Abstract
Background
Radiofrequency (RF) is commonly recognized treatment option for fat reduction, utilizing heat‐induced adipocyte deletion. HIFEM treatment has been proven to be an effective tool for body shaping.
Objectives
To document the structural changes in human subcutaneous tissue induced by the combination of RF treatment with the HIFEM procedure.
Methods
Four subjects (51.50 ± 6.35 years, 22.59 ± 3.21 kg/m2) received three 30‐minute abdominal treatments consisting of RF therapy and HIFEM. One subject (57 years, 23.60 kg/m2) served as a control. Punch biopsies were collected at baseline, 1‐week, and 1‐month post‐treatment. Samples were sliced and stained with H&E. Waist circumference, digital photographs, satisfaction, and therapy comfort were assessed. Subjects were monitored for any adverse event, and fat temperatures were measured.
Results
Baseline samples showed a healthy appearance of adipocytes, composed of round‐shaped unilocular cells of uniform size. At follow‐up, treated adipocytes demonstrated nuclear and shape changes with consequent fat reduction. Adipocytes were found to be flattened/shrunken and of smaller size (−33.5% at 1 week; −31.7% at 1‐month) along with occasional ruptures of the cytoplasmic membrane. In contrast to baseline, pyknotic nuclei with condensed nuclear chromatin were seen at 1‐week and 1‐month post‐treatment. The control samples showed no treatment‐related changes. Waist circumference decreased by an average of 2.20 cm in the treated patients. No adverse events were observed. The fat temperature reached 42‐45°C, during treatment; the therapy was comfortable with high patient satisfaction.
Conclusions
Results suggest the efficacy and safety of the therapy combining RF and HIFEM. The adipocyte deletion and shrinkage resulted in overall reduction of fat tissue.
Keywords: apoptosis, HIFEM, histology, radiofrequency
1. INTRODUCTION
Human subcutaneous fat tissue has a layered structure primarily consisting of fine collagen fibrous septa meshed with clusters of round‐shape cells termed adipocytes. 1 Their cytoplasm is occupied by a large single lipid droplet, which serves as an energy reservoir, and is responsible for adipocytes spherical shape. Based on the amount of stored lipids, the adipocyte volume fluctuates throughout their lifetime. 2 , 3
The continuous imbalance of caloric intake leads to the gradual accumulation of the adipose tissue since the excessive energy, which is not utilized by the body, is being stored in the lipid droplet in the form of triglyceride content. 2 , 3 A sedentary lifestyle and an unbalanced diet are considered the main contributors to increased fat storages in healthy human. 4 Besides the health risks and a need of considerable medical attention that often arise in the case of an overweight body, 5 the social aspects of a patient's well‐being should not be underestimated as well. Aesthetically displeasing localized fat deposits are distressful for many and may result in the loss of self‐esteem or trauma from embarrassment. Therefore, noninvasive body contouring has received increased attention in the recent years as a convenient solution for improving body image.
Noninvasive fat tissue reduction is energy‐dependent and may be achieved either by reducing the fat stores or permanently removing adipocytes. 2 The first effect occurs when adipocytes are appropriately stimulated with energies of lower intensities, such as during low‐level laser therapy (LLLT). 6 The catabolic process referred to as lipolysis is initiated, leading to a breakdown of the triglycerides stored in fat vacuoles into glycerol and free fatty acids (FFA) released into the vasculature. 7 , 8 However, although the decrease in adipocytes volume may be achieved by lipolysis, the cells are still viable and present in the same amount as before the therapy.
In addition to lipolysis, the adipose tissue can be eliminated permanently via various regulated cell death programs such as pyroptosis, necroptosis, and apoptosis. 9 Apoptosis is a complex intracellular pathway that allows the organism to control cell number and tissue size. 3 , 10 Morphologically, the most distinctive signs of apoptotic processes are cell shrinkage accompanied by nuclear changes in the form of chromatin condensation (pyknosis) and nuclear fragmentation (karyorrhexis). 2 , 11 Also, late plasma membrane disintegration may occur in case of extrinsic pathway of apoptosis. 9 Consequently, the cell remnants are digested by the immune cells during a phagocytic clearance. 12
A specific amount of energy transformed into heat is required to induce fat apoptosis, elevating fat temperatures to 42‐45°C. At the same time, it is necessary not to exceed the upper limit of 45°C in the long‐term to avoid a risk of necrosis. 1 , 13 , 14 , 15 To safely heat the adipose tissue, various devices and technologies utilizing radiofrequency (RF) energy for fat apoptosis have been developed. 13 , 14 , 16 , 17 Due to the extensive research in the recent past, there exists a clear association between RF heating and adipocyte apoptosis documented in vivo in humans, 17 and correspondingly in porcine animal model. 14
Nonetheless, body contouring may also be achieved via a nonthermal manner due to the muscle toning delivered by a high‐intensity focused electromagnetic field (HIFEM) procedure. 18 , 19 , 20 HIFEM utilizes strong alternating magnetic fields, and based on the law of electromagnetic induction, it induces electric currents that depolarize neuromuscular tissue causing supramaximal muscle contractions. Such contractions demand a considerable amount of energy that is supplied in the form of FFA from adipose tissue via lipolysis.
The different modus operandi of the thermal (RF) and nonthermal (HIFEM) body contouring technologies allows for its combined use. An assumption arises that RF‐induced fat loss may even be further promoted by the metabolic effect of HIFEM on fat when used simultaneously. Therefore, this study aims to document structural changes in human subcutaneous tissue induced by a novel device utilizing the simultaneous delivery of synchronized radiofrequency and HIFEM energy.
2. MATERIALS AND METHODS
This was a prospective single‐center study. Inclusion criteria were specified as BMI < 35.0 kg/m2, no pregnancy or breastfeeding, injury in the treated area, active participation in any other concurrent therapy, and general contraindications for HIFEM and RF application to the human body. Five subjects were enrolled while four subjects (51.50 ± 6.35 years; BMI of 22.59 ± 3.21 kg/m2) were assigned for active treatments, and one subject served as a control (57 years; BMI of 23.60 kg/m2). At the time of enrollment, all study subjects were instructed about the study and associated procedures in detail. Written informed consent was obtained from all participants, and the study adhered to the ethical principles for medical research involving human subjects (1975 Declaration of Helsinki).
In total, the participants received three 30‐minute abdominal treatments with a frequency of 1 treatment per week. No anesthesia or pretreatment, preparation was needed for the procedure. A device (Emsculpt NEO, BTL Industries INC., Boston MA) simultaneously delivering synchronized RF and HIFEM energies were used in this study. It utilizes a novel interspaced design of RF electrode that allows emitting radiofrequency and HIFEM fields in a synchronized manner, meaning that the apparatus for delivering both energies is embedded in one single applicator. Based on the patient's physical constitution, one or two applicators were placed over the treated area and affixed by an elastic fixation belt. The output power of RF was set to 100% in the active treatments and 5% for sham treatments. The intensity of HIFEM (0%‐100%) in active group was cautiously adjusted according to the patient's feedback, ending up at a maximum tolerable level. HIFEM intensity in sham treatments was again set to 5%.
Punch biopsies (4 mm in diameter) of adipose tissue, after 1% lidocaine local anesthesia, were obtained from each subject at baseline, 1 week after the last treatment, and at 1‐month follow‐up. The baseline samples were collected at least 5 cm away from the treatment site to avoid bleeding in the treated area and minimize patient discomfort during the therapy. Three tissue samples were collected from each subject (15 samples total). All specimens were preserved in a fixation solution (phosphate‐buffered formalin), dehydrated, cleared, embedded in paraffin wax, and sectioned into thin (6 μm) slices using a microtome (120 micrographs in total). The slices were stained by the hematoxylin and eosin to finalize the processing for examination under the light microscope (Leica DM1000; Leica Microsystems, Germany). To evaluate structural changes of fat tissue, slices were subjected to detailed analysis by a certified histopathologist. The area of adipocytes (μm2) in the examined slices was measured using Leica Application Suite Core Software and statistically analyzed by a two‐tailed paired t test with α = 5%.
To verify the temperature distribution in the subcutaneous layer, the direct temperature measurements were taken using a flexible optic probe (LumaSense Technologies Inc, Germany) in one subject from the active group. Ultrasound‐guided insertion of the probe into mid‐depth of subcutaneous tissue was performed, and the temperature changes were monitored during the whole 30‐minute treatment period.
Aside from histology, the secondary outcomes included documentation of treatment area by digital photographs, waist circumference, weight measurements, 5‐point Likert scale satisfaction questionnaire, and 10‐point visual analog scale (VAS) therapy comfort questionnaire assessed at baseline, 1‐week, and 1‐month follow‐up. Any occurrence of adverse events was monitored throughout the study.
3. RESULTS
All subjects successfully finished their treatments and follow‐up visits. There were no adverse events reported. The only documented side effects were transient muscle soreness the day after the therapy and erythema that resolved within a few hours. Subjects rated their therapy comfort very high with average scores of 0.83 (active) and 0 (sham) at 10‐point VAS (0 = no discomfort, 10 = unbearable discomfort).
Histological examination of baseline and control samples revealed healthy unilocular cells of round/polygonal shape. There were no deviations from the usual structure of adipose tissue (see Figure 1). On the contrary, the samples of treated tissue taken at 1 week showed visible shape alternations of fat cells and occasional fat ruptures. The evidence of ongoing programmed cell death processed was documented by the presence of pyknotic nuclei manifested by hypercondensation of chromatin and destructive fragmentation of the nucleus (Figure 2). Those findings persisted up to 1 month since the fat tissue still demonstrated an elevated level of adipocyte deletion and structural changes manifested as the flattening and plasma membrane disintegration (Figure 3). No such changes were found after the administration of sham treatment. Furthermore, no signs of necrosis were present after the active or sham treatments.
FIGURE 1.
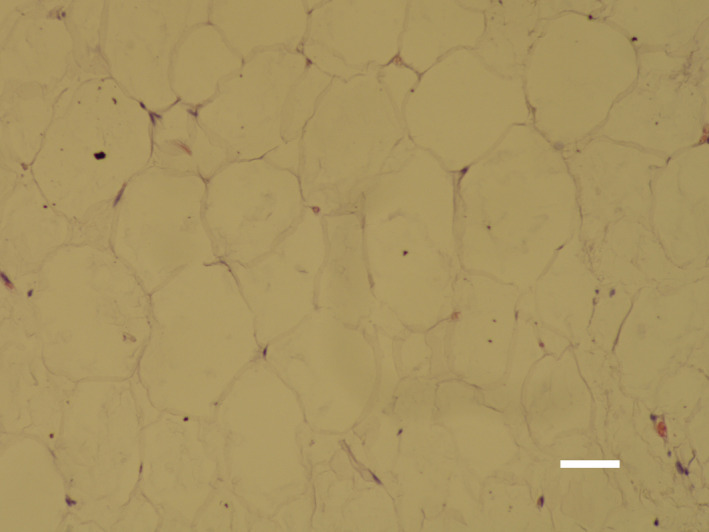
Subcutaneous tissue at baseline is consisted of healthy unilocular cells of round/polygonal shape (bar = 40 μm)
FIGURE 2.
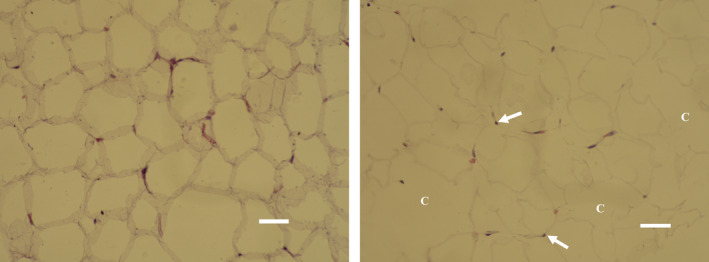
Elimination of fat cells (C), noticeable shape alternations, and pyknotic nuclei (depicted by an arrow) found in the treated adipose tissue at 1 wk (right, bar = 30 μm). No changes were found in control/sham subject (left, bar = 30 μm)
FIGURE 3.
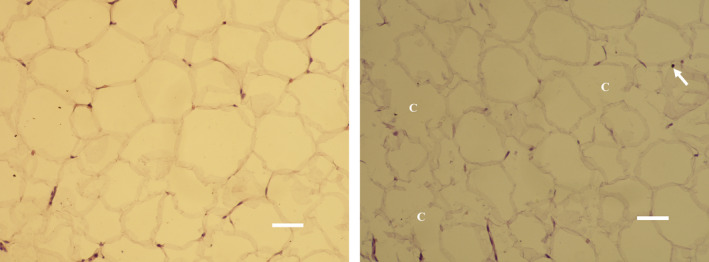
Elimination of fat cells (C), pyknotic nuclei (arrow), and shrinkage of adipocytes were found at 1 mo in treated subjects (right, bar = 30 μm). No changes were present after the sham treatment (left, bar = 30 μm)
The outcomes of adipocyte size measurements can be seen in Figure 4. The fat cells in control samples were of a uniform size (approximately 1750 μm2) throughout the study. The baseline measurements in the active group showed similar values, as seen in the control subjects. However, post‐treatment samples showed a significant reduction in the adipocyte's size. At 1‐week follow‐up, the reduction in active group was 33.5% (−583 μm2; P = .009), which was sustained at 31.7% (−552 μm2; P = .01) at 1 month post‐treatment. The loss of adipocytes content followed by its shrinkage/flattening was also apparent in examined histological samples. See Figures 2 and 3.
FIGURE 4.
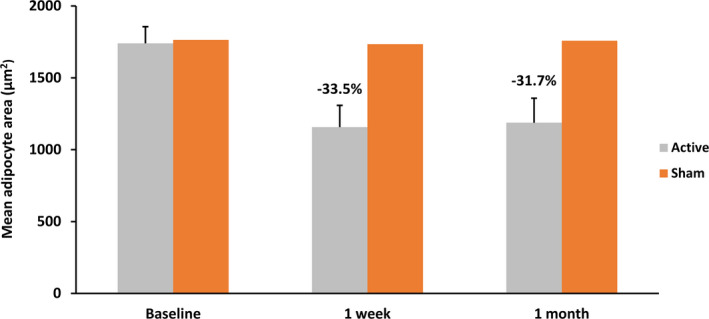
Adipocytes showed a significant (P < .05) decrease in size after the active treatment compared with sham at 1 wk and 1 mo. The error bars represent the variability of results among the subjects
Fiber optic probe measurements showed a rapid increase in fat temperature in the first four minutes when the level of 42°C was achieved. The steady temperature range of 43‐45°C was maintained for most of the therapy. In summary, the effective temperature needed to induce structural changes in adipose tissue was held most of the treatment time (Figure 5).
FIGURE 5.
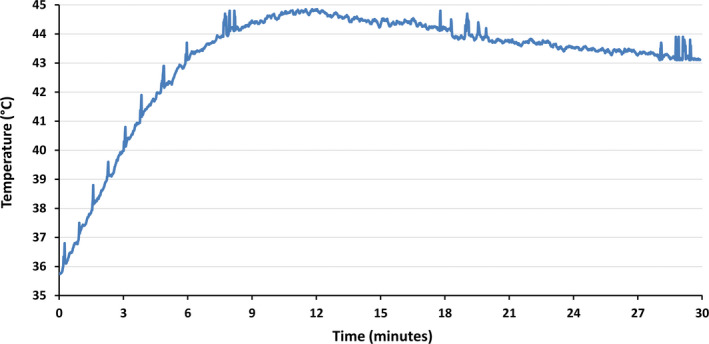
Rapid increase in fat temperature was seen, exceeding 42°C after the fourth minute. For most of the therapy time, the temperature was maintained at the level needed to induce structural changes to adipose tissue
Weight fluctuations in the active group were insignificant (−0.2 kg at 1 week and −0.3 kg at 1 month, respectively). The changes in fat tissue observed by the microscopic evaluation were projected into a visible improvement of abdominal body contour (see Figure 6). The average waist circumference reduction in the active group was −2.20 cm with a maximum 5.4 cm reduction. Concurrently, the control subject showed no improvement in waist circumference. Active treatment resulted in a much greater satisfaction rate with therapy outcomes. Patients who received therapy with maximum intensities agreed (4) or strongly agreed (5) with the statement that the appearance of their abdominal area improved while the sham subject disagreed (2).
FIGURE 6.
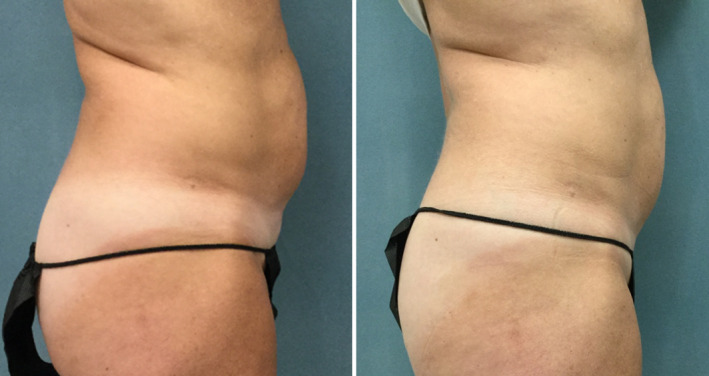
Illustration of treatment outcomes by digital photographs (57 y, 22.98 kg/m2). There is a noticeable improvement of the abdominal body contour seen at 1 mo (right) compared with baseline (left)
4. DISCUSSION
The study investigated the effects of a novel technology utilizing simultaneous delivery of synchronized radiofrequency heating and HIFEM stimulation on subcutaneous adipose tissue. Histological evaluation based on H&E staining showed prominent alterations within the adipose tissue at 1 week and 1 month post‐treatment in subjects who received active treatment. No changes in the tissue were seen in the sham patient.
The results indicate that the simultaneous treatment triggers lipolysis along with fat cell elimination. The reduced adipocyte size is strong evidence for lipolysis as the adipocytes lose their intracellular content due to the breakdown of triglycerides into glycerol and FFA, which are subsequently liberated into the blood stream. The intracellular content has been reduced by 33.5% at 1 week and by 31.7% at 1 month compared with baseline. Also, the mean cross‐sectional area at both follow‐ups shows relatively small variation (see Figure 4), indicating that decrease in adipocytes size was uniform across all cells rather than it was a matter of some extreme values or outliers. Supposedly, the lipolytic effect of the simultaneous treatment may be attributed to both modalities, HIFEM and RF, as radiofrequency heating is known to trigger the lipolysis, and the supramaximal contractions induced by HIFEM were documented to result in increased metabolic activity, also leading to the fat lipolysis. 21
Regarding adipocyte deletion, the presence of pyknotic nuclei, alternations of adipocytes shape, and absence of the inflammation are strong indicators of apoptotic changes happening in the tissue, since the pyknosis is irreversible and leads to nuclear fragmentation and cell death. Thus, the increased presence of pyknotic nuclei suggests elevated number of cells that entered the apoptotic process. This is also supported by the temperature measurements which have shown that the temperature in the fat tissue reaches 42°C after 4 minutes and remains between 43 and 45°C during the most of the treatment, effectively triggering the adipocyte apoptosis. 16 On the other hand, although the observed plasma membrane disintegration might be a consequence of apoptotic‐related decomposition, it also may indicate the presence of distinct regulated cell death pathways like pyroptosis. 9 However, the examination of the fat tissue at molecular level needs to prove this hypothesis which is beyond the scope of this study.
The efficacy of the simultaneous application of HIFEM and synchronized RF seen herein is in agreement with previous research that has shown a 29.8% reduction in the subcutaneous fat layer, as measured by ultrasound imaging system, and similar histological changes as seen in this study. 22 Future research can be used to look at programmed cell death markers for adipocytes 9 , 23 as well as to determine the duration of induced changes in a broader patient sample.
Individuals willing to improve their abdominal body contour may benefit from a mutual combination of the synchronized RF and HIFEM energies. This study documents that the simultaneous use of both investigated technologies is safe and results in noticeable body shaping effect, achieved primarily by the reduction in size and numbers of fat cells.
CONFLICT OF INTEREST
This study was funded by a research grant from BTL Industries Inc.
Goldberg DJ. Deletion of adipocytes induced by a novel device simultaneously delivering synchronized radiofrequency and hifem: Human histological study. J Cosmet Dermatol.2021;20:1104–1109. 10.1111/jocd.13970
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jimenez Lozano JN, Vacas‐Jacques P, Anderson RR, Franco W. Effect of Fibrous Septa in Radiofrequency Heating of Cutaneous and Subcutaneous Tissues: Computational Study: EFFECT OF FIBROUS SEPTA IN RF HEATING. Lasers Surg Med. 2013;45(5):326‐338. [DOI] [PubMed] [Google Scholar]
- 2. Sorisky A, Magun R, Gagnon A. Adipose cell apoptosis: death in the energy depot. Int J Obes. 2000;24(S4):S3‐S7. [DOI] [PubMed] [Google Scholar]
- 3. Prins JB, Walker NI, Winterford CM, Cameron DP. Apoptosis of Human Adipocytes in Vitro. Biochem Biophys Res Commun. 1994;201(2):500‐507. [DOI] [PubMed] [Google Scholar]
- 4. Jebb SA, Moore MS. Contribution of a sedentary lifestyle and inactivity to the etiology of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31(Supplement 1):S534. [DOI] [PubMed] [Google Scholar]
- 5. Field AE, Coakley EH, Must A, et al. Impact of Overweight on the Risk of Developing Common Chronic Diseases During a 10‐Year Period. Arch Intern Med. 2001;161(13):1581. [DOI] [PubMed] [Google Scholar]
- 6. Caruso‐Davis MK, Guillot TS, Podichetty VK, et al. Efficacy of low‐level laser therapy for body contouring and spot fat reduction. Obes Surg. 2011;21(6):722‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duncan RE, Ahmadian M, Jaworski K, Sarkadi‐Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53(6):482‐491. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28(1):9‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeyakumar SM, Vajreswari A, Sesikeran B, Giridharan NV. Vitamin A supplementation induces adipose tissue loss through apoptosis in lean but not in obese rats of the WNIN/Ob strain. J Mol Endocrinol. 2005;35(2):391‐398. [DOI] [PubMed] [Google Scholar]
- 11. Xu X, Lai Y, Hua Z‐C. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1):BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erwig L‐P, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15(2):243‐250. [DOI] [PubMed] [Google Scholar]
- 13. Boisnic S, Divaris M, Nelson AA, Gharavi NM, Lask GP. A clinical and biological evaluation of a novel, noninvasive radiofrequency device for the long‐term reduction of adipose tissue. Lasers Surg Med. 2014;46(2):94‐103. [DOI] [PubMed] [Google Scholar]
- 14. McDaniel D, Fritz K, Machovcova A, Bernardy J. A focused monopolar radiofrequency causes apoptosis: a porcine model. J Drugs Dermatol. 2014;13(11):1336‐1340. [PubMed] [Google Scholar]
- 15. Fenn AJ. Breast Cancer Treatment by Focused Microwave Thermotherapy. Burlington, MA: Jones & Bartlett Learning; 2007. [Google Scholar]
- 16. Franco W, Kothare A, Ronan SJ, Grekin RC, McCalmont TH. Hyperthermic injury to adipocyte cells by selective heating of subcutaneous fat with a novel radiofrequency device: Feasibility studies. Lasers Surg Med. 2010;42(5):361‐370. [DOI] [PubMed] [Google Scholar]
- 17. McDaniel D, Lozanova P. Human adipocyte apoptosis immediately following high frequency focused field radio frequency: case study. J Drugs Dermatol. 2015;14(6):622‐623. [PubMed] [Google Scholar]
- 18. Kent DE, Jacob CI. Simultaneous Changes in Abdominal Adipose and Muscle Tissues Following Treatments by High‐Intensity Focused Electromagnetic (HIFEM) Technology‐Based Device: Computed Tomography Evaluation. J Drugs Dermatol. 2019;18(11):1098‐1102. [PubMed] [Google Scholar]
- 19. Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: Safety and efficacy study of a dual tissue effect based non‐invasive abdominal body shaping: MRI EVALUATION OF ELECTROMAGNETIC THERAPY. Lasers Surg Med. 2019;51:40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinney BM, Kent DE. MRI and CT Assessment of Abdominal Tissue Composition in Patients After High‐Intensity Focused Electromagnetic Therapy Treatments: One‐Year Follow‐Up. Aesthet Surg J. 2020;40:NP686‐NP693. [DOI] [PubMed] [Google Scholar]
- 21. Halaas Y, Bernardy J. Mechanism of nonthermal induction of apoptosis by high‐intensity focused electromagnetic procedure: Biochemical investigation in a porcine model. J Cosmet Dermatol. 2020;19(3):605‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Food and Drug Administration . 510(k) Premarket Notification: K192224. Published online December 5, 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192224.pdf. Accessed September 24, 2020.
- 23. Kosacka J, Koch K, Gericke M, et al. The polygenetically inherited metabolic syndrome of male WOKW rats is associated with enhanced autophagy in adipose tissue. Diabetol Metab Syndr. 2013;5(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


