Abstract
Introduction
Emicizumab is a subcutaneously (SC) administered prophylactic agent for persons with haemophilia A (PwHA). As part of its clinical development, a new instrument was required to measure treatment satisfaction.
Aim
Describe development of the Satisfaction Questionnaire with Intravenous or Subcutaneous Hemophilia Injection (SQ‐ISHI) and its subsequent testing with HAVEN 3 study participants to measure patient satisfaction with emicizumab.
Methods
To develop the SQ‐ISHI, we conducted four rounds of in‐person interviews at five qualitative research facilities. Participants aged ≥12 years with moderate or severe haemophilia A, receiving intravenous factor VIII (FVIII) prophylaxis, provided feedback to optimize content understanding, ease of completion and item relevance. The final SQ‐ISHI was completed by HAVEN 3 participants who previously received FVIII prophylaxis; baseline scores were compared with those at Week 21 or 25 of emicizumab prophylaxis.
Results
Sixty‐three HAVEN 3 participants were eligible to complete the questionnaire and rate their satisfaction on a scale of 0 (‘not at all satisfied’) to 10 (‘extremely satisfied’). Mean ‘overall satisfaction’ with previous FVIII prophylaxis at baseline was 6.9 (95% confidence interval [CI]: 6.2 to 7.7) increasing to 8.8 (95% CI: 8.4 to 9.3) at follow‐up (Week 21/25 of treatment with emicizumab). The greatest improvement was observed in satisfaction with treatment half‐life (mean score at baseline: 5.8 [95% CI: 4.9 to 6.6] vs 8.6 [95% CI: 8.0 to 9.2] at follow‐up).
Conclusion
These results demonstrate that emicizumab prophylaxis leads to greater treatment satisfaction compared with FVIII prophylaxis, reflecting in part the low treatment burden of emicizumab associated with its infrequent, SC administration.
Keywords: emicizumab, haemophilia A, patient‐reported outcomes, questionnaire, SQ‐ISHI, treatment satisfaction
1. INTRODUCTION
Haemophilia A (HA) is a bleeding disorder characterized by deficiency of coagulation protein factor VIII (FVIII). Therapeutic options for persons with haemophilia A (PwHA) without FVIII inhibitors include plasma‐derived or recombinant FVIII concentrates, which require intravenous (IV) infusions at least 2–3 times per week to achieve adequate prophylaxis. 1
The short half‐lives, need for IV infusion, and treatment storage can negatively impact treatment adherence and subsequent clinical outcomes and health‐related quality of life (HRQoL). 2 , 3 During a US Food and Drug Administration panel discussion, PwHA highlighted the need for products that shift the focus towards outcomes that are important to them, such as ease of administration and longer‐acting treatments. 4
Emicizumab, a novel, bispecific, humanized monoclonal antibody, bridges activated factor IX (FIXa) and factor X (FX), restoring the function of missing activated FVIII in PwHA. 5 , 6 , 7 , 8 , 9 Emicizumab is administered subcutaneously (SC) either once weekly, every 2 weeks, or every 4 weeks. 5 , 6 , 9 The Phase 3 HAVEN 3 study demonstrated the efficacy and safety of emicizumab in adult/adolescent PwHA without FVIII inhibitors and showed superior bleed prevention in individuals receiving FVIII prophylaxis who switched to emicizumab. 6
Various instruments are currently available to measure treatment satisfaction. The Treatment Satisfaction Questionnaire for Medication (TSQM) measures side effects, efficacy and convenience; however, it lacks the scope to assess certain concepts relevant to PwHA. 10 The Haemophilia Treatment Satisfaction questionnaire for adults (Hemo‐SatA), was designed to measure haemophilia‐specific treatment satisfaction. 11 However, its length (34 questions), completion time (15 min) and inability to measure the impact of treatment on daily life, limits its deployment. The HaemoPREF questionnaire measures the impact of treatment, treatment‐related risk and influence on others, and has been validated in a real‐world setting in PwHA. 12 , 13 It has only 14 questions, making it less burdensome than the Hemo‐SatA, but its utility is focused on IV clotting factor treatments. Therefore, there was a need to develop a disease‐specific questionnaire for the assessment of treatment satisfaction with IV and SC treatments.
Here, we describe development of the Satisfaction Questionnaire with Intravenous or Subcutaneous Hemophilia Injection (SQ‐ISHI) and initial results in PwHA in the HAVEN 3 study.
2. MATERIALS AND METHODS
2.1. SQ‐ISHI development
For the initial development of the SQ‐ISHI, we included elements of TSQM and Hemo‐SATA, as well as the Rituximab Administration Symptom Questionnaire (RASQ; another well‐used questionnaire designed to assess satisfaction in patients receiving IV and SC treatment). 10 , 11 , 14 Literature was searched to generate potential questions and concepts considered important when assessing IV and SC treatment administration in PwHA.
A patient global impression of change item assessing treatment satisfaction (PGIC‐S) was included. This was to ensure that an increase in overall satisfaction could be detected even among respondents with high or maximum baseline scores on individual SQ‐ISHI items. 15 , 16
2.2. SQ‐ISHI interviews
To assess the relevance of the SQ‐ISHI, RTI Health Solutions conducted face and content validity assessments in adolescents and adult PwHA. In‐person interviews were conducted at research facilities in five US cities prior to initiation of the HAVEN 3 study. Participants were aged ≥12 years with moderate or severe congenital HA and were receiving FVIII prophylaxis at least twice weekly. All participants were required to be able to read, speak, and understand English. Informed consent was given by adults or caregivers of children/adolescents; assent was given by adolescents.
Interviews were conducted in two parts: concept elicitation and cognitive debriefing. As part of the concept elicitation, participants were asked open‐ended questions about their current FVIII treatment such as: ‘What do you like about your FVIII product?’ and ‘What do you dislike about your FVIII product?’. They were also asked how their current FVIII treatment could be improved (e.g. mode/frequency of administration). In addition, participants were shown a 5‐min video describing SC emicizumab administration (including a description of how to use the vial and syringe), and their overall reactions to SC injections were recorded. For the cognitive debriefing, after completing the paper‐based SQ‐ISHI, participants were asked to assess how easily they completed the draft questionnaire, the relevance and importance of the items, and to identify any concepts important to treatment satisfaction that may be missing. Four interview rounds were performed and feedback from four to five participants was collected at each round. Each individual participated only once. With the same participants, four rounds of cognitive debriefing took place and each response was assessed, with changes to items or wording occurring as needed prior to the next round. The final SQ‐ISHI included 15 items and the PGIC‐S item; no further changes were made as the items were deemed clear and important to treatment satisfaction.
2.3. SQ‐ISHI administration in HAVEN 3
In the HAVEN 3 study, Arm D comprised participants who had received prior FVIII prophylaxis and subsequently received emicizumab 1.5 mg/kg weekly maintenance therapy during the study; 6 only participants from Arm D (n = 63) completed the 15‐item SQ‐ISHI. The SQ‐ISHI was completed by participants twice: Week 1 (baseline) and either at Week 21 or Week 25. Administration was completed using a site‐based hand‐held electronic tablet, without observation by clinic staff, to protect participant privacy and data integrity. Flexibility in the timing of the second administration was intended to minimize participant burden in the context of multiple additional evaluations in the trial.
Due to a tablet programming error, participants were able to complete the questionnaire at both Week 21 and Week 25, with some participants doing so. As a result, data from the follow‐up administration from each respondent (Week 21 or 25) were utilized to form a derived Week 25 (hereafter referred to as the follow‐up analysis). This comprised the original Week 21 assessment, or the original Week 25 assessment if only this was available and the original Week 21 assessment was not available. If both Week 21 and Week 25 were available, only Week 21 was included; where both assessments were missing, the result was set as missing and the participant not evaluable.
Descriptive analyses of absolute values and change from baseline at the follow‐up (Week 21/25) assessment (with 95% confidence intervals [CIs]) for each item of the SQ‐ISHI (without grouping items into a composite score), along with the categorical analysis of the overall satisfaction item, are reported. The extent to which scores shifted from the baseline assessment was evaluated using a 2‐point change cut‐off, which has been identified in previous studies in a variety of diseases, employing an 11‐point numeric response scale (NRS) as the amount of change that participants think is clinically meaningful. 17 , 18 For each individual item of the SQ‐ISHI and the overall score, respondents were categorized by whether their beliefs about the treatment improved, stayed the same, or worsened.
3. RESULTS
3.1. SQ‐ISHI development
Overall, 19 males participated in four rounds of qualitative interviews (Figure 1); their characteristics are presented in Table S1.
FIGURE 1.
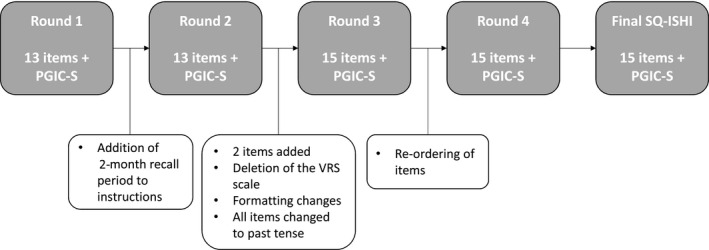
Summary of SQ‐ISHI development review rounds. Abbreviations: PGIC‐S, patient global impression of change item assessing treatment satisfaction; SQ‐ISHI, Satisfaction Questionnaire—Intravenous Subcutaneous Hemophilia Injection; VRS, verbal response scale
3.2. Concept elicitation
All 19 participants stated that they were generally satisfied with their current HA treatment. Reasons for satisfaction were most often related to the efficacy of their treatment (n = 14, 74%). Participants were asked about their ‘ideal’ treatment or if there was anything they would improve about their current treatment if they could. The majority of respondents referred to the desire for fewer infusions (n = 13, 68%) and a desire for a non‐injectable treatment (n = 7, 37%) and for not having to find a vein (n = 5, 26%).
Participant responses to the 5‐min video describing SC administration varied. Positive comments were related to speed and convenience (n = 5, 26%), avoiding vein access (n = 4, 21%), and less pain (n = 3, 16%). The majority had at least one concern related to efficacy (n = 9, 47%), the preparation process (n = 9, 47%), pain (n = 8, 42%), bruising or scarring (n = 3, 16%), or safety (n = 3, 16%).
3.3. Cognitive debriefing
All participants completed the instrument in approximately 2–5 min, without difficulty, confusion or questions. In Rounds 1 and 2, an initial 13‐item version of the SQ‐ISHI was tested, along with a PGIC‐S item. No specific recall period was referenced on the SQ‐ISHI tested in Round 1; in Round 2, instead, the participants were instructed to think about their most recent treatment. Based on Round 1 feedback, a 2‐month recall period was included in the instructions for subsequent rounds and maintained in the final SQ‐ISHI.
Two types of response scales were tested in a rotating order in Round 1 and Round 2: a 5‐point verbal response scale (VRS) and an 11‐point NRS. The VRS included three sets of response options: ‘none’, ‘mild’, ‘moderate’, ‘severe,’ and ‘very severe’ when asked about injection discomfort; ‘very satisfied’, ‘satisfied’, ‘neither satisfied nor dissatisfied’, ‘dissatisfied’, and ‘very dissatisfied’ when asked about overall satisfaction; and ‘not at all’, ‘a little bit’, ‘somewhat’, ‘quite a bit’, and ‘very much’ for all other questions. The NRS ranged from 0 to 10 with anchors at each end, where 0 represented ‘not at all’ (e.g. ‘not at all impacted’, ‘not at all satisfied’) and 10 represented ‘extremely’ (e.g. ‘significantly impacted’, ‘extremely satisfied’). Both scales tested well; participants were easily able to respond with an answer to each item using either scale. In both rounds, when asked which scale they preferred, results were mixed, with participants voicing strengths for each scale. Due to the broader option of responses available with the NRS, this scale was retained for testing in Rounds 3 and 4, and maintained in the final SQ‐ISHI.
Based on Round 1 and 2 feedback, two new items were added. The first explored satisfaction with spontaneity, a concept related to the participants’ ability to do what they wanted, when they wanted, without regard to their haemophilia treatment. The second item focused on satisfaction with treatment half‐life. The resulting instrument contained 15 items, all of which were retained after testing in Rounds 3 and 4.
Formatting changes to improve clarity included: underlining key words in the questions, adjusting phrases to past tense, and reordering the questions to group together those questions with positive anchors at different ends (i.e. left and right side) of the scale.
The concepts that the items assessed included: the treatment's ease of administration, convenience, influence on daily life, and the participant's confidence and satisfaction. As the first 11 items used words like ‘difficult’, ‘bother’, ‘worry’ and ‘impact’ to assess the concepts, lower scores were reflective of less difficulty, bother, and impact. In contrast, the last four items used words like ‘confident’ and ‘satisfied’ to assess the concept and thus higher scores were reflective of more confidence and satisfaction.
3.4. Utility of the SQ‐ISHI in HAVEN 3 study participants
Overall, 63 males from Arm D of the HAVEN 3 study were eligible to complete the SQ‐ISHI. Mean age was 36.4 years (range: 13–68) and the majority (75%) were white (Table S2). 6
The questionnaire completion rate at Week 1 was 57/63 (90%) and at Week 21 was 50/63 (79%). At Week 25, 52/63 (83%) questionnaires were completed, the majority of these completed by participants who had already completed the questionnaire at Week 21. In total, 60/63 (95%) participants completed the questionnaire at least once during Week 21 or Week 25; 54 participants responded at baseline and Week 21 and/or Week 25.
Scores for each of the SQ‐ISHI items are presented in Table 1. At baseline, participants treated with IV FVIII reported the highest (indicating worse) scores on items reflecting difficulty, impact, or bother from the frequency of treatments, discomfort with injections, travel impact, and how time‐consuming treatment was. Consistent with this, at baseline, participants reported only moderate satisfaction with the half‐life of their treatment, and were only moderately satisfied with their previous FVIII prophylaxis. Mean overall satisfaction score at baseline was 6.9 (95% CI: 6.2 to 7.7) on a scale ranging from 0 (‘not at all satisfied’) to 10 (‘extremely satisfied’). At the follow‐up analysis, participants treated with SC emicizumab reported less difficulty, impact, worry, and bother, and greater confidence and satisfaction on all items, including overall satisfaction, which increased to a mean score of 8.8 (95% CI: 8.4 to 9.3). The greatest improvements were observed in satisfaction with treatment half‐life (change from baseline 2.9 [95% CI: 1.8 to 4.0]) and overall satisfaction (change from baseline 2.0 [95% CI: 1.3 to 2.7]) where increased scores indicate greater confidence/satisfaction, and bother with the frequency of treatments (change from baseline −2.5 [95% CI: −3.4 to −1.7]), where a decreased score indicates less impairment (Figure 2A,B; Table 1).
TABLE 1.
SQ‐ISHI items: baseline score and change from baseline at follow‐up in HAVEN 3 study participants a
| Concept/Item | Baseline score, mean (95% CI) | Change from baseline score, mean (95% CI) |
|---|---|---|
| Items 1–11: higher scores indicate greater impairment | ||
| 1. Injection discomfort | 2.9 (2.1 to 3.7) | −1.3 (−2.1 to −0.5) |
| 2. Vein access difficulty | 2.4 (1.7 to 3.2) | −1.5 (−2.4 to −0.6) |
| 3. Injection difficulty | 2.5 (1.6 to 3.4) | −1.4 (−2.2 to −0.6) |
| 4. Injection worry | 1.9 (1.1 to 2.6) | −1.0 (−1.8 to −0.3) |
| 5. Time consuming | 2.8 (2.1 to 3.4) | −1.7 (−2.3 to −1.0) |
| 6. Bother | 3.3 (2.5 to 4.2) | −2.5 (−3.4 to −1.7) |
| 7. Preparation difficulty | 1.3 (0.8 to 1.8) | −0.6 (−1.1 to −0.1) |
| 8. Travel impact | 3.0 (2.0 to 4.0) | −1.8 (−3.2 to −0.5) |
| 9. Daily activities impact | 2.4 (1.7 to 3.1) | −1.7 (−2.4 to −1.0) |
| 10. Free time activity impact | 2.5 (1.7 to 3.3) | −1.4 (−2.2 to −0.6) |
| 11. Taking treatment as prescribed | 1.8 (1.1 to 2.4) | −1.1 (−1.7 to −0.4) |
| Items 12–15: higher scores indicate greater confidence/satisfaction | ||
| 12. Confidence to prevent bleeds | 7.8 (7.0 to 8.5) | 0.9 (0.2 to 1.6) |
| 13. Satisfaction with spontaneity | 6.8 (6.0 to 7.6) | 1.5 (0.5 to 2.5) |
| 14. Satisfaction with treatment half‐life | 5.8 (4.9 to 6.6) | 2.9 (1.8 to 4.0) |
| 15. Overall satisfaction | 6.9 (6.2 to 7.7) | 2.0 (1.3 to 2.7) |
Each concept/item was represented by a detailed question, which is not stated here for proprietary reasons.
n = 54, except ‘8. Travel Impact’ where n = 30.
Abbreviations: CI, confidence interval.
Follow‐up analysis: data included from the original Week 21 assessment, if available, or the original Week 25 if this was available and the original Week 21 assessment was not available. If both Week 21 and Week 25 assessments were missing, then it was set as missing.
FIGURE 2.
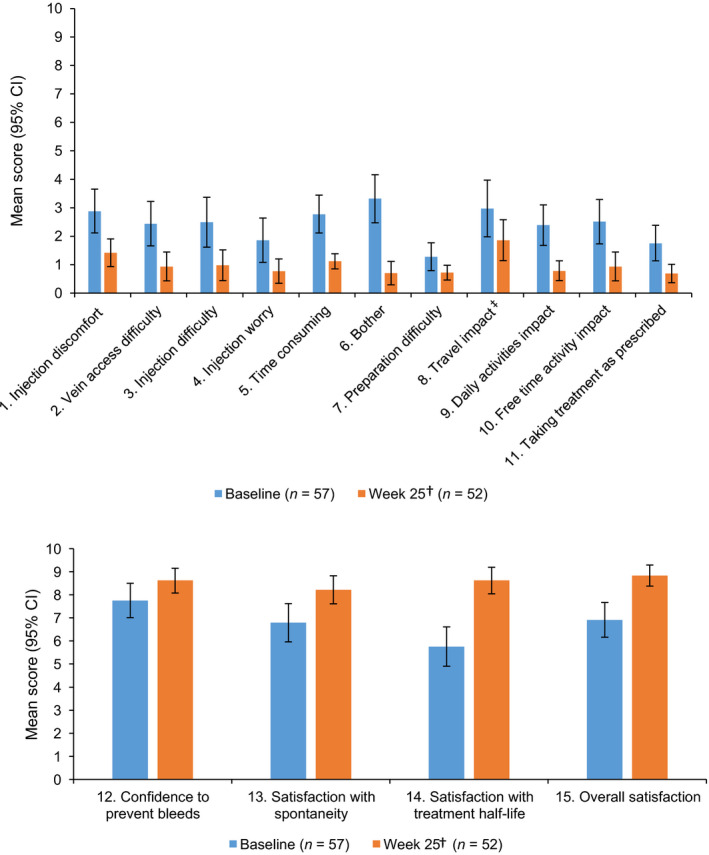
Mean scores for each SQ‐ISHI item at baseline and follow‐up for items related to treatment impact (A) and treatment confidence/satisfaction (B)†. Mean scores presented for items related to treatment impact (A), where higher scores indicate greater impact, and items related to confidence/satisfaction with treatment (B), where higher scores indicate greater confidence/satisfaction. †Follow‐up analysis: data included from the original Week 21 assessment, if available, or the original Week 25 if this was available and the original Week 21 assessment was not available. If both Week 21 and Week 25 assessments were missing, then it was set as missing. ‡Participants could answer ‘not applicable’ for travel impact; n = 39 at baseline, n = 42 at Week 25. Abbreviations: CI, confidence interval; SQ‐ISHI, Satisfaction Questionnaire—Intravenous Subcutaneous Hemophilia Injection
The proportion of respondents reporting a meaningful ≥2‐point improvement in scores 17 , 18 was generally highest in items related to disease impact: ‘travel impact’ (50%), ‘time consuming’ (48%) and ‘bother’ and ‘satisfaction with spontaneity’ (both 46%). Overall, 50% of participants reported a ≥2‐point improvement in overall satisfaction and 54% reported similar improvements in ‘satisfaction with treatment half‐life’. At the follow‐up analysis, between 39% and 70% of respondents reported no change (defined as a difference in item score between −1 and 1) across the SQ‐ISHI items (Figure 3). Across all items, the proportion of participants reporting meaningful improvements in SQ‐ISHI items was between 26% and 54%, with the exception of ‘injection worry’ and ‘preparation difficulty’, where 2‐point improvements were only seen in 22% of participants. Across all items, the proportion of respondents reporting worsening of SQ‐ISHI items was between 4% and 13%.
FIGURE 3.
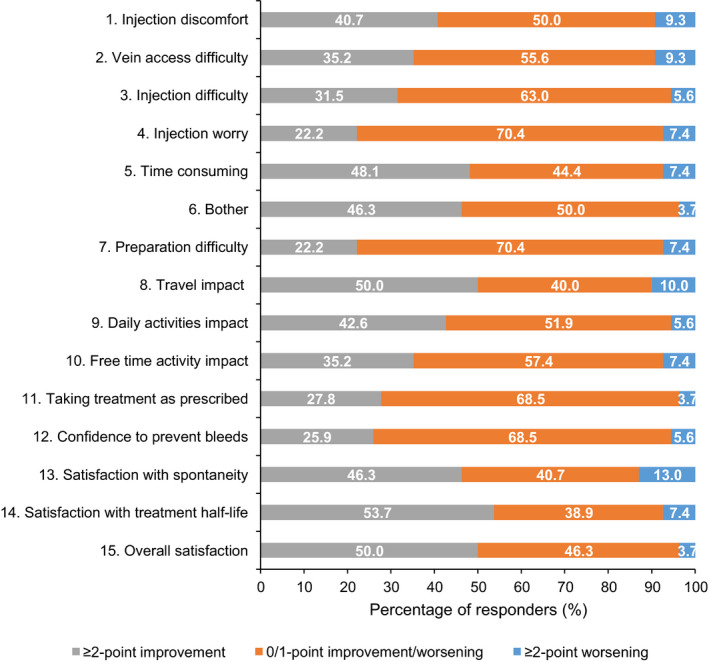
Shift from baseline in scores by ≥2‐point improvement or worsening to follow‐up analysis† for SQ‐ISHI items. ≥2‐point improvement is considered clinically relevant. †Follow‐up analysis: data included from the original Week 21 assessment if available or the original Week 25 if available and original Week 21 not available. If both Week 21 and Week 25 assessments were missing, then it was set as missing. ‡Participants could answer ‘not applicable’ for travel impact. Abbreviations: SQ‐ISHI, Satisfaction Questionnaire—Intravenous Subcutaneous Hemophilia Injection
The PGIC‐S was included to assess participants' satisfaction with their current haemophilia treatment (i.e. emicizumab) compared with their treatment before the beginning of the study (FVIII). Fifty‐five of 60 respondents (92%) reported being ‘much more’ or ‘a lot more’ satisfied with their haemophilia treatment at follow‐up. No participants reported being less satisfied, although two (3%) noted no difference (Table 2). Results at Week 21 alone were consistent with the follow‐up results (pooled Week 21/25), with no apparent bias (but increased imprecision in the pooled analysis).
TABLE 2.
Patient global impression of change in treatment satisfaction (PGIC‐S) in HAVEN 3 study participants
| ‘Overall satisfaction’, n (%) |
Arm D: Emicizumab 1.5 mg/kg per week |
|---|---|
| Follow‐up analysis a | |
| n | 60 |
| Much more satisfied | 44 (73.3) |
| A lot more satisfied | 11 (18.3) |
| A little more satisfied | 3 (5.0) |
| No difference | 2 (3.3) |
| A little less satisfied | 0 |
| A lot less satisfied | 0 |
| Much less satisfied | 0 |
Percentages are based on n, where n is the number of participants who responded to the item.
Follow‐up analysis: data included from the original Week 21 assessment, if available, or the original Week 25 if this was available and the original Week 21 assessment was not available. If both Week 21 and Week 25 assessments were missing, then it was set as missing.
4. DISCUSSION
Here, interview participants reported that the primary drivers of haemophilia treatment satisfaction were related to the treatment efficacy, as well as the ease and reduced burden of a simple and fast preparation and administration process.
The study found that the SQ‐ISHI offers advantages over previous treatment satisfaction questionnaires. It includes the specificity to haemophilia that the TSQM lacks, a shorter completion time than the Hemo‐SatA, and the ability to evaluate both IV and SC methods of administration unlike the HaemoPREF. 10 , 11 , 12 , 13
Treatment satisfaction is an important factor in helping to increase treatment adherence rates. 19 , 20 Adherence to prophylaxis is key for optimal clinical outcomes and, in turn, can improve the HRQoL of PwHA: for example, good adherence to prophylaxis reduces the incidence of joint bleeds and arthropathy. 21 , 22 , 23 The results of the SQ‐ISHI in the HAVEN 3 study demonstrate that participants receiving emicizumab prophylaxis had greater treatment satisfaction across all areas compared with their prior IV FVIII prophylaxis. At the start of emicizumab treatment, participants reported moderately high levels of satisfaction in most of the items assessed by the SQ‐ISHI. Given the high baseline levels, it was potentially difficult to observe positive improvements following emicizumab treatment due to ceiling effects. Despite this, the majority of items relating to treatment impact did show improvement. Accordingly, on the PGIC‐S, over 90% of participants reported being ‘much more’ or ‘a lot more' satisfied with emicizumab treatment at follow‐up compared with prior FVIII standard‐of‐care treatment. Thus, the inclusion of the PGIC‐S was a valuable part of the study, making it possible to put into context the results of each item at follow‐up (even if scores on the individual items were at the highest levels at baseline).
A significant factor in treatment burden is the need for frequent IV infusions: in this study, baseline scores for items relating to injection were all below three points. Although most participants had received coagulation factor concentrates with standard half‐lives, subgroup analysis showed no difference in satisfaction between products with standard versus extended half‐lives. Treatment with emicizumab eliminates the need for frequent infusions and IV access, and this was reflected in the improvement reported by participants in items related to difficulty with vein access and injection, and injection worry. Thus, although participants reported moderately high levels of satisfaction with IV treatment at baseline, they recognized greater satisfaction with the SC administration.
Overall, 26%–54% of participants reported meaningful improvements in SQ‐ISHI items, with the exception of ‘injection worry’ and ‘preparation difficulty’ (both 22%). This could be due to participants continuing to worry about injections or difficulty preparing the treatment, or having high baseline scores and not being able to improve by 2 points. As 63%–70% of respondents reported no change on these items and the average baseline scores were <2 for each of them, it is most likely that the majority of participants were not able to improve by 2 points.
The study does, however, have some limitations. Firstly, the tablet programming error, which led to some follow‐up assessments being collected at Week 21 and Week 25 and thus resulted in data being pooled, needs to be considered when interpreting these data. Secondly, the sensitivity of the PGIC‐S in terms of the time it was administered should be noted, as it was based on participant recall to answer how they felt about their treatment currently compared with how they felt before the study start. However, with a lifetime of prior treatment, the participants were unlikely to have forgotten their past experiences. This study was focused on the development and initial experience with the SQ‐ISHI and consequently did not include psychometric validation or comparison with an alternative questionnaire such as Haemophilia Quality of Life Questionnaire for Adults (Haem‐A‐QoL). Future studies could be designed to address these gaps.
5. CONCLUSIONS
The SQ‐ISHI is a content‐validated, treatment satisfaction measure for IV and SC treatments for HA, which allows investigators to assess aspects of satisfaction with both types of treatment that are of importance to this population. The results of the analysis of SQ‐ISHI in HAVEN 3 study participants demonstrate that SC emicizumab prophylaxis leads to greater treatment satisfaction compared with IV FVIII treatment for a variety of parameters, including satisfaction with time required and impact on daily activities.
DISCLOSURES
CK has received research funding from Novo Nordisk and compensation for consultation or advisory role with Spark Therapeutics, Pfizer, and Genentech, Inc.; PCT is employed by F. Hoffmann‐La Roche Ltd/Genentech, Inc. and owns stock in the company; AP has received compensation for consultation or advisory role with Shire/Takeda, Sunovion, I‐Mab, Sigilon, and UniQure, and research funding from Genentech, Inc. and Shire/Takeda; MN is employed by F. Hoffmann‐La Roche Ltd; AC‐B is employed by Genentech, Inc. and owns stock and other ownership interests in F. Hoffmann‐La Roche Ltd; MC has received honoraria for advisory boards with Shire, Octapharma, Grifols, Pfizer, Bayer, F. Hoffmann‐La Roche Ltd, Bioverativ, Hema Biologics, Spark Therapeutics, BioMarin, and Global Blood Therapeutics, speakers' bureau fees from Shire, F. Hoffmann‐La Roche Ltd/Genentech, Inc, Bayer, Novo Nordisk, BioMarin, Global Blood Therapeutics, research support from Shire and Pfizer, acted as site investigator/sib‐I for clinical trials for Pfizer, F. Hoffmann‐La Roche Ltd/Genentech, Inc, Novo Nordisk, Global Blood Therapeutics, Sancillio, Amgen, and BioMarin, and holds stock in Alnylam; NOC has received honoraria from SOBI, compensation for consultation or advisory role with UniQure, speakers' bureaus fees from F. Hoffmann‐La Roche Ltd and SOBI, and research funding from SOBI; IP‐P is an employee of Genentech, Inc.; and JNM has received research grants from Bayer, Biogen, BioMarin, CSL, Novo Nordisk, SOBI, F. Hoffmann‐La Roche Ltd, and UniQure; is a member of scientific advisory committee of Amgen, Bayer, Biotest, Biogen, Baxalta, CSL Behring, Catalyst Biosciences, Novo Nordisk, F. Hoffmann‐La Roche Ltd, and Spark Therapeutics, UniQure; and is a member of a speaker bureau for Alnylam, Bayer, Biotest, Biogen, Novo Nordisk, Pfizer, SOBI, Shire, F. Hoffmann‐La Roche Ltd, ISTH, and WFH.
AUTHOR CONTRIBUTIONS
Peter Trask was involved in study design and data analysis and interpretation. Markus Niggli, Ido Paz‐Priel, Michael Callaghan, Christine Kempton and Johnny Mahlangu were involved in study design, data acquisition, and data analysis and interpretation. Avrita Campinha‐Bacote was involved in data analysis and interpretation. Aric Parnes and Niamh O'Connell were involved in data acquisition and data analysis and interpretation.
Supporting information
Table S1‐S2
ACKNOWLEDGEMENTS
The authors would like to thank T. Michelle Brown, PhD, and Dana DiBenedetti, PhD, from RTI Health Solutions for their contributions to the SQ‐ISHI development and validation. Editorial assistance for the development of this manuscript, under the direction of the authors, was provided by Alex Coulthard, BSc, and Robert Harrison, PhD, of Gardiner‐Caldwell Communications, Macclesfield, UK, and funded by F. Hoffmann‐La Roche Ltd.
Funding information
F. Hoffmann‐La Roche Ltd; Chugai Pharmaceutical Co., Ltd
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/).For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
REFERENCES
- 1. Lambert T, Benson G, Dolan G, et al. Practical aspects of extended half‐life products for the treatment of haemophilia. Ther Adv Hematol. 2018;9(9):295‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tischer B, Marino R, Napolitano M. Patient preferences in the treatment of hemophilia A: impact of storage conditions on product choice. Patient Prefer Adherence. 2018;12:431‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. FDA . The Voice of the Patient: Hemophilia A, Hemophilia B, von Willebrand Disease and Other Heritable Bleeding Disorders. 2016. Available at: https://www.fda.gov/media/99237/download. Accessed Dec 2020. [Google Scholar]
- 5. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in Hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809‐818. [DOI] [PubMed] [Google Scholar]
- 6. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811‐822. [DOI] [PubMed] [Google Scholar]
- 7. Shima M, Hanabusa H, Taki M, et al. Factor VIII‐mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374(21):2044‐2053. [DOI] [PubMed] [Google Scholar]
- 8. Young G, Liesner R, Chang T, et al. A multicenter, open‐label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised phase 3 study. Lancet Haematol. 2019;6(6):e295‐e305. [DOI] [PubMed] [Google Scholar]
- 10. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearney S, Raffini LJ, Pham TP, et al. Health‐related quality‐of‐life and treatment satisfaction of individuals with hemophilia A treated with turoctocog alfa pegol (N8‐GP): a new recombinant extended half‐life FVIII. Patient Prefer Adherence. 2019;13:497‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Teal S, Brohan E, Hettema Y, et al. Development and psychometric evaluation of a novel tool for assessing patient perception and preference for haemophilia treatment (HaemoPREF). Haemophilia. 2014;20(5):666‐673. [DOI] [PubMed] [Google Scholar]
- 13. Bonanad S, Schulz M, Gordo A, et al. HaemoPREF: further evaluation of patient perception and preference for treatment in a real world setting. Haemophilia. 2017;23(6):884‐893. [DOI] [PubMed] [Google Scholar]
- 14. Banderas B, Skup M, Shields AL, Mazar I, Ganguli A. Development of the Rheumatoid Arthritis Symptom Questionnaire (RASQ): a patient reported outcome scale for measuring symptoms of rheumatoid arthritis. Curr Med Res Opin. 2017;33(9):1643‐1651. [DOI] [PubMed] [Google Scholar]
- 15. Sprangers MA, Schwartz CE. Integrating response shift into health‐related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507‐1515. [DOI] [PubMed] [Google Scholar]
- 16. Rapkin BD, Schwartz CE. Toward a theoretical model of quality‐of‐life appraisal: Implications of findings from studies of response shift. Health Qual Life Outcomes. 2004;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283‐291. [DOI] [PubMed] [Google Scholar]
- 18. Farrar JT, Pritchett YL, Robinson M, Prakash A, Chappell A. The clinical importance of changes in the 0 to 10 numeric rating scale for worst, least, and average pain intensity: analyses of data from clinical trials of duloxetine in pain disorders. J Pain. 2010;11(2):109‐118. [DOI] [PubMed] [Google Scholar]
- 19. Sweileh WM, Ihbesheh MS, Jarar IS, et al. Self‐reported medication adherence and treatment satisfaction in patients with epilepsy. Epilepsy Behav. 2011;21(3):301‐305. [DOI] [PubMed] [Google Scholar]
- 20. Zyoud SH, Al‐Jabi SW, Sweileh WM, et al. Health‐related quality of life associated with treatment adherence in patients with hypertension: a cross‐sectional study. Int J Cardiol. 2013;168(3):2981‐2983. [DOI] [PubMed] [Google Scholar]
- 21. Royal S, Schramm W, Berntorp E, et al. Quality‐of‐life differences between prophylactic and on‐demand factor replacement therapy in European haemophilia patients. Haemophilia. 2002;8(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 22. Garcia‐Dasi M, Aznar JA, Jimenez‐Yuste V, et al. Adherence to prophylaxis and quality of life in children and adolescents with severe haemophilia A. Haemophilia. 2015;21(4):458‐464. [DOI] [PubMed] [Google Scholar]
- 23. Manco‐Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115‐2124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
Qualified researchers may request access to individual patient‐level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/).For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).


