Abstract
Objectives
Children with type 1 diabetes (T1D) are not included in guidelines regarding diagnosis criteria for celiac disease (CD) without a diagnostic biopsy, due to lack of data. We explored whether tissue transglutaminase antibodies (anti‐tTG) that were ≥ 10 times the upper limit of normal (10× ULN) predicted CD in T1D.
Methods
Data from the Swedish prospective Better Diabetes Diagnosis study was used, and 2035 children and adolescents with T1D diagnosed between 2005–2010 were included. Of these, 32 had been diagnosed with CD before T1D. The children without CD were repeatedly screened for CD using anti‐tTG antibodies of immunoglobulin type A. In addition, their human leukocyte antigen (HLA) were genotyped. All children with positive anti‐tTG were advised to undergo biopsy. Biopsies were performed on 119 children and graded using the Marsh‐Oberhüber classification.
Results
All of the 60 children with anti‐tTG ≥10x ULN had CD verified by biopsies. The degree of mucosal damage correlated with anti‐tTG levels. Among 2003 screened children, 6.9% had positive anti‐tTG and 5.6% were confirmed CD. The overall CD prevalence, when including the 32 children with CD before T1D, was 7.0% (145/2035). All but one of the children diagnosed with CD had HLA‐DQ2 and/or DQ8.
Conclusions
As all screened children and adolescents with T1D with tissue transglutaminase antibodies above 10 times the positive value 10x ULN had CD, we propose that the guidelines for diagnosing CD in screened children, when biopsies can be omitted, should also apply to children and adolescents with T1D as a noninvasive method.
Keywords: biopsy, celiac disease, screening, tissue transglutaminase antibodies, type 1 diabetes
Abbreviations
- 10x ULN
10 times the upper limit of normal
- Anti‐tTG
antibodies against tissue‐transglutaminase
- BDD
better diabetes diagnosis
- CD
celiac disease
- CI
confidence interval
- EliA
enzyme‐linked immuno assay
- ELISA
enzyme‐linked immunosorbent assay
- ESPGHAN
European society for pediatric gastroenterology, hepatology and nutrition
- HLA
human leukocyte antigen
- IgA
immunglobulin A
- TD1
type 1 diabetes
- ULN
upper limit of normal
1. INTRODUCTION
Celiac disease (CD) affects 3%–16% of children with type 1 diabetes mellitus (T1D) worldwide. 1 , 2 , 3 , 4 , 5 In Sweden, the prevalence of CD in children with T1D is between 9%–10%, 6 , 7 , 8 compared with 1% in the general population. 9 An explanation for the higher prevalence is that both T1D and CD are associated with the human leucocyte antigen (HLA) class II genes. 1 , 10
In a classical presentation, CD often is correlated with gastrointestinal dysfunction, 9 but it is mostly asymptomatic in children with T1D and the disease is usually detected by screening. 1 , 4 , 6 , 11 In general, CD diagnoses have been based on clinical features, serology, genetics and biopsies. Most guidelines have recognized biopsies as the gold standard for CD diagnosis, even in children with T1D. 1 , 12 , 13 , 14 , 15
In 2012, revised guidelines from the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) suggested small bowel biopsies could be omitted for symptomatic pediatric patients over 2 years of age if concentrations of antibodies against tissue transglutaminase (anti‐tTG) were ≥10 times the upper limit of normal (10× ULN). 16 Other criteria were positive endomysium antibodies in another blood sample and positive risk HLA allele. Both these autoantibodies were of immunoglobuline type A (IgA), because children with IgA deficiency were always recommended the biopsy procedure. The recommendations for the diagnosis of CD in the ESPGHAN 2012 guidelines were divided into two algorithms, one for children with symptoms, and another for screened children, where the pathway for the latest did not allow the no‐biopsy approach. 16
A large Swedish study of 12‐year‐old school children found that all the screened children with anti‐tTG levels of ≥10× ULN had biopsy‐proven CD. 17 Other studies of asymptomatic children with high anti‐tTG levels have found that biopsies could safely be omitted. Some of these children had T1D, but they were not assessed separately. 18 , 19 A small Finnish study, 20 a retrospective Australian study 21 and a Dutch collaboration 22 confirmed that anti‐tTG had a good positive predictive value for biopsy‐proven CD in children with T1D. Nevertheless, the 2020 European Society for Pediatric Gastroenterology, Hepatology and Nutrition guidelines for diagnosing celiac disease (CD), without a biopsy, do not include type 1 diabetes (T1D) due to lack of data. 23
This lack of data prompted us to explore whether high levels of anti‐tTG (≥10x ULN) predicted CD in children and adolescents with T1D, addressing the possibility to include them in future guidelines. We wanted, as well, to analyze the agreement between celiac autoantibodies levels and biopsy results.
2. METHOD
2.1. Study design and subjects
Our study was based on data from Better Diabetes Diagnosis (BDD), an ongoing Swedish national prospective population‐based cohort study that started in May 2005. 24 The study investigates children under the age of 18 years with newly diagnosed diabetes. The main aim of BDD is to improve the classification of diabetes and increase knowledge on the underlying factors behind diabetes. The secondary aims include exploring co‐morbidities and risk factors.
The American Diabetes Association criteria for classifying T1D were used to determine the clinical diagnosis of diabetes 25 and the T1D diagnoses were re‐evaluated after 1 year. All of the children who were included in this study met the criteria for T1D.
The present study included 2035 children diagnosed with T1D between May 2005 and December 2010 (Figure 1), and it was approved by the Ethics Committee in Lund (number 2014/476). In addition, the original BDD study was approved by the Ethics Committee at the Karolinska Institutet in Stockholm (number 04–826/1, with amendments in 2006, 2007, 2009 and 2011).
FIGURE 1.
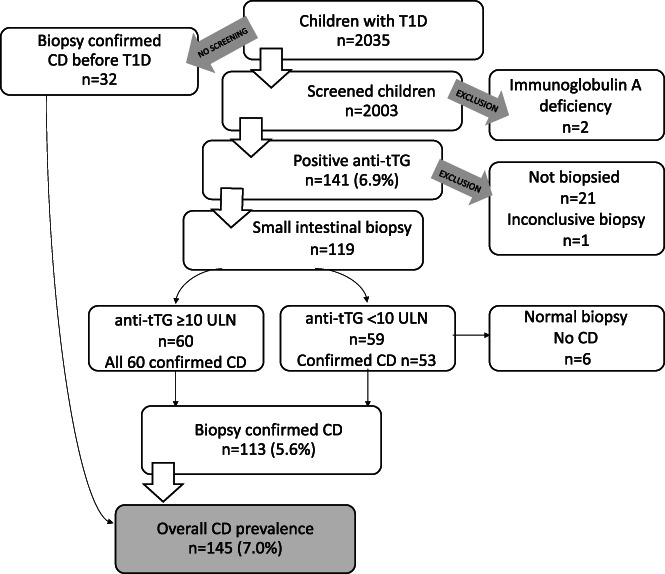
Flowchart summarizing the diagnostic process of celiac disease (CD) in children with type 1 diabetes (T1D) with antibodies against tissue‐transglutaminase (anti‐tTG) and biopsies. The levels of anti‐tTG were given as under 10 times the upper limit of normal (<10x ULN) or equal and above 10 times this limit (≥10x ULN)
2.2. Screening and blood analysis
All the children were screened for CD using anti‐tTG when their diabetes was diagnosed 26 and then on a yearly basis. Teams from 13 Pediatric Diabetes Centers were involved in the current study: Göteborg, Helsingborg, Jönköping, Kristianstad, Linköping, Lund, Malmö, Norrköping, Stockholm, Västerås, Ystad, Örebro and Östersund. These centers collected the results of anti‐tTG and intestinal biopsies from patients investigated for CD. The autoantibody levels were grouped according to the last positive value before each patient's biopsy.
Anti‐tTG levels of IgA type were analyzed using two different Thermo Fisher Scientific systems (Legal Manufacture Phadia AB, Uppsala, Sweden). One was an enzyme‐linked immuno assay (EliA), the EliA Celikey IgA, with the level of positivity set at >10 U/ml. The other was an enzyme‐linked immunosorbent assay (ELISA), the Celikey Tissue transglutaminase IgA Antibody Assay, with the level of positivity set at >8 U/ml.
Furthermore, total IgA was tested to rule out immunodeficiency that would not detect anti‐tTG of this type. Children with IgA deficiency were excluded, as the recommendation for these children were always to have duodenal biopsies. 16 , 23
2.3. Celiac disease criteria
The general recommendation from ESPGHAN at the time of the study was that all the children who were screened and had a positive anti‐tTG result should undergo a biopsy to confirm or rule out CD. 27 According to the ESPGHAN 2012 revised guidelines for diagnostic criteria, 16 CD could be diagnosed without being confirmed with a duodenal biopsy in children over 2 years of age if they had clear symptoms. That was frequently not the case in children with T1D who were screened for CD. 1 These symptoms needed to be combined with IgA anti‐tTG antibodies that were ≥ 10x ULN, verified by endomysium antibody positivity and the presence of HLA‐DQ2 and/or HLA‐DQ8. Children with anti‐tTG levels that were < 10x ULN were always recommended to undergo a biopsy to confirm CD. 16
2.4. HLA typing
The HLA profile was analyzed for all children included in the BDD study. Blood samples were obtained at clinical onset of T1D and further processed by the Clinical Research Centre at Malmö, which is part of Skåne University Hospital. The HLA‐DQ genotype was determined by polymerase chain reaction. 24 Genes associated with the risk of CD were 0201–02 (DQ2), 05–02 (DQ2), 03–02 (DQ2), Z‐0302/0304 (DQ8), Z‐0302 (DQ8) and 05–0302 (DQ8). 26
2.5. Upper gastrointestinal biopsies and histological classifications
Participants with positive serology were recommended to have duodenal biopsies. They were obtained according to local clinical routines, mostly by endoscopy and sometimes by suction capsule, and further assessed by local pathologists. The histological results were reviewed and scored by the same person, according to the revised Marsh‐Oberhüber classification. 28
The 32 children with confirmed CD prior to T1D diagnosis were excluded from further analysis, but they were taken into consideration when the statistics on prevalence were analyzed. In addition, 21 of the children with positive antibodies were excluded, mainly because their families did not want them to undergo a biopsy. Some biopsy data was also missing. One child was excluded due to inconclusive biopsy results.
2.6. Statistics
The analyses were carried out using SPSS software, version 25 (IBM Corp., New York, USA). The data are presented with descriptive statistics and the 95% confidence interval (95% CI) has been provided where necessary. The scatter plot was created using GraphPad Prism 7.0 (GraphPad Software, California, USA).
3. RESULTS
3.1. Celiac disease and anti‐tTG serology
Celiac autoimmunity with positive anti‐tTG was found in 141/2003 (6.9%, 95% CI 5.8–8.0) of the screened children. CD was confirmed in 113/119 children who underwent biopsies and this equated to 5.6% (95% CI 4.6–6.7) of the 2003 screened children.
When the 32 children with known CD at T1D diagnosis were added back into the 2003 children included in the main analysis, this gave an overall prevalence of CD of 145/2035 (7.0%, 95% CI 6.0–8.2), as shown in Figure 1.
3.2. Celiac disease and considerations about age and sex
The age when the children were diagnosed with CD varied between 0.5–17.9 years. For the 32 children with CD before T1D the age of CD diagnosis ranged from 0.5 to 14.9, the mean age for diagnosis CD was 4.3, and the median age was 4.75. For the 113 screened T1D children, the age of CD diagnosis ranged from 2 to 17.9, the mean age for diagnosis CD was 9.9 years, and the median was 9.1.
The distribution of the age of CD diagnosis was divided into age groups according to an earlier an BDD study. 26 The results were as following: 16% were 0–4 years of age, 37% 5–9 years, 34% 10–14, and 13% 15–17.9 years old.
Regarding the sex of the children with CD, the female to male ratio was similar. The female to male ratio of children diagnosed with CD before T1D onset (n = 32) was 17:15 and in children diagnosed with CD after the screening (n = 2003) it was 55:58. Among the children with positive anti‐tTG and normal biopsy only one child was a girl. Thus, of the 145 children with CD and T1D, 72 were girls and 73 were boys (n = 2035).
3.3. Anti‐tTG and mucosal damage
The degree of mucosal damage correlated with anti‐tTG levels. All 60 children with an anti‐tTG of ≥10x ULN, had their CD diagnosis verified by a biopsy, regardless of the method used to analyze anti‐tTG.
3.3.1. EliA method with a cut‐off positivity level of >10 U/ml
All children with an anti‐tTG of ≥10x ULN (≥100 U/ml), and even with lower cut‐off of 70–99 U/ml, were diagnosed with CD.
Of the 80 participants with positive EliA results (>10 U/ml) who underwent a biopsy, 74 were diagnosed with CD. The frequency of CD was 76% in the group with the lowest anti‐tTG values of between 10–39 U/ml, and four children had a normal biopsy (Marsh 0). In the group with anti‐tTG values of between 40–69 U/ml, one child with a tTG level of 58 U/ml had a biopsy (Marsh 0). Another child had Marsh 1 and the remainder had Marsh 3a‐3c (Figure 2).
FIGURE 2.
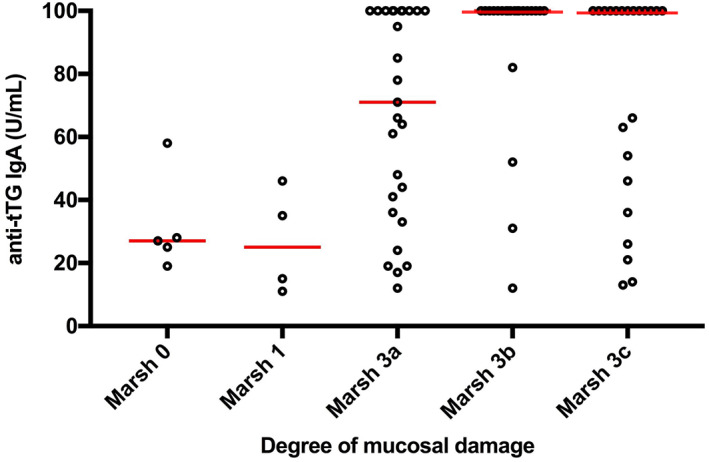
Degrees of mucosal damage in relation to antibodies against tissue‐transglutaminase (anti‐tTG) using the enzyme‐linked immuno assay (EliA) method. The lines represent the median. Levels of anti‐tTG correlated to the degree of mucosal damage
There were 41 patients with serological levels of ≥100 U/ml and their biopsies all contained high‐grade mucosal lesions, corresponding to Marsh 3a‐3c. Marsh 3 lesions were also found in 14/21 (67%) children with low positive tTG levels (10–39 U/ml) and Marsh 1 lesions were found in 3/21 (14%) at this low anti‐tTG levels (Figure 2).
Although a wide distribution of anti‐tTG values was seen, the frequency of villous atrophy was higher when the levels of tTG increased. Marsh 3 lesions were present in 67%, 85%, 100% and 100% of the participants with anti‐tTG values between 10–39, 40–69, 70–99 and ≥ 100 U/ml, respectively.
3.3.2. ELISA method with a cut‐off positivity level of >8 U/ml
All children with anti‐tTG ≥10x ULN (≥80 U/ml) were diagnosed with CD.
Among children positive for anti‐tTG, there were 19/39 children with an anti‐tTG of ≥80 U/ml and 18/39 of these had villous atrophy (Marsh 3a‐3c) (Figure 3). One patient had Marsh 1 score on the first histological evaluation and was diagnosed with CD based on clinical features and after the biopsy was reconsidered.
FIGURE 3.
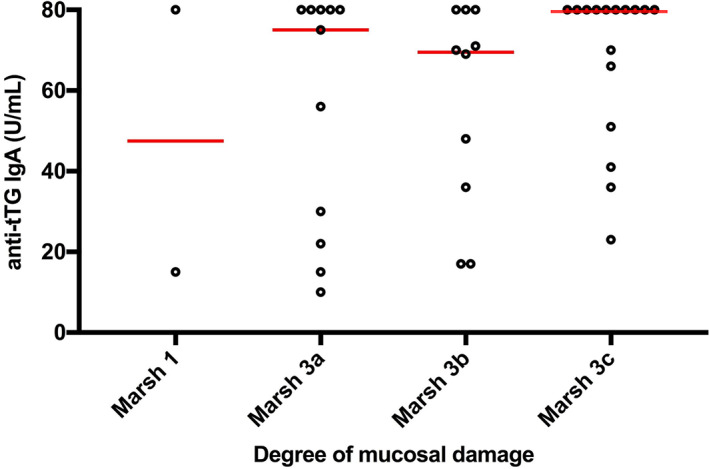
Degrees of mucosal damage in relation to antibodies against tissue‐transglutaminase (anti‐tTG) using the enzyme‐linked immunosorbent assay (ELISA) method. The lines represent the median. Levels of anti‐tTG correlated to the degree of mucosal damage
The frequency of CD was also very high in the groups with lower levels of anti‐tTG. All 13 patients with anti‐tTG level between 30–79 U/ml had Marsh 3 lesions. In the seven children with a low positive anti‐tTG of 8–29 U/ml, there was one child with intra‐epithelial lymphocytes and six had villous atrophy. Figure 3 shows the degree of enteropathy in relation to anti‐tTG levels. Marsh 3 was seen in 86%, 100%, 100% and 95% of the groups with anti‐tTG values between 8–29, 30–54, 55–79 and ≥ 80 U/ml, respectively.
3.4. HLA typing
With the exception of one patient with Down syndrome, all the children with CD had high‐risk genes, namely HLA‐DQ2 and/or DQ8. Genetic analyses could not be performed for three children, as their blood samples were missing from the BDD database. The child with Down syndrome, who lacked both HLA‐DQ2 and DQ8, had HLA genotype DQ7/DQ9.
The most frequent genotype was HLA‐DQ2/DQ8 (42.4%), followed by DQ8/DQX (21.1%), DQ2/DQ2 (12.1%), DQ2/DQX (8.4%), DQ8/DQ8 (15.1%) and DQX/DQ9 (0.9%).
4. DISCUSSION
Our study results indicate that CD can be diagnosed without biopsies in children and adolescents with T1D who have a high level of anti‐tTG. All the children with T1D with anti‐tTG exceeding 10x ULN were diagnosed with CD. Interestingly, all children with an anti‐tTG that exceeded 7x ULN were also diagnosed with CD. Overall, the serological level of anti‐tTG was related to the degree of gluten‐induced enteropathy.
Our findings confirm that children and adolescents with T1D should be included in the ESPGHAN guidelines for CD diagnosis by screening. In addition, to being able to follow the protocol and avoid endoscopy with general anesthesia, children and adolescents with T1D would benefit from avoiding the difficulties of a fasting period. Furthermore, it would also reduce healthcare costs, as previously reported. 29
In line with other smaller studies, our study investigated associations between anti‐tTG levels and the degree of intestinal damage or enteropathy in children with T1D screened for CD. Our results are in accordance with Popp et al, who showed that none of their 7/181 Finnish symptom‐free children with T1D and CD and high anti‐tTG levels would have needed a biopsy to confirm CD, because all those with an anti‐tTG of ≥10x ULN had duodenal villous atrophy (Marsh 3). 20 A larger Brazilian study randomly screened 887/3247 children with T1D and found higher anti‐tTG levels in children with pathological biopsies, but did not specify if the anti‐tTG levels were ≥ 10x ULN. 2 An Australian study retrospectively investigated 936 children with T1D to see if CD could be diagnosed without duodenal biopsies. 21 The authors reported that 35 children had high levels of anti‐tTG and confirmed CD, but the association with the degree of enteropathy was not studied or presented. 21 In addition, a multicenter study including children from the Netherlands, recently showed that high levels of CD biomarkers could give accurate CD diagnosis. 22 This Dutch cooperation, included a retrospective evaluation on 63 children with T1D and CD, using six different types of tTG assays, and showed that the cut‐off value 11 times the ULN had a sensitivity of 96%, and a positive predictive value of 94%. Our findings were based on a cohort study that was more than twice the size of the studies mentioned above, the children were prospectively and consecutively included in the BDD study 24 and they were followed in clinical settings across Sweden. We therefore believe that our results had better external validity and could be generalized to a higher degree to other groups outside the study population.
The correlation between anti‐tTG levels and intestinal damage has been evaluated in population‐based pediatric studies and the results and conclusions are in agreement with ours. 17 Webb et al prospectively screened more than 13,000 Swedish school children for CD when they were 12 years old and all 64 with anti‐tTG levels of ≥10x ULN were diagnosed with CD, mostly with Marsh 3 type lesions. 17 Furthermore, the gastrointestinal symptoms associated with CD showed poor diagnostic predictability for CD in this large cohort. 17 Other studies have confirmed that celiac symptoms were as common in children with CD detected by screening as in children with normal biopsy results. 30 , 31 One previous Stockholm‐based study of children with T1D also reported this finding 6 and therefore it was not assessed by the current study. Small subgroups of children with T1D were included in two prospective multinational studies that validated the accuracy of diagnosing CD in screened children without biopsies. 18 , 19 More than half of the children in these studies were asymptomatic, but the anti‐tTG levels and the degree of enteropathy in children with T1D were not presented separately. The ESPGHAN 2020 guidelines state that biopsies can be omitted in screened, asymptomatic children if their anti‐tTG levels are ≥10x ULN, but the guidelines excluded T1D due to insufficient data. 23 In the same line as the Brazilian, 2 the Dutch 22 and the Australian 21 studies mentioned above, our study addressed this lack of data and we propose that children and adolescents with T1D should be included in any future revisions of the guidelines.
We also found that more pronounced mucosal damage was associated with increasing anti‐tTG levels, implying a possible lower cut‐off for CD diagnoses. These findings agreed with the results of the Australian cohort, 21 but not with the Dutch study. 22 The ESPGHAN 2020 guidelines indicate that CD can be diagnosed without biopsies when anti‐tTG exceeds 10x ULN, but there was insufficient data to lower the cut‐off levels. 23 If we had applied the safety guidelines to our cohort, the cut‐off level could have been ≥7x ULN. The predictive value of gluten‐induced abnormalities was low for low serological levels, especially when EliA was used. As a result, individuals with low anti‐tTG levels would still require biopsies to diagnose or rule out CD, as suggested by the ESPGHAN 2020 guidelines. 23 Moreover, it is unclear why children with low ant‐tTG levels sometimes present with high degrees of mucosal damage, as seen in some of the children in our study. Further studies need to investigate whether the criteria to omit biopsies can safely be applied to screened individuals with anti‐tTG titers under 10x ULN.
We are aware that one of the criticisms towards a no‐biopsy approach has been that some publications showed that T1D children could have transient elevation of autoantibodies without mucosal damage. None of the studies we have reviewed 32 , 33 , 34 showed that children with high levels of tTG had their autoantibody levels normalized after a period of time during a diet with gluten. Therefore, the concern should focus on children with low or moderate levels of tTG. In our study, the serological markers were correlated to the enteropathy. Furthermore, according to a previous validation study on biopsies in Sweden, whereas in low degrees of mucosal damage, the evaluations between pathologists had some disagreements, there were very good agreements and a high specificity in the pathologists' assessments of biopsies with villous atrophy. 35
Moreover, the value of symptoms regarding accuracy of CD diagnosis, and adherence to the treatment with a life‐long gluten‐free diet, has been focus in several studies. In screening Swedish healthy children, Rosen et al 30 showed that symptoms are not a useful criteria to determine if CD is present or not, and similar findings were seen in a T1D cohort of Swedish children. 6 At the same time, an argument against screening has been that children may have a lower adherence to the life‐long gluten‐free diet required as treatment, if they are found through mass‐screening and asymptomatic. 36 Another possible argument, could be that a low compliance may apply as well to children diagnosed without a biopsy. Thus, this aspect regarding compliance needs to be further evaluated, and could be done in the future, comparing T1D children without symptoms diagnosed through screening, with otherwise healthy asymptomatic screened children, diagnosed according to the ESPGHAN 2020.
Although the genetic risk in CD is largely influenced by HLA‐DQ2/DQ8 alleles, 26 one child CD in our study did not have the HLA risk alleles DQ2/DQ8. That exception was a child with Down syndrome, a condition that has been associated with a higher prevalence of autoimmune diseases and CD, 9 possibly because altered gene expression on chromosome 21 increases the risk for autoimmunity. This could explain why the classical risk genotypes were missing. The child had a DQ7/DQ9 genotype and the HLA allele DQ9 has been suggested as a susceptibility factor for CD. 37 Thus, we agree with the ESPGHAN 2020 guidelines that HLA assessment should no longer be mandatory for a CD diagnosis, 23 because it provides no further information on children with T1D. The 2018 consensus guidelines from the International Society for Pediatric and Adolescent Diabetes also state that HLA assessment is no longer needed. 14
The main strength of our study was the prospective multicenter data collection. The BDD T1D cohort comprised over 2000 Swedish children and adolescents screened for CD, with good internal validity due to high national coverage and very few exclusions, missing data or cases lost to follow up. 24 The association between anti‐tTG levels and the degree of intestinal mucosal enteropathy were explored by using a large number of biopsies. We believe that the present study is the largest of its kind. The prevalence of celiac autoimmunity was similar to comparable populations, 8 , 26 as well as the overall 7.0% prevalence for CD, 6 , 7 suggesting that our cohort was representative. Likewise, the children received standard medical care, meaning that the results had good external validity and the data and results can be generalized to other settings. Having said that, the study only included children living in Sweden and that should be borne in mind when extrapolating our findings to children living in other parts of the world.
One limitation of our study was that the biopsies were assessed by several different pathologists. On the other hand, they were thoroughly reviewed by the same person using the revised Marsh‐Oberhüber classification. Furthermore, a previous study including all the 28 pathology departments in Sweden validated the good specificity of biopsy result, especially with villous atrophy. 35 Only one child with low tTG had an inconclusive biopsy, without doing a new procedure, and that child was excluded. Another child was diagnosed with CD based on high anti‐tTG levels, re‐assessment of the biopsies and clinical follow up. That child had high anti‐tTG (≥80 U/ml) and had initially been assessed with low‐grade enteropathy (Marsh 1), based only just two biopsies, instead of the five recommended by the ESPGHAN 2020 guidelines. At least four biopsies from the distal duodenum and one from the duodenal bulb are recommended because enteropathy can have a patchy distribution in CD. 23 As a result, it was possible that villous atrophy zones were missed. One less plausible explanation could be that the child was diagnosed early in the development of CD and that the mucosal inflammation could have progressed to villous atrophy due to the continued gluten load.
Another possible limitation of the study was that the blood samples and biopsies were not obtained at the same time. However, the participants were instructed to continue with the same diet and we still found gluten‐induced enteropathy in all patients, but one, with high transglutaminase serology when the intestinal biopsies were performed. Moreover, we speculate that the real overall prevalence may be higher. On one hand, because children that had IgA deficiency were not included in the aim of our study, and on the other hand, because 22 children with positive CD biomarkers were not fully investigated, mainly due to parents' decision not to proceed with the biopsy procedure.
5. CONCLUSIONS
In summary, we believe that our findings indicate that guidelines with a no‐biopsy approach for diagnosing CD in screened children, should also apply to children and adolescents with T1D. In our study, CD was diagnosed in all the screened children and adolescents with T1D who had anti‐tTG above 10x ULN, which indicates that CD diagnosis can be predicted by high levels of antibodies against tissue transglutaminase in children and adolescents with T1D.
6.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13165.
ACKNOWLEDGMENTS
The authors are grateful to the children and parents who participated in the study. The authors also thank the participating Swedish Pediatric Diabetes Centers who contributed to the study. Without the efforts of all the pediatricians and pediatric diabetes nurses it would not have been possible to perform this study. Special thanks go to Qefsere Brahimi, BMI, for assisting with the data collection, Professor Åke Lenmark for his expertise in the BDD group and Fredrik Norström, Ph.D., for his help with the statistical analysis.
Cerqueiro Bybrant M, Udén E, Frederiksen F, et al. Celiac disease can be predicted by high levels of tissue transglutaminase antibodies in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2021;22:417–424. 10.1111/pedi.13165
Funding information Barndiabetesfonden; Skåne County Council's Research and Development Foundation
REFERENCES
- 1. Sud S, Marcon M, Assor E, Palmert MR, Daneman D, Mahmud FH. Celiac disease and pediatric type 1 diabetes: diagnostic and treatment dilemmas. Int J Pediatr Endocrinol. 2010;2010:161285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Punales M, Bastos MD, Ramos ARL, et al. Prevalence of celiac disease in a large cohort of young patients with type 1 diabetes. Pediatr Diabetes. 2019;20(4):414‐420. [DOI] [PubMed] [Google Scholar]
- 3. Pham‐Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for celiac disease in type 1 Diabetes: a systematic review. Pediatrics. 2015;136(1):e170‐e176. [DOI] [PubMed] [Google Scholar]
- 4. Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta‐analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123‐1132. [DOI] [PubMed] [Google Scholar]
- 5. Craig ME, Prinz N, Boyle CT, et al. Prevalence of celiac disease in 52,721 youth with type 1 diabetes: International comparison across three continents. Diabetes Care. 2017;40(8):1034‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bybrant MC, Ortqvist E, Lantz S, Grahnquist L. High prevalence of celiac disease in Swedish children and adolescents with type 1 diabetes and the relation to the Swedish epidemic of celiac disease: a cohort study. Scand J Gastroenterol. 2014;49(1):52‐58. [DOI] [PubMed] [Google Scholar]
- 7. Larsson K, Carlsson A, Cederwall E, et al. Annual screening detects celiac disease in children with type 1 diabetes. Pediatr Diabetes. 2008;9(4 Pt 2):354‐359. [DOI] [PubMed] [Google Scholar]
- 8. Adlercreutz EH, Svensson J, Hansen D, et al. Prevalence of celiac disease autoimmunity in children with type 1 diabetes: regional variations across the Øresund strait between Denmark and southernmost Sweden. Pediatr Diabetes. 2015;16(7):504‐509. [DOI] [PubMed] [Google Scholar]
- 9. Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med. 2019;17(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Redondo MJ, Steck AK, Pugliese A. Genetics of type 1 diabetes. Pediatr Diabetes. 2018;19(3):346‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fröhlich‐Reiterer EE, Hofer S, Kaspers S, et al. Screening frequency for celiac disease and autoimmune thyroiditis in children and adolescents with type 1 diabetes mellitus–data from a German/Austrian multicentre survey. Pediatr Diabetes. 2008;9(6):546‐553. [DOI] [PubMed] [Google Scholar]
- 12. McCarty TR, O'Brien CR, Gremida A, Ling C, Rustagi T. Efficacy of duodenal bulb biopsy for diagnosis of celiac disease: a systematic review and meta‐analysis. Endoscopy International Open. 2018;6(11):E1369‐E1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubio‐Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA, American College of G . ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108(5):656‐676. quiz 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mahmud FH, Elbarbary NS, Fröhlich‐Reiterer E, et al. ISPAD clinical practice consensus guidelines 2018: other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(Suppl 27):275‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kordonouri O, Maguire AM, Knip M, et al. ISPAD clinical practice consensus guidelines 2006‐2007. Other complications and associated conditions. Pediatr Diabetes. 2007;8(3):171‐176. [DOI] [PubMed] [Google Scholar]
- 16. Husby S, Koletzko S, Korponay‐Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136‐160. [DOI] [PubMed] [Google Scholar]
- 17. Webb C, Norstrom F, Myleus A, et al. Celiac disease can be predicted by high levels of anti‐tissue transglutaminase antibodies in population‐based screening. J Pediatr Gastroenterol Nutr. 2015;60(6):787‐791. [DOI] [PubMed] [Google Scholar]
- 18. Wolf J, Petroff D, Richter T, et al. Validation of antibody‐based strategies for diagnosis of pediatric celiac disease without biopsy. Gastroenterology. 2017;153(2):410‐419 e417. [DOI] [PubMed] [Google Scholar]
- 19. Werkstetter KJ, Korponay‐Szabo IR, Popp A, et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology. 2017;153(4):924‐935. [DOI] [PubMed] [Google Scholar]
- 20. Popp A, Mihu M, Munteanu M, et al. Prospective antibody case finding of coeliac disease in type‐1 diabetes children: need of biopsy revisited. Acta Paediatr. 2013;102(3):e102‐e106. [DOI] [PubMed] [Google Scholar]
- 21. Joshi KK, Haynes A, Davis EA, D'Orsogna L, McLean‐Tooke A. Role of HLA‐DQ typing and anti‐tissue transglutaminase antibody titers in diagnosing celiac disease without duodenal biopsy in type 1 diabetes: a study of the population‐based pediatric type 1 diabetes cohort of Western Australia. Pediatr Diabetes. 2019;20(5):567‐573. [DOI] [PubMed] [Google Scholar]
- 22. Wessels M, Velthuis A, van Lochem E, et al. Raising the cut‐off level of anti‐tissue transglutaminase antibodies to detect celiac disease reduces the number of small bowel biopsies in children with type 1 Diabetes: a retrospective study. J Pediatr. 2020;223:87‐92 e81. [DOI] [PubMed] [Google Scholar]
- 23. Husby S, Koletzko S, Korponay‐Szabo I, et al. European society Paediatric gastroenterology, Hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. 2020;70(1):141‐156. [DOI] [PubMed] [Google Scholar]
- 24. Persson M, Becker C, Elding Larsson H, et al. The better Diabetes diagnosis (BDD) study ‐ a review of a nationwide prospective cohort study in Sweden. Diabetes Res Clin Pract. 2018;140:236‐244. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42‐S47. [DOI] [PubMed] [Google Scholar]
- 26. Cerqueiro Bybrant M, Grahnquist L, Ortqvist E, et al. Tissue transglutaminase autoantibodies in children with newly diagnosed type 1 diabetes are related to human leukocyte antigen but not to islet autoantibodies: a Swedish nationwide prospective population‐based cohort study. Autoimmunity. 2018;51(5):221‐227. [DOI] [PubMed] [Google Scholar]
- 27. Walker‐Smith JAGS, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Report of working Group of European Society of Paediatric gastroenterology and nutrition. Arch Dis Child. 1990;65(8):909‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59(10):1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paul SP, Sandhu BK, Spray CH, Basude D, Ramani P. Evidence supporting serology‐based pathway for diagnosing celiac disease in asymptomatic children from high‐risk groups. J Pediatr Gastroenterol Nutr. 2018;66(4):641‐644. [DOI] [PubMed] [Google Scholar]
- 30. Rosén A, Sandström O, Carlsson A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics. 2014;133(2):211‐218. [DOI] [PubMed] [Google Scholar]
- 31. van der Pals M, Myleus A, Norstrom F, et al. Body mass index is not a reliable tool in predicting celiac disease in children. BMC Pediatr. 2014;14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rinawi F, Badarneh B, Tanous O, Bashir H, Tennenbaum‐Rakover Y, Peleg S. Elevated anti‐tissue transglutaminase antibodies in children newly diagnosed with type 1 diabetes do not always indicate coeliac disease. Acta Paediatr. 2019;108(1):149‐153. [DOI] [PubMed] [Google Scholar]
- 33. Simell S, Hoppu S, Hekkala A, et al. Fate of five celiac disease‐associated antibodies during normal diet in genetically at‐risk children observed from birth in a natural history study. Am J Gastroenterol. 2007;102(9):2026‐2035. [DOI] [PubMed] [Google Scholar]
- 34. Castellaneta S, Piccinno E, Oliva M, et al. High rate of spontaneous normalization of celiac serology in a cohort of 446 children with type 1 diabetes: a prospective study. Diabetes Care. 2015;38(5):760‐766. [DOI] [PubMed] [Google Scholar]
- 35. Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fabiani E, Taccari LM, Ratsch IM, Di Giuseppe S, Coppa GV, Catassi C. Compliance with gluten‐free diet in adolescents with screening‐detected celiac disease: a 5‐year follow‐up study. J Pediatr. 2000;136(6):841‐843. [PubMed] [Google Scholar]
- 37. Bodd M, Tollefsen S, Bergseng E, Lundin KE, Sollid LM. Evidence that HLA‐DQ9 confers risk to celiac disease by presence of DQ9‐restricted gluten‐specific T cells. Hum Immunol. 2012;73(4):376‐381. [DOI] [PubMed] [Google Scholar]


