page number(s) in the dossier as well as a verifiable justification(s)/reasons(s) for the statement. Otherwise, the applicant is asked to confirm that no claims for confidentiality are made for this application.
On 9 September 2020, EFSA received the missing information concerning the application. The list of documents submitted to EFSA is available in Section 5. After checking the content of the full dossier, EFSA considered that the application EFSA‐Q‐2020‐00450 was valid on 7 October 2020. According to Regulation (EC) No 1069/2009, EFSA shall respect the deadline of 6 months to deliver the scientific opinion. Therefore, the opinion must be delivered by 7 April 2021.
According to Section 2D, Chapter IV of Annex IV of Commission Regulation (EU) No 142/20112, for the biodiesel production process, as an alternative processing method, the Competent Authority may authorise a process using the following process parameters:
‘D. Biodiesel production process
-
Starting material
For this process, a fat fraction derived from animal by‐products of all categories may be used.For this process, a fat fraction derived from animal by‐products of all categories may be used.
-
Processing method
Biodiesel production shall be carried out according to the following processing standards:-
Unless fish oil or rendered fat are used which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No 853/2004 3 , respectively, the fat fraction derived from animal by‐products must be first processed using:
- in the case of Category 1 or 2 materials, processing method 1 (pressure sterilisation) as set out in Chapter III; and
- in the case of Category 3 materials, any of the processing methods 1–5 or processing method 7 or, in the case of material derived from fish, processing methods 1 to 7 as set out in Chapter III;
-
The processed fat must then be processed further using one of the following methods:
- a process whereby the processed fat must be separated from the protein and, in the case of fat from ruminant origin, insoluble impurities in excess of 0.15% by weight must be removed, and the processed fat must be subsequently submitted to esterification and transesterification. However, esterification is not required for processed fat derived from Category 3 material. For esterfication the pH must be reduced to less than 1 by adding sulphuric acid (H 2 SO 4 ) or an equivalent acid and the mixture must be heated to 72°C for at least 2 h during which it must be intensely mixed. Transesterification must be carried out by increasing the pH to about 14 with potassium hydroxide or with an equivalent base at 35–50°C for at least 15 min. Transesterfication shall be carried out twice under the conditions described in this point using a new base solution. This process must be followed by refinement of the products including vacuum distillation at 150°C, leading to biodiesel;
- a process using equivalent process parameters authorised by the Competent Authority.’
-
An alternative method for biodiesel production was submitted by the applicant who has developed a new patented process (RepCat® process) for the production of biodiesel (fatty acid methyl esters, FAME) from waste material. This process can also use ABP tallow as feedstock. Briefly, the RepCat® process consists of a conversion unit, where esterification and transesterification take place in a single step, using a catalyst and in the presence of methanol, followed by water/methanol recovery and vacuum distillation of the end‐product, biodiesel, and the co‐product glycerine. Although the applicant refers to glycerine as another end product of the alternative process, according to point 2 (b) Section 3 Chapter IV of Annex IV of Commission Regulation (EU) No 142/2011, glycerine is described as a derived material resulting from processing, in accordance with the biodiesel production process. Therefore, in the current opinion glycerine is considered as a derived product or co‐product, following the regulation.
As set out in Article 20 of Regulation (EC) No 1069/2009, EFSA is required to assess whether the method submitted ensures that any risks to public or animal health are reduced to a degree which is at least equivalent, for the relevant ABP category, to the processing method laid down in the legislation.
1.2. Additional information
In 2017, the EFSA Panel on Biological Hazards (BIOHAZ) published a scientific opinion, following a request from the Federal Ministry of Health and Women's Affairs of Austria, on behalf of the company BDI – BioEnergy International AG, to evaluate an alternative method for the production of biodiesel from rendered fat of all categories of animal by‐products, also called BDI‐RepCat® Process (EFSA BIOHAZ Panel, 2017). The method was based on a single step conversion unit with esterification and transesterification, in the presence of methanol, followed by water/methanol recovery and vacuum distillation of biodiesel and glycerine, similar to the current application. The Panel could not conclude about the equivalence of the alternative method with the approved biodiesel production process for a twofold reason: (i) the level of risk reduction achieved had not been demonstrated using experimental trials run under conditions equivalent to the ones described for the RepCat® process; (ii) insufficient evidence was provided regarding the possible effect of methanol in stabilising prion molecules, which may impact on the level of Transmissible Spongiform Encephalopathy (TSE) hazard reduction achievable.
Based on the information provided in the current application, the differences between the alternative method evaluated in the previous EFSA scientific opinion (EFSA BIOHAZ Panel, 2017) and the current one, in terms of treatment conditions, are the minimum treatment time, temperature and pressure applied in the RepCat® process, during the conversion phase (Table 1).
Table 1.
Comparison of processing parameters of the approved method in the legislation (Commission Regulation (EU) No 142/2011) and the alternative methods (submitted by the applicant in 2017 and 2020)
| Methods and their parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commission Regulation (EU) No 142/2011 Section 2 D, Chapter IV, Annex IV | RepCat® process, 2017 | RepCat® process 2020 | ||||||||||
| Process | pH | T | t | p | pH | T | t | p | pH | T | t | p |
| Pressure sterilisation a | n.s. | 133 | 20 | 3 bar | n.s. | 133 | 20 | 3 bar | n.s. | 133 | 20 | 3 bar |
| Removal of insoluble impurities b | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Esterification c | < 1d | 72 | ≥ 120e | n.s. | n.s. | ≥ 220 | ≥ 30h | ≥ 80 bar | n.s. | ≥ 200 | ≥ 15i | ≥ 70 bar |
| Transesterification c | 14f | 35–50 | ≥ 15g | n.s. | ||||||||
| Vacuum distillation | n.s. | 150 | n.s. | n.s. | n.s. | ≥ 150 | n.s. | ≤ 10 mbar | n.s. | ≥ 150 | n.s. | ≤ 10 mbar |
n.s: not specified; T: temperature in centigrade; t: time in minutes; p: pressure in bar or mbar as indicated.
According to method 1 as specified in Commission Regulation (EU) No 142/2011.
To obtain cleaned raw material with solid content < 0.15% w/w.
For the RepCat® method, esterification and transesterification are combined into a single step.
By addition of sulfuric acid or equivalent acid.
Mixed intensely.
By addition of potassium hydroxide or an equivalent base.
Carried out twice using a new base solution.
Mixing with addition of methanol to 15% w/w.
Mixing with addition of methanol to 10–15% w/w and traces of MgO as catalyst.
During the assessment process, it was deemed necessary to obtain additional information on the alternative method without extending the time required for the assessment. EFSA asked the applicant to clarify:
if the percentage of methanol added is 15% (as indicated in the Section ‘level of risk reduction’ of the application).
the rationale of deleting ‘methanol dosage’, indicated as a critical control point (CCP 2) in the 2017 application.
the animal by‐product categories from which the starting materials derive.
The applicant submitted additional information that was considered as part of the application and reviewed during the assessment.
2. Data and methodologies
2.1. Data
The data used in the assessment were provided by the applicant as requested in Annex VII of Commission Regulation (EU) No 142/2011 and its amendment by Regulation (EU) No 749/20114. A process flow diagram (Figure 1) and a Hazard Analysis and Critical Control Points (HACCP) plan were included in the application dossier. The report submitted by the Austrian Competent Authority (CA) related to the application was also considered. Relevant scientific papers provided by experts of the Working Group (WG) were also considered during the assessment.
Figure 1.
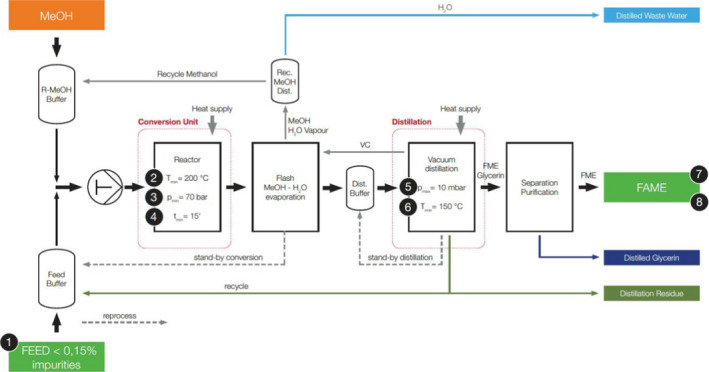
- The numbers (1–8) indicate the critical control points (CCPs), which are described in Section 3.5.1.
2.2. Methodologies
The EFSA BIOHAZ Panel evaluated the application for a new alternative biodiesel production process by individually assessing the following steps as set out in the ‘Statement on technical assistance on the format for applications for new alternative methods for animal by‐products’ (EFSA BIOHAZ Panel, 2010a). These steps are:
full description of the process;
full description of the material to be treated;
hazard identification;
level of risk reduction;
HACCP plan;
risk associated with interdependent processes;
risk associated with the intended end use of the products.
The applicant is required to document as fully as possible the different aspects of each of these steps. According to the CA assessment, the application meets the requirements as laid down in the EFSA Statement (EFSA BIOHAZ Panel, 2010a).
As set out in subparagraph 5 of Article 20 of Regulation (EC) No 1069/2009, EFSA shall assess whether the method submitted ensures that the risks to public or animal health are:
‘controlled in a manner which prevents their proliferation before disposal in accordance with this Regulation or the implementing measures thereof; or
reduced to a degree which is at least equivalent, for the relevant category of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1).’
This requirement for applications is elaborated in Commission Regulation (EU) No 142/2011, implementing Regulation (EC) No 1069/2009, and amended by Regulation (EU) No. 749/2011. According to point 2 d, Chapter II, Annex VII of Commission Regulation (EU) No. 142/2011, any application for the evaluation of alternative methods shall ‘show that the most resistant biological hazards associated with the category of materials to be processed are reduced in any products generated during the process, including the wastewater, at least to the degree achieved by the processing standards laid down in this Regulation for the same category of animal by‐products. The degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable’.
According to the EFSA Statement (EFSA BIOHAZ Panel, 2010a) and to point 3, Chapter II, Annex VII of Commission Regulation (EU) No 142/2011, validated direct measurements as referred to above shall mean:
-
‘measuring the reduction of viability/infectivity of endogenous indicator organisms during the process, where the indicator is:
-
−
consistently present in the raw material in high numbers,
-
−
not less resistant to the lethal aspects of the treatment process, but also not significantly more resistant, than the pathogens for which it is being used to monitor,
-
−
relatively easy to quantify and relatively easy to identify and to confirm; or
-
−
using a well‐characterised test organism or virus introduced in a suitable test body into the starting material.’
The EFSA Statement (EFSA BIOHAZ Panel, 2010a) asserts that ‘results should be accompanied by evidence’. Such evidence ‘includes, for measurements, information on the methodology used, nature of samples that have been analysed and evidence that samples are representative (e.g. number of samples, number of tests performed and selection of measuring points). If several treatment steps are involved, an assessment should be performed on the degree to which individual titre reduction steps are additive, or whether early steps in the process may compromise the efficacy of subsequent steps. In any case it is necessary to provide the sensitivity and specificity of the detection methods applied. Data on the repeatability and statistical variability of the measures obtained during the experiments should also be presented.’ It also states that ‘Generally, the level of risk reduction for human and animal health which can be achieved by the process should be evaluated on the basis of direct measurements (validation).’
Should ‘no direct measurements of the risk reduction be available (i.e. no validation as defined above is feasible), modelling or comparison with other processes may be acceptable if:
the factors leading to the risk reduction are well known;
the model of risk reduction is well established; and
continuous direct measurements of the factors leading to the risk reduction are provided for the full‐scale process which demonstrate that these factors are homogeneously applied throughout the treated batch.’
In point 2 d, ‘Level of risk reduction’ of Section 2.1.2.1 ‘Content of applications’ of the EFSA Statement (EFSA BIOHAZ Panel, 2010a), it is stated that ‘in principle, the new proposed process should be able to reduce the amount of the most resistant biological hazards associated with the category of the material to be processed for a defined final use to an acceptable level’. Although Chapter II of Annex VII of Commission Regulation (EU) No 142/2011 adopted the proposal of the EFSA opinion to use ‘the level of risk reduction’ and ‘the level of reduction of the most resistant biological hazards’ interchangeably, it is acknowledged that these are different terms and that the purpose of the evaluation of alternative methods is not the estimation of the level of any risk, but the level of hazard reduction.
There are no hazard reduction standards for proposed alternative methods for biodiesel production using ABP. However, in previous EFSA opinions (EFSA BIOHAZ Panel, 2015, 2017) dealing with proposed alternative processing methods for Category 1 ABP, the BIOHAZ Panel concluded that a reduction of 6 log10 in prion infectivity by the alternative method is required to consider it at least equivalent to the method approved in the legislation. This is in addition to the inactivation achieved by the pressure sterilisation method (method 1) that is required prior to the application of the alternative method to Category 1 and 2 ABP. Where direct measurement of infectivity in the final product is not possible, proxy measurements, such as the demonstration of residual prion protein using Western blot (WB), have previously been considered acceptable, although it has been acknowledged that they are not ideal (e.g. SSC, 2001; EFSA BIOHAZ Panel, 2015). The use of rodent‐adapted experimental prion strains, such as hamster 263K, which is similar to the hamster‐adapted strain Sc237, and mouse ME7, as proxies for naturally occurring strains in inactivation studies has also been widely accepted historically (EFSA BIOHAZ Panel, 2020a), although a growing body of data is now challenging this assumption (see Appendix A).
In the absence of more relevant data, the use of strains other than classical bovine spongiform encephalopathy (C‐BSE), and the use of WB demonstrating the presence of PrPSc, as a proxy for infectivity, have therefore been considered acceptable in previous EFSA opinions (EFSA BIOHAZ Panel, 2015, 2017, 2020b) to evaluate the equivalence of alternative methods for the production of renewable fuels using Category 1 material. The same methodology is used in the evaluation of the current application.
Some of the sections of the current application, such as those dealing with the material to be treated and hazard identification, apply both to the application made by the same company in 2017 and to the current one; these sections have not been evaluated again as they were already considered in the 2017 assessment (EFSA BIOHAZ Panel, 2017), as per the information provided in Appendix B. Therefore, it was decided that in this current opinion, only those steps that differ between the two alternative methods will be considered. These differences relate mainly to the unified esterification/transesterification process and are shown in Table 1. A new publication (Mohammadi et al., 2020) submitted by the applicant regarding the effect of methanol on prion inactivation was also considered.
3. Assessment
In the current chapter, the sections defined as ‘provided by the applicant’ present the description extracted verbatim from the application, edited for clarity and abridged in places for brevity.
3.1. Description of the alternative method (RepCat® process)
3.1.1. Description of the alternative method as provided by the applicant5
The RepCat® process is an automatically controlled and closed system. All critical process parameters (temperatures, pressures, flow, etc.) are controlled through a process control system and are recorded and stored electronically. In contrast to the already permitted standard biodiesel process (according to Section 2 D, Chapter IV, Annex IV of Commission Regulation (EU) No 142/2011), no salt, i.e. potassium sulfate, is produced. In the case of glycerine, this co‐product is treated with the same process steps as the biodiesel, i.e. high temperature and high pressure, and final vacuum distillation.
The by‐product distillation residue is equivalent to that of the already authorised biodiesel process and can be recycled in the process. The wastewater is a distilled product without any solids and will be discharged into a wastewater treatment plant.
Category 1 ABP tallow must be sterilised according to method 1 of the Section 2 D Chapter IV Annex IV of Commission Regulation (EU) No 142/2011 prior to entering the RepCat® process. Any remaining PrPSc will be further reduced by the pressurised, high temperature process, as shown by direct measurements in laboratory‐scale method replication studies (Mohammadi et al., 2020), and the subsequent vacuum distillation.
3.1.1.1. Conversion step
The starting material is rendered fat, including Category 1 ABP tallow.
This feedstock is first transferred from the tank farm into the feed buffer of the process. Afterwards it is mixed with methanol and traces of MgO catalyst. The feedstock is heated to the reaction temperature (min. 200°C) via several heat exchangers and transferred into the continuous tube reactor, for conversion, by means of a high‐pressure pump (pressure higher than 70 bar). The reaction temperature is held for a minimum of 15 min at a minimum temperature of 200°C. After the conversion, the pressure is released by discharging the treated product into the flash vessel, which causes the methanol and water (by‐products from the esterification reaction) to evaporate. The product vapours are recovered and separated into water and methanol by a distillation column. Methanol is completely reused in the process and transferred to the recycled methanol buffer. The distilled water (which is free of any solids according to Section 2 Chapter 1 Annex IV of Commission Regulation (EU) No 142/2011) is discharged into a wastewater treatment plant. The intermediate product (a mixture of biodiesel and glycerine) is transferred into the distillation buffer vessel.
Temperature and pressure are monitored with temperature and pressure indicators. The retention time is controlled by the flow into the conversion unit, where 15 min represents the shortest time, i.e. the worst‐case condition.
If critical parameters are not achieved, the intermediate product is transferred back to the feed buffer vessel (stand‐by conversion).
3.1.1.2. Distillation
The biodiesel/glycerine mixture is transferred from the distillation buffer to the multi‐stage distillation column, where it is distilled under vacuum at a minimum temperature of 150°C and maximum 10 mbar. Afterwards, the biodiesel is separated from the glycerine by gravimetric settling.
3.2. Material to be treated
3.2.1. Material to be treated as provided by the applicant6
The starting material for the new biodiesel process is rendered fat including ABP Category 1 tallow, as defined in Article 8 of Regulation (EC) No 1069/2009.
The tallow must have been sterilised according to processing method 1 (pressure sterilisation) as set out in Chapter III of Annex IV Commission Regulation (EU) No 142/2011.
The solid content must be below 0.15% w/w before entering the biodiesel process.
3.2.2. BIOHAZ Panel assessment of the material to be treated
The assessment of the material to be treated remains unchanged from that undertaken when assessing the 2017 application (provided in Appendix B).
3.3. Hazard identification
3.3.1. Hazard identification as provided by the applicant7
Category 1 ABP materials can contain different biological hazards, including some highly heat‐resistant bacterial spores or viruses. However, PrPSc is considered to be the most relevant hazard. Tests of the method, at laboratory scale, regarding the hazard reduction were performed on PrPSc. Due to the extreme thermostability of PrPSc, it can be assumed that even thermoresistant viruses and bacterial spores are completely inactivated if the new method assures inactivation of PrPSc.
3.3.2. BIOHAZ Panel assessment of the hazard identification
The assessment of the hazard identification remains unchanged from that undertaken when assessing the 2017 application (provided in Appendix B).
3.4. Level of risk reduction
3.4.1. Level of risk reduction as provided by the applicant8
The most resistant hazard identified was prions. Prion reduction was tested directly using the prion strains RML (Rocky Mountain Laboratories) and sporadic Creutzfeldt‐Jakob‐Disease (sCJD). The applicant states that RML prions are well‐characterised test agents, and that CJD prions were used as they are considered to be ‘more than 100,000‐fold more difficult to inactivate than the 263K strain’ (a widely used hamster‐adapted strain); therefore, large parts of the experiments were conducted with sCJD prions (Mohammadi et al., 2020).
Two stages are considered important for prion risk reduction within the RepCat® process: conversion and distillation. In the conversion unit, the level of prion reduction was validated by experimental data and was found to be ≥ 6 log10. In the vacuum distillation step, the reduction was already tested by Mittelbach et al. (2007) and found to be 3 log10. According to the applicant, the total risk reduction in the RepCat® process is therefore expected to be ≥ 9 log10.
3.4.1.1. Conversion
Using process worst‐case conditions of 200°C at 70 bar for 15 min in the presence of 15% w/w methanol and 0.2% w/w MgO catalyst, a CJD prion reduction below the detection limit of 6 log10 was found. A detailed description of the experimental trials was supplied in the form of the publication by Mohammadi et al. (2020), describing commissioned studies.
In addition to the prion reduction in the tallow, the reduction of prions in the by‐product glycerine was also assessed at the worst‐case process conditions of 200°C at 70 bar for 15 min, and a CJD prion reduction below the assay detection limit of 6 log10 was found.
To exclude the possibility that higher temperature and pressure for a longer time may be less effective for prion reduction, tallow in the presence of 15% w/w methanol and 0.2% w/w MgO catalyst, as well as glycerine, were also tested at 220°C at 80 bar for 30 min, using CJD and RML prions. In both cases, no CJD or RML prions were found after the conversion. The reductions were therefore below the detection limit of 6 log10.
It was also shown that methanol has no influence on the PrPSc reduction (Table 2).
Table 2.
Level of prion reduction described in the literature, as provided by the applicant
3.4.1.2. Vacuum distillation
The distillation of biodiesel under vacuum at 150°C is already part of the standard biodiesel process according to Section 2 Chapter IV of Commission Regulation (EU) No 142/2011.
In the vacuum system of the distillation process, volatile compounds (VC) are condensed. They consist mainly of methanol and water. They are transferred in a completely closed system into the flash reactor, from where they are transferred to the distillation column where the VC are separated into recycled methanol and distilled wastewater.
The reduction of prions during the biodiesel vacuum distillation was tested by Mittelbach et al. (2007). In this work, a reduction of 3 log10 was found for the distilled product as well as for the distillation residue using direct measurements in laboratory scale studies.
3.4.2. BIOHAZ Panel assessment of the level of risk reduction
3.4.2.1. Conversion
The applicant included in the dossier five published experimental studies: Müller and Riesner (2005), Müller et al. (2006, 2008); Mittelbach et al. (2007) and Mohammadi et al. (2020).
The three papers by Müller et al. explore the reduction in prion signal and infectivity using tallow spiked with prion rods (Sc27‐30) derived from hamster 263K, a strain which has been widely used in prion inactivation studies (see Appendix A). They cite literature supporting the high starting titres available when using this strain, and comparative data indicating that it behaves very similarly to BSE when heat‐treated. The studies, extrapolating from both the WB signal and direct measurement of infectivity using hamster bioassay of PrP recovered by methanol/chloroform precipitation from the treated product, to assess the impact of bench‐scale processing methods on prion survival, indicate that a reduction in prion activity of at least 6 log10 is achievable under certain conditions. However, as discussed in a previous assessment (EFSA BIOHAZ Panel, 2017), ‘these describe inactivation of TSE prions in tallow after it has been subjected to oleochemical manufacturing processes of hydrolytic fat splitting, not production of Biodiesel. Therefore, they are not considered to be directly relevant to the RepCat process’.
In addition to the use of higher temperatures and higher pressure, the RepCat® process adds methanol to the conversion process, which is not accounted for in these studies.
Mohammadi et al. (2020) describe three studies that used RML (a mouse adapted goat scrapie strain), 22L (a mouse adapted sheep scrapie strain) and/or sCJD(MM1) (a human isolate) to assess the PrPSc signal reduction following heating in the presence of methanol, and under simulated RepCat® treatment. No explanation is given for the subselection of prion source(s) for each study.
Study one: It evaluates whether the presence of 15% methanol renders prions more resistant to proteinase K (PK) digestion, because of its potential to alter tertiary protein structure in a way that could compromise the effectiveness of protease treatments and/or the subsequent immunochemical detection of the PrPSc. Such effects could lead to a potential over‐estimation of the prion reduction capability of this biodiesel production method. However, a recovery rate of 95%, with no alteration to the WB profile, was achieved for both RML and 22L spiked samples, thereby demonstrating that detection of these strains is not adversely affected by the presence of 15% methanol.
Study two: using all three strains, it showed that the addition of 15% methanol to spiked tallow subjected to 134°C with 2 bar pressure for 60 min did not change the expected outcome for any of the prion strains used, i.e. there was a loss of prion signal for all three strains following autoclaving and PK digestion whether or not methanol was present. This indicates that the methanol had not altered the tertiary structure of the PrPSc in a way that might be protective against the degradative effects of temperature and/or pressure.
Study three: sCJD and RML were both used in the assessment of the replicated industrial process. Giles et al. (2017), cited by the publications provided by the applicant in support of the selection of sCJD for use in this study, stated that sCJD(MM) was 105‐fold more resistant to inactivation by various methods than 263K (Peretz et al., 2006). This was similar to the outcome for BSE which was reported to be 106‐fold more resistant than 263K (Giles et al., 2008). While this indicates that it may not be equivalent to BSE, it can be concluded that sCJD is not less appropriate than 263K as a proxy for inactivation studies (see Appendix A).
Unlike other inactivation studies where the starting titres of infectivity or detectable PrPSc and the subsequent log reductions were directly measured, the prion titres of the brain homogenates used by Mohammadi et al. (2020) were not determined. Instead, the sensitivity of the WB detection method was calibrated by its ability to detect PrPSc (associated with known amounts of protein) in 10‐fold dilutions of the ‘spiked’ brain homogenate added to the substrate. This established a ‘lowest detectable’ threshold of PrPSc. The Mohammadi et al. (2020) study shows that the RepCat® process, with parameters of 200°C, 70 bar, 15 min, resulted in a complete loss of recoverable PrPSc. This was calculated to represent a reduction in excess of 6 log10 based on extrapolation from the analysis of the final product, and the previously determined detection limits of the assay.
3.4.2.2. Vacuum distillation
The assessment of this phase of the process remains unchanged from that undertaken when assessing the 2017 application (provided in Appendix B).
3.4.2.3. Conclusion
The data provided support the assertion that the RepCat® process can achieve at least a 6 log10 reduction in detectable PrPSc.
3.5. HACCP plan
3.5.1. HACCP plan as provided by the applicant9
The applicant states that the Category 1 animal fat is already processed through a steam sterilisation process (method 1 as described in Section 2 D Chapter IV Annex IV of Commission Regulation (EU) No 142/2011) and that as the content of solids (i.e. insoluble impurities) must be below 0.15% w/w, a very low risk can be expected from the feedstock for the RepCat® process. However, since the process involves a high‐temperature and high‐pressure conversion and a distillation step, any remaining risk after the RepCat® process will be reduced.
The reaction takes place in a closed industrial system without any human or animal exposure during processing, except for sample taking, to test the triglycerides and solids content.
The effectiveness of the production system is monitored at several measuring points including monitoring the flow of liquids, temperature and pressure. These values are visualised in a process control system as well as recorded and saved automatically. In case of deviations from the critical process conditions, the system automatically switches to a stand‐by mode until all conditions are within the specified parameters. However, even if these conditions are fulfilled, the operation mode must be re‐started by an operator.
In addition, the conversion of the feedstock is checked by laboratory analyses of the remaining fat in the distilled biodiesel in terms of triglycerides and impurities, measured by the approved analytical methods EN 1410510 and EN 1266211, respectively.
Unauthorised persons and animals do not have access to the processing plant (Table 3).
Table 3.
Critical control points (CCPs) of the new biodiesel process (CCP numbers are stated in the flow chart in Figure 1)
| CCP no | Critical control point | Critical limits* | Corrective actions |
|---|---|---|---|
| 1 | Delivery of ABP tallow | Solids < 0.15% w/w | If solids are higher than 0.15% w/w, the tallow is reprocessed |
| 2 | Temperature during conversion | T ≥ 200°C | If temperature is below 200°C, automatic stand‐by of process and reprocessing |
| 3 | Pressure during conversion | p ≥ 70 bar | If pressure is below 70 bar, automatic stand‐by of process and reprocessing |
| 4 | Retention time | t ≥ 15 min, by flow rate | Any failure would increase the retention time |
| 5 | Vacuum in distillation column | p ≤ 10 mbar | If pressure is higher than 10 mbar, automatic stand‐by of process and reprocessing |
| 6 | Temperature for distillation | T ≥ 150°C | If temperature is below 150°C, automatic stand‐by of process and reprocessing |
| 7 | Remaining fat content | Triglycerides < 0.05% w/w** | If triglycerides are higher than 0.05% w/w, reprocess |
| 8 | Solids | < 24 mg/kg | If solids are higher than 24 mg/kg, reprocess |
The parameters ‘T’, ‘p’, ‘t’ have been modified from the original version including the equal symbol (=), for consistency with the provided flow chart presented in Figure 1. The headings have been rephrased in order to align them with the HACCP concepts.
Remaining fat components are measured as triglycerides according to EN 14105 and should be below 0.05% w/w. However, much lower values are expected, but cannot be measured accordingly due to the poor reproducibility of the analytical method at these low concentrations, as stated in EN ISO 4259.12
3.5.1.1. Detailed description
Besides the quality of the feedstock (CCP 1, sterilised according to method 1 acc. Commission Regulation (EU) No 142/2011 and with a solid content < 0.15% w/w), the process parameters temperature, pressure and flow rate (CCP 2‐4) are essential to guarantee the PrPSc reduction.
Measuring devices monitor all critical process parameters. Their signals are controlled, visualised and processed as well as recorded in a process control system. In case of deviating conditions, the product will be transferred back into the feed buffer (refer to Figure 1 ‘stand‐by conversion’).
To ensure a 3 log10 reduction of PrPSc level in the distillation step, the pressure and temperature are controlled (CCP 5 and 6, respectively) during the distillation. However, due to the nature of the distillation, no unprocessed product will leave the distillation column. In case of deviating conditions, the product will be transferred back into the distillation buffer (refer to Figure 1 ‘stand‐by distillation’).
As a final quality control of the process, the conversion of the fat is monitored by measuring the remaining triglyceride content (CCP 7) in the biodiesel. Additionally, the content of solids is measured (CCP 8) and must be below 24 mg/kg in the biodiesel. Periodic samples are taken in the biodiesel pipeline after the distillation unit on the way to the tank farm. In the tank farm, the biodiesel will be buffered in a quality buffer tank (day‐tank). If an analysis of the samples shows a result above the triglyceride or solids limit, the day‐tank has to be reprocessed (Table 4).
Table 4.
Overview of the CCPs as provided by the applicant
| CCP No 1 | The first parameter that must be checked is the content of solids in the fat, which must be below 0.15% w/w. Every production plant is equipped with a laboratory for feedstock analyses, production control and determination of the final product quality. Therefore, the determination of solids can be performed on site. The solid content must be below 0.15% w/w, so, before usage, this content must be measured regularly, e.g. before each unloading. If the value is above the limit, the feedstock must be rejected for biodiesel production and must be reprocessed until the solid content is below the limit. |
| CCP No 2 | The temperature of the reaction mixture for the conversion unit is provided by steam or thermal oil via a heat exchanger. The temperature is measured and automatically controlled as it is also needed to ensure the completeness of the reaction. If the temperature is below the limit of 200°C, the material is not sent for distillation and is automatically transferred from the flash vessel back into the feed buffer tank. It is reprocessed when the temperature is below 200°C. |
| CCP No 3 | The pressure is provided by means of a high‐pressure pump. The pressure is automatically controlled and needed for the reaction. If the pressure is below the limit of 70 bar, the material is not further processed and automatically transferred from the flash vessel back to the feed buffer. It is reprocessed when the pressure is below 70 bar. |
| CCP No 4 | The reaction time is given by the retention time during the conversion process. The flow is performed by a high‐pressure pump (refer to CCP No 3) and controlled by a flow indicator which is connected to the process control system for data monitoring and visualisation. The maximum flow is limited by the process control system. A higher throughput and therefore a shorter retention time are not possible. However, any failure in the pump would cause an increased retention time, which does not adversely affect the PrPSc reduction. |
| CCP No 5 | The distillation is performed under vacuum by means of several vacuum pumps. The vacuum is monitored by pressure indicators. If the pressure is too high (above 10 mbar), the distillation will switch automatically to a stand‐by mode. The products are transferred back into the distillation buffer. |
| CCP No 6 | Temperature is required for the evaporation of the biodiesel. The temperature is monitored by temperature indicators. If the temperature is too low (below 150°C), the distillation will switch automatically to a stand‐by mode and the products are transferred back into the distillation buffer. |
| CCP No 7 | As a final control, the quality of the biodiesel has to be analysed for the parameter ‘triglycerides’, determined according to EN 14105 for maximum 0.05%. If the results exceed the limit, the biodiesel has to be reprocessed in the distillation unit in order to reduce the triglyceride content. |
| CCP No 8 | As a final control, the quality of the biodiesel is analysed for the parameter ‘solids’, determined according to EN 12662 to be below 24 mg/kg (limit according to EN 1421413). If the results exceed the limit, the biodiesel has to be reprocessed in the distillation unit in order to reduce the solid content. |
3.5.2. BIOHAZ Panel assessment of the HACCP plan
The applicant provided a HACCP plan detailing the key parameters to be fulfilled at each identified critical control point (CCP) as well as corrective actions which are undertaken in case of failure of one of the parameters, as detailed by the applicant.
The parameter to fulfil in CCP1 (i.e. solids (% w/w)) is verified in the laboratory for feedstock analysis in the production plant. If the solid content is above the limit, the feedstock must be reprocessed but the applicant did not specify if this is done in the processing plant or by returning the fat to the supplier.
Parameters to fulfil CCPs 2–4 (i.e. temperature (°C), pressure (bar) and retention time (min), respectively) are measured by electronic devices. Their signals are controlled, visualised, processed and recorded in a process control system. The applicant stated that in case of deviating conditions, the product will be automatically transferred back into the feed buffer. However, it is not specified if all CCP2‐ to CCP4‐related parameters are recorded and verified in real time.
Parameters to fulfil in CCPs 5 and 6 (i.e. pressure (mbar) and temperature (°C), respectively) are visualised in a process control system, recorded and saved automatically. If the expected values are not achieved (e.g. pressure is above 10 mbar and/or temperature is below 150°C), the distillation will switch to a stand‐by mode and the products are transferred back into the distillation buffer.
Parameters to fulfil in CCPs 7 and 8 (i.e. triglycerides < 0.05% and solids < 24 mg/kg) are measured by taking periodic samples: if they show results above the limits, the day‐tank is reprocessed. The applicant used the generic term of periodic samples, but the corrective measures refer to the day‐tank.
The applicant highlighted that the reaction takes place in a fully automated and closed industrial system without any contact with humans during processing, except for taking the samples to verify CCP 7 and CCP 8.
3.6. Risk associated with interdependent processes
3.6.1. Risk associated with interdependent processes as provided by the applicant14
In general, the RepCat® process is a closed system, and sampling is reduced to a minimum. However, as methanol is used in the process, which is considered toxic according to Regulation (EC) No 1272/2008 (CLP),15 safety procedures according to Council Directive 89/391/EEC16 are in place. Direct contact with skin, ingestion or inhalation is not expected.
3.6.1.1. Transportation
The transportations of feedstock, the product and the by‐products are performed with closed tank trucks. For loading and unloading, closed pipelines are used.
3.6.1.2. Storage
Feedstock, as well as the final product, the by‐products and the wastewater are stored in closed storage tanks.
3.6.1.3. Wastewater
Wastewater is recovered from condensation as a distilled product without solids. Any carryover of solids with a mesh size higher than 6 mm can be excluded, especially as no solids of this size are present in the animal fat and its initial solid content is below 0.15% w/w. An oil phase can be present, which will be separated from the wastewater.
Wastewater from cleaning processes will be collected; any fat will be separated in a grease trap and reused as feedstock. The remaining wastewater will be treated in a wastewater treatment plant. The wastewater meets the requirements set out in Section 2, Chapter I, Annex IV of the Commission Regulation (EU) No 142/2011.
3.6.1.4. Oil phase from wastewater
The oil phase from the wastewater consists of lipophilic substances from the conversion process, mainly short‐chain fatty acid methyl esters of short‐chain fatty acids. This stream can be recycled in the process and transferred back to the feed buffer. This oil phase can be used as a biofuel.
3.6.1.5. Distillation residue
The distillation residue can be recycled and transferred back to the feed buffer. This enables the process to keep the amount of residue that is leaving the process as low as possible. Nevertheless, the distillation residue from a biodiesel process is already described in point 2 (b) (i) Section 3 Chapter IV Annex IV of the Commission Regulation (EU) No 142/2011 and can be used as a biofuel. No additional risks are foreseen due to the new process.
3.6.1.6. Volatile compounds
Volatile compounds are condensed in the vacuum system of the distillation unit. They consist mainly of methanol and water. This liquid is recycled internally and transferred in a closed system to the flash vessel, where it is separated into wastewater and recycled methanol. This product does not leave the process.
3.6.1.7. Glycerine
Glycerine, obtained from biodiesel production of Category 1 material is, according to 2 (b) (iii) Section 3 Chapter IV Annex IV of the Commission Regulation (EU) No 142/2011, foreseen as feedstock for biogas.
3.6.2. BIOHAZ Panel assessment of the risk associated with interdependent processes
The applicant provided a description of the risks associated with the interdependent processes and the procedures that would be implemented for dealing with these risks.
The procedures for the transport and storage of ABP and derived products are briefly described. It is not mentioned whether they will be undertaken in compliance with the requirements set out in Article 21 of Regulation (EC) No 1069/2009, Article 17 and Annex VIII of Commission Regulation (EU) No 142/2011 and various other parts of these Regulations.
According to the applicant, wastewater from the process is distilled; hence, it is free of impurities as required in Section 2 Chapter I Annex IV of Commission Regulation (EU) No 142/2011. As the feedstock is free of impurities from ABP (< 0.15% w/w), the same will apply to the wastewater from the cleaning processes. This wastewater is collected and any fat will be separated in a grease trap and reused as feedstock. All wastewater streams will be treated in a wastewater treatment plant (WWTP). The procedures for dealing with wastewater meet the requirements set out in Section 2, Chapter I, Annex IV, of Commission Regulation (EU) No 142/2011. It is assumed that any cleaning step involved will follow the relevant regulation.
The procedures for dealing with other by‐products of the alternative process, such as the oil phase from wastewater, the distillation residue and volatile compounds (methanol and water) are in compliance with Article 12 of Commission Regulation (EC) No 1069/2009 and with Section 3, Chapter IV of Annex IV of Commission Regulation (EU) No 142/2011. Measures to mitigate any risks that may arise from interdependent processes are also set out in the HACCP plan accompanying the application. Considering the nature of these by‐products and the procedures for dealing with them, exposure of animals or humans to prions resulting from/related to interdependent processes would not be expected.
As stated in the EFSA ‘Scientific Opinion on the abiotic risks for public and animal health of glycerine as co‐product from the biodiesel production from Category 1 animal by‐products (ABP) and vegetable oils’ (EFSA CONTAM Panel, 2010), crude glycerine (typically containing 20% water and residual esterification catalyst) is nowadays mostly obtained as a co‐product of saponification and transesterification from biodiesel production. It should be noted that in the application, glycerine is described in the section on the risk associated with the intended end use of the products, as a possible feedstock for biogas. However, even though glycerine is extracted in the final step of the process (vacuum distillation) by splitting the mixture biodiesel/glycerine, the risk to be assessed with regard to the intended end product should only consider biodiesel, whereas the risk associated with glycerine should be assessed in this section, together with other interdependent processes.
According to Mohammadi et al. (2020), under worst‐case conditions (200°C at 70 bar for 15 min), which are applied during the RepCat® process, at least a 6 log10 reduction of PrPSc in the co‐product glycerine was confirmed, demonstrating that the process can also achieve at least a 6 log10 reduction of the TSE hazards in the glycerine.
3.7. Risk associated with the intended end use of the product
3.7.1. Risk associated with the intended end use of the product from the process as provided by the applicant17
The intended end use of the product biodiesel is as biofuel as already described in point 2 (b) (i) Section 3 Chapter IV Annex IV of the Commission Regulation (EU) No 142/2011. Due to the new process, no additional risks are foreseen.
3.7.2. BIOHAZ Panel assessment of the risk associated with the intended end use of the product from the process
In this process, the end product is biodiesel. Considering the nature of the final product, a very low level of biological risk for humans and animals associated with the intended end use is expected.
As discussed in the EFSA BIOHAZ opinion (2020b) on the evaluation of another alternative method for the production of biodiesel using Category 1 material, biodiesel can be blended with standard diesel for use in private and commercial vehicles and will be dispensed at retail filling stations or directly at wholesale fuel facilities. As discussed by Mittelbach et al. (2007), two hypothetical routes for the infection of humans with BSE exist as a consequence of any residual risk associated with biodiesel: oral and subcutaneous. It is highly unlikely that biodiesel would be intentionally ingested and, equally, prions will not be inhaled ‘since proteins are not known to evaporate’ (Mittelbach et al., 2007). The subcutaneous route represents a more viable potential route of exposure if the barrier created by the skin in some way suffered a loss of integrity such as an open cut or wound. If a wound or trauma had compromised the integrity of the skin, the volume of biodiesel that could penetrate the underlying cells would be in the range of less than a millilitre. Exposure to prions via filling a vehicle with biodiesel is therefore not expected. A third route of exposure can be the uptake of prions via mucous membranes. For example, successful transmission of scrapie to susceptible sheep by experimental introduction of the scrapie agent via the conjunctival and nasal mucosa has been reported (Hamir et al., 2008). It has also been speculated that human TSE, kuru, can be spread by ‘conjunctival, nasal, and skin contamination by highly infectious brain tissue’ (Liberski et al., 2019). This hypothetical route is even more unlikely to occur for biodiesel than the oral or subcutaneous routes. Animal exposure to prions in biodiesel could possibly occur, either by ingestion, subcutaneous or mucosal routes.
The environmental and safety measures applied in wholesale fuel facilities and at retail fuel stations mean that exposure of humans and animals to prions via contact with sufficient biodiesel would not be expected during normal operations, but there might be a greater risk associated with diesel spills or leakage from storage tanks or vehicles at farm level. Similarly, since in the case of any spillage or major incident (explosion, accidental submersion) at wholesale or retail outlets, protective measures would be taken to remove spilled diesel due to other risks associated with the material (combustion, etc.), exposure of humans or animals to prions via biodiesel would not be expected. Nevertheless, in the event that any human or animal is exposed to biodiesel through any of these above‐mentioned routes, the lack of prions in the biodiesel, following the processing method, renders the end product safe from the TSE hazard point of view.
4. Conclusions
The method under assessment (RepCat® process) for the production of biodiesel from rendered animal fats, including animal by‐product (ABP) Category 1 tallow, consists of esterification and transesterification at ≥ 200°C, ≥ 70 bar, ≥ 15 min in a single step, using MgO as a catalyst and in the presence of methanol (10–15%), followed by vacuum distillation (at ≥ 150°C, ≤ 10 mbar) of the end‐product biodiesel.
Since the starting material includes Category 1 ABP tallow, the applicant considered that, of any biological hazards that may be present, prions would be the most resistant. The BIOHAZ Panel agrees with the approach used by the applicant of focusing on prions.
Previous EFSA Opinions established that a reduction in prion infectivity, or detectable PrPSc, of at least 6 log10 should be achieved to consider a method at least equivalent, for the relevant category of ABP (i.e. Category 1), to the processing method laid down in the legislation.
The applicant provided evidence in the form of published studies in which it was shown that methanol does not affect the recovery or detection of prions from a biodiesel substrate. It was also shown that the RepCat® esterification and transesterification process can achieve a reduction in sCJD(MM1) prions of at least 6 log10, as estimated through Western blot, from the complete loss of recoverable PrPSc, and that vacuum distillation can provide a further reduction of 3 log10.
The application includes sufficient information on the HACCP plan and about the risks of interdependent processes and those associated with the intended end use of biodiesel. The measures proposed in the dossier to deal with these risks are compliant with the relevant legislation.
The alternative method is capable of achieving at least the 6 log10 reduction in detectable PrPSc that enables it to be considered at least equivalent to the processing method laid down in the legislation for the production of biodiesel from raw materials including category 1 ABP.
5. Documentation provided to EFSA
Application for the use of Category 1 material for the biofuel production under the conditions of the BDI RepCat® process. Submitted by the company BDI–BioEnergy International GmbH to the Austrian Competent Authority and then submitted to EFSA on 10 June 2020; consolidated version submitted by the company BDI–BioEnergy International GmbH to EFSA on 9 September 2020.
Report of the Austrian Competent Authority related to the application for new alternative biodiesel process for rendered fat of Cat. 1. Submitted by the Austrian Federal Ministry of Social Affairs, Health, Care and Consumer Protection on 10 June 2020.
5.1. List of references provided by the applicant
Müller H and Riesner D, 2005. Thermal degradation of prions in the presence of fats: implication for oleochemical processes. European Journal of Lipid Science and Technology, 107, 833–839.
Müller H, Stitz L and Riesner D, 2006. Risk assessment for fat derivatives in case of contamination with BSE. European Journal of Lipid Science and Technology, 108, 812–826.
Mittelbach M, Pokits B, Müller H, Müller M and Riesner D, 2007 Risk assessment for prion protein reduction under the conditions of the biodiesel production process. European Journal of Lipid Science and Technology, 109, 79–90.
Müller H, Stitz L and Riesner D, 2008. Prion decontamination during the oleochemical process of fat hydrogenation. European Journal of Lipid Science and Technology, 110, 392–399.
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp.
Mohammadi B, Raudner R, Shafiq M, Ahn E, Altmeppen HC and Glatzel M, 2020. Influence of methanol on prion reduction during high temperature and high pressure oleochemical processes. European Journal of Lipid Science and Technology, 122, 2000136.
Glossary
- Bar
Non‐international system of units (SI unit) for pressure, accepted for use but not encouraged. One hundred thousand pascals are called a bar (100,000 Pa = 1 bar). Commonly used for technical applications. https://www.nist.gov/pml/special-publication-811/nist-guide-si-chapter-5-units-outside-si
- Bioassay
A bioassay is a biological assay used to estimate the potency of agents by observing their effects on living animals (in vivo) or tissue/cell culture systems (in vitro). TSE bioassay experiments are conducted using living animals as tissue/cell culture bioassays are inefficient and unreliable.
- Biodiesel
Renewable fuel comprised of mono‐alkyl esters of long chain fatty acids derived from vegetable oils or animal fats. https://www.biodiesel.org/what-is-biodiesel/biodiesel-basics
- By‐product
An incidental or secondary product made in the manufacture or synthesis of a certain product.
- Catalyst
Catalyst, in chemistry, is any substance that increases the rate of a reaction without itself being consumed. https://www.britannica.com/science/catalyst
- CJD
Creutzfeldt‐Jakob disease (CJD), rare fatal degenerative disease of the central nervous system. CJD occurs throughout the world at an incidence of one in every one million people. There are three major types of CJD: familial (fCJD), sporadic (sCJD) and acquired (aCJD). https://www.britannica.com/science/Creutzfeldt-Jakob-disease
- Co‐product
A product with commercial relevance obtained during the manufacture or synthesis of another certain product, with common steps in the production process.
- Derived material
Any material, different from the final product, obtained during a process of manufacture or synthesis.
- Distillation
Separation of different components in a liquid by evaporation and condensation using various boiling points of the substances to be separated. https://www.britannica.com/science/distillation
- Esterification
The reaction between an alcohol (R‐COH) and a carboxylic acid (R’‐COOH) forming in the presence of a catalyst an ester (R‐COO-R’) and water (H2O). Typical alcohols used in esterification are methanol and ethanol. A reaction with free fatty acids results in fatty acid alkyl esters and water. https://www.britannica.com/science/alcohol/Esterification#ref998542
- FAME
Fatty acid methyl ester. An ester obtained by reactions of fatty acids with methanol. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=4986
- Glycerine
C3H8O3, a co‐product from, among other sources, animal by‐products (ABP) and vegetable oils.
- Insoluble impurities
Solid material which remains non‐soluble in analytical solvent (commonly light petroleum) and can be isolated by filtration and weighed.
- PrP
A host‐encoded, hydrophobic glycoprotein known as the prion protein. To discriminate it from the pathogenic isoform it can also be denoted as cellular prion protein (PrPC). In this case the abbreviation PrP may be used to include the sum of PrPC and PrPSc.
- PrPSc
Abnormal isoform of PrPC resulting from a post‐translational modification of the cellular prion protein (PrPC). PrPSc is the only macromolecule demonstrated to be specifically associated with TSE diseases.
- RML strain
A mouse adapted classical scrapie strain derived from the Rocky Mountains Laboratory, USA. Also referred to as RML prions.
- sCJD (MM1)
One of the major subtypes of sporadic Creutzfeldt–Jakob disease (CJD) as classified by PRNP codon 129 genotype and PrPSc isotype. sCJD has a variable clinical and pathological phenotype; PRNP sequencing and Western blot analysis for PrPSc have allowed a subclassification of sCJD based on the combinations of the PRNP codon 129 polymorphism (MM, MV, or VV) and the PrPSc isoform (type 1 and type 2) (Ironside et al., 2017).
- Tallow
Animal fat obtained after rendering of animal by‐products. https://www.daera-ni.gov.uk/articles/animal-by-products-specific-guidance
- Transesterification
The reaction between an alcohol (R’’‐OH) and an ester (R‐COO-R’) forming in the presence of a catalyst a different ester (R‐COO-R’’) and a different alcohol (R’‐OH) with exchanged R groups. A reaction with triglycerides results in fatty acid alkyl esters and glycerol. https://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/transesterification-to-biodiesel
Abbreviations
- ABP
animal by‐products
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- C‐BSE
classical BSE
- CA
Competent Authority
- CCP
Critical Control Point
- CJD
Creutzfeldt–Jakob disease
- HACCP
Hazard Analysis and Critical Control Points
- H‐BSE
H‐type bovine spongiform encephalopathy
- L‐BSE
L‐type bovine spongiform encephalopathy
- PK
proteinase K
- TSE
transmissible spongiform encephalopathy
- VC
volatile compounds
- WB
Western blot
- WG
working group
- WWTP
wastewater treatment plant
Appendix A – Methodological limitations of prion‐related hazard reduction assessments
1.
Inactivation studies based on experimentally derived laboratory prion strains, and using WB to assess the presence of detectable PrPSc following treatment, are increasingly being considered suboptimal as predictors of the resistance of natural TSE agents when they are subjected to any particular inactivation protocol, due to the inherently limited analytical sensitivity and biological relevance of WB for the detection of prion infectivity (Marín‐Moreno et al., 2019; EFSA BIOHAZ Panel, 2020a,b). However, similar methods have been used in previous inactivation studies and appear in official guidelines (EMA, 2019). The latter acknowledges that most studies aimed at following the partition/removal of PrPSc and/or infectivity during plasma fractionation processes also use ‘rodent‐adapted TSE agent (263K hamster strain) brain homogenate and microsomal brain fractions as a spike and rely on direct [PrPSc] immunodetection tools (WB or conformation‐dependent immunoassay) to demonstrate a drop in the TSE agent content in processed fractions and on bioassay infectivity measurements to confirm the results’.
Historically, EFSA opinions have used the terms ‘infectivity’ and ‘the presence of detectable abnormal form of PrP (PrPSc) as a proxy for infectivity’ interchangeably, but data now support the view that they cannot be considered biologically interchangeable. The presence of PrPSc in tissues is generally indicative of the presence of TSE infectivity, but there is not an absolute correlation between the amount of detectable PrP and the amount of detectable infectivity. It has been demonstrated that high titres of TSE infectivity can be present in brain tissue from animals that show clinical and vacuolar signs of TSE disease but contain low or undetectable levels of PrPSc (Barron et al., 2007). Also, in many cases, during the incubation phase of the disease, infectivity (as detected by the rodent bioassay) can be demonstrated before detectable accumulation of PrPSc (as determined using existing in vitro detection methods). In addition, the structure of the polymer aggregates of different strains results in different levels of infectivity as shown for fast hamster prions (Riesner et al., 1996) and confirmed more recently by Tixador et al. (2010), in what has been described as the decoupling between infectivity measured by bioassay and the amount of PK‐resistant PrPSc estimated by WB, with respect to the size of particles.
While a few well‐characterised rodent‐adapted strains were widely used for decontamination/inactivation studies at the start of the bovine spongiform encephalopathy (BSE) epidemic, it has subsequently been shown that individual strains can respond very differently to different physico‐chemical processes, and extrapolation from one strain to another should be carried out with extreme caution, unless there are robust parallel studies that allow direct comparisons (EFSA BIOHAZ Panel, 2020a). This also applies to prions derived from naturally occurring TSE. Thus, it cannot be assumed that even prions from the same host species, e.g. classical BSE (C‐BSE), or atypical BSE (L‐BSE and H‐BSE), will respond similarly to such processes. In fact, the unique comparative study that has been undertaken for these strains and their response to Method 1 sterilisation indicated substantial differences in outcome (Chapman et al., 2020).
The BIOHAZ Panel has performed evaluations of several applications involving the use of Category 1 material for the production of fuels, and other oleochemical processes (EFSA BIOHAZ Panel, 2010b, 2011, 2015, 2017, 2020b). The evaluation published in 2015 included an extensive description of the opinions published prior to that. In the more recent opinions, it was noted that there was a limitation of data in the scientific literature that hampered the assessment of the required infectivity reduction in the proposed processes in some of the cases (EFSA BIOHAZ Panel, 2015, 2017, 2020b).
In 2020, an alternative method for production of biodiesel from processed fats from Category 1, 2 and 3 animal by‐products was assessed (EFSA BIOHAZ Panel, 2020b). Experimental data from studies commissioned to quantify the required reduction in detectable PrPSc in material spiked with a TSE strain and measured by WB were presented. The proposed method was considered to be at least equivalent to the methods previously approved to produce biodiesel from all categories of animal by‐products, although it was subject to the methodological caveats described above.
New in vitro methods are now being developed that may offer improved alternatives to bioassays. However, until the relevant guidance documents are updated with regard to the need to use situation‐specific strains, and comparatively evaluated quantification methods, the BIOHAZ Panel considers that assessments using proxy strains such as rodent‐adapted prion strains and WB detection methods remain a necessarily acceptable approach. Despite the methodological caveats that may lead to an under‐/overestimation of the actual level of hazard reduction, they still enable comparison of new biodiesel methodologies relative to pre‐existing approved methods that have been assessed by similar means.
Appendix B – Extract from 2017 EFSA BIOHAZ Panel Scientific Opinion
1.
Extract verbatim from the EFSA BIOHAZ Panel ‘Scientific Opinion on the evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT)’ (EFSA BIOHAZ Panel, 2017):
‘3.3 Full description of the material to be treated
Category 1 ABP tallow, pretreated according to Method 1, is used as feedstock with a maximum permitted level of insoluble impurities of 0.15%.
3.4 Hazard identification
Although the Applicant did not present the complete hazard identification, taking into consideration the type of process, TSEs must be considered the most relevant hazards. This application is specifically aimed at using Category 1 animal fat, a high‐risk material due to the potential presence of TSE agents. The method proposed by the Applicant is suitable for all kinds of animal fats as a feedstock. Besides TSE agents, Category 1 material can contain other biological hazards (including some highly heat‐resistant bacterial spores and viruses). However, given the high resistance to destruction, and, in particular, the high thermostability of the infectious agents causing TSEs (Somerville et al., 2009), it is assumed that if the alternative method ensures the inactivation of the TSE agent, then all microorganisms, including spore‐forming bacteria and thermoresistant viruses, will be completely inactivated. Therefore, the focus will be on the risk reduction in relation to TSE agents.
3.5.3 Assessment of the level of risk reduction (distillation)
[…] The distillation phase of the RepCat process is equivalent to the distillation step of the approved biodiesel production process and similar to the one described by Mittelbach et al. (2007). A 3 log10 reduction factor in PrP27–30 was obtained by these authors, and therefore, a similar level of PrP27–30 reduction could be expected for the distillation phase of the proposed process.’
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Fernández Escámez P, Spiropoulos J, Iulietto MF, Ortiz‐Peláez A and Alvarez‐Ordóñez A, 2021. Scientific Opinion on the evaluation of the application for new alternative biodiesel production process for rendered fat including Category 1 animal by‐products (BDI‐RepCat® process, AT). EFSA Journal 2021;19(4):6511, 26 pp. 10.2903/j.efsa.2021.6511
Requestor: Austrian Competent Authority
Question number: EFSA‐Q‐2020‐00450
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel wishes to thank Katrin Bote for the support provided to this scientific output.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © as provided by the applicant, BDI‐BioEnergy International.
Adopted: 10 March 2021
Notes
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by‐products Regulation). OJ L 300, 14.11.2009, p. 1–33.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, p. 1–254.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55–205.
Commission Regulation (EU) No 749/2011 of 29 July 2011 amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 198, 30.7.2011, p. 3–22.
The content of the Section ‘Description of the alternative method as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
The content of the Section ‘Material to be treated as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
The content of the Section ‘Hazard identification as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
The content of the Section ‘Level of risk reduction as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
The content of the Section ‘HACCP plan as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
EN 14105 Fat and oil derivatives – Fatty Acid Methyl Esters (FAME) – Determination of free and total glycerol and mono‐, di‐, triglyceride contents. European Committee for Standardization https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:66921,6288&cs=13D4CCC2A103219F3DFD99A2A608B9EC4
EN 12662 Liquid petroleum products – Determination of total contamination in middle distillates, diesel fuels and fatty acid methyl esters. European Committee for Standardization https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:33063,6003&cs=12EE1A7C749CBAC7C22E59E8EA06314B7
EN ISO 4259 Petroleum and related products — Determination and application of precision data in relation to methods of test, International Organization for Standardization. https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_LANG_ID,FSP_PROJECT:25,41369&cs=11729CF659FCD931E474AA87C6B156ED5
EN 14214 Liquid petroleum products – Fatty acid methyl esters (FAME) for use in diesel engines and heating applications – Requirements and test methods, European Committee for Standardization. https://standards.cen.eu/dyn/www/f?p=204:110:0::::FSP_PROJECT,FSP_ORG_ID:68664,6003&cs=13028D9E9E251D55C039E49EE3F0DD41E
The content of the Section ‘Risk associated with interdependent processes as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Council Directive 89/391/EEC of 12 June 1989 on the introduction of measures to encourage improvements in the safety and health of workers at work. OJ L 183, 29.6.1989, p. 1–8.
The content of the Section ‘Risk associated with intended end use as provided by the applicant’ is extracted verbatim from the application, edited for clarity and abridged in places for brevity.
References
- Barron R, Campbell S, King D, Bellon A, Chapman K, Williamson R and Manson J, 2007. High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo . The Journal of Biological Chemistry, 282, 35878–35886. 10.1074/jbc.M704329200 (https://www.jbc.org/article/S0021-9258(20)62125-1/fulltext) [DOI] [PubMed] [Google Scholar]
- Chapman GE, Lockey R, Beck KE, Vickery C, Arnold M, Thorne L, Thorne JK, Walker SR, van Keuken L, Casalone C, Griffiths PC, Simmons MM, Terry LA and Spiropoulos J, 2020. Inactivation of H‐type and L‐type bovine spongiform encephalopathy following recommended autoclave decontamination procedures. Transboundary and Emerging Diseases, 67, 1872–1878. 10.1111/tbed.13513 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010a. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp. 10.2903/j.efsa.2009.1680 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010b. Scientific Opinion on the Neste Oil Application for a new alternative method of disposal or use of Animal By‐Products. EFSA Journal 2010;8(10):1825, 9 pp. 10.2903/j.efsa.2010.1825 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the capacity of oleochemical processes to minimise possible risks linked to TSE in Category 1 animal by‐products. EFSA Journal 2011;9(2):1976, 26 pp. 10.2903/j.efsa.2011.1976 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a continuous multiple‐step catalytic hydro‐treatment for the processing of rendered animal fat (Category 1). EFSA Journal 2015;13(11):4307, 23 pp. 10.2903/j.efsa.2015.4307 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Alvarez Ordóñez A, Griffin J, Spiropoulos J, Vanopdenbosch E, Correia S and Fernández Escámez PS, 2017. Scientific Opinion on the evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT). EFSA Journal 2017;15(11):5053, 22 pp. 10.2903/j.efsa.2017.5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Bolton DJ, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman LM, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Andréoletti O, Griffin J, Spiropoulos J, Ortiz‐ Peláez A and Alvarez‐Ordóñez A, 2020a. Scientific Opinion on the potential BSE risk posed by the use of ruminant collagen and gelatine in feed for non‐ruminant farmed animals. EFSA Journal 2020;18(10):6267, 68 pp. 10.2903/j.efsa.2020.6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Andréoletti O, Fernández Escamez P, Griffin J, Spiropoulos J, Ashe S, Ortiz‐Peláez A and Alvarez‐Ordóñez A, 2020b. Scientific Opinion on the evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products (submitted by College Proteins). EFSA Journal 2020;18(4):6089, 29 pp. 10.2903/j.efsa.2020.6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on the abiotic risks for public and animal health of glycerine as co‐product from the biodiesel production from Category 1 animal by‐products (ABP) and vegetable oils. EFSA Journal 2010;8(12):1934, 22 pp. 10.2903/j.efsa.2010.1934 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2019. CHMP position statement on Creutzfeldt–Jakob disease and plasma‐derived and urine‐derived medicinal products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/chmp-position-statement-creutzfeldt-jakob-disease-plasma-derived-urine-derived-medicinal-products_en-0.pdf
- Giles K, Glidden DV, Beckwith R, Seoanes R, Peretz D, DeArmond SJ and Prusiner SB, 2008. Resistance of bovine spongiform encephalopathy (BSE) prions to inactivation. PLoS Pathogens, 4. 10.1371/journal.ppat.1000206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles K, Woerman AL, Berry DB and Prusiner SB, 2017. Bioassays and inactivation of prions. Cold Spring Harbor Perspectives in Biology, 9. 10.1101/cshperspect.a023499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamir AN, Kunkle RA, Richt JA, Miller JM and Greenlee JJ, 2008. Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Veterinary Pathology, 45, 7–11. 10.1354/vp.45-1-7 [DOI] [PubMed] [Google Scholar]
- Ironside JW, Ritchie DL and Head MW, 2017. Prion diseases. Handbook of Clinical Neurology, 145, 393–403. 10.1016/B978-0-12-802395-2.00028-6 [DOI] [PubMed] [Google Scholar]
- Liberski PP, Gajos A, Sikorska B and Lindenbaum S, 2019. Kuru, the first human prion disease. Viruses, 11, 232. 10.3390/v11030232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐Moreno A, Aguilar‐Calvo P, Moudjou M, Espinosa JC, Béringue V and Torres JM, 2019. Thermostability as a highly dependent prion strain feature. Scientific Reports, 9, 11396. 10.1038/s41598-019-47781-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbach M, Pokits B, Müller H, Müller M and Riesner D, 2007. Risk assessment for prion protein reduction under the conditions of the biodiesel production process. European Journal of Lipid Science and Technology, 109, 79–90. 10.1002/ejlt.200600172 [DOI] [Google Scholar]
- Mohammadi B, Raudner R, Shafiq M, Ahn E, Altmeppen HC and Glatzel M, 2020. Influence of methanol on prion reduction during high temperature and high pressure oleochemical processes. European Journal of Lipid Science and Technology, 122, 2000136. 10.1002/ejlt.202000136 [DOI] [Google Scholar]
- Müller H and Riesner D, 2005. Thermal degradation of prions in the presence of fats: implication for oleochemical processes. European Journal of Lipid Science and Technology, 107, 833–839. 10.1002/ejlt.200501189 [DOI] [Google Scholar]
- Müller H, Stitz L and Riesner D, 2006. Risk assessment for fat derivatives in case of contamination with BSE. European Journal of Lipid Science and Technology, 108, 812–826. 10.1002/ejlt.200600068 [DOI] [Google Scholar]
- Müller H, Stitz L and Riesner D, 2008. Prion decontamination during the oleochemical process of fat hydrogenation. European Journal of Lipid Science and Technology, 110, 392–399. 10.1002/ejlt.200700171 [DOI] [Google Scholar]
- Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, Safar JG, Glidden DV, McCulloch C, Nguyen H‐OB, Scott M, DeArmond SJ and Prusiner SB, 2006. Inactivation of prions by acidic sodium dodecyl sulfate. Journal of Virology, 80, 322–331. 10.1128/jvi.80.1.322-331.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesner D, Kellings K, Post K, Wille H, Serban H, Groth D, Baldwin MA and Prusiner SB, 1996. Disruption of prion rods generates 10‐nm spherical particles having high alpha‐helical content and lacking scrapie infectivity. Journal of Virology, 70, 1714–1722. 10.1128/JVI.70.3.1714-1722.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RA, Fernie K, Smith A, Andrews R, Schmidt E and Taylor DM, 2009. Inactivation of a TSE agent by a novel biorefinement system. Process Biochemistry, 44, 1060–1062. 10.1016/j.procbio.2009.06.002 [DOI] [Google Scholar]
- SSC (Scientific Steering Committee), 2001. Revised opinion and report on: the safety of tallow obtained from ruminant slaughter by‐products, adopted by the Scientific Steering Committee at its meeting of 28‐29 June 2001. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_ssc_out219_en.pdf
- Tixador P, Herzog L, Reine F, Jaumain E, Chapuis J, Le Dur A, Laude H and Béringue V, 2010. The physical relationship between infectivity and prion protein aggregates is strain‐dependent. PLOS Pathogens, 6. 10.1371/journal.ppat.1000859 [DOI] [PMC free article] [PubMed] [Google Scholar]


