Abstract
Aim
To evaluate healthcare professionals' performance and treatment fidelity in the Cardiac Care Bridge (CCB) nurse‐coordinated transitional care intervention in older cardiac patients to understand and interpret the study results.
Design
A mixed‐methods process evaluation based on the Medical Research Council Process Evaluation framework.
Methods
Quantitative data on intervention key elements were collected from 153 logbooks of all intervention patients. Qualitative data were collected using semi‐structured interviews with 19 CCB professionals (cardiac nurses, community nurses and primary care physical therapists), from June 2017 until October 2018. Qualitative data‐analysis is based on thematic analysis and integrated with quantitative key element outcomes. The analysis was blinded to trial outcomes. Fidelity was defined as the level of intervention adherence.
Results
The overall intervention fidelity was 67%, ranging from severely low fidelity in the consultation of in‐hospital geriatric teams (17%) to maximum fidelity in the comprehensive geriatric assessment (100%). Main themes of influence in the intervention performance that emerged from the interviews are interdisciplinary collaboration, organizational preconditions, confidence in the programme, time management and patient characteristics. In addition to practical issues, the patient's frailty status and limited motivation were barriers to the intervention.
Conclusion
Although involved healthcare professionals expressed their confidence in the intervention, the fidelity rate was suboptimal. This could have influenced the non‐significant effect of the CCB intervention on the primary composite outcome of readmission and mortality 6 months after randomization. Feasibility of intervention key elements should be reconsidered in relation to experienced barriers and the population.
Impact
In addition to insight in effectiveness, insight in intervention fidelity and performance is necessary to understand the mechanism of impact. This study demonstrates that the suboptimal fidelity was subject to a complex interplay of organizational, professionals' and patients' issues. The results support intervention redesign and inform future development of transitional care interventions in older cardiac patients.
Keywords: cardiology, caregivers, frailty, nurses/midwives/nursing, process assessment, qualitative research, transitional care
1. INTRODUCTION
The 30‐day rehospitalization and mortality rates of older patients with acute myocardial infarction or heart failure are high: 20% and 8% respectively (Ko et al., 2020). The burden of hospitalization among older patients is considerable, and geriatric conditions are often overlooked while the focus mainly lies on the disease (Dodson et al., 2019). These factors increase the risk of adverse events such as readmissions (Bell & Saraf, 2016; Vitale et al., 2019). In the phase in which patients are discharged, the risk of adverse events increases again (Naylor et al., 2011), while medication regimes and treatment advices are often not well understood or mixed‐up with previous advices (Schoonover et al., 2014), and signs of physical deterioration are often detected too late (van Seben et al., 2019). Lastly, older cardiac patients are often not referred to traditional cardiac rehabilitation programmes because they are too intensive, or, when patients are referred, they often do not participate due to the intensity, travel issues and hindering comorbidities (Ruano‐Ravina et al., 2016). The cardiac rehabilitation uptake is only 20%–30% among older patients. However, the risks of recurring events and mortality of non‐participators are increased (Zullo et al., 2018).
To reduce the previously mentioned risks and to overcome the shortcomings in the continuity of care, we developed the Cardiac Care Bridge (CCB) nurse‐coordinated, interdisciplinary, transitional care programme, and evaluated it in a multicentre randomized trial in 306 frail, older (≥70 years) hospitalized cardiac patients in the Netherlands (Verweij et al., 2018) (Jepma et al., submitted). The intervention included case management, disease management and home‐based cardiac rehabilitation, integrated in the process from hospital to home. The transitional care model focuses on continuity of care when patients transfer between healthcare settings (Naylor et al., 2011, 2017), and is mostly based on a case management approach with a broad focus on patients' needs (Naylor et al., 2011). A follow‐up after 6 months did not show a statistically significant difference on the main composite outcome of readmission and mortality (Jepma et al., submitted).
2. Background
Complex care interventions with multiple interacting components such as the CCB intervention, are often studied in a traditional randomized trial design to explore its effectiveness. However, to interpret the results, it is important to investigate to what extent the intervention protocol is delivered as designed (treatment fidelity) and what factors may have influenced the intervention performance (Craig et al., 2008, 2013; McGee et al., 2018). Studies on treatment fidelity are often integrated in process evaluations alongside effectiveness studies of complex interventions, and explore causal assumptions, implementation success and flaws, contextual factors and the mechanisms of impact of the intervention (Furness et al., 2018; Moore et al., 2015). In brief: the why, who, what, where, how and how much should be integrated in the evaluation of complex interventions (Conn & Groves, 2011; Craig et al., 2013). The ‘why’ is addressed in the introduction section and the items who, what and where are described in the CCB intervention protocol and are summarized in Appendix S1 (Verweij et al., 2018). Exploration of how and how much of the intervention was performed, supports interpretation of the study results and informs future intervention (re)design and implementation. Therefore, it is necessary to evaluate the CCB study results by assessing the level of treatment fidelity and the healthcare professionals' perspective on the CCB intervention performance.
3. THE STUDY
3.1. Aim
The aim of the study is to analyse the CCB study results by assessing the level of treatment fidelity and the healthcare professionals' perspective on the CCB intervention performance.
3.2. Design
A mixed‐methods concurrent, primarily qualitative study was conducted alongside the CCB study. Data were collected and analysed before the CCB study results on effectiveness were known, to avoid a potential bias in the interpretation of the data (Moore et al., 2014). This process evaluation was based on the Medical Research Council Process Evaluation framework, which has operationalized implementation theories including RE‐AIM (Moore et al., 2015). The RE‐AIM implementation theory formed the theoretical basis of the CCB intervention implementation. (Glasgow et al., 1999, 2019). To induce change by the CCB intervention, we applied implementation strategies based on leading theories of change, such as motivational, educational and facilitating strategies (Waltz et al., 2019). Figure 1 provides the logic model of the CCB intervention that structured the process evaluation (Moore et al., 2015).
FIGURE 1.
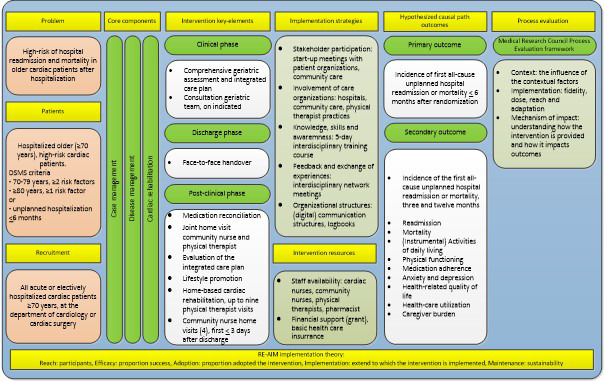
Logic model of the CCB intervention (Buurman et al., 2016; Dolansky et al., 2011; Doll et al., 2015; Feltner et al., 2014; Glasgow et al., 2019; Glasgow et al., 1999; Kwan et al., 2013; Le Berre et al., 2017; Minneboo et al., 2017; Moore et al., 2014; Naylor et al., 2011; Naylor et al., 2017; Oerkild et al., 2012; Verhaegh et al., 2014; Waltz et al., 2019; Zullo et al., 2018). Abbreviation: DSMS, Dutch Safety Management System [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. The CCB intervention and patients
Patients were eligible for inclusion in the CCB study if they were admitted to the department of cardiology or thoracic surgery, were at high risk of adverse events according to the Dutch Safety and Management System criteria (Heim et al., 2015) or experienced a hospital readmission in the 6 months prior to the index admission, and if the Mini Mental State Examination was scored ≥15 (see Figure 2).
FIGURE 2.
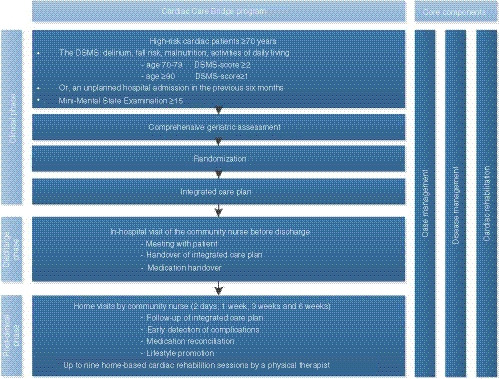
CCB transitional care programme (Jepma et al., submitted) [Colour figure can be viewed at wileyonlinelibrary.com]
Eligible patients all received a comprehensive geriatric assessment at baseline and were randomized into either the CCB intervention or usual care. The CCB intervention consisted of three core components, case management, disease management and cardiac rehabilitation, provided in three phases, the clinical, discharge and post‐clinical phase. The clinical phase included a geriatric assessment based integrated care plan and geriatric team consultation based on findings from the geriatric assessment. The discharge phase included an in‐hospital face‐to‐face handover with the community‐based registered nurse (community nurse). In the post‐clinical phase, four home visits from the community nurse were performed, focused on medication reconciliation, lifestyle promotion, evaluation of the care plan and early detections of physical deterioration. A CCB‐affiliated pharmacist assisted the community nurses with medication reconciliation. Physical therapists provided home‐based cardiac rehabilitation, with a total of nine visits. Figure 2 provides an overview of the intervention. Full study details are published elsewhere (Verweij et al., 2018). Appendix S2 provides a description of the standard care system in the Netherlands.
To implement the CCB intervention, a 5‐day interdisciplinary training programme on case management, disease management and home‐based cardiac rehabilitation was organized for all participating healthcare professionals. Managers of involved healthcare organizations were asked to provide education time for the participating staff. Additional intervention costs on top of the usual care costs were reimbursed by the study.
In total, 306 patients were recruited in six hospitals in the Netherlands from June 2017 until March 2019, of whom 153 were randomized into the intervention group. The included patients had a mean age of 82 years (standard deviation 6); 51% was male and 58% was admitted for heart failure. About their risk profile, 45% had an unplanned hospital readmission in the 6 months prior to the index hospitalization, 56% were at risk of delirium, 47% had fallen in the 6 months prior to the hospitalization, 39% had ADL limitations and 33% were at risk of malnutrition. There were no significant differences in baseline characteristics (Jepma et al., submitted).
3.4. Sample/participants (CCB healthcare professional)
This process evaluation focused on the experiences and performance of CCB healthcare professionals, including cardiac nurses, community nurses and primary care physical therapists. Other collaborating disciplines were not included in this process evaluation, because they performed usual care and did not adjust work processes. CCB healthcare professionals were purposefully sampled to reach maximal variation in work regions, work experience and experience with the CCB intervention (Moore et al., 2015; Moore et al., 2014; Polit & Beck, 2015). They were invited to participate if they treated at least one CCB patient. Invitations were sent by email and a telephone reminder was made after 2 weeks without response. All 19 invited healthcare professionals participated in the interviews.
3.5. Data collection on CCB care delivered
Data were collected on the three key functions of the Medical Research Council framework for Process Evaluation, defined as: (1) ‘context’ (the influence of the contextual factors on providing CCB care), (2) ‘implementation’ (fidelity, dose, reach and adaptation) and (3) ‘mechanism of impact’ (understanding how the CCB intervention is provided and how the intervention impacts outcomes). Fidelity has been defined as CCB care delivered as intended (Mars et al., 2013; Wilson et al., 2009). Intervention dose has been defined as the number of delivered intervention key elements per individual. The intervention reach has been defined as the number of patients who received the CCB intervention and adaptation has been defined as the manner in which CCB healthcare professionals performed the intervention in relation to the study protocol (Wilson et al., 2009).
Quantitative data to assess key function (2) ‘implementation’ (fidelity, dose and reach) were prospectively collected alongside the CCB study, according to predefined quality indicators on the intervention key elements see Table 1 (Appendix S3 CCB quality indicator example). Data sources were hospital chart files and self‐reported logbooks from home visits of the community nurses and physical therapists.
TABLE 1.
Fidelity, dose and reach in the CCB intervention key elements
| Intervention key elements | N | % |
|---|---|---|
| Clinical phase | ||
| CGA and CGA‐based integrated care plan | 153/153 | 100 |
| Geriatric consultation based on indication a | 11/66 | 17 |
| Discharge phase | ||
| Handover | ||
| Face‐to‐face | 49/134 | 37 |
| Telephone | 19/134 | 14 |
| Written | 66/134 | 49 |
| Post‐clinical phase | ||
| Community nurse home visits b | 82/133 | 62 |
| First home visit within 72 hr after discharge | 76/133 | 57 |
| Number of community nurse home visits | Median 3 | IQR 2–4 |
| Medication reconciliation including the Red Flag instrument (28) | 118/133 | 89 |
| Follow‐up of the integrated care plan | 71/132 | 54 |
| Lifestyle promotion | 91/132 | 69 |
| Joint home visit of the physical therapist and community nurse | 33/81 | 41 |
| Home‐based cardiac rehabilitation c | 70/116 | 60 |
| Number of home‐based rehabilitation sessions | Median 4 | IQR 2–6 |
| Mean patient‐specific fidelity percentage | 153 | 67 |
Abbreviations: CGA, comprehensive geriatric assessment; IQR, interquartile range.
Geriatric team consultation was indicated in case of ≥5 geriatric problems, of which ≥1 problem had to be within the psychological domain.
Four home visits according to the CCB protocol.
Max. nine home‐based rehabilitation session, according to the CCB protocol.
Qualitative data on key functions, (1) ‘context’, (2) ‘implementation’ (adaptation) and (3) ‘mechanism of impact’, were collected using semi‐structured interviews. Interviews were held in a private room at a location of the healthcare professional's preference and were conducted during the CCB study period between June 2017 and October 2018, by three researchers (Ms. LV (MSc.), Mr. MT (MSc.) and Ms. DS (MSc.)). The topic list was based on the key functions and the CCB logic model (Figure 1) (Appendix S4, Topic list) (Moore et al., 2015; Moore et al., 2014). During the interviews, notes were made, and at the end of the interviews, a verbal summary of the main topics was provided to the participants to verify the interpretation of the collected data (Braun & Clarke, 2014). The interviews lasted between 30 and 60 min each. The interviews were audio recorded and transcribed ad verbatim.
3.6. Ethical considerations
Ethical approval was provided by the Medical Ethics Committee of the Amsterdam UMC, University of Amsterdam (Protocol ID: MEC2016_024). Written informed consent was obtained from all interviewed CCB healthcare professionals.
3.7. Data analysis
Descriptive statistics were analysed for key function (2) ‘implementation’. The intervention fidelity was calculated per intervention patient. The denominator of the key elements was set on the number of feasible key elements for an individual. Intervention key elements missed due to, for example, hospital readmission, mortality, or disabilities that withheld patients from participation in, for instance, the home‐based cardiac rehabilitation, were not counted in the denominator. The mean fidelity rate was calculated per intervention key element. In addition, we calculated an overall unweighted average of the patient‐specific adherence percentage across all intervention patients. Outcomes were presented as number with a percentage, and as median with an interquartile range. Missing data from logbooks were interpreted as ‘care not delivered’. Analysis was performed in IBM SPSS Statistics version 23 (Armork, New York, USA).
Qualitative data analysis followed the phases of thematic analysis, a six‐phase guidance to systematically analyse qualitative data (Braun & Clarke, 2014). Two members of the research team (LV, DS) independently analysed the data. The first phase comprised of the open coding of the collected data. After every two interviews, codes were compared, and differences were discussed to reach consensus. Main themes were formed from matching codes by LV and DS, to reflect the data. Interviews were stopped when theoretical saturation was reached and no new codes and themes were formed (van Rijnsoever, 2017; Vasileiou et al., 2018). MAX‐QDA 12 Standard (Berlin, Germany) was used in the analysis.
After the collection of quantitative and qualitative data, the findings on the intervention performance were integrated with the information from the interviews. The quantitative data supported the interpretation of the qualitative data and vice versa. This manuscript was reported according to the COREQ‐checklist for the reporting of qualitative research (Tong et al., 2007).
4. RESULTS
4.1. Intervention fidelity, dose and reach
Data on performance about the key elements of the intervention were collected for all intervention patients. Table 1 provides an overview of the intervention fidelity, dose and reach of the intervention key elements in the clinical, discharge and post‐clinical phase.
In the clinical phase, the geriatric assessment and integrated care plan were performed with all patients. Referral to the geriatric team, based on the geriatric assessment indication, was reported in only a few patients (17%). In the discharge phase, a face‐to‐face handover was performed in 37%. Alternatively, handovers by telephone (14%) or in writing (49%) were performed. In the post‐clinical phase, 62% of the community nurses home visits were performed and in 57% in 3 days (interquartile range 2–4) after discharge. In 60% of the patients, home‐based cardiac rehabilitation sessions were delivered as intended. The number of eligible patients for cardiac rehabilitation (N = 116) was lower than the number of eligible patients for the community nurse home visits (N = 133), mainly due to patients' physical or mental inabilities. The mean individual patient fidelity rate across all key elements that patients were entitled to, was 67%.
4.2. Interviews with healthcare professionals
In total, 19 CCB healthcare professionals were interviewed, including 5 cardiac nurses, 6 community nurses and 7 physical therapists. Most of the participants were female (90%), and they had a median age of 37 years (interquartile range 27–54). Their median work experience was 20 years (interquartile range 6–30); see Table 2.
TABLE 2.
Characteristics of interviewed CCB healthcare professionals
| Respondent | Age | Gender | Profession | Education | Work experience, years | N CCB patients treated |
|---|---|---|---|---|---|---|
| R1 | 24 | Female | Cardiac nurse | Bachelor | 1 | 20 |
| R2 | 27 | Female | Cardiac nurse | Bachelor | 7 | 15 |
| R3 | 24 | Female | Cardiac nurse | Master | 4 | 10 |
| R4 | 54 | Female | Cardiac nurse | Bachelor | 34 | 30 |
| R5 | 37 | Female | Cardiac nurse | Vocational | 9 | 20 |
| R6 | 37 | Female | Community nurse | Vocational | 22 | 5 |
| R7 | 62 | Female | Community nurse | Vocational | 41 | 15 |
| R8 | 44 | Female | Community nurse | Bachelor | 20 | 4 |
| R9 | 45 | Female | Community nurse | Bachelor | 24 | 10 |
| R10 | 49 | Female | Community nurse | Bachelor | 20 | 15 |
| R11 | 52 | Female | Community nurse | Vocational | 20 | 10 |
| R12 | 23 | Female | Physical therapist | Master | 2 | 1 |
| R13 | 25 | Female | Physical therapist | Bachelor | 2 | 2 |
| R14 | 34 | Female | Physical therapist | Master | 10 | 1 |
| R15 | 58 | Female | Physical therapist | Master | 35 | 1 |
| R16 | 57 | Female | Physical therapist | Bachelor | 30 | 1 |
| R17 | 28 | Male | Physical therapist | Bachelor | 6 | 3 |
| R18 | 36 | Male | Physical therapist | Bachelor | 8 | 4 |
| R19 | 59 | Female | Physical therapist | Bachelor | 36 | 8 |
The themes derived from the interviews are framed and summarized in the key functions (1) ‘context’, (2) ‘implementation’ and (3) ‘mechanism of impact’, and integrated in the information on the intervention key elements. The main themes were (1) interdisciplinary collaboration, (2) organizational preconditions, (3) confidence in the CCB intervention, (4) time management and (5) influence of patient characteristics on the intervention.
4.2.1. Key function 1. Context
Contextual factors that could have affected the intervention performance were summarized in the themes ‘interdisciplinary collaboration’ and ‘organizational preconditions'.
Theme 1. Interdisciplinary collaboration
In the intervention period, the community nurse intensified the collaboration with nurse‐specialists, general practitioners, a CCB‐affiliated pharmacist and outpatient clinics. CCB healthcare professionals met each other during training sessions, meetings and face‐to‐face handovers. This reduced barriers to interprofessional communication in case of questions, observed physical deterioration or other symptoms (quote 1).
Quote 1 “… the fact that you know each other, makes it easier to contact…” (Respondent 6 community nurse)
The collaboration between physical therapists and community nurses was considered valuable to motivate patients when working on the same goals from different perspectives. Although the joint visits were performed only in 41% of the cases, which was mainly due to different work schedules, all interviewed healthcare professionals mentioned the value of the collaboration and integrated alternative communication routes such as contact by telephone (quote 2); see Table 1.
Quote 2 “I think we, the physical therapist and I, accomplished a lot. There was a woman, … She went for groceries with her walker the first day after discharge; and there she sat in the middle of the street. She simply overestimated her situation… Together with the physical therapist we enabled her to do the groceries again; then, you feel satisfied….” (Respondent 8 community nurse)
Theme 2. Organizational preconditions
Cardiac nurses experienced the geriatric assessment as an important precondition of the intervention, although time‐consuming. They mentioned time limitation and a lack of consistency in their work schedules as barriers to the performance. Furthermore, cardiac nurses did not always recognize the advantage of consulting a geriatric team about patient care, and thought they were able to address the observed geriatric problem themselves (quote 3).
Quote 3 “The protocol says to consult a geriatric team if indicated, but I think… it takes a lot of time, and what does the geriatric team actually additionally do?” (Respondent 1 cardiac nurse)
A high hospital turnover was mentioned as an additional reason for not consulting geriatric teams. These barriers resulted in the limited number of referrals (17%) of indicated patients to geriatric teams; see Table 1.
The CCB healthcare professionals mentioned the high in‐hospital turnover and the registration burden as general barriers to perform the intervention key elements. Cardiac nurses were, for example, responsible for the geriatric assessment as part of the intervention, as well as for the regular nursing assessment. In addition, healthcare professionals did not have enough time to plan the face‐to‐face handover (quote 4).
Quote 4 “As soon as they (patients) are a little recovered, they are discharged; we kind of throw them out. It sounds very worrisome, but … [silence] There is enormous pressure on the beds, because new patients are already queued at the front door….” (Respondent 4 cardiac nurse)
Physical therapists mentioned the high costs and limited reimbursement of the home‐based rehabilitation as a barrier. The CCB study reimbursed the rehabilitation costs if this was not covered by the patient's insurance policy. Nevertheless, the physical therapists had to invest more time to obtain the reimbursement and expressed their concerns about the feasibility.
4.2.2. Key function 2. Implementation
Relevant themes that could have affected the implementation of the programme were: ‘belief in the effectiveness of the programme’, ‘time management’ and ‘influence of patients characteristics’.
Theme 3. Confidence in the programme
Cardiac nurses considered the assessment of geriatric problems in‐hospital as a valuable intervention in this frail population to identify geriatric conditions and to develop the care plan. Nevertheless, they considered the time after discharge as the most important part of the CCB intervention. All community nurses believed they contributed to the prevention of adverse events, such as readmission due to the early recognition of signs of heart failure decompensation or other deteriorating conditions (quote 5).
Quote 5 “…people say that they know very well when they are decompensating (in heart failure), but when the early signs appear, most people don't respond adequately… People remain very passive and do not act, they do not realize that their situation is deteriorating again.” (Respondent 10 community nurse)
The physical therapists noticed improvement over time in the physical condition of treated patients. They mentioned the confidence of the patient in their ability to achieve results as an important factor of success, and they mentioned anxiety to exercise and to experience physical complaints as an important barrier to training success (quote 6).
Quote 6 “Yes, I think it is a good idea to guide patients after hospitalization… They can train with me until a level that they have enough energy and power. And so, they are not afraid to exercise anymore. Yes, anxiety is very important.” (Respondent 12 physical therapist)
Theme 4. Time management
The geriatric assessment and included physical tests were time‐consuming, and often went at the expense of activities such as the geriatric team consultation. Cardiac nurses also mentioned logistic barriers: for example, patients had to leave for diagnostic tests or relatives were visiting.
The community nurses highly valued collaboration with the cardiac nurses, and vice versa. Belief in the added value of the face‐to‐face handover was a common statement. The healthcare professionals experienced it as a valuable method to communicate about the patients' condition (quote 7).
Quote 7 “…you have the opportunity to ask questions, which make uncertainties about the treatment clear. So yes, so during the first home visit you can immediately start. Thereby, meeting the patient was also very important, so they already knew who was coming after discharge.” (Respondent 8 community nurse)
Nevertheless, the handover was only done face‐to‐face in 37% of the cases. Travel distances to the hospital of up to 30 min led to a low performance rate. These situations forced alternative work strategies, such as handover by telephone, which was performed 14% of the time, and written handovers, in 49% of the cases.
The median time period until the first home visit was 3 days (interquartile range 2–4); see Table 1. Some community nurses decided on alternatives, such as calling patients at the day of discharge, or the day after discharge in case they were not able to perform a home visit in 2 days.
The community nurses mentioned that with every patient they visited, something failed in the medication process. They were proactive and contacted the hospital, the general practitioner or the CCB pharmacist. The process of medication verification and problem solving was time‐consuming but highly valued by nurses, and performed with 89% (see Table 1). The community nurses also valued the collaboration with the CCB pharmacist because of the quick access and problem solving in case of medication problems.
4.2.3. Key function 3. Mechanism of impact
Patient characteristics such as the high level of frailty and comorbidities were mentioned as important contributors to the intervention's impact.
Theme 5. Influence of patient's characteristics
The physical therapists noticed that once patients had set a goal, they were motivated to exercise and practice. However, motivating patients was a struggle sometimes, according to the therapists. Some patients declined participation in home‐based cardiac rehabilitation (quote 9). In total, 60% of eligible patients participated in the home‐based rehabilitation session, with a median number of training sessions of 4 (interquartile range 2–6); see Table 1.
Quote 9 “There was a woman who didn't want me to come over. So, I contacted the community nurse and we had a joint visit… Then everything seemed to be good. Afterwards when I stood there in front of her door, she wouldn't let me in.” (Respondent 13 physical therapist)
Goal setting was mentioned as an important contributor to convince patients of the added value of physical therapy (quote 10). However, many patients found it difficult to formulate goals.
Quote 10 “…He (patient) thought it all took too much time. But when we finally found out that sportfishing was very important for him, we (community nurse, physical therapist) focused on that goal.” (Respondent 8 community nurse)
Physical therapists mentioned that the intensity of two training sessions per week was not feasible for every patient due to their condition, such as tiredness or poor health. The high level of frailty of the population was of large influence on the execution of the intervention. Physical therapists observed that patients often had comorbidities that limited them in their level of activity and therefore made patient‐tailored adjustments to the CCB protocol.
5. DISCUSSION
This process evaluation explored the delivered CCB intervention key elements and the considerations about the intervention fidelity from CCB healthcare professionals' perspectives. We found that the overall proportion of intervention fidelity was suboptimal and intervention key elements were often not performed as intended. CCB healthcare professionals mentioned various causes, such as time limitation, logistical barriers and patient characteristics. With the incorporation of alternative work processes such as alternative handovers and adjusted rehabilitation programmes, they adjusted the CCB intervention to the circumstances and individual case of the patients. The CCB healthcare professionals expressed their confidence in the intervention's contribution to patients' wellbeing and the ability to prevent hospital readmissions and mortality. However, they also expressed doubts on the feasibility of individual intervention components about, for example, the intensity of the home‐based rehabilitation programme in relation to the study population, the planning of joint home visits and interdisciplinary collaboration.
The CCB study showed a non‐significant effect on the primary composite outcome of readmission and mortality at 6 months follow‐up (Jepma et al., submitted). Although CCB healthcare professionals expressed their confidence and believe in the intervention, this was not reflected in the results on effectiveness. The current process evaluation unravelled at least a part of the black box about the non‐significant results. The suboptimal intervention fidelity could have influenced the lack of intervention effect. However, in a previous study on a transitional care intervention in heart failure patients with a fairly good intervention fidelity, no intervention effect was found either (Van Spall et al., 2019). In contrast, recent systematic reviews on the topic showed positive effects on readmission and mortality rates (Feltner et al., 2014; Van Spall et al., 2017). Besides intervention fidelity, the conflicting results could also be caused by an older and frail patient population in the CCB study.
About the performance on intervention key elements, the cardiac nurses expressed the additional value of the geriatric assessment, although they had to overcome logistical barriers and timing issues while the geriatric assessment was performed on top of the regular nursing assessment. It was remarkable that the cardiac nurses expressed low priority about the consultation of geriatric teams. Although education on the additional value of in‐hospital geriatric team consultation was part of the CCB training programme, a sceptical view on the actual contribution was mentioned, and cardiac nurses mentioned that they thought they were able to act on observed geriatric problems. Apparently, the current procedure in the CCB intervention, with protocolized geriatric team consultation, did not provide enough impulse for close collaboration (Verweij et al., 2018). An alternative approach in which geriatric teams work proactively on hospital wards, may overcome with these barriers. For example, in‐hospital geriatric co‐management with a proactive approach showed promising results (Van Grootven et al., 2017). This approach prevents that the collaboration is dependent on levels of priority among hospital staff in consulting geriatric teams, and the approach enables focusing on preventive instead of reactive strategies.
The community nurses mentioned early detection of physical deterioration and medication reconciliation as the most important study components. The risk of readmission is especially high in the first 30 days after discharge (Dharmarajan et al., 2013), and can potentially be reduced by high‐intensity transitional care interventions, including a home visit in 3 days after discharge (Verhaegh et al., 2014). Therefore, an early (≤3 days) community nurses' home visit was included in the CCB intervention. During the study period, community nurses were in close contact with the CCB‐affiliated pharmacist and experienced quick access, effective problem solving and efficient referral to other disciplines about medication problems. The contributing value of intensive medication guidance in the transition of care is reported in the study of Daliri et al. (Daliri et al., 2019). They found that better information transfer to primary care providers and the involvement of the community‐based pharmacist after discharge, led to significantly less medication‐related problems. Currently, community‐based pharmacists do not have a structural role in community care in the Netherlands. Since up to 49% of the older patients experience medication‐related problems after discharge, and community nurses are often involved in the post‐discharge phase, it is a promising collaboration to further explore (Garcia‐Caballos et al., 2010). Many medication‐related problems are caused by inadequate patient information (Cua & Kripalani, 2008; Eibergen et al., 2018) or a lack of a proper handover to primary caregivers (Kattel et al., 2016; Kripalani et al., 2007). The potential of these interventions is high in the prevention of 30‐day readmission rates (Daliri et al., 2020). However, in the CCB intervention, no additional effect was found.
Although the beneficial effects of cardiac rehabilitation in older patients have been documented, the participation rate is still very low (14% in Medicare beneficiaries), which is caused by factors such as comorbidities and functional limitations (Zullo et al., 2018). Therefore, a home‐based cardiac rehabilitation programme was integrated in the CCB programme (Verweij et al., 2018). In total, 60% of the CCB intervention patients participated in the cardiac rehabilitation programme. Physical therapists mentioned it was challenging to motivate patients to participate, but found that patients' personal goal setting was an important motivating factor. This was also reported by Tinetti et al. (2016) who emphasized the importance of ‘patient goal directed care’ to achieve results. However, patients' health status, tiredness and anxiety were mentioned as hindering. These factors could be part of a ‘post‐hospital syndrome’ that was possibly manifested in the frail older cardiac population in the CCB study (Mesquita et al., 2015). Especially older cardiac patients are at high risk of developing this complex mechanism (Mesquita et al., 2015), which, among others, is triggered by the underlying disease in combination with different kind of stressors during hospital stay (Krumholz, 2013). As a result, patients become deconditioned and cognitive functions may decrease. This potentially influenced the decreased motivation for the home‐based cardiac rehabilitation programme.
From a healthcare professional's perspective, the fairly low fidelity rate to the CCB key elements (total mean fidelity rate of 67%) could be explained by several factors, such as time limitations and other logistical barriers. However, they expressed their beliefs in the intervention and started implementing CCB intervention aspects in daily work routines. Several initiatives grew towards structural implementation, such as standard community nurse home visits of heart failure patients in collaboration with CCB participating hospitals. This eventually led to the early termination of the CCB study (Jepma et al., submitted). Another point of concern is the influence of the CCB population characteristics such as the high age, the high level of comorbid diseases and the level of frailty, on the intervention fidelity, which should not be underestimated (Jepma et al., submitted). The included population, those who were in an advanced stage of disease and beyond the point of no return, might have benefitted more from advance care planning and end‐of‐life transitional care interventions (Saunders et al., 2019; Wong et al., 2016). The feasibility of the intervention components needs to be reconsidered from this perspective as well.
6. LIMITATIONS
By using a mixed‐methods design, we were able to form an integrated conclusion on the intervention outcome (Moore et al., 2015; Polit & Beck, 2015). However, the quantitative data from the logbooks were subject to a limitation of the study. The data were reported by the CCB healthcare professionals, who could have failed registration or could have registered without actually having performed the key element (Polit & Beck, 2015). Missing data were interpreted as ‘care not delivered’, which potentially led to under‐registration of the key elements. This could affect the conclusion on the influence of the limited fidelity rates on the CCB main outcome of no effect. However, in the interviews, healthcare professionals mentioned various barriers in the performance of various key elements which makes the lower fidelity rates reliable. Furthermore, the data of the in‐hospital intervention performance was collected from the hospital chart file, which was a reliable source. We therefore believe that the reported key element reflects the reality of the CCB intervention fidelity. Another point of concern is related to the logistical barriers to perform face‐to‐face handovers and joint home visits, as expressed by the healthcare professionals. Although the involved staff was equipped with tablets and could have chosen to use modern communication routes, they rather called each other to discuss the case or waited for the written handover. Optimization of the use of modern communication routes could have overcome the fairly low fidelity rates in the communication between healthcare professionals.
Despite these limitations, the current findings enable adjustments to the CCB intervention, such as proactive geriatric team consultation, alternatives for the face‐to‐face handover and a patient‐tailored cardiac rehabilitation programme to overcome the barriers and adjust the intervention to the needs of the CCB patient population, or otherwise to reconsider the target population carefully.
7. CONCLUSION
CCB healthcare professionals expressed their confidence in the CCB intervention and its contribution to prevent hospital readmissions and mortality. However, the intervention fidelity was suboptimal and intervention key elements were often not performed as intended. The low fidelity rate could have influenced the non‐significant effect of the CCB intervention on the primary composite outcome of readmission and mortality 6 months after randomization. However, besides the intervention fidelity, the patient's frail health status and the motivation to participate in the intervention might have influenced the outcome. For future purposes, the feasibility of intervention key elements as well as the target population need to be reconsidered.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
AUTHOR CONTRIBUTIONS
All authors have agreed on the final version and meet at least one of the following criteria (recommended by the ICMJE): (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/jan.14786.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Verweij L, Spoon DF, Terbraak MS, et al. The Cardiac Care Bridge randomized trial in high‐risk older cardiac patients: A mixed‐methods process evaluation. J Adv Nurs. 2021;77:2498–2510. 10.1111/jan.14786
Funding information
This work was supported by the Netherlands Organization for Health Research and Development (ZonMw) as part of the ‘From knowledge to Action II program’ (grant number 520002002) and is partly financed by the Netherlands Organization for Scientific Research (NWO) grant number 023.008.024.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Bell, S. P. , & Saraf, A. A. (2016). Epidemiology of multimorbidity in older adults with cardiovascular disease. Clinics in Geriatric Medicine, 32(2), 215–226. 10.1016/j.cger.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, V. , & Clarke, V. (2014). What can “thematic analysis” offer health and wellbeing researchers? International Journal of Qualitative Studies on Health and Well‐being, 9, 26152. 10.3402/qhw.v9.26152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurman, B. M. , Parlevliet, J. L. , Allore, H. G. , Blok, W. , van Deelen, B. A. J. , Moll van Charante, E. P. , de Haan, R. J. , & de Rooij, S. E. (2016). Comprehensive geriatric assessment and transitional care in acutely hospitalized patients: The transitional care bridge randomized clinical trial. JAMA Internal Medicine, 176(3), 302–309. 10.1001/jamainternmed.2015.8042 [DOI] [PubMed] [Google Scholar]
- Conn, V. S. , & Groves, P. S. (2011). Protecting the power of interventions through proper reporting. Nursing Outlook, 59(6), 318–325. 10.1016/j.outlook.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, P. , Dieppe, P. , Macintyre, S. , Michie, S. , Nazareth, I. , & Petticrew, M. (2008). Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ, 337, a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., & Petticrew M. (2013). Developing and evaluating complex interventions: The new Medical Research Council guidance. International Journal of Nursing Studies, 50(5), 587–592. 10.1016/j.ijnurstu.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Cua, Y. M. , & Kripalani, S. (2008). Medication use in the transition from hospital to home. Annals of the Academy of Medicine, Singapore, 37(2), 136. [PMC free article] [PubMed] [Google Scholar]
- Daliri, S. , Boujarfi, S. , el Mokaddam, A. , Scholte op Reimer, W. J. M. , ter Riet, G. , den Haan, C. , Buurman, B. M. , & Karapinar‐Çarkit, F. (2020). Medication‐related interventions delivered both in hospital and following discharge: A systematic review and meta‐analysis. BMJ Quality & Safety, 30(2), 146–156. 10.1136/bmjqs-2020-010927 [DOI] [PubMed] [Google Scholar]
- Daliri, S. , Hugtenburg, J. G. , ter Riet, G. , van den Bemt, B. J. F. , Buurman, B. M. , Scholte op Reimer, W. J. M. , van Buul‐Gast, M.‐C. , & Karapinar‐Çarkit, F. (2019). The effect of a pharmacy‐led transitional care program on medication‐related problems post‐discharge: A before‐After prospective study. PLoS ONE, 14(3), e0213593. 10.1371/journal.pone.0213593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarajan, K. , Hsieh, A. F. , Lin, Z. , Bueno, H. , Ross, J. S. , Horwitz, L. I. , Barreto‐Filho, J. A. , Kim, N. , Bernheim, S. M. , Suter, L. G. , Drye, E. E. , & Krumholz, H. M. (2013). Diagnoses and timing of 30‐day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA, 309(4), 355–363. 10.1001/jama.2012.216476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson, J. A. , Hajduk, A. M. , Murphy, T. E. , Geda, M. , Krumholz, H. M. , Tsang, S. , Nanna, M. G. , Tinetti, M. E. , Goldstein, D. , Forman, D. E. , Alexander, K. P. , Gill, T. M. , & Chaudhry, S. I. (2019). Thirty‐day readmission risk model for older adults hospitalized with acute myocardial infarction. Circulation: Cardiovascular Quality and Outcomes, 12(5), e005320. 10.1161/circoutcomes.118.005320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolansky, M. A. , Zullo, M. D. , Boxer, R. S. , & Moore, S. M. (2011). Initial efficacy of a cardiac rehabilitation transition program: Cardiac TRUST. Journal of Gerontological Nursing, 37(12), 36–44. 10.3928/00989134-20111103-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll, J. A. , Hellkamp, A. , Thomas, L. , Ho, P. M. , Kontos, M. C. , Whooley, M. A. , Boyden, T. F. , Peterson, E. D. , & Wang, T. Y. (2015). Effectiveness of cardiac rehabilitation among older patients after acute myocardial infarction. American Heart Journal, 170(5), 855–864. 10.1016/j.ahj.2015.08.001 [DOI] [PubMed] [Google Scholar]
- Eibergen, L. , Janssen, M. J. A. , Blom, L. , & Karapinar‐Carkit, F. (2018). Informational needs and recall of in‐hospital medication changes of recently discharged patients. Research in Social and Administrative Pharmacy, 14(2), 146–152. 10.1016/j.sapharm.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Feltner, C. , Jones, C. D. , Cené, C. W. , Zheng, Z.‐J. , Sueta, C. A. , Coker‐Schwimmer, E. J. L. , Arvanitis, M. , Lohr, K. N. , Middleton, J. C. , & Jonas, D. E. (2014). Transitional care interventions to prevent readmissions for persons with heart failure: A systematic review and meta‐analysis. Annals of Internal Medicine, 160(11), 774–784. 10.7326/m14-0083 [DOI] [PubMed] [Google Scholar]
- Furness, C. , Howard, E. , Limb, E. , Cook, D. G. , Kerry, S. , Wahlich, C. , Victor, C. , Ekelund, U. , Iliffe, S. , Ussher, M. , Whincup, P. , Fox‐Rushby, J. , Ibison, J. , DeWilde, S. , & Harris, T. (2018). Relating process evaluation measures to complex intervention outcomes: Findings from the PACE‐UP primary care pedometer‐based walking trial. Trials, 19(1), 58. 10.1186/s13063-017-2428-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Caballos, M. , Ramos‐Diaz, F. , Jimenez‐Moleon, J. J. , & Bueno‐Cavanillas, A. (2010). Drug‐related problems in older people after hospital discharge and interventions to reduce them. Age and Ageing, 39(4), 430–438. 10.1093/ageing/afq045 [DOI] [PubMed] [Google Scholar]
- Glasgow, R. E. , Harden, S. M. , Gaglio, B. , Rabin, B. , Smith, M. L. , Porter, G. C. , Ory, M. G. , & Estabrooks, P. A. (2019). RE‐AIM planning and evaluation framework: Adapting to new science and practice with a 20‐year review. Front Public Health, 7, 64. 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow, R. E. , Vogt, T. M. , & Boles, S. M. (1999). Evaluating the public health impact of health promotion interventions: The RE‐AIM framework. American Journal of Public Health, 89(9), 1322–1327. 10.2105/ajph.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, N. , van Fenema, E. M. , Weverling‐Rijnsburger, A. W. E. , Tuijl, J. P. , Jue, P. , Oleksik, A. M. , Verschuur, M. J. , Haverkamp, J. S. , Blauw, G. J. , van der Mast, R. C. , & Westendorp, R. G. J. (2015). Optimal screening for increased risk for adverse outcomes in hospitalised older adults. Age and Ageing, 44(2), 239–244. 10.1093/ageing/afu187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattel, S. , Manning, D. M. , Erwin, P. J. , Wood, H. , Kashiwagi, D. T. , & Murad, M. H. (2016). Information transfer at hospital discharge: A systematic review. Journal of Patient Safety, 16(1), e25–e33. 10.1097/pts.0000000000000248 [DOI] [PubMed] [Google Scholar]
- Ko, D. T. , Khera, R. , Lau, G. , Qiu, F. , Wang, Y. , Austin, P. C. , & Krumholz, H. M. (2020). Readmission and mortality after hospitalization for myocardial infarction and heart failure. Journal of the American College of Cardiology, 75(7), 736–746. 10.1016/j.jacc.2019.12.026 [DOI] [PubMed] [Google Scholar]
- Kripalani, S. , LeFevre, F. , Phillips, C. O. , Williams, M. V. , Basaviah, P. , & Baker, D. W. (2007). Deficits in communication and information transfer between hospital‐based and primary care physicians: Implications for patient safety and continuity of care. JAMA, 297(8), 831–841. 10.1001/jama.297.8.831 [DOI] [PubMed] [Google Scholar]
- Krumholz, H. M. (2013). Post‐hospital syndrome – An acquired, transient condition of generalized risk. New England Journal of Medicine, 368(2), 100–102. 10.1056/NEJMp1212324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, J. L. , Lo, L. , Sampson, M. , & Shojania, K. G. (2013). Medication reconciliation during transitions of care as a patient safety strategy: A systematic review. Annals of Internal Medicine, 158(5 Pt 2), 397–403. 10.7326/0003-4819-158-5-201303051-00006 [DOI] [PubMed] [Google Scholar]
- Le Berre, M. , Maimon, G. , Sourial, N. , Gueriton, M. , & Vedel, I. (2017). Impact of transitional care services for chronically ill older patients: A systematic evidence review. Journal of the American Geriatrics Society, 65(7), 1597–1608. 10.1111/jgs.14828 [DOI] [PubMed] [Google Scholar]
- Mars, T. , Ellard, D. , Carnes, D. , Homer, K. , Underwood, M. , & Taylor, S. J. (2013). Fidelity in complex behaviour change interventions: A standardised approach to evaluate intervention integrity. British Medical Journal Open, 3(11), e003555. 10.1136/bmjopen-2013-003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, D. , Lorencatto, F. , Matvienko‐Sikar, K. , & Toomey, E. (2018). Surveying knowledge, practice and attitudes towards intervention fidelity within trials of complex healthcare interventions. Trials, 19(1), 504. 10.1186/s13063-018-2838-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, E. T. , Cruz, L. N. , Mariano, B. M. , & Jorge, A. J. (2015). Post‐hospital syndrome: A new challenge in cardiovascular practice. Arquivos Brasileiros De Cardiologia, 105(5), 540–544. 10.5935/abc.20150141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneboo, M. , Lachman, S. , Snaterse, M. , Jørstad, H. T. , ter Riet, G. , Boekholdt, S. M. , Scholte op Reimer, W. J. M. , Peters, R. J. G. , Riezebos, R. K. , van Liebergen, R. , van der Spank, A. , van Dantzig, J. M. , de Milliano, P. , van Hessen, M. , Kragten, J. A. , Jaarsma, W. , den Hartog, F. R. , Bartels, G. L. , Aengevaeren, W. , … de Vries, C. J. (2017). Community‐based lifestyle intervention in patients with coronary artery disease: The RESPONSE‐2 trial. Journal of the American College of Cardiology, 70(3), 318–327. 10.1016/j.jacc.2017.05.041 [DOI] [PubMed] [Google Scholar]
- Moore, G. F. , Audrey, S. , Barker, M. , Bond, L. , Bonell, C. , Hardeman, W. , Moore, L. , O'Cathain, A. , Tinati, T. , Wight, D. , & Baird, J. (2015). Process evaluation of complex interventions: Medical Research Council guidance. BMJ, 350, h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, G. F. , Suzanne, A. , Barker, M. , Bond, L. , Bonell, C. , & Hardeman, W. (2014). Process evaluation of complex interventions, UK Medical Research Council (MRC) guidance. Retrieved from http://decipher.uk.net/wp‐content/uploads/2014/11/MRC‐PHSRN‐Process‐evaluation‐guidance.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor, M. D. , Aiken, L. H. , Kurtzman, E. T. , Olds, D. M. , & Hirschman, K. B. (2011). The care span: The importance of transitional care in achieving health reform. Health Affairs, 30(4), 746–754. 10.1377/hlthaff.2011.0041 [DOI] [PubMed] [Google Scholar]
- Naylor, M. D. , Shaid, E. C. , Carpenter, D. , Gass, B. , Levine, C. , Li, J. , Malley, A. , McCauley, K. , Nguyen, H. Q. , Watson, H. , Brock, J. , Mittman, B. , Jack, B. , Mitchell, S. , Callicoatte, B. , Schall, J. , & Williams, M. V. (2017). Components of comprehensive and effective transitional care. Journal of the American Geriatrics Society, 65(6), 1119–1125. 10.1111/jgs.14782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerkild, B. , Frederiksen, M. , Hansen, J. F. , & Prescott, E. (2012). Home‐based cardiac rehabilitation is an attractive alternative to no cardiac rehabilitation elderly patients with coronary heart disease: Results from a randomised clinical trial. British Medical Journal Open, 2(6), e001820. 10.1136/bmjopen-2012-001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polit, D. F. , & Beck, C. T. (2015). Nursing research, generating and assessing evidence for nursing practice (10th ed.). Wolters Kluwer. [Google Scholar]
- Ruano‐Ravina, A. , Pena‐Gil, C. , Abu‐Assi, E. , Raposeiras, S. , van 't Hof, A. , Meindersma, E. , Bossano Prescott, E. I. , & González‐Juanatey, J. R. (2016). Participation and adherence to cardiac rehabilitation programs. A systematic review. International Journal of Cardiology, 223, 436–443. 10.1016/j.ijcard.2016.08.120 [DOI] [PubMed] [Google Scholar]
- Saunders, S. , Killackey, T. , Kurahashi, A. , Walsh, C. , Wentlandt, K. , Lovrics, E. , Scott, M. , Mahtani, R. , Bernstein, M. , Howard, M. , Tanuseputro, P. , Goldman, R. , Zimmermann, C. , Aslakson, R. A. , Isenberg, S. R. , Aslakson, R. , Ast, K. , Carroll, T. , Dzeng, E. , … Wong, S. (2019). Palliative care transitions from acute care to community‐based care – A systematic review. Journal of Pain and Symptom Management, 58(4), 721–734.e721. 10.1016/j.jpainsymman.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Schoonover, H. , Corbett, C. F. , Weeks, D. L. , Willson, M. N. , & Setter, S. M. (2014). Predicting potential postdischarge adverse drug events and 30‐day unplanned hospital readmissions from medication regimen complexity. Journal of Patient Safety, 10(4), 186–191. 10.1097/pts.0000000000000067 [DOI] [PubMed] [Google Scholar]
- Tinetti, M. E. , Naik, A. D. , & Dodson, J. A. (2016). Moving from disease‐centered to patient goals‐directed care for patients with multiple chronic conditions: Patient value‐based care. JAMA Cardiology, 1(1), 9–10. 10.1001/jamacardio.2015.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, A. , Sainsbury, P. , & Craig, J. (2007). Consolidated criteria for reporting qualitative research (COREQ): A 32‐item checklist for interviews and focus groups. International Journal for Quality in Health Care, 19(6), 349–357. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- Van Grootven, B. , Flamaing, J. , Dierckx de Casterlé, B. , Dubois, C. , Fagard, K. , Herregods, M.‐C. , Hornikx, M. , Laenen, A. , Meuris, B. , Rex, S. , Tournoy, J. , Milisen, K. , & Deschodt, M. (2017). Effectiveness of in‐hospital geriatric co‐management: A systematic review and meta‐analysis. Age and Ageing, 46(6), 903–910. 10.1093/ageing/afx051 [DOI] [PubMed] [Google Scholar]
- van Rijnsoever, F. J. (2017). (I Can't Get No) Saturation: A simulation and guidelines for sample sizes in qualitative research. PLoS ONE, 12(7), e0181689. 10.1371/journal.pone.0181689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seben, R. , Reichardt, L. A. , Aarden, J. J. , van der Schaaf, M. , van der Esch, M. , Engelbert, R. H. H. , Twisk, J. W. R. , Bosch, J. A. , Buurman, B. M. , Kuper, I. , de Jonghe, A. , Leguit‐Elberse, M. , Kamper, A. D. , Posthuma, N. , Brendel, N. , & Wold, J. (2019). The course of geriatric syndromes in acutely hospitalized older adults: The hospital‐ADL study. Journal of the American Medical Directors Association, 20(2), 152–158.e152. 10.1016/j.jamda.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Van Spall, H. G. C. , Lee, S. F. , Xie, F. , Oz, U. E. , Perez, R. , Mitoff, P. R. , Maingi, M. , Tjandrawidjaja, M. C. , Heffernan, M. , Zia, M. I. , Porepa, L. , Panju, M. , Thabane, L. , Graham, I. D. , Haynes, R. B. , Haughton, D. , Simek, K. D. , Ko, D. T. , & Connolly, S. J. (2019). Effect of patient‐centered transitional care services on clinical outcomes in patients hospitalized for heart failure: The PACT‐HF randomized clinical trial. JAMA, 321(8), 753–761. 10.1001/jama.2019.0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Spall, H. G. C. , Rahman, T. , Mytton, O. , Ramasundarahettige, C. , Ibrahim, Q. , Kabali, C. , Coppens, M. , Brian Haynes, R. , & Connolly, S. (2017). Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: A systematic review and network meta‐analysis. European Journal of Heart Failure, 19(11), 1427–1443. 10.1002/ejhf.765 [DOI] [PubMed] [Google Scholar]
- Vasileiou, K. , Barnett, J. , Thorpe, S. , & Young, T. (2018). Characterising and justifying sample size sufficiency in interview‐based studies: Asystematic analysis of qualitative health research over a 15‐year period. BMC Medical Research Methodology, 18(1), 148. 10.1186/s12874-018-0594-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaegh, K. J. , MacNeil‐Vroomen, J. L. , Eslami, S. , Geerlings, S. E. , de Rooij, S. E. , & Buurman, B. M. (2014). Transitional care interventions prevent hospital readmissions for adults with chronic illnesses. Health Affairs, 33(9), 1531–1539. 10.1377/hlthaff.2014.0160 [DOI] [PubMed] [Google Scholar]
- Verweij, L. , Jepma, P. , Buurman, B. M. , Latour, C. H. M. , Engelbert, R. H. H. , ter Riet, G. , Karapinar‐Çarkit, F. , Daliri, S. , Peters, R. J. G. , & Scholte op Reimer, W. J. M. (2018). The cardiac care bridge program: Design of a randomized trial of nurse‐coordinated transitional care in older hospitalized cardiac patients at high risk of readmission and mortality. BMC Health Services Research, 18(1), 508. 10.1186/s12913-018-3301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, C. , Jankowska, E. , Hill, L. , Piepoli, M. , Doehner, W. , Anker, S. D. , & Coats, A. J. (2019). Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. European Journal of Heart Failure, 21(11), 1299–1305. 10.1002/ejhf.1611 [DOI] [PubMed] [Google Scholar]
- Waltz, T. J. , Powell, B. J. , Fernández, M. E. , Abadie, B. , & Damschroder, L. J. (2019). Choosing implementation strategies to address contextual barriers: Diversity in recommendations and future directions. Implementation Science, 14(1), 42. 10.1186/s13012-019-0892-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, D. K. , Griffin, S. , Saunders, R. P. , Kitzman‐Ulrich, H. , Meyers, D. C. , & Mansard, L. (2009). Using process evaluation for program improvement in dose, fidelity and reach: The ACT trial experience. International Journal of Behavioral Nutrition and Physical Activity, 6, 79. 10.1186/1479-5868-6-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, F. K. , Ng, A. Y. , Lee, P. H. , Lam, P. T. , Ng, J. S. , Ng, N. H. , & Sham, M. M. (2016). Effects of a transitional palliative care model on patients with end‐stage heart failure: A randomised controlled trial. Heart, 102(14), 1100–1108. 10.1136/heartjnl-2015-308638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo, M. D. , Dolansky, M. A. , Josephson, R. A. , & Cheruvu, V. K. (2018). Older adult attendance in cardiac rehabilitation: Impact of functional status and postacute care after acute myocardial infarction in 63092 Medicare beneficiaries. Journal of Cardiopulmonary Rehabilitation and Prevention, 38(1), 17–23. 10.1097/hcr.0000000000000264 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


