Abstract
We evaluated the role of early response after 3 weeks of neoadjuvant treatment (NAT) assessed by ultrasound (US), magnetic resonance imaging (MRI) and Ki‐67 dynamics for prediction of pathologic complete response (pCR) in different early breast cancer subtypes. Patients with HR+/HER2+, HR−/HER2− and HR−/HER2+ tumors enrolled into three neoadjuvant WSG ADAPT subtrials underwent US, MRI and Ki‐67 assessment at diagnosis and after 3 weeks of NAT. Early response was defined as complete or partial response (US, MRI) and ≥30% proliferation decrease or <500 invasive tumor cells (Ki‐67). Predictive values and area under the receiver operating characteristic (AUC) curves for prediction of pCR (ypT0/is ypN0) after 12‐week NAT were calculated. Two hundred twenty‐six had MRI and 401 US; 107 underwent both MRI and US. All three methods yielded a similar AUC in HR+/HER2+ (0.66‐0.67) and HR−/HER2− tumors (0.53‐0.63), while MRI and Ki‐67 performed better than US in HR−/HER2+ tumors (0.83 and 0.79 vs 0.56). Adding MRI+/‐Ki‐67 increased AUC of US in HR−/HER2+ tumors to 0.64 to 0.75. MRI and Ki‐67 demonstrated highest sensitivity in HR−/HER2− (0.8‐1) and HR−/HER2+ tumors (1, both). Negative predictive value was similar for all methods in HR+/HER2+ (0.71‐0.74) and HR−/HER2− tumors (0.85‐1), while it was higher for MRI and Ki‐67 compared to US in HR−/HER2+ subtype (1 vs 0.5). Early response assessed by US, MRI and Ki‐67 is a strong predictor for pCR after 12‐week NAT. Strength of pCR prediction varies according to tumor subtype. Adding MRI+/‐Ki‐67 to US did not improve pCR prediction in majority of our patients.
Keywords: breast cancer, magnetic resonance imaging, neoadjuvant therapy, pathologic complete response, ultrasound
Short abstract
What's new?
In breast cancer, a pathologic complete response (pCR) after neoadjuvant therapy (NAT) can predict long‐term outcome. However, rates of pCR differ according to EBC subtype. So what is the most useful approach to determine early response? In this prospective study, the authors compared MRI, ultrasound, and Ki‐67 status after three weeks of NAT. They found that MRI and Ki‐67 had higher sensitivity than ultrasound in HR‐/HER2+ and HR‐/HER2‐ tumors, while all three methods were similar for HR+/HER2+ tumors. These findings could guide identification of candidates for therapy de‐escalation or escalation.
Abbreviations
- AUC
area under curve
- BL
baseline
- CR
complete response
- ECR
early clinical response
- HER2
human epidermal growth factor receptor 2
- HR
hormone receptor
- MRI
magnetic resonance imaging
- NAT
neoadjuvant therapy
- NPV
negative predictive value
- pCR
pathological complete response
- PD
progressive disease
- PPV
positive predictive value
- PR
partial response
- SD
stable disease
- SENS
sensitivity
- SPEC
specificity
- T‐DM1
trastuzumab emtansine
- US
ultrasound
1. INTRODUCTION
Neoadjuvant therapy (NAT) is a widely used option for patients with early breast cancer (EBC) if an indication for chemotherapy is given. 1 NAT enables individualization of post‐NAT therapy according to response at surgery. Moreover, it is used to downgrade the tumor size thereby increasing the rate of breast conserving surgery. Clinical and pathologic complete response (pCR) predicts long‐term outcome; however, pCR rates differ according to EBC subtype. Highest pCR rates are observed in human epidermal growth factor receptor 2‐positive (HER2+) and hormone receptor‐negative (HR−)/HER2− tumors. 2 , 3 pCR in these subtypes is a surrogate marker for disease‐free survival and overall survival. 4 , 5 , 6 , 7
NAT is standard of care in HER2+ or triple‐negative (HR−/HER2−) EBC. 1 The ongoing development and introduction of more effective treatments, especially anti‐HER2 treatments, have resulted in a continuous increase of pCR rates in these three subtypes. 8 , 9 Modern individualized therapy strategies try to use information from in vivo testing of tumor sensitivity (SENS) already during NAT for response‐guided therapy management. Imaging has been used for detection of response and resistance and for the extent of possible residual disease to guide subsequent surgery. 10 , 11 It is well established that assessment of response, as well as assessment of residual tumor by conventional imaging (mammography or ultrasound [US]) is less accurate than assessment by dynamic contrast material‐enhanced magnetic resonance imaging (MRI). 12 , 13 , 14
A growing mounting body of indicates that it is worthwhile to identify early responders and nonresponders thus allowing early modulation of the individual therapeutic strategy. 15 , 16 , 17 Previous studies already reported that MRI findings after or during NAT could predict pCR. 18 , 19 , 20 , 21 , 22 For instance, the I‐Spy trial investigators showed the greatest relative benefit for a sequential MRI examination before the second cycle of NAT. 19
The ongoing Adjuvant Dynamic Marker‐Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early Breast Cancer (ADAPT, NCT01779206) trial, performed by the West German Study Group (WSG), aims to individualize therapy to avoid over‐ and undertreatment of patients with different EBC subtypes. 23 Assessment of early response after only 3 weeks of treatment measured by sequential Ki‐67 in luminal tumors and measured by sequential Ki‐67, US and MRI in HER2+ and HR−/HER2− tumors is one of the primary objectives of the trial. Three subtype‐specific ADAPT subtrials are neoadjuvant trials: ADAPT Triple Negative, ADAPT HR+/HER2+ and ADAPT HR−/HER2+.
In our study, we prospectively analyzed the value of MRI and US in assessing early response after one cycle of NAT for prediction of pCR in HR+/HER2+, HR−/HER2− and HR−/HER2+ EBC. The main objective was to characterize the predictive impact of early clinical response (ECR) measured by MRI (mRECIST) and/or US (RECIST 1.1) for achieving pCR in the different EBC subtypes. Additionally, ECR according to imaging was compared to tumor tissue changes in core biopsies after 3 weeks of NAT. This is the first study to our knowledge that provides prospective data for early response assessed by MRI and US in different breast cancer subtypes in the neoadjuvant setting.
2. SUBJECTS AND METHODS
2.1. Study design
ADAPT is a prospective, multicenter, controlled, nonblinded, randomized, investigator‐initiated umbrella trial. The results of the neoadjuvant substudies have been published elsewhere (Supplementary Figure 1). 23 , 24 , 25 , 26 Briefly, ADAPT triple positive (HR+/HER2+, NCT01817452) compares a trastuzumab‐chemotherapy conjugate (trastuzumab emtansine [T‐DM1])+/‐endocrine therapy vs a chemotherapy free arm with trastuzumab+ET. In ADAPT HR‐/HER2+ (NCT01779206), dual blockade+/‐chemotherapy (paclitaxel weekly) was tested. The trial was closed prematurely due to a clinically relevant superiority of the chemotherapy arm. In ADAPT HR−/HER2− (NCT01815242), treatment consisted of neoadjuvant nab‐paclitaxel+gemcitabine or nab‐paclitaxel+carboplatin. pCR was assessed in all subtrials after 12 weeks of NAT and was defined as no histopathological evidence of residual invasive tumor cells, both in breast and axillary lymph nodes (ypT0/is, ypN0). After NAT, surgery was performed within 3 weeks; if investigators opted for further NAT, prior histologic confirmation of non‐pCR by core needle biopsy was obligatory. Recommended poststudy therapy (neoadjuvant and/or adjuvant) followed national guidelines. Patients underwent mandatory US and optional MRI prior to therapy (baseline [BL]), after 3 weeks of NAT, and at the end of treatment. In addition, US was performed every 4 weeks during NAT to monitor tumor response. For the purpose of this analysis, only patients with MRI and/or US performed at BL and after 3 weeks of NAT were analyzed. Core biopsies were performed at BL (time of diagnosis) and on treatment (3‐week biopsy, Supplementary Figure 1). Protocols of the ADAPT studies were approved by national authorities, local ethics committees and/or institutional review boards.
2.2. Patient eligibility and enrollment
Eligible patients were women ≥18 years with histologically confirmed unilateral, primary invasive breast carcinoma and centrally confirmed HR (estrogen receptor‐ and/or progesterone receptor‐positive ≥1% of tumor nuclei or HR‐negative <1% of tumor nuclei) and HER2 status. Eastern Cooperative Oncology Group Performance Status ≤1 or Karnofsky Performance Status ≥80%, adequate hematologic parameters (ADAPT TN) and normal organ function were required for inclusion. Exclusion criteria have been published previously. 23 , 24 , 25 , 26 Written informed consent was obtained from all patients prior to enrollment in the study.
2.3. Histologic assessment of response
Patients underwent core biopsy at the time of diagnosis and on‐treatment (at Week 3). Ki‐67 was assessed by central pathology using rabbit monoclonal anti‐Ki‐67 antibody 30‐9 (Ventana Medical Systems, Tucson, AZ) on at least 500 invasive tumor cells. Early Ki‐67 response was evaluated as a composite endpoint including a ≥30% proliferation decrease in Ki‐67 compared to the BL or low cellularity (<500 invasive tumor cells) at Week 3. Results for the three substudies (ADAPT HER2+/HR−, ADAPT HER2+/HR+ and ADAPT TN) have been described elsewhere. 24 , 25 , 26
2.4. MRI technique
MRI examinations were performed at 43 sites thus representing a cross section of the radiology landscape in Germany. MRI was performed at BL according to a standardized image protocol using the 1.5 T or 3.0 T MR systems with a dedicated breast multichannel surface coil.
To ensure comparable quality of images obtained at each site, the imaging protocol was provided by the central MRI reading site (Department of Diagnostic and Interventional Radiology, University Hospital, RWTH Aachen, Germany) and consisted of well‐established sequences without the need for any additional special hardware or software. Only sites producing images of required quality (as assessed by the central reading site) were eligible to perform breast MRI within the study (5 centers were not eligible and 12 centers decided not to participate).
The standardized imaging protocol at 1.5 T consisted of an axial bilateral two‐dimensional multisection gradient‐echo dynamic series (repetition time ms/echo time ms, 250/4.6; flip angle, 90°) with a full 512 × 512 acquisition matrix and a section thickness of 3 mm. The dynamic sequence war performed prior to and four times after bolus injection of macrocyclic gadolinium agent, gadobutrol (Gadovist/Gadavist, Bayer AG, Leverkusen, Germany, 0.1 mmol/kg body weight) followed by a saline flush. In addition, an axial T2‐weighted fast spin‐echo sequence without fat suppression and with identical anatomic parameters was performed. The sequences at 3.0 T MR systems were analogous.
2.5. MRI image interpretation
All MRI images were read and interpreted at the central reading site by two experienced specialized breast radiologists (Simone Schrading and Christiane K. Kuhl, respectively). Readers were not aware of study arm, clinical information and histological details. MRI images were read according to a standardized reading protocol based on the fifth edition of BI‐RADS. 27 Each lesion in each MRI was described by size, morphological and enhancement criteria. To evaluate lesion changes during NAT, careful correlation of every lesion between the MRI BL and subsequent findings was performed.
Tumor size was measured in the longest diameter on the unsubstracted images and the same measurement direction was used for all subsequent MRI examinations. In addition, breast cancer volume of the enhancing tumor part (enhancing tumor volume) as well as whole tumor volume (enhancing and nonenhancing part) was determined by lesion segmentation (Intellispace, Philips, Best, the Netherlands). Morphological criteria contained description of lesion type (mass, nonmass, focus), shape, margin, growth direction, internal architecture and signal intensity in T2‐weigted and T1‐weighted precontrast images (according to BI‐RADS, fifth edition). 27 Contrast enhancement rates of enhancing lesions were assessed visually in consensus by the two radiologists, in line with clinical practice. Areas with the strongest and earliest enhancement were selected and evaluated in the first postcontrast sequence (early enhancement) and last postsequence (late enhancement). Early enhancement was interpreted as no enhancement, slow, moderate or strong enhancement. Late phase enhancement was considered equivalent to the curve type with steady, plateau and wash‐out curve. For multifocal or multicentric breast cancer, only the strongest‐enhancing breast lesion was measured and used for analysis in order to avoid data clustering. In case the tumor was no longer visible after therapy, the site of the lesion was carefully identified using anatomic landmarks in nonsubtracted T1‐ and T2‐weighted images.
2.6. Assessment of response by MRI
All imaging criteria of the breast tumor were intraindividually compared in all examinations for a given patient. MRI was used to assess response after one cycle of NAT in relation to the MRI findings at BL. Early response was defined according to mRECIST criteria as previously published. 19 , 28 Complete response (CR) by MRI was said to be present if, in the dynamic series, the cancer no longer showed enhancement until the late dynamic phase (no enhancement beyond that of the fibroglandular tissue). If enhancement rates, kinetics and tumor size were unchanged compared to BL examination, this was called stable disease (SD). Progressive disease (PD) was defined as an increase in tumor size, that is, a ≥20% increase of the longest diameters of the cancer. Any residual enhancement that did not meet the criteria for CR, SD or PD was considered partial response (PR). Patients showing CR or PR by MRI after one cycle of NAT were classified as having an ECR and all other patients as nonresponders (no ECR).
2.7. US imaging protocol
Patients had breast US at BL and after one cycle of NAT. US was performed at each study site by specialized breast gynecologists. The entire breast was systematically examined using a radial or linear approach with high resolution at least 7.5‐MHz probes. If possible, the tumor was measured in one to three diameters, and measurements were registered in study case report forms. The lesions were described and tumor size measured. To evaluate lesion changes after one cycle of NAT, a correlation with the BL lesion was performed. The tumor was marked with a clip before the first cycle of NAT to reliably identify the tumor region at the subsequent examinations.
2.8. Assessment of response by US
Patients showing CR or PR according to RECIST 1.1 after one cycle of NAT were classified as having ECR by US, patients with SD and PD as having no ECR. 29 , 30 CR was defined as disappearance of all target lesions and reduction in short axis to <10 mm of any pathological lymph nodes (target or nontarget) and PR was defined as a ≥30% decrease of the longest diameter of target lesion compared to the longest diameter at BL. PD was defined as a ≥20% increase of at least 5 mm of the longest diameter of the target lesions compared to the longest recorded diameter at BL. Patients with small changes in the sum of diameters not qualifying for PR or PD since BL were classified as SD.
2.9. Statistical methods
Differences between MRI and US in prediction of ECR were analyzed by comparing positive predictive value (PPV, defined as probability that pCR was actually achieved after ECR on imaging), negative predictive value (NPV, defined as probability that non‐pCR was documented when no ECR was observed), SENS, and specificity (SPEC). The 95% confidence intervals for binomial proportions were used since all considered events were coded as binary variables.
Multiple logistic regression model was derived by (forward) stepwise selection to perform a multiple binomial logistic regression. Presence of pCR was the dependent variable. Parameters included (independent variables) were age (grouped as: <40, 40‐49, 50‐59, ≥60), clinical tumor stage (cT1, cT2, cT3, cT4), clinical nodal status (cN0, cN1, cN2‐3), tumor grade (central grade: 1, 2, 3), menopausal status (postmenopausal, premenopausal), Ki‐67 (grouped by quartiles), treatment (arm) as well as HR− and HER status. Both the entry and the stay level were set to 0.1. The goodness of fit was evaluated with Hosmer‐Lemeshow test. In addition, a standard binomial logistic regression was performed for each of the mentioned parameters with presence of pCR as the dependent variable.
All statistical data analyses were performed with SAS software (version 9.4, SAS Institute).
3. RESULTS
3.1. Patient characteristics
From October 2012 until December 2015, 845 patients at 58 centers in Germany were randomized in the ADAPT subtrial matching their EBC subtype. Three hundred seventy‐five patients were randomized into the ADAPT HR+/HER2+ study, 336 into the ADAPT HR−/HER2− study and 134 into the ADAPT HR−/HER2+ study (Figure 1). Five hundred twenty patients underwent only MRI, only US or both at BL and after one cycle of NAT. MRI was performed in 226 (MRI group) and US in 401 patients (US group) of whom 107 had both MRI and US (MRI and US group).
FIGURE 1.
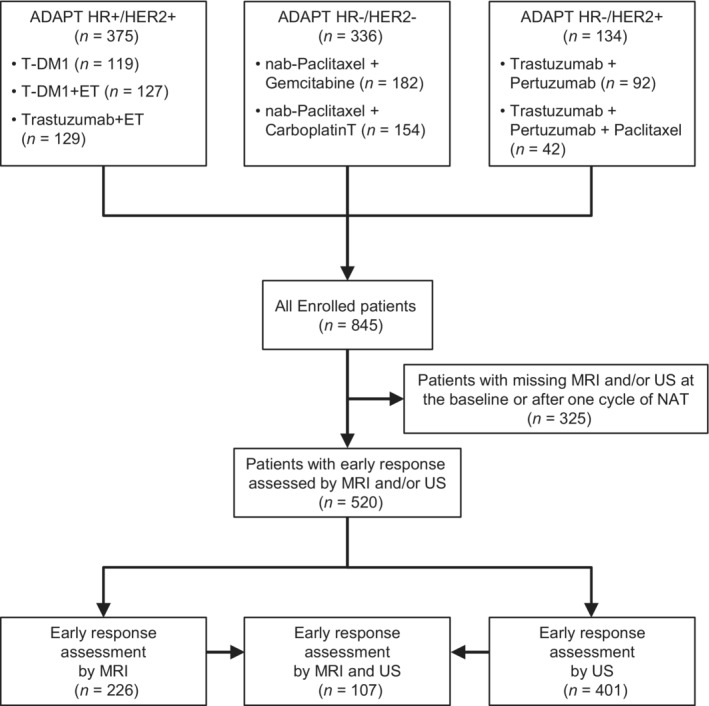
CONSORT diagram
Tumor characteristics were well balanced in the three groups. Median age was 51 years with 48% of patients being premenopausal (Table 1); 44.6% of patients had cT1, 50% cT2 tumors; 70.7% had cN0 and 25.5% cN1 tumors. Age, menopausal status, cTN status, central grade and clinical BL characteristics in the US group, the MRI group and the US/MRI group were generally in line with characteristics from the parent subtrial populations.
TABLE 1.
Patient characteristics
| MRI group | US group | MRI and US group | All patients | |
|---|---|---|---|---|
| Number of patients | 226 | 401 | 107 | 520 |
| Age at initial visit (years) | ||||
| Mean | 51.28 | 51.60 | 49.88 | 51.82 |
| SD | 10.96 | 11.49 | 10.76 | 11.38 |
| Median | 52.00 | 51.00 | 50.00 | 51.00 |
| Min | 25.00 | 21.00 | 26.00 | 21.00 |
| Max | 77.00 | 78.00 | 77.00 | 78.00 |
| N.D. | 2 | 2 | 1 | 3 |
| Central grade, N (%) | ||||
| 1 | 4 (1.77) | 7 (1.75) | 2 (1.87) | 9 (1.73) |
| 2 | 95 (42.04) | 181 (45.14) | 47 (43.93) | 229 (44.04) |
| 3 | 125 (55.31) | 211 (52.62) | 57 (53.27) | 279 (53.65) |
| N.D. | 2 (0.88) | 2 (0.50) | 1 (0.93) | 3 (0.58) |
| Clinical baseline characteristics, N (%) | ||||
| cT | ||||
| 1 | 96 (42.48) | 178 (44.39) | 42 (39.25) | 232 (44.62) |
| 2 | 121 (53.54) | 198 (49.38) | 59 (55.14) | 260 (50.00) |
| 3 | 8 (3.54) | 23 (5.74) | 6 (5.61) | 25 (4.81) |
| 4 | 1 (0.44) | 2 (0.50) | — | 3 (0.58) |
| cN | ||||
| 0 | 150 (66.37) | 290 (72.32) | 72 (67.29) | 368 (70.77) |
| 1 | 66 (29.20) | 99 (24.69) | 32 (29.91) | 133 (25.58) |
| 2 | 9 (3.98) | 11 (2.74) | 2 (1.87) | 18 (3.46) |
| 3 | 1 (0.44) | 1 (0.25) | 1 (0.93) | 1 (0.19) |
| Menopausal status | ||||
| Premenopausal | 106 (46.90) | 198 (49.38) | 53 (49.53) | 251 (48.27) |
| Postmenopausal | 107 (47.35) | 178 (44.39) | 45 (42.06) | 240 (46.15) |
| Unknown/unclear | 13 (5.75) | 25 (6.23) | 9 (8.41) | 29 (5.58) |
| BC subtype and therapy, N (%) | ||||
| HR+/HER2+ | 96 (42.48) | 258 (64.34) | 66 (61.68) | 288 (55.38) |
| pCR rate, N (%) | 34 (35.42) | 78 (30.23) | 23 (34.85) | 89 (30.90) |
| Ki‐67 response rate, N (%) | 58 (60.42) | 145 (56.20) | 42 (63.64) | 161 (55.90) |
| T‐DM1 | 33 (14.60) | 84 (20.95) | 23 (21.50) | 94 (18.08) |
| T‐DM1+ET | 30 (13.27) | 86 (21.45) | 20 (18.69) | 96 (18.46) |
| Trastuzumab+ET | 33 (14.60) | 88 (21.95) | 23 (21.50) | 98 (18.85) |
| HR−/HER2− | 87 (38.50) | 93 (23.19) | 25 (23.36) | 155 (29.81) |
| pCR rate, N (%) | 29 (33.33) | 30 (32.26) | 5 (20.00) | 54 (34.84) |
| Ki‐67 response rate, N (%) | 39 (44.83) | 42 (45.16) | 13 (52.00) | 68 (43.87) |
| nab‐paclitaxel+gemcitabine | 50 (22.12) | 56 (13.97) | 17 (15.89) | 89 (17.12) |
| nab‐paclitaxel+carboplatin | 37 (16.37) | 37 (9.23) | 8 (7.48) | 66 (12.69) |
| HR‐/HER2+ | 43 (19.03) | 50 (12.47) | 16 (14.96) | 77 (14.81) |
| pCR rate, N (%) | 20 (46.51) | 29 (58.00) | 9 (56.25) | 40 (51.95) |
| Ki‐67 response rate, N (%) | 13 (30.23) | 20 (40.00) | 4 (25.00) | 29 (37.66) |
| Trastuzumab+pertuzumab | 32 (14.16) | 35 (8.73) | 12 (11.21) | 55 (10.58) |
| Trastuzumab+pertuzumab+paclitaxel | 11 (4.87) | 15 (3.74) | 4 (3.73) | 22 (4.23) |
Most patients were derived from the HR+/HER2+ (55.4%) subtrial, followed by the HR−/HER2− (29.8%) and the HR−/HER2+ (14.8%) subtrials (Table 1). The percentage of patients with HR+/HER2+ EBC was numerically higher in the US group (64.3%) than in the MRI group (43.5%). The MRI group had more HR−/HER2− tumors (38.5%) than the US group (23.2%); in the HR−/HER2+ group, there were more patients with MRI (19%) than US (12.5%). In our imaging cohort, a comparable share of patients with HR+/HER2+ tumors received T‐DM1+/‐endocrine therapy or trastuzumab+ET (Table 1). In HR−/HER2+ group, more patients had neoadjuvant trastuzumab+pertuzumab than trastuzumab+pertuzumab+paclitaxel whereas in HR−/HER2− group, patients more often had neoadjuvant nab‐paclitaxel+gemcitabine than nab‐paclitaxel+carboplatin therapy.
3.2. Pathological outcomes
3.2.1. pCR rates
Approximately one third of all patients had a pCR (34.2% in US group, 36.7% in MRI group and 34.6% in the MRI and US group). pCR rates in patients with HR+/HER2+, HR−/HER2−and HR−/HER2+ tumors were 30.2%, 32.3% and 58% in US group, 35.4%, 33.3% and 46.5% in MRI group, and 34.9%, 20% and 56.3% in MRI and US group (Table 1).
3.2.2. Ki‐67 response rates
Ki‐67 response at 3 weeks was seen in 51.6% of patients in the US group, 48.7% in the MRI group, and 55.1% in the MRI and US group. The percentages of patients with HR+/HER2+, HR−/HER2− or HR−/HER2+ tumors and Ki‐67 response were 56.2%, 45.2% and 40% in the US group, 60.4%, 44.8% and 30.2% in the MRI group and 63.6%, 52% and 25% in the MRI and US group (Table 1).
3.3. Imaging response rates
3.3.1. MRI response
In the MRI group, CR, PR, SD and PD were documented in 4.9%, 58%, 36.3% and 0.9% of patients, respectively (Table 2). According to mRECIST criteria, 142 (62.8%) of 226 patients had ECR (Table 3). The pCR rate was higher in MRI early responders (43%, 61/142 patients) than in nonresponders (26.2%, 22/84 patients). ECR correctly predicted pCR more often in HR−/HER2+ (53.3%, 16/30 patients) than in HR+/HER2+ (40.4%, 21/52) and HR−/HER2− tumors (40%, 24/60 patients). Representative breast MRI images at BL and at Week 3 in patients with and without MRI early response are shown in Supplementary Figures 2 and 3, respectively.
TABLE 2.
Tumor response rates
| Group | MRI group (N = 226) | US group (N = 401) | US and MRI group (N = 107) | |
|---|---|---|---|---|
| Assessment | MRI (mRECIST) | US (RECIST 1.1) | MRI (mRECIST) | US (RECIST 1.1) |
| Tumor response rate, N (%) | ||||
| CR | 11 (4.87) | 14 (3.49) | 2 (1.87) | 3 (2.80) |
| PR | 131 (57.96) | 187 (46.63) | 62 (57.94) | 56 (52.34) |
| SD | 82 (36.28) | 129 (32.17) | 41 (38.32) | 27 (25.23) |
| PD | 2 (0.88) | 6 (1.50) | 2 (1.87) | — |
| N.D. | 0 (0) | 65 (16.21) | — | 21 (19.63) |
TABLE 3.
Rates of pCR and Ki‐67 response in patients with ECR in the MRI group and in the US group
| pCR | MRI subgroup (N = 226) | US subgroup (N = 401) | Ki‐67 response | MRI subgroup (N = 226) | US subgroup (N = 401) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ECR | No ECR | ECR | No ECR | ECR | No ECR | ECR | No ECR | ||
| Overall | |||||||||
| pCR a | 61 | 22 | 71 | 66 | Ki‐67 response a | 67 | 43 | 88 | 119 |
| (42.96) | (26.19) | (46.10) | (26.72) | (47.18) | (51.19) | (57.14) | (48.18) | ||
| No pCR a | 78 | 61 | 80 | 177 | No Ki‐67 response a | 36 | 31 | 29 | 91 |
| (54.93) | (72.62) | (51.95) | (71.66) | (25.35) | (36.90) | (18.83) | (36.84) | ||
| Missing a | 3 | 1 | 3 | 4 | Missing a | 39 | 10 | 37 | 37 |
| (2.11) | (1.19) | (1.95) | (1.62) | (27.46) | (11.90) | (24.03) | (14.98) | ||
| Total b | 142 | 84 | 154 | 247 | Total b | 142 | 84 | 154 | 247 |
| (62.83) | (37.17) | (38.40) | (61.60) | (62.83) | (37.17) | (38.40) | (61.60) | ||
| HR+/HER2+ | |||||||||
| pCR a | 21 | 13 | 38 | 40 | Ki‐67 response a | 30 | 28 | 63 | 82 |
| (40.38) | (29.55) | (36.54) | (25.97) | (57.69) | (63.64) | (60.58) | (53.25) | ||
| No pCR a | 29 | 31 | 64 | 113 | No Ki‐67 response a | 8 | 14 | 21 | 53 |
| (55.77) | (70.45) | (61.54) | (73.38) | (15.38) | (31.82) | (20.19) | (34.42) | ||
| Missing a | 2 | 0 | 2 | 1 | Missing a | 14 | 2 | 20 | 19 |
| (3.85) | — | (1.92) | (0.65) | (26.92) | (4.55) | (19.23) | (12.34) | ||
| Total b | 52 | 44 | 104 | 154 | Total b | 52 | 44 | 104 | 154 |
| (54.17) | (45.83) | (40.31) | (59.69) | (54.17) | (45.83) | (40.31) | (59.69) | ||
| HR−/HER2− | |||||||||
| pCR a | 24 | 5 | 13 | 17 | Ki‐67 response a | 28 | 11 | 11 | 31 |
| (40.00) | (18.52) | (61.90) | (23.61) | (46.67) | (40.74) | (52.38) | (43.06) | ||
| No pCR a | 35 | 21 | 7 | 52 | No Ki‐67 response a | 22 | 13 | 5 | 34 |
| (58.33) | (77.78) | (33.33) | (72.22) | (36.67) | (48.15) | (23.81) | (47.22) | ||
| Missing a | 1 | 1 | 1 | 3 | Missing a | 10 | 3 | 5 | 7 |
| (1.67) | (3.70) | (4.76) | (4.17) | (16.67) | (11.11) | (23.81) | (9.72) | ||
| Total b | 60 | 27 | 21 | 72 | Total b | 60 | 27 | 21 | 72 |
| (68.97) | (31.03) | (22.58) | (77.42) | (68.97) | (31.03) | (22.58) | (77.42) | ||
| HR−/HER2+ | |||||||||
| pCR a | 16 | 4 | 20 | 9 | Ki‐67 response a | 9 | 4 | 14 | 6 |
| (53.33) | (30.77) | (68.97) | (42.86) | (30.00) | (30.77) | (48.28) | (28.57) | ||
| No pCR a | 14 | 9 | 9 | 12 | No Ki‐67 response a | 6 | 4 | 3 | 4 |
| (46.67) | (69.23) | (31.03) | (57.14) | (20.00) | (30.77) | (10.34) | (19.05) | ||
| Missing a | 0 | 0 | 0 | 0 | Missing a | 15 | 5 | 12 | 11 |
| — | — | — | — | (50.00) | (38.46) | (41.38) | (52.38) | ||
| Total b | 30 | 13 | 29 | 21 | Total b | 30 | 13 | 29 | 21 |
| (69.77) | (30.23) | (58.00) | (42.00) | (69.77) | (30.23) | (58.00) | (42.00) | ||
Abbreviations: ECR, early clinical response; MRI, magnetic resonance imaging; pCR, pathological complete response; US, ultrasound.
Rates of pCR, no pCR and patients with missing data among the patients with and without imaging response.
Share of patients with and without imaging response.
3.3.2. US response
In the US group, 3.5% of patients showed CR, 46.6% PR, 32.2% SD, 1.5% PD and for 16.2% tumor response could not be determined because of missing values (Table 2). One hundred fifty‐four of 401 patients (38.4%) had ECR by US according to RECIST 1.1 criteria (Table 3). Overall, pCR rate was higher in US early responders (46.1%, 71/154 patients) than in nonresponders (26.7%, 66/247 patients; Table 3). ECR by US correctly predicted pCR more often in HR−/HER2+ (69%, 20/29 patients) and HR−/HER2− (61.9%, 13/21 patients) than in HR+/HER2+ tumors (36.5%, 38/104 patients).
3.4. Association between imaging response and proliferation response
Overall, the percentage of patients with both Ki‐67 response and ECR was 42.7% (67/142 patients) in the MRI group and 57.1% (88/154 patients) in the US group (Table 3). The rate of Ki‐67 response among patients with ECR by both MRI and US was highest in HR+/HER2+ and lowest in HR−/HER2+ tumors.
3.5. Prediction of pCR by both US and MRI
3.5.1. MRI and US response
Among the 107 patients with both imaging assessments, ECR was seen more often by MRI than by US (59.8%, 64 patients, vs 35.5%, 38 patients; Supplementary Table 1). Across BC subtypes, the difference between patients with ECR according to MRI and to US was larger in HR−/HER2− (72% and 12%, respectively) than in HR−/HER2+ (75% and 50%) or HR+/HER2+ (51.5% and 40.9%) tumors. The pCR rate among MRI responders was 43.8% (28/64 patients) and 52.6% among US responders (20/38 patients). US and MRI correctly predicted pCR in 9/12 (75%) and 5/8 (62.5%) of HR‐/HER2+ tumors, 5/18 (27.8%) and 2/3 (66.7%) of HR−/HER2− tumors and 14/34 (41.2%) and 13/27 (48.2%) of HR+/HER2+ tumors.
Ki‐67 response was more often observed in patients with ECR by US than by MRI among all patients (65.8%, 25/38, vs 56.3%, 36/64 responders), in HR+/HER2+ (74.1%, 20/27, vs 64%, 22/34 responders), in HR−/HER2− (66.7%, 2/3, vs 61.1%, 11/18 responders) and in HR−/HER2+ tumors (37.5%, 3/8 vs 25%, 3/12 responders; Supplementary Table 1).
Overall, PPV and NPV were 0.47 and 0.73 in US group and 0.44 and 0.73 in MRI group, respectively (Table 4). For both modalities, numerically highest PPV values were observed in the HR−/HER2+ subgroup (0.69 for US and 0.53 for MRI) while NPV values were highest in HR−/HER2− tumors (0.75 for US and 0.81 for MRI). Among patients with both US and MRI, US showed numerically highest PPV in HR+/HER2+ and HR−/HER2− tumors while MRI and Ki‐67 yielded the highest PPV in HR−/HER2+ EBC (Table 4). All methods showed similar NPV in HR+/HER2+ (0.71‐0.74) and HR−/HER2− tumors (0.85‐1), while MRI and Ki‐67 yielded higher NPV than US in HR−/HER2+ tumors (1, both, vs 0.5). MRI and Ki‐67 demonstrated higher SENS than US in HR−/HER2− (1 and 0.8, respectively, vs 0.4) and HR−/HER2+ tumors (1, both, vs 0.56). Ki‐67 was the single most sensitive method in HR+/HER2+ EBC (0.79 vs 0.57‐0.61).
TABLE 4.
PPV, NPV, SENS and SPEC for prediction of pCR by US, MRI and Ki‐67
| US and MRI group (N = 107) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| US group (N = 401) | MRI group (N = 226) | US | MRI | Ki‐67 a | ||||||
| Value | Estimator | CL | Estimator | CL | Estimator | CL | Estimator | CL | Estimator | CL |
| Overall | ||||||||||
| PPV | 0.4702 | 0.3886‐0.5530 | 0.4388 | 0.3549‐0.5255 | 0.5405 | 0.3692‐0.7051 | 0.4444 | 0.3192‐0.5751 | 0.3793 | 0.2551‐0.5163 |
| NPV | 0.7284 | 0.6678‐0.7833 | 0.7349 | 0.6266‐0.8258 | 0.7500 | 0.6302‐0.8471 | 0.7857 | 0.6319‐0.8970 | 0.8148 | 0.6192‐0.9370 |
| SENS | 0.5182 | 0.4313‐0.6044 | 0.7349 | 0.6266‐0.8258 | 0.5405 | 0.3692‐0.7051 | 0.7568 | 0.5880‐0.8823 | 0.8148 | 0.6192‐0.9370 |
| SPEC | 0.6887 | 0.6282‐0.7448 | 0.4388 | 0.3549‐0.5255 | 0.7500 | 0.6302‐0.8471 | 0.4853 | 0.3622‐0.6097 | 0.3793 | 0.2551‐0.5163 |
| HR+/HER2+ | ||||||||||
| PPV | 0.3725 | 0.2788‐0.4739 | 0.4200 | 0.2819‐0.5679 | 0.5000 | 0.2993‐0.7007 | 0.4242 | 0.2548‐0.6078 | 0.3659 | 0.2212‐0.5306 |
| NPV | 0.7386 | 0.6615‐0.8062 | 0.7045 | 0.5480‐0.8324 | 0.7436 | 0.5787‐0.8696 | 0.7188 | 0.5325‐0.8625 | 0.7143 | 0.4190‐0.9161 |
| SENS | 0.4872 | 0.3723‐0.6031 | 0.6176 | 0.4356‐0.7783 | 0.5652 | 0.3449‐0.7681 | 0.6087 | 0.3854‐0.8029 | 0.7895 | 0.5443‐0.9395 |
| SPEC | 0.6384 | 0.5630‐0.7092 | 0.5167 | 0.3839‐0.6477 | 0.6905 | 0.5291‐0.8238 | 0.5476 | 0.3867‐0.7015 | 0.2778 | 0.1420‐0.4519 |
| HR−/HER2− | ||||||||||
| PPV | 0.6500 | 0.4078‐0.8461 | 0.4068 | 0.2807‐0.5425 | 0.6667 | 0.0943‐0.9916 | 0.2778 | 0.0969‐0.5348 | 0.3077 | 0.0909‐0.6143 |
| NPV | 0.7536 | 0.6351‐0.8495 | 0.8077 | 0.6065‐0.9345 | 0.8571 | 0.6366‐0.9695 | 1.0000 | 0.5407‐1.0000 | 0.9091 | 0.5872‐0.9977 |
| SENS | 0.4333 | 0.2546‐0.6257 | 0.8276 | 0.6423‐0.9415 | 0.4000 | 0.0527‐0.8534 | 1.0000 | 0.4782‐1.0000 | 0.8000 | 0.2836‐0.9949 |
| SPEC | 0.8814 | 0.7707‐0.9509 | 0.3750 | 0.2492‐0.5145 | 0.9474 | 0.7397‐0.9987 | 0.3158 | 0.1258‐0.5655 | 0.5263 | 0.2886‐0.7555 |
| HR−/HER2+ | ||||||||||
| PPV | 0.6897 | 0.4917‐0.8472 | 0.5333 | 0.3433‐0.7166 | 0.6250 | 0.2449‐0.9148 | 0.7500 | 0.4281‐0.9451 | 0.7500 | 0.1941‐0.9937 |
| NPV | 0.5714 | 0.3402‐0.7818 | 0.6923 | 0.3857‐0.9091 | 0.5000 | 0.1570‐0.8430 | 1.0000 | 0.3976‐1.0000 | 1.0000 | 0.1581‐1.0000 |
| SENS | 0.6897 | 0.4917‐0.8472 | 0.8000 | 0.5634‐0.9427 | 0.5556 | 0.2120‐0.8630 | 1.0000 | 0.6637‐1.0000 | 1.0000 | 0.2924‐1.0000 |
| SPEC | 0.5714 | 0.3402‐0.7818 | 0.3913 | 0.1971‐0.6146 | 0.5714 | 0.1841‐0.9010 | 0.5714 | 0.1841‐0.9010 | 0.6667 | 0.0943‐0.9916 |
Abbreviations: CL, exact 95% confidence limits (Clopper‐Pearson); NPV, negative predictive value, defined as P(pCR = 0|R = 0); PPV, positive predictive value, defined as P(pCR = 1|R = 1); SENS, sensitivity, defined as P(R = 1|pCR = 1); SPEC, specificity, defined as P(R = 0|pCR = 0 where P(A|B) denotes the conditional probability of event A given that event B has occurred and R is a placeholder for ECR or Ki‐67 response.
Ki‐67 response data was available for 86 out of 107 patients with MRI and US.
Area under curve (AUC) analysis of all patients with US and MRI demonstrated that both methods had a comparable accuracy for detecting pCR (AUC = 0.65 and 0.62, respectively) which corresponded to the accuracy of Ki‐67 (AUC = 0.60; Figure 2). Performance of US, MRI and Ki‐67 assessment was similar in patients with HR−/HER2− EBC (AUC = 0.67, 0.66 and 0.66, respectively). In HR+/HER2+ tumors, accuracy for detecting pCR was slightly higher for US (AUC = 0.63) than for MRI and Ki‐67 (AUC = 0.58 and 0.53, respectively). Ki‐67 and MRI performed better than US in HR−/HER2+ tumors (AUC = 0.83 and 0.79 vs 0.56).
FIGURE 2.
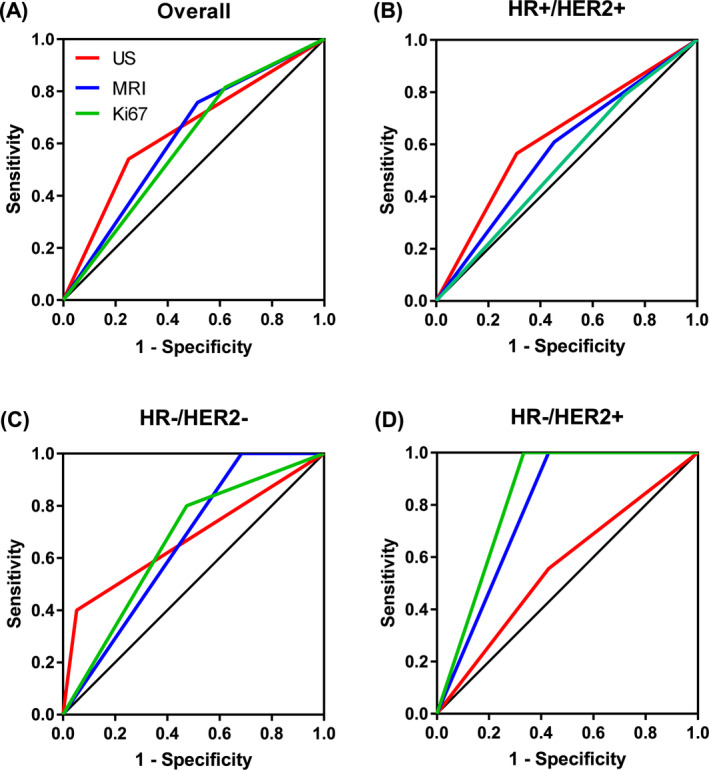
ROC curves for detecting pCR by US, by MRI and by Ki‐67. Data are shown for all patients with MRI and US (A) and for patients with HR+/HER2+ (B), HR−/HER2− (C) and HR−/HER2+ (D) tumors. MRI and US data were available for 107 patients and Ki‐67 data were available for 86 patients. HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MRI, magnetic resonance imaging; pCR, pathological complete response; ROC, receiver operating characteristic; US, ultrasound
We also analyzed AUC of combined assessments to test whether adding Ki‐67, MRI or both Ki‐67 and MRI to US yielded additional benefit. To this end, definition of response required all methods to demonstrate ECR. Additional Ki‐67 assessment improved AUC of US by 0.0315 (in HR+/HER2+) to 0.0463 (in HR−/HER2− tumors; Supplementary Table 2). AUC of US was improved by 0.0715 in HR−/HER2+, reduced by 0.0737 in HR−/HER2− and not changed by additional MRI in HR+/HER2+ tumors. Addition of both MRI and Ki‐67 to US improved AUC by 0.0833 in HR−/HER2+ tumors and reduced it by 0.0731 and 0.0737 in HR+/HER2+ and HR−/HER2− tumors, respectively.
4. DISCUSSION
pCR after completion of a 12‐ to 24‐week standard NAT is a well‐established prognostic factor. Early response/resistance after a short window of treatment (2‐4 weeks) is less well studied but may be clinically even more relevant as it could help to guide individualized de‐escalation/escalation strategies. Assessment of early response is therefore one of the primary objectives of the ADAPT umbrella trial. In ADAPT HR+/HER2−, early response to a 3‐week endocrine treatment measured using a sequential Ki‐67 evaluation together with the BL Oncotype DX recurrence score is used to guide chemotherapy indication, since posttherapeutic Ki‐67 is an established predictor of endocrine responsiveness. 31 , 32 , 33 For the HER2+ and HR−/HER2− subtypes, the respective ADAPT subprotocols prespecified sequential assessment using Ki‐67, MRI and US after a short 3‐week window of treatment in a 12‐week NAT regimen. Correlation of early Ki67 response with pCR was among the objectives of these neoadjuvant subprotocols. Previously published ADAPT trial data demonstrated a substantial number of early responses (67% in HR+/HER2+, 41.3% in HR−/HER2+, and 44.4% in HR−/HER2− tumors) as well as good correlation with pCR (35.7% in HR+/HER2+, 44.7% in HR−/HER2+ and 44.4% in HR−/HER2− tumors. 24 , 26 , 34
To our knowledge, the data presented in our study are the first evaluating the role of US, MRI, Ki‐67 and US combined with MRI and/or Ki‐67 for detection of early response and prediction of pCR in the HER2+ and HR−/HER2− subgroups. Data have been generated and combined from the three ADAPT subtrials. AUC curves for all patients with US and MRI demonstrated that both methods had a comparable accuracy for predicting pCR, while Ki‐67 assessment showed lower accuracy. Performance of US, MRI and Ki‐67 assessment was comparable in HR+/HER2+ and HR−/HER2− tumors indicating that all three methods are similarly accurate for pCR prediction in these subtypes. Furthermore, MRI, Ki‐67 or both assessments performed in addition to US improved correct identification of response in tumors with or without pCR in maximally 7.4% of patients. Therefore, in HR+/HER2+ and HR−/HER2− tumors US assessment would be the first choice in the daily clinical practice since it is widely available, less costly compared to MRI and offers the opportunity for a potential second core biopsy.
In HR−/HER2+ EBC, however, accuracy of US was markedly lower compared to that of single Ki‐67 or MRI assessment. Although combination of US with MRI (with or without Ki‐67) improved AUC, the combined approach was able to correctly identify pCR or no pCR in up to 8.3% of patients. Therefore, these findings suggest that in HR−/HER2+ EBC, identification of patients most likely to achieve pCR should be performed by MRI assessment than by US. Nevertheless, the number of HR−/HER2+ patients included in this analysis was small which combined with imbalance in treatment types and pCR rates precludes drawing of the definitive conclusion.
Selection of patients for therapy de‐escalation requires a highly sensitive method allowing early identification of response among those patients who will later have a pCR. In our study, MRI and Ki‐67 yielded highest SENS, particularly in HR−/HER2+ EBC in which both methods identified ECR in all tumors with pCR. Conversely, SENS of US was markedly lower in HR−/HER2+ tumors (0.56). Therefore, US did not detect ECR in 44% of patients with pCR. These numbers demonstrate the risk that patients who could potentially benefit from de‐escalation strategies may not be reliably identified by US, at least in this subtype.
Another trial evaluating early response by sequential MRI (BL and at least 2 weeks after the first cycle, prior to the second NAT cycle) was reported by the I‐Spy investigators. 19 They compared MRI vs clinical assessment in an unselected population of 216 patients with EBC. MRI (size) was superior to clinical examination at all time points, showing the greatest relative benefit at the second examination, with low additional information after the second MRI. This implies that an early MRI control may be the best time point if there is a limited access to MRI for more examinations. Analysis of I‐Spy MRI data was performed in a unicenter setting with a prespecified MRI protocol. Our results were generated by central analysis of data from multiple experienced centers using a prespecified MRI protocol. Early evaluation of protocol adherence showed that local standards differed, resulting in a clinical meaningful percentage of centers that had to be excluded and in heterogeneous quality of local MRI imaging. Taking all of these findings this into consideration, US appears as the optimal method for early response assessment in daily clinical practice while MRI and Ki‐67 (in TN and HER2+) should be reserved for a clinical trial setting with integrated rigorous quality assurance measures.
Nevertheless, detection of early resistance has clinically important information. In the GeparTrio trial, US monitoring was performed in 832 patients at BL, at Week 6, and at the end of treatment. 35 The study protocol prespecified the switch to a noncross resistant chemotherapy regimen in case of no response after two cycles of conventional chemotherapy. This switch of therapy resulted in better outcomes, underlining the importance of correctly identified nonresponders. In this context, NPV (noECR/non‐pCR) values indicate that at least 71% of patients with non‐pCR would have already been identified as early nonresponders, irrespectively of method and BC subtype. A notable exception are HR−/HER2+ tumors, where in our analysis either MRI or Ki‐67 yielded much a higher NPV than US (100% or 100%, vs 50%). Although these values seem clinically meaningful, further research is warranted to better optimize selection of candidates for therapy escalation. Interestingly, NPV for MRI and US obtained in this analysis were generally slightly higher than previously reported for post‐NAT assessments. 36 , 37
Our study has certain limitations. First, of the 401 patients in US group and 226 in MRI group, only 107 patients had both imaging methods performed. This limited the number of patients for a head to head comparison of US and MRI data and could have influenced the relative value of these methods for prediction of pCR. Second, an impact of therapy type on the imaging accuracy cannot be excluded. For instance, it was shown that MRI may underestimate residual tumor size in taxane‐containing treatments. 38 Considering that all our patients with HR−/HER2− tumors received a taxane, there is a risk that several patients in this subgroup could be false‐positive on MRI. Lastly, the comparisons of our results to other published data may be confounded by the heterogeneity of subtypes, pCR rates, targeted therapies and chemotherapy regimens in our study and prior publications.
5. CONCLUSIONS
Early response is a strong predictor of pCR. We evaluated early response by proliferation response in an early on‐treatment biopsy and a more conservative approach by conventional imaging. Our data demonstrate subtype specific effects with similar accuracy of MRI, US and Ki‐67 in HR+/HER2+ and HR−/HER2− tumors, and superiority of MRI and Ki‐67 in the HR−/HER2+ subtype. Therefore, US assessment would be the first choice in daily clinical practice for HR+/HER2+ and HR−/HER2− tumors; in HR−/HER2+ tumors, MRI may be considered. Even though our data may some limitations (heterogeneity, small sample size) but in a multicentric setting adding MRI+/‐Ki‐67 to US does not improve prediction of pCR in a clinically meaningful number of patients. Together with published evidence, our results highlight the need for identification of strong biomarkers for early response and molecular imaging modalities in order to optimally guide neoadjuvant breast cancer therapy in daily clinical practice.
CONFLICT OF INTEREST
Oleg Gluz acts as Co‐Director West German Study Group (WSG), received honoraria from Genomic Health/Exact Sciences, Roche, Celgene, Pfizer, Novartis, NanoString Technologies, AstraZeneca, served in consulting/advisory role for Celgene, Genomic Health/Exact Sciences, Lilly, MSD, Novartis, Pfizer, Roche, and received travel support from Roche. Rachel Würstlein served in consulting/advisory role as well as on speakers' bureau for and received travel support from Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Clinsol, Daiichi‐Sankyo, Eisai, Genomic Health, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Nanostring, Novartis, Odonate, Onkowissen, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Seattle Genetics, Tesaro Bio, Teva, Viatris. Sherko Kümmel acts as Co‐Director West German Study Group (WSG), received personal fees from Lilly, Roche, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, AstraZeneca, SOMATEX Medical Technologies, MSD, Pfizer, Puma Biotechnology, PFM medical, and nonfinancial support from Roche, Daiichi Sankyo, Sonoscope. Michael Braun received honoraria from AstraZeneca, Exact Sciences, Novartis, Pfizer, Roche, Teva, travel support from AstraZeneca, Celgene, Medac, Novartis, Roche and served in consulting/advisory role for AstraZeneca, Exact Sciences, Novartis, Puma, Roche. Bahriye Aktas reports a potential financial conflict of interest as follows: Pfizer Pharma GmbH, Roche Pharma AG, Novartis Pharma GmbH, AstraZeneca GmbH, PharmaMar GmbH, MSD Merck Sharp & Dohme GmbH, Onkowissen.de GmbH, Lilly Deutschland GmbH, Promedicis GmbH. Cornelia Kolberg‐Liedtke has ownership interest in Theraklion, Phaon Scientific (both applicable for immediate family member), received honoraria from Roche, AstraZeneca, Celgene, Novartis, Pfizer, Lilly, Hexal, Amgen, SonoScape, and an immediate family member received honoraria from Pfizer, Novartis, Roche, Genomic Health, Amgen, AstraZeneca, Riemser, Carl Zeiss MediTec, TEVA Pharmaceuticals Industries, Theraklion, Janssen‐Cilag, GlaxoSmithKline, LIV Pharma, served in consulting/advisory role for Roche, Novartis, Pfizer, Celgene, Phaon Scientific, and an immediate family member served in consulting/advisory role for Pfizer, Novartis, SurgVision, CarlZeissMeditec, Amgen, Onkowissen, received research funding from Roche, Novartis, Pfizer, and received travel support from Roche, Daiichi Sankyo, Novartis, and an immediate family member received research funding from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo. Nadia Harbeck acts as Co‐Director West German Study Group (WSG) and reports a potential financial conflict of interest as follows: AstraZeneca, Lilly, Novartis, Pfizer, Pierre Fabre, Roche/Genentech, Merck Sharp & Dohme, Seattle Genetics. Ulrike Nitz acts as Co‐Director West German Study Group (WSG), received honoraria from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Novartis pharma, Pfizer Pharmaceuticals, Roche/Genentech, Teva, served in consulting/advisory role for Genomic Health, Roche, provided expert testimony for Genomic Health, received travel support from Genomic Health, Pfizer Pharmaceuticals, Roche, and her institution received research funding from Agendia, Amgen, Celgene, Genomic Health, NanoString Technologies, Roche, Sanofi.
ETHICS STATEMENT
The ADAPT trial was approved by the Ethics Committee of the University of Cologne, Germany. Trial registration numbers for the mentioned subtrials are NCT01817452 (HR+/HER2+), NCT01779206 (HR−/HER2+) and NCT01815242 (HR−/HER2−). Written informed consent was obtained from each patient prior to registration.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The analysis of MRI data presented in this article was funded by Bayer AG Germany. ADAPT HER2+/HR+ and WSG‐ADAPT HER2+/HR− trials were financially supported by Hoffmann la Roche, the WSG‐ADAPT TN trial was financially supported by Celgene and Teva. Medical writing and editorial support were provided by Lukasz Wujak, Lukasz Wujak MedComms, Warsaw, Poland, and was funded by WSG GmbH, Moenchengladbach, Germany.
Graeser M, Schrading S, Gluz O, et al. Early response by MR imaging and ultrasound as predictor of pathologic complete response to 12‐week neoadjuvant therapy for different early breast cancer subtypes: Combined analysis from the WSG ADAPT subtrials. Int. J. Cancer. 2021;148:2614–2627. 10.1002/ijc.33495
Monika Graeser and Simone Schrading contributed equally to this study.
Funding information Bayer; Celgene; Roche; Teva Pharmaceutical Industries
DATA AVAILABILITY STATEMENT
Data used for this analysis are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Arnaout A, Lee J, Gelmon K, et al. Neoadjuvant therapy for breast cancer: updates and proceedings from the Seventh Annual Meeting of the Canadian Consortium for Locally Advanced Breast Cancer. Curr Oncol. 2018;25:e490‐e498. [Google Scholar]
- 2. Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology. 2017;285:358‐375. [DOI] [PubMed] [Google Scholar]
- 3. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta‐analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342‐3354. [DOI] [PubMed] [Google Scholar]
- 4. von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796‐1804. [DOI] [PubMed] [Google Scholar]
- 5. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long‐term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164‐172. [DOI] [PubMed] [Google Scholar]
- 6. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin Oncol. 2008;26:1275‐1281. [DOI] [PubMed] [Google Scholar]
- 7. Symmans WF, Wei C, Gould R, et al. Long‐term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McFarland DC, Naikan J, Rozenblit M, Mandeli J, Bleiweiss I, Tiersten A. Changes in pathological complete response rates after neoadjuvant chemotherapy for breast carcinoma over five years. J Oncol. 2016;2016:4324863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hennigs A, Riedel F, Marme F, et al. Changes in chemotherapy usage and outcome of early breast cancer patients in the last decade. Breast Cancer Res Treat. 2016;160:491‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maur M, Guarneri V, Frassoldati A, Conte PF. Primary systemic therapy in operable breast cancer: clinical data and biological fall‐out. Ann Oncol. 2006;17(Suppl 5):v158‐v164. [DOI] [PubMed] [Google Scholar]
- 11. Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014;23:526‐537. [DOI] [PubMed] [Google Scholar]
- 12. Keune JD, Jeffe DB, Schootman M, Hoffman A, Gillanders WE, Aft RL. Accuracy of ultrasonography and mammography in predicting pathologic response after neoadjuvant chemotherapy for breast cancer. Am J Surg. 2010;199:477‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Croshaw R, Shapiro‐Wright H, Svensson E, Erb K, Julian T. Accuracy of clinical examination, digital mammogram, ultrasound, and MRI in determining postneoadjuvant pathologic tumor response in operable breast cancer patients. Ann Surg Oncol. 2011;18:3160‐3163. [DOI] [PubMed] [Google Scholar]
- 14. Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. 2005;184:868‐877. [DOI] [PubMed] [Google Scholar]
- 15. von Minckwitz G, Blohmer JU, Costa SD, et al. Response‐guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623‐3630. [DOI] [PubMed] [Google Scholar]
- 16. von Minckwitz G, Kummel S, Vogel P, et al. Intensified neoadjuvant chemotherapy in early‐responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst. 2008;100:552‐562. [DOI] [PubMed] [Google Scholar]
- 17. Guarneri V, Dieci MV, Bisagni G, et al. De‐escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2‐week letrozole: results of the PerELISA neoadjuvant study. Ann Oncol. 2019;30:921‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minarikova L, Bogner W, Pinker K, et al. Investigating the prediction value of multiparametric magnetic resonance imaging at 3 T in response to neoadjuvant chemotherapy in breast cancer. Eur Radiol. 2017;27:1901‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hylton NM, Blume JD, Bernreuter WK, et al. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy—results from ACRIN 6657/I‐SPY TRIAL. Radiology. 2012;263:663‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu HD, Zhang YQ. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer using diffusion‐weighted imaging and dynamic contrast‐enhanced magnetic resonance imaging. Neoplasma. 2017;64:430‐436. [DOI] [PubMed] [Google Scholar]
- 21. Michishita S, Kim SJ, Shimazu K, et al. Prediction of pathological complete response to neoadjuvant chemotherapy by magnetic resonance imaging in breast cancer patients. Breast. 2015;24:159‐165. [DOI] [PubMed] [Google Scholar]
- 22. Nagashima T, Sakakibara M, Nakamura R, et al. Dynamic enhanced MRI predicts chemosensitivity in breast cancer patients. Eur J Radiol. 2006;60:270‐274. [DOI] [PubMed] [Google Scholar]
- 23. Hofmann D, Nitz U, Gluz O, et al. WSG ADAPT ‐ adjuvant dynamic marker‐adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: study protocol for a prospective, multi‐center, controlled, non‐blinded, randomized, investigator initiated phase II/III trial. Trials. 2013;14:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harbeck N, Gluz O, Christgen M, et al. De‐escalation strategies in human epidermal growth factor receptor 2 (HER2)‐positive early breast cancer (BC): final analysis of the West German study group adjuvant dynamic marker‐adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2‐ and hormone receptor‐positive phase II randomized trial‐efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J Clin Oncol. 2017;35:3046‐3054. [DOI] [PubMed] [Google Scholar]
- 25. Nitz UA, Gluz O, Christgen M, et al. De‐escalation strategies in HER2‐positive early breast cancer (EBC): final analysis of the WSG‐ADAPT HER2+/HR‐ phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab +/− weekly paclitaxel. Ann Oncol. 2017;28:2768‐2772. [DOI] [PubMed] [Google Scholar]
- 26. Gluz O, Nitz U, Liedtke C, et al. Comparison of neoadjuvant nab‐paclitaxel+carboplatin vs nab‐paclitaxel+gemcitabine in triple‐negative breast cancer: randomized WSG‐ADAPT‐TN trial results. J Natl Cancer Inst. 2018;110:628‐637. [DOI] [PubMed] [Google Scholar]
- 27. ACR . ACR BI‐RADS Atlas® 5th Edition, vol. 2019.
- 28. Marinovich ML, Sardanelli F, Ciatto S, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669‐677. [DOI] [PubMed] [Google Scholar]
- 29. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 30. Semiglazov V. RECIST for response (clinical and imaging) in neoadjuvant clinical trials in operable breast cancer. J Natl Cancer Inst Monogr. 2015;2015:21‐23. [DOI] [PubMed] [Google Scholar]
- 31. Dowsett M, Ebbs SR, Dixon JM, et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER‐2 in breast cancer—a study from the IMPACT trialists. J Clin Oncol. 2005;23:2477‐2492. [DOI] [PubMed] [Google Scholar]
- 32. Robertson J, Dowsett M, Bliss J, et al. Abstract GS1‐03: peri‐operative aromatase inhibitor treatment in determining or predicting longterm outcome in early breast cancer – the POETIC* trial (CRUK/07/015). Cancer Res. 2018;78:GS1‐03‐GS1‐03. [Google Scholar]
- 33. Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short‐term presurgical endocrine therapy for primary breast cancer. JNCI. 2007;99:167‐170. [DOI] [PubMed] [Google Scholar]
- 34. Nitz UA, Gluz O, Christgen M, et al. De‐escalation strategies in HER2‐positive early breast cancer (EBC): final analysis of the WSG‐ADAPT HER2+/HR‐ phase II trial: efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab +/− weekly paclitaxel. Ann Oncol. 2017;28:2768‐2772. [DOI] [PubMed] [Google Scholar]
- 35. Marinovich ML, Houssami N, Macaskill P, von Minckwitz G, Blohmer JU, Irwig L. Accuracy of ultrasound for predicting pathologic response during neoadjuvant therapy for breast cancer. Int J Cancer. 2015;136:2730‐2737. [DOI] [PubMed] [Google Scholar]
- 36. De Los Santos JF, Cantor A, Amos KD, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119:1776‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumgartner A, Tausch C, Hosch S, et al. Ultrasound‐based prediction of pathologic response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2018;39:19‐23. [DOI] [PubMed] [Google Scholar]
- 38. Denis F, Desbiez‐Bourcier AV, Chapiron C, Arbion F, Body G, Brunereau L. Contrast enhanced magnetic resonance imaging underestimates residual disease following neoadjuvant docetaxel based chemotherapy for breast cancer. Eur J Surg Oncol. 2004;30:1069‐1076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data used for this analysis are available upon reasonable request to the corresponding author.


