Abstract
Aim
When glycaemic control for people with type 2 diabetes is not achieved with metformin and sulfonylurea alone, adding another oral anti‐diabetes drug, such as a sodium–glucose co‐transporter 2 (SGLT2) or dipeptidyl peptidase‐4 (DPP‐4) inhibitor, is an alternative to starting insulin. The aim of this study is to determine the cost‐effectiveness of dapagliflozin (an SGLT2 inhibitor) compared with DPP‐4 inhibitors when added to metformin and sulfonylurea in people with type 2 diabetes in the Netherlands.
Methods
A cost–utility analysis is performed using the Cardiff diabetes model, a fixed‐time increment stochastic simulation model informed by ‘United Kingdom Prospective Diabetes Study 68’ risk equations. The base‐case analysis uses a 40‐year time horizon, a Dutch societal perspective and differential discounting (4% for costs, 1.5% for effects). Inputs are obtained from the literature and Dutch price lists. Univariate and probabilistic sensitivity analysis are performed.
Results
Dapagliflozin is dominant compared with DPP‐4 inhibitors, resulting in a €990 cost saving and a 0.28 quality‐adjusted life year gain over 40 years. Cost savings are associated mainly with treatment costs and a reduced incidence of micro‐ and macrovascular complications, among others nephropathy, myocardial infarction and stroke. Results are robust to changes in input parameters.
Conclusions
Dapagliflozin is a cost‐saving alternative to DPP‐4 inhibitors when added to metformin and sulfonylurea. The incidence of micro‐ and macrovascular complications is lower for people treated with dapagliflozin. Uncertainty around this outcome is low.
What’s new?
When glycaemic control for people with type 2 diabetes is not achieved with metformin and sulfonylurea alone, adding another oral anti‐diabetes drug, like a sodium–glucose co‐transporter 2 inhibitor or dipeptidyl peptidase‐4 (DPP‐4) inhibitor, is an alternative to starting insulin.
Dapagliflozin results in a €990 cost saving and a 0.28 quality‐adjusted life year gain over 40 years per person compared with DPP‐4 inhibitors, from a Dutch societal perspective.
The incidence of micro‐ and macrovascular complications (nephropathy, myocardial infarction, stroke) is lower for people treated with dapagliflozin, resulting in lower costs and better outcomes.
Dapagliflozin should be preferred over DPP‐4 inhibitors based on modelled outcomes as well as costs.
1. INTRODUCTION
Diabetes is one of the most prevalent diseases in the Netherlands with ~ 1.1 million patients in 2017, 91% of whom have type 2 diabetes. 1 Diabetes prevalence is expected to rise to ~ 1.5 million cases in the Netherlands by 2040. 2 In 2016, the economic burden of type 2 diabetes was €5.9 billion: €1.3 billion in direct healthcare costs, €1.1 billion in direct complication costs and €3.5 billion in indirect costs such as productivity losses. 3 Consequently, type 2 diabetes poses a substantial and increasing economic burden to the Netherlands, as it does in many other countries. 4
Current type 2 diabetes management is aimed at preventing diabetes‐related complications, among others by controlling blood‐glucose levels. When lifestyle interventions prove ineffective, Dutch primary care guidelines recommend a three‐step treatment approach: (1) starting metformin, (2) adding sulfonylurea and (3) adding insulin. 5 Because the first two steps are often insufficient to control blood glucose levels in the long term, many people resort to insulin treatment. In cases where insulin treatment is undesirable, current Dutch primary care guidelines recommend the use of dipeptidyl peptidase‐4 (DPP‐4) inhibitors for people with a BMI < 30 kg/m2, and the use of a DPP‐4 inhibitor or glucagon‐like peptide‐1 (GLP‐1) receptor agonist for people with a BMI ≥ 30 kg/m2. 5 However, in contrast to Dutch guidelines, international guidelines recommend considering addition of a sodium–glucose cotransporter 2 (SGLT2) inhibitor such as dapagliflozin to treatments with metformin. Furthermore, sulfonylurea should be prescribed only to people without atherosclerotic cardiovascular disease when cost is a critical concern. 6 Note that the Dutch diabetes guidelines, both for primary and specialty care, are currently being revised to reflect the latest evidence regarding SGLT2 inhibitors and GLP‐1 receptor agonists.
Given the current registration and reimbursement of dapagliflozin in the Netherlands, clinicians could consider adding it to treatment with metformin plus sulfonylurea (or metformin alone), as an alternative to DPP‐4 inhibitors. Randomized controlled trials show that the addition of dapagliflozin to metformin plus sulfonylurea is safe and effective. 7 , 8 A network meta‐analysis by Lozano‐Ortega et al. 9 concluded that SGLT2 inhibitors are at least as effective as other classes of anti‐diabetes agents at controlling blood glucose levels, while providing the additional benefits of weight loss and reducing systolic blood pressure (SBP). Only SGLT2 inhibitors and GLP‐1 receptor agonists led to a weight loss and decrease in SBP, all other treatments showed either an increase or no change. The network meta‐analysis presented in UK National Institute for Health and Care Excellence (NICE) technology appraisal 418 provides similar results. It reports a similar HbA1c effect between dapagliflozin (−2.23 mmol/mol, −0.85%) and DPP‐4 inhibitors (−2.22 mmol/mol, −0.79%). Dapagliflozin additionally results in weight loss and a decrease in SBP. 10
In the Netherlands, the reimbursement of dapagliflozin is restricted to people with type 2 diabetes who cannot be treated with the combination of metformin plus sulfonylurea alone, do not use insulin and will use dapagliflozin as dual or triple therapy in combination with metformin plus sulfonylurea. 11 Metformin plus sulfonylurea is a common treatment in the Netherlands. A 5‐year longitudinal study showed 84% of people with type 2 diabetes start biguanides (metformin). The next most common treatment (in 19% of cases) is biguanides + sulfonylurea. 12 Despite reimbursement of dapagliflozin when added to metformin plus sulfonylurea, use of dapagliflozin in the Netherlands is limited, partially due to cost considerations. 13 Dutch physicians are continuously challenged to deliver the highest standard of care within a limited budget. However, although ‘new’ diabetes treatments are often considered expensive, their cost‐effectiveness may be favourable when they result in savings elsewhere in the treatment pathway, 14 , 15 , 16 , 17 for example by preventing cardiovascular events.
Although use of SGLT2 inhibitors in Dutch clinical practice is limited, the proportion of patients using DPP‐4 inhibitors has increased from 1% in 2008 to 7% in 2013, after which the proportion stabilized. 18 This modelling study evaluates the cost‐effectiveness of dapagliflozin compared with DPP‐4 inhibitors (sitagliptin, linagliptin, vildagliptin and saxagliptin) when added to metformin plus sulfonylurea in people with type 2 diabetes in the Netherlands. The cost‐effectiveness of dapagliflozin has not been evaluated previously in this setting. The aim of this study is to inform treatment choices and clinical guideline development.
2. METHODS
2.1. The model
To determine the cost‐effectiveness of dapagliflozin compared with DPP‐4 inhibitors in this treatment setting, the Cardiff diabetes model is used. This fixed‐time increment stochastic microsimulation model (see structure in Fig. 1) has been described and validated previously, 19 , 20 and is one of the two most often applied diabetes models within Health Technology Assessment submissions. 21
Figure 1.
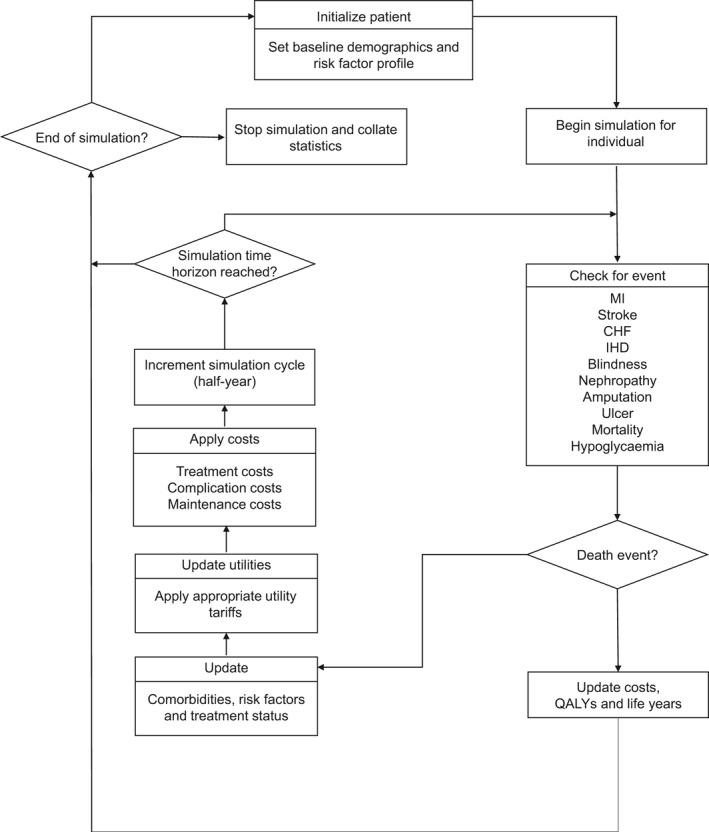
Cardiff type 2 diabetes model structure. MI, myocardial infarction; CHF, congestive heart failure; IHD, ischaemic heart disease; QALY, quality‐adjusted life year
The model uses the United Kingdom Prospective Diabetes Study 68 risk equations, consisting of seven Weibull proportional hazards models derived from a cohort of 5102 people with diabetes in the UK. 22 They estimate the likelihood of clinical events dependent on risk factors, including HbA1c levels, SBP, cholesterol levels and total bodyweight. Risk factors change over time as a result of treatment and natural progression, see Appendix S1. Treatment‐related changes are triggered when starting a new treatment after passing a predefined HbA1c threshold (see below).
There is a lack of data on the durability of treatment‐induced weight loss or gain in people with type 2 diabetes. The model assumes that patients who lose or gain weight due to treatment will maintain this change for the rest of their lives. Scenario analyses are performed to evaluate the impact of a more conservative assumption: complete loss of the first‐line weight effect within 1 or 5 years.
Modelled outcomes include survival, macrovascular events (myocardial infarction, stroke, ischaemic heart disease, congestive heart failure), microvascular events (amputation, end‐stage renal disease, blindness), treatment‐related adverse events (including hypoglycaemia), quality of life, costs and cost‐effectiveness. The model is adjusted to the Dutch situation, using Dutch sources for input parameters wherever possible. The simulations use a cohort of 10 000 people.
Following Dutch pharmacoeconomic guidelines, a societal perspective is taken including direct healthcare costs as well as productivity costs (see Table 1 for terminology). Discount rates are 4% for costs and 1.5% for effects, a lifetime horizon is used (implemented as 40 years) and cycle length is 6 months. 23 Given the chronic nature of the disease, 6‐month time increments allow for accurate estimation of the impact of clinical events, without making the simulation overly granular and slow.
Table 1.
Terminology
| Term | Meaning |
|---|---|
| Perspective | The perspective of an economic evaluation is the viewpoint from which the intervention’s costs and consequences are evaluated. When a societal perspective is taken, not only direct medical care costs are included (e.g. cost of the intervention and follow‐up treatment) but also broader costs to society (e.g. productivity losses resulting from poor health or premature death) [S14]. |
| Base‐case analysis | The base‐case analysis is the main analysis and reflects those model inputs that the authors consider most relevant. This is the analysis for which results are provided in detail. |
| Cycle length | Cycle length refers to the simulated time increments based on which costs and consequences are being calculated. |
| Deterministic sensitivity analyses | In deterministic, univariate sensitivity analyses, parameter values are varied manually to test the sensitivity of the model’s results to specific parameters or sets of parameters. The aim is to evaluate parameter uncertainty [S15]. |
| Discount rates | Discount rates are used to calculate the present value of future costs and consequences, to adequately reflect time preference [S14]. |
| (Dis)utility | Utility is a measure of quality of life, usually expressed on a scale from 0 to 1. Disutility is a loss in utility, resulting in reduced quality of life. |
| Friction cost method | Method to determine the costs of productivity losses, based on the period an employer needs to replace a sick employee 23 . |
| Probabilistic sensitivity analyses | In probabilistic sensitivity analyses, parameters are varied simultaneously, with multiple sets of parameter values being sampled from a priori defined probability distributions. The aim is to evaluate parameter uncertainty. |
| Quality‐adjusted life year | A quality‐adjusted life year is a preference‐based measure of health outcome that combines length of life and health‐related quality of life in a single metric. It is calculated by multiplying length of life with utility [S14]. |
| Time horizon | The time horizon refers to the length of time over which costs and consequences are being evaluated [S14]. |
The model considers multiple, consecutive treatment lines. People are assumed to switch to the next treatment line when they pass a predefined HbA1c threshold of 64 mmol/mol (8.0%). 24 Once this happens, dapagliflozin and DPP‐4 inhibitors are replaced by basal insulin glargine, matching Dutch clinical practice. The next step, after failing on basal insulin plus metformin and sulfonylurea, is an intensified insulin regimen. This includes bolus insulin and does not contain sulfonylurea. The intensified regimen is continued until death or end of the model time horizon.
2.2. The patient population
Baseline characteristics reflect a population of people with type 2 diabetes with insufficient glycaemic control [HbA1c between 53 and 91 mmol/mol (7.0% and 10.5%)] on metformin plus sulfonylurea. Baseline characteristics are largely obtained from Matthaei et al. 25 and the NICE technology appraisal 418 10 (Table 2). They are assumed to be representative of the Dutch type 2 diabetes population with insufficient glycaemic control on metformin plus sulfonylurea. A Dutch cohort 26 was used for ethnicity distribution and smoking in Dutch people with type 2 diabetes. Note that baseline characteristics are sampled independently and therefore the model does not account for the correlation between, for example, BMI, smoking and blood pressure. This may impact on the incidence of clinical events and uncertainty measured in the probabilistic sensitivity analyses (see below).
Table 2.
Patient characteristics at baseline on dual therapy with metformin and sulfonylurea
| Characteristic | Study | |
|---|---|---|
| Demographics | ||
| Age (years) | 61.0 (0.6) | Matthaei et al. (2015) 25 |
| Proportion female | 0.5 (0.0) | |
| Diabetes duration (years) | 9.5 (0.4) | |
| Height (m) | 1.68 (0.00) | NICE (2016) 10 |
| Proportion Afro‐Caribbean | 0.00 (0.00) | Hertroijs et al. (2017) 26 |
| Proportion Indian | 0.05 (0.00) | |
| Proportion smokers | 0.20 (0.00) | |
| Clinical risk factors | ||
| HbA1c (mmol/mol) | 66 | Matthaei et al. (2015) 25 |
| HbA1c (%) | 8.2 (0.1) | |
| Total cholesterol (mmol/l) | 175.9 (2.8) | |
| HDL cholesterol (mmol/l) | 46.4 (0.8) | |
| SBP (mmHg) | 135.4 (0.1) | |
| Weight (kg) | 89.4 (1.2) | |
| Clinical history | ||
| Atrial fibrillation | 0.0063 (0.0004) | NICE (2016) 10 |
| Peripheral vascular disease | 0.0047 (0.0003) | |
| Ischaemic heart disease | 0.0970 (0.0014) | |
| Myocardial infarction | 0.0250 (0.0008) | |
| Congestive heart failure | 0.0230 (0.0007) | |
| Stroke | 0.0180 (0.0006) | |
| Amputation | 0.0040 (0.0003) | |
| Blindness | 0.0220 (0.0007) | |
| End‐stage renal disease | 0.0100 (0.0005) | |
Values are given as mean (se).
2.3. Model inputs
2.3.1. Treatment effects
Treatment effects and their sources are provided in Table 3 (first line) and Table S1 (later lines). The evaluation takes a conservative approach by only including SGLT2‐related adverse events. The yearly percentage of people discontinuing treatment due to adverse events is assumed to be 1.8% for metformin plus sulfonylurea and dapagliflozin 25 and 2.9% for metformin plus sulfonylurea and a DPP‐4 inhibitor. 27
Table 3.
First line treatment effects and natural progression
| MET + SU + Dapa | MET + SU + DPP‐4 | Distribution in PSA | |||
|---|---|---|---|---|---|
| ΔHbA1c (mmol/mol | −2.23 (2.17) | NICE 10 | −2.22 (2.16) | NICE 10 | Normal |
| ΔHbA1c (%) | −0.85 (0.18) | −0.79 (0.06) | |||
| HbA1c delay in creep a | 0.00 (0.00) | Assumption | 0.00 (0.00) | Assumption | Not sampled |
| ΔSBP (mmHg) | −3.13 (4.33) | NICE 10 | 1.85 (5.17) | NICE 10 | Normal |
| ΔTotal cholesterol (mg/dl) | 6.05 (3.22) | Matthaei et al. (2015) 25 | 8.61 (0.86) | Schernthaner et al. (2013) 27 | Normal |
| ΔHDL cholesterol (mg/dl) | 3.20 (0.86) | Matthaei et al. (2015) 25 | 0.27 (0.40) | Schernthaner et al. (2013) 27 | Normal |
| ΔWeight (kg) | −2.20 (0.88) | NICE 10 | 0.12 (0.33) | NICE 10 | Normal |
| Years weight change maintained | 1.00 (0.00) | Assumption | 1.00 (0.00) | Assumption | Not sampled |
| Natural annual weight gain (kg) | 0.20 (0.00) | Assumption | 0.20 (0.00) | Assumption | Not sampled |
| Years to loss of effect | 0.00 (0.00) | Assumption | 0.00 (0.00) | Assumption | Not sampled |
| Probability of symptomatic hypoglycaemia | 0.12 (0.03) | Matthaei et al. (2015) 25 | 0.16 (0.02) | Hermansen et al. (2007) [S18] | Normal |
| Probability of nocturnal hypoglycaemia | 0.00 (0.00) | Assumption | 0.00 (0.00) | Assumption | Normal |
| Probability of severe hypoglycaemia | 0.00 (0.00) | Matthaei et al. (2015) 25 | 0.00 (0.00) | Hermansen et al. (2007) [S18] | Normal |
| Probability of UTI | 0.10 (0.03) | Matthaei et al. (2015) 25 | 0.06 (0.01) | Schernthaner et al. (2013) 27 | Normal |
| Probability of GI | 0.10 (0.03) | Matthaei et al. (2015) 25 | 0.02 (0.01) | Schernthaner et al. (2013) 27 | Normal |
| Probability of discontinuation | 0.02 (0.00) | Matthaei et al. (2015) 25 | 0.03 (0.00) | Schernthaner et al. (2013) 27 | Not sampled |
Values are given as mean (se).
Dapa, dapagliflozin; DPP‐4, dipeptidyl peptidase‐4 inhibitors; GI, genital infection; MET, metformin; PSA, probabilistic sensitivity analysis; SU, sulfonylurea; UTI, urinary tract infection.
Period following treatment before HbA1c progresses naturally.
2.3.2. Health‐related quality of life and costs
In the absence of Dutch utility values, age‐specific baseline utility is based on EQ‐5D measurements from a national survey by the UK Department of Health. 28 Disutilities associated with diabetes‐related events, treatment‐related events and BMI are obtained from published literature and are applied additively (Table 4).
Table 4.
Disutilities
| Input | Source | Distribution in PSA | |
|---|---|---|---|
| Diabetes‐related event disutility | |||
| Ischaemic heart disease | 0.04 (0.01) | Sullivan et al. 2016 [S19] (no Dutch source identified) | Beta |
| Myocardial infarction | 0.05 (0.01) | Sullivan et al. 2016 [S19] (no Dutch source identified) | Beta |
| Congestive heart failure | 0.20 (0.02) | Kraai et al. 2013 [S20], based on a mean utility of 0.68 in the total group | Beta |
| Stroke | 0.11 (0.01) | Visser et al. 2016 [S21], based on a mean utility of 0.77 in the control group | Beta |
| Amputation | 0.16 (0.02) | Redekop et al. (2004) [S22], based on a mean utility of 0.68 for diabetes with previous foot amputation and 0.84 for diabetes without foot ulcer or amputation | Beta |
| Blindness | 0.10 (0.01) | Langelaan et al. [S23] based on a mean utility of 0.64 for patients with diabetic retinopathy or other retinal vascular disease, subtracted from 0.74 (quality of life for Dutch people with type 2 diabetes [S24]) | Beta |
| End‐stage renal disease | 0.15 (0.01) | Mazairac et al. 2013 [S25], based on a mean utility of 0.735 (0.74 and 0.73 for haemodiafiltration and haemodialysis, respectively) | Beta |
| Treatment‐related event disutilities | |||
| Symptomatic hypoglycaemia | 0.01 (0.00) | Currie et al. (2006) [S26] (no Dutch source identified) | Not sampled |
| Nocturnal hypoglycaemia | 0.01 (0.00) | Currie et al. (2006) [S26] (no Dutch source identified) | Not sampled |
| Severe hypoglycaemia | 0.05 (0.00) | Currie et al. (2006) [S26] (no Dutch source identified) | Not sampled |
| UTI | 0.02 (0.00) | Van’t Hout et al. 2014 [S27], based on the decrement after the first UTI | Not sampled |
| GI | 0.02 (0.00) | Van’t Hout et al. 2014 [S27]: assumed equal to urinary tract infection, due to a lack of Dutch data on the disutility associated with genital tract infections. | Not sampled |
| BMI disutilities | |||
| BMI (per unit increase) | 0.01 (0.00) | Caro et al. (2007) [S28] (no Dutch source identified) | Beta |
| BMI (per unit decrease) | −0.01 (0.00) | Caro et al. (2007) [S28] (no Dutch source identified) | Beta |
GI, genital infection; PSA, probabilistic sensitivity analysis; UTI, urinary tract infection.
Costs are measured from a societal perspective and include medication costs, dispensing fees, consumables (e.g. needles, test strips, lancets), direct costs of disease and treatment‐related events, productivity costs and travel costs. These are provided in Table 5. Dutch costs were used whenever available. DPP‐4 inhibitor costs were a weighted average based on the Dutch market shares of sitagliptin, linagliptin, vildagliptin and saxagliptin. 29 Productivity costs are measured using the friction cost method with a maximum friction period of 85 days 23 (see Table 1 for terminology). Historic costs are inflated to 2018 using the consumer price index provided by Statistics Netherlands. 30
Table 5.
Costs
| Input | Source | Distribution in PSA | |
|---|---|---|---|
| Annual treatment costs, including dispensing fees | |||
| MET + SU + Dapa | €622 (€62) | National Health Care Institute (2016) 29 | Not sampled |
| MET + SU + DPP‐4 | €611 (€61) | National Health Care Institute (2016) 29 | Not sampled |
| MET + SU + insulin glargine, including consumables a | €881 (€88) | National Health Care Institute (2016) 29 | Not sampled |
| MET + insulin glargine + insulin bolus, including consumables b | €2239 (€224) | National Health Care Institute (2016) 29 | Not sampled |
| Direct costs of disease and treatment‐related events | |||
| Ischaemic heart disease | €5832 (€583) in Y1, €1767 (€177) in Y >1 | Clarke et al. (2008) [S29] | Gamma |
| Myocardial infarction | €19 708 (€1971) in Y1, €1198 (€120) in Y >1 | Greving et al. (2011) [S30] | Gamma |
| Myocardial infarction (fatal) | €17 540 (€1754) in Y1 | Greving et al. (2011) [S30] | Gamma |
| Congestive heart failure | €10 899 (€1090) in Y ≥1 | Postmus et al. (2011) [S31] | Gamma |
| Congestive heart failure (fatal) b | €9700 (€970) in Y1 | Assumption | Gamma |
| Stroke | €38 120 (€3812) in Y1, €4793 (€479) in Y >1 | Baeten et al. (2010) [S32] | Gamma |
| Stroke (fatal) | €21 729 (€2173) in Y1 | Baeten et al. (2010) [S32] | Gamma |
| Amputation | €16 790 (€1679) in Y1, €630 (€63) in Y >1 | Niessen et al. (2003) [S33] | Gamma |
| Amputation (fatal) c | €9570 (€957) in Y1 | Assumption | Gamma |
| Blindness | €2662 (€266) in Y ≥1 | Niessen et al. (2003) [S33] | Gamma |
| End‐stage renal disease | €87 699 (€8770) in Y ≥1 | De Vries et al. (2016) [S34] | Gamma |
| Symptomatic hypoglycaemia | €30 (€3) | Jönsson et al. (2006) [S35] | Not sampled |
| Nocturnal hypoglycaemia | €0 (€0) | Jönsson et al. (2006) [S35] | Not sampled |
| Severe hypoglycaemia | €537 (€54) | Jönsson et al. (2006) [S35] | Not sampled |
| UTI and GI | €86 (€9) | NHG (2013) [S36] | Not sampled |
| Discontinuation | €74 (€7) | Hakkaart‐van Roijen et al. (2016) [S37] | Not sampled |
| Annual indirect costs of disease and treatment‐related eventsd | |||
| Ischaemic heart disease | €1102 (€110) | Clarke et al. (2008) [S29] | Not sampled |
| Myocardial infarction | €8289 (€829) | Isaaz et al. (2010) [S38] | Not sampled |
| Congestive heart failure | €8289 (€829) | Ericson et al. (2011) [S39] | Not sampled |
| Stroke | €8289 (€829) | Lindgren et al. (2008) [S40] | Not sampled |
| Amputation | €6120 (€612) | Fisher et al. (2003) [S41] | Not sampled |
| Blindness | €8289 (€829) | Frick et al. (2003) [S42] | Not sampled |
| End‐stage renal disease | €8289 (€829) | Naim et al. (2010) [S43] | Not sampled |
Dapa, dapagliflozin; DPP‐4i, dipeptidyl peptidase‐4 inhibitors; GI, genital infection; MET, metformin; PSA, probabilistic sensitivity analysis; SU, sulfonylurea; UTI, urinary tract infection; Y1, year 1; Y >1, subsequent years; Y ≥1, year 1 and subsequent years.
Consumables include needles, test strips and lancets. Insulin costs in the table are based on a mean weight of 83.42 kg. In the model, insulin costs are calculated per simulated person, dependent on weight and weight change over time: €0.016/kg/day for insulin glargine, €0.034/kg/day for insulin glargine + insulin bolus.
Costs for fatal congestive heart failure are unknown. The ratio of costs for fatal compared with non‐fatal congestive heart failure was assumed to be equal to the ratio of costs for fatal compared with non‐fatal myocardial infarction.
Costs for fatal amputation are unknown. The ratio of costs for fatal compared with non‐fatal amputation was assumed to be equal to the ratio of costs for fatal compared with non‐fatal stroke.
Productivity losses are applied below the age of 66.
2.4. Sensitivity analyses
In addition to the base‐case analysis, deterministic univariate sensitivity analyses and probabilistic sensitivity analyses are performed to quantify the impact of parameter uncertainty. Tables 3, 4, 5 and Table S1 report which parameters are sampled in the probabilistic analyses, and which distributions are applied. Additionally, scenario analyses are performed including: (1) price decreases for DPP‐4 inhibitors, (2) a shorter time horizon, (3) analysis from a medical instead of societal perspective, (4) the use of Neutral Protamine Hagedorn insulin instead of insulin glargine consistent with Dutch guidelines [S1], (5) loss of weight effects after 1 or 5 years, (6) alternative HbA1c thresholds, (7) an equal effect on first year HbA1c reduction for dapagliflozin and DPP‐4 inhibitors, (8) alternative event costs, (9) alternative HbA1c reductions associated with the second treatment line, (10) twice the baseline proportion of patients with clinical history, and (11) alternative values for all disutilities and costs obtained from non‐Dutch sources.
3. RESULTS
3.1. Base case
People receiving dapagliflozin experience a reduced incidence of micro‐ and macrovascular events compared with people receiving DPP‐4 inhibitors. They also gain less weight and experience a reduced number of hypoglycaemic events, in exchange for a higher number of urinary tract infections and genital infections. Within a period of 40 years, people receiving dapagliflozin accrue on average 15.86 quality‐adjusted life years (QALY), compared with 15.59 QALY for people receiving DPP‐4 inhibitors. This results in a 0.28 QALY gain with dapagliflozin. This is mainly due to quality of life improvement associated with a lower BMI and the reduced incidence of micro‐ and macrovascular events. Combined with a €990 cost saving (total cost of €52 587 with dapagliflozin vs. €53 577 with DPP‐4 inhibitors) the incremental cost–utility ratio is −€3564 per QALY gained, meaning dapagliflozin is dominant (cheaper and more effective) to DPP‐4 inhibitors (Table 6). Cost savings are mainly associated with treatment costs and the reduced incidence of micro‐ and macrovascular complications. Note that treatment costs include all treatment lines, including insulin treatments for people who are no longer regulated on dapagliflozin or DPP‐4 inhibitors. For graphs of modelled risk factors over time, see Figs S1–S4. The peaks in Fig. S1 represent the modelled time to basal insulin and time to bolus insulin, as blood glucose levels will decrease once a new treatment line is initiated.
Table 6.
Base case incidence of events, costs, quality‐adjusted life years and incremental cost–utility ratio, 40‐year time horizon
| Dapagliflozin | DPP‐4 | Increment | ||||
|---|---|---|---|---|---|---|
| Events (n) | Costs (€) | Events (n) | Costs (€) | Events (n) | Costs (€) | |
| Microvascular complications | ||||||
| Blindness | 632 | 10 720 950 | 632 | 10 735 902 | 0 | −14 952 |
| Nephropathy | 425 | 114 905 112 | 431 | 119 590 568 | −6 | −4 685 456 |
| Amputation | 568 | 5 863 718 | 572 | 5 976 472 | −4 | −112 754 |
| Macrovascular complications | ||||||
| Ischaemic heart disease | 1172 | 15 806 158 | 1187 | 16 250 270 | −15 | −444 111 |
| Myocardial infarction | 2904 | 40 269 542 | 2936 | 41 244 101 | −32 | −974 559 |
| Congestive heart failure | 1092 | 50 604 512 | 1090 | 51 157 396 | −3 | −552 885 |
| Stroke | 974 | 36 347 339 | 987 | 37 538 215 | −14 | –1 190 876 |
| Hypoglycaemia a | 135 684 | 3 814 852 | 136 618 | 3 846 536 | −934 | −31 684 |
| UTI and GI | 208 | 619 409 | 5884 | 249 081 | 3676 | 370 327 |
| Treatment | 246 916 445 | 249 177 816 | −2 261 371 | |||
| Total | 525 868 037 | 535 766 358 | −9 898 320 | |||
| Average costs per person | 52 587 | 53 577 | −990 | |||
| Average number of QALYs accrued per person | 15.86 | 15.59 | 0.28 | |||
| Average number of LYs | 19.12 | 19.07 | 0.05 | |||
| ICUR (€/QALY) | −3564 (dominant) | |||||
DPP‐4, dipeptidyl peptidase‐4 inhibitor; GI, genital infection; ICUR, incremental cost‐utility ratio; LY, life year; QALY, quality‐adjusted life year; UTI, urinary tract infection.
Includes symptomatic, nocturnal and severe hypoglycaemic events.
3.2. Sensitivity and scenario analyses
Univariate sensitivity analyses show that the incremental cost–utility ratio remains dominant under all tested parameter variations (Table 7; Fig. S5). Variations in SBP at baseline have the strongest effect on the incremental cost–utility ratio. Cost savings with dapagliflozin are highest for people with high baseline SBP. If the price of DPP‐4 inhibitors drops due to generic entry (expected shortly in the Netherlands), dapagliflozin would still be cost‐saving (with a 50% price reduction) or cost‐effective (with a 75% or 90% price reduction) (Table S2). At a willingness‐to‐pay threshold of €20 000 per QALY, the probabilistic sensitivity analysis indicates that dapagliflozin is cost‐effective compared with DPP‐4 inhibitors in 99.9% of simulations (Figs 2 and 3).
Table 7.
Main outcomes for the univariate sensitivity analyses
| Δ Cost (€) | Δ QALY | ICUR (€/QALY) | |
|---|---|---|---|
| Base case | −990 | 0.28 | −3564 |
| Sensitivity analyses | |||
| Baseline SBP, upper threshold (+25%) | −1495 | 0.26 | −5721 |
| Baseline SBP, lower threshold (−25%) | −438 | 0.24 | −1844 |
| Discount rates, upper threshold (6%) | −830 | 0.17 | −4764 |
| Discount rates, lower threshold (0%) | −1460 | 0.29 | −5112 |
| BMI utility, upper threshold (+25%) | −987 | 0.34 | −2933 |
| BMI utility, lower threshold (−25%) | −987 | 0.23 | −4374 |
| All event costs, upper threshold (+25%) | −1180 | 0.28 | −4200 |
| All event costs, lower threshold (−25%) | −793 | 0.28 | −2823 |
| Weight treatment effect, upper threshold (+25%) | −1066 | 0.34 | −3167 |
| Weight treatment effect, lower threshold (−25%) | −922 | 0.23 | −4092 |
| Baseline age, upper threshold (70 years) | −604 | 0.20 | −3039 |
| Baseline age, lower threshold (40 years) | −1291 | 0.33 | −3912 |
| HbA1c treatment effects, upper threshold (+25%) | −900 | 0.28 | −3269 |
| HbA1c treatment effects, lower threshold (−25%) | −1041 | 0.28 | −3757 |
| Baseline weight, upper threshold (+25%) | −907 | 0.25 | −3635 |
| Baseline weight, lower threshold (−25%) | −860 | 0.24 | −3546 |
ICUR, incremental cost‐utility ratio; QALY, quality‐adjusted life year.
Figure 2.
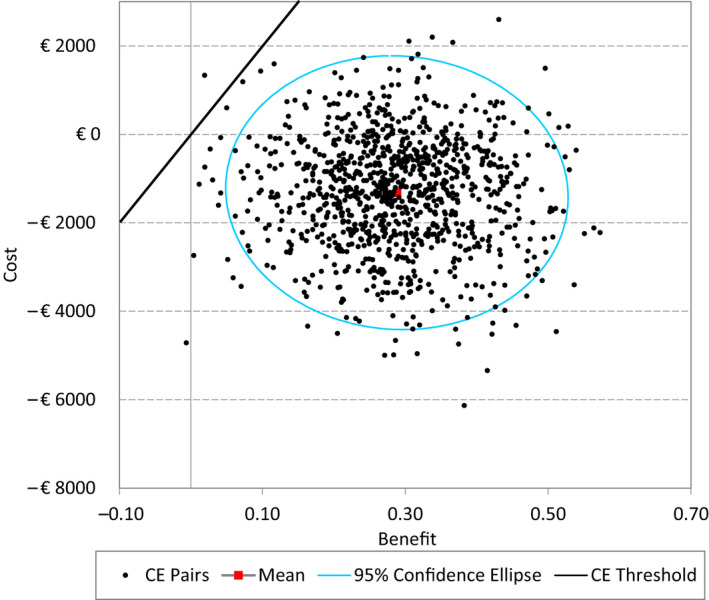
Probabilistic sensitivity analysis, cost‐effectiveness plane
Figure 3.
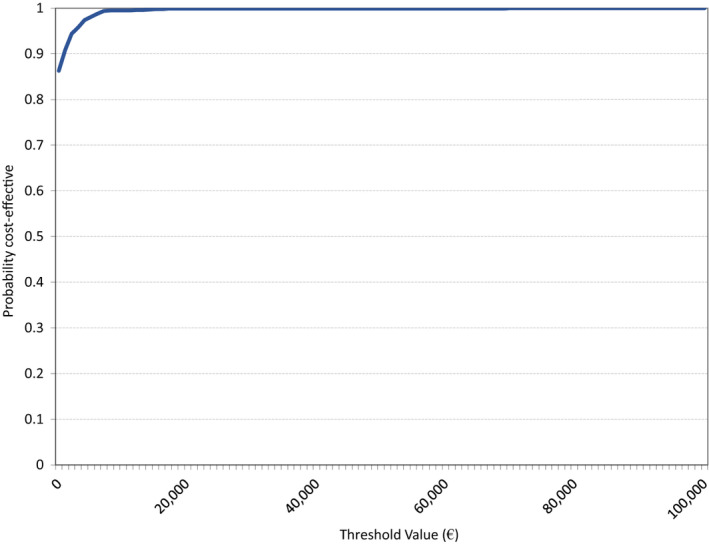
Probabilistic sensitivity analysis, cost‐effectiveness acceptability curve
4. DISCUSSION
Dapagliflozin is a cost‐saving alternative to DPP‐4 inhibitors when added to metformin plus sulfonylurea. This holds true at short as well as long time horizons, even when the price for DPP‐4 inhibitors is halved. Incidence of micro‐ and macrovascular complications is lower for people treated with dapagliflozin. People receiving dapagliflozin do experience a higher number of urinary tract infections and genital infections, against a reduced number of hypoglycaemic events. Additionally, QALY gains with dapagliflozin are due to its impact on weight, caused both by the treatment with dapagliflozin itself (associated with weight reduction), and the delay in treatment with insulin (associated with weight gain). Based on deterministic and probabilistic sensitivity analyses, uncertainty around these outcomes is low.
The difference in total costs is largely due to higher treatment costs in the DPP‐4 inhibitor arm and the lower cardiovascular and renal event costs with dapagliflozin. Costs of insulin treatment are relatively high, resulting in increasing treatment costs over time. On average, people in the DPP‐4 inhibitor arm required basal insulin earlier compared with people in the dapagliflozin arm, due to the larger HbA1c effect with dapagliflozin. Similarly, on average, people in the DPP‐4 inhibitor arm required a combination of basal and bolus insulin earlier compared with people in the dapagliflozin arm.
Previous cost‐effectiveness studies did not compare dapagliflozin with DPP‐4 inhibitors when added to metformin and sulfonylurea. Charokopou et al. 15 did, however, compare dapagliflozin with DPP‐4 inhibitors as dual therapy with metformin. They find a small QALY gain with dapagliflozin, driven by treatment‐induced weight differences between the treatment arms. By contrast, they present higher costs associated with dapagliflozin treatment. Although in both studies drug acquisition costs are higher for dapagliflozin compared with DPP‐4 inhibitors, in Charokopou et al. this difference is not offset by higher treatment costs for DPP‐4 inhibitors in later treatment lines. Costs associated with insulin use were assumed to be lower in the study by Charokopou et al., which would serve as potential explanation.
Our economic evaluation has several limitations. Only two types of adverse events were included (genital and urinary tract infections), both occurring more frequently in treatment with dapagliflozin. A potentially more severe adverse event associated with dapagliflozin is ketoacidosis, which was excluded from the analysis. Because ketoacidosis is a rare adverse event in people with type 2 diabetes, it is not expected to significantly impact cost‐effectiveness estimates, even though utility and cost impact on patients experiencing this event would be large.
However, none of the DPP‐4 inhibitor‐associated adverse events were included. These include, among others, nasopharyngitis, upper respiratory tract infection and headache [S2,S3]. If DPP‐4 inhibitor‐associated adverse events would have been included, QALY gains and cost savings with dapagliflozin compared with DPP‐4 inhibitors would likely be higher.
Utility is modelled in an additive fashion, such that a combination of multiple disutilities may cause an individual person’s utility to drop below zero. Low utilities are especially common at long time horizons, given ageing, the assumed weight gain of people over time, and the associated weight‐related disutility. It may be more realistic to place a cap on the maximum weight‐related disutility a person experiences, because the impact of weight gain on quality of life may become smaller over time or after a certain threshold. Unfortunately, not much is known about the association between weight and utility, and further data collection would allow for model improvements. Capping weight‐related utility would likely benefit the DPP‐4 inhibitor arm, because dapagliflozin is associated with weight reduction.
Another limitation of the current model is the use of United Kingdom Prospective Diabetes Study 68 data to inform all risk equations. This data set is relatively old and only allows for the prediction of the first event in any single category of diabetes‐related complications. 22 Concerns regarding the generalizability of model results to the Dutch situation are discussed in Appendix S2.
The current cost‐effectiveness analysis compares dapagliflozin with DPP‐4 inhibitors as add‐on to metformin plus sulfonylurea, international guidelines suggest that the optimal position of dapagliflozin may be earlier in the treatment pathway, as add‐on to metformin instead of metformin plus sulfonylurea. The cost‐effectiveness of dapagliflozin vs. sulfonylurea from a Dutch perspective will be evaluated in a future study. Next to the current economic evaluation and the comparison of metformin + dapagliflozin vs. metformin + sulfonylurea, various other comparisons would be valuable to inform treatment choices and clinical guideline development. These include comparisons between the various SGLT2 inhibitors (e.g. dapagliflozin vs. canagliflozin, empagliflozin and ertugliflozin), as well as comparisons against the various other treatment options such as GLP‐1 receptor agonists. The current evaluation was focused on the comparison with DPP‐4 inhibitors given the relatively common use of these drugs in Dutch clinical practice. 18
This study indicates that dapagliflozin is effective and cost‐saving compared with DPP‐4 inhibitors in combination with metformin and sulfonylurea in the treatment of Dutch people with type 2 diabetes who are inadequately controlled on metformin and sulfonylurea alone.
COMPETING INTERESTS
SN and NvdL are employed by AstraZeneca, the market authorization holder of dapagliflozin and saxagliptin. SvO has performed an internship at AstraZeneca.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ad Antonisse, Carolien Zanstra and Marlies Dopmeijer for their help with modelling assumptions and paper review.
Funding information
AstraZeneca BV funded salaries from authors NvdL and SN, and provided internship reimbursement for SvO.
REFERENCES
- 1. RIVM . Prevalentie diabetes naar leeftijd en geslacht, 2016. Available at https://www.volksgezondheidenzorg.info/onderwerp/diabetes‐mellitus/cijfers‐context/huidige‐situatie#node‐prevalentie‐diabetes‐naar‐leeftijd‐en‐geslacht Last accessed 1 May 2020. [Google Scholar]
- 2. RIVM . VTV, 2018. Available at https://www.vtv2018.nl/en/diseases Last accessed 1 May 2020. [Google Scholar]
- 3. Peters M, Huisman E, Schoonen M, Wolffenbuttel B. The current total economic burden of diabetes mellitus in the Netherlands. Neth J Med. 2017;75:281‐297. [PubMed] [Google Scholar]
- 4. Bommer C, Heesemann E, Sagalova V, Manne‐Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost‐of‐illness study. Lancet Diabetes Endocrinol. 2017;5:423‐430. [DOI] [PubMed] [Google Scholar]
- 5. NHG . NHG‐Standaard Diabetes Mellitus Type 2, 2013. Available at https://www.nhg.org/standaarden/volledig/nhg‐standaard‐diabetes‐mellitus‐type‐2 Last accessed 1 May 2020. [Google Scholar]
- 6. Davies MJ. Management of hyperglycaemia in type 2 diabetes: the 2018 consensus report by ADA/EASD Insights from one of the authors. Br J Diabetes. 2018;18:137‐140. [Google Scholar]
- 7. Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S. Dapagliflozin improves glycemic control and reduces body weight as add‐on therapy to metformin plus sulfonylurea: a 24‐week randomized, double‐blind clinical trial. Diabetes Care. 2015;38:365‐372. [DOI] [PubMed] [Google Scholar]
- 8. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lozano‐Ortega G, Goring S, Bennett H, Bergenheim K, Sternhufvud C, Mukherjee J. Network meta‐analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr Med Res Opin. 2016;32:807‐816. [DOI] [PubMed] [Google Scholar]
- 10. National Institute of Health and Care Excellence . Dapagliflozin in Triple Therapy Regimens for Treating Type 2 Diabetes, 2016. Available at https://www.nice.org.uk/guidance/ta418/resources/dapagliflozin‐in‐triple‐therapy‐for‐treating‐type‐2‐diabetes‐pdf‐82604609944261 Last accessed 1 May 2020. [Google Scholar]
- 11. ZIN . Kosten en regelgeving – regeling zorgverzekering, 2018. Available at https://www.farmacotherapeutischkompas.nl/algemeen/regeling‐zorgverzekering Last accessed 1 July 2018. [Google Scholar]
- 12. Van den Heuvel JM, Farzan N, van Hoek M. Maitland‐van der Zee A‐H, Ahmadizar F. Mining treatment patterns of glucose‐lowering medications for type 2 diabetes in the Netherlands. BMJ Open Diabetes Res Care. 2020;8:e000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Visser J. Artsen zuinig met nieuwe diabetesmiddelen, 2016. Available at https://www.medischcontact.nl/nieuws/laatste‐nieuws/artikel/‐artsen‐zuinig‐met‐nieuwe‐diabetesmiddelen‐.htm Last accessed 1 May 2020. [Google Scholar]
- 14. Van Haalen HG, Pompen M, Bergenheim K, McEwan P, Townsend R, Roudaut M. Cost effectiveness of adding dapagliflozin to insulin for the treatment of type 2 diabetes mellitus in the Netherlands. Clin Drug Investig. 2014;34:135‐146. [DOI] [PubMed] [Google Scholar]
- 15. Charokopou M, McEwan P, Lister S, Callan L, Bergenheim K, Tolley K, et al. The cost‐effectiveness of dapagliflozin versus sulfonylurea as an add‐on to metformin in the treatment of Type 2 diabetes mellitus. Diabet Med. 2015;32:890‐898. [DOI] [PubMed] [Google Scholar]
- 16. Sabapathy S, Neslusan C, Yoong K, Teschemaker A, Johansen P, Willis M. Cost‐effectiveness of canagliflozin versus sitagliptin when added to metformin and sulfonylurea in type 2 diabetes in Canada. J Popul Ther Clin Pharmacol. 2016;23:e151‐e168. [PubMed] [Google Scholar]
- 17. Ravasio R, Pisarra P, Porzio R, Comaschi M. Economic evaluation of canagliflozin versus glimepiride and sitagliptin in dual therapy with metformin for the treatment of type 2 diabetes in Italy. Glob Reg Health Technol Assess. 2016;3:92‐101. [Google Scholar]
- 18. Heintjes E, Houben E, Beekman‐Hendriks W, Lighaam E, Cremers S, Penning‐van Beest F, et al. Trends in mortality, cardiovascular complications, and risk factors in type 2 diabetes. Neth J Med. 2019;77:317‐329. [PubMed] [Google Scholar]
- 19. McEwan P, Peters JR, Bergenheim K, Currie CJ. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost‐utility model (DiabForecaster). Curr Med Res Opin. 2006;22:121‐129. [DOI] [PubMed] [Google Scholar]
- 20. The Mount Hood 4 Modeling Group . Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care. 2007;30:1638. [DOI] [PubMed] [Google Scholar]
- 21. Charokopou M, Sabater F, Townsend R, Roudaut M, McEwan P, Verheggen B. Methods applied in cost‐effectiveness models for treatment strategies in type 2 diabetes mellitus and their use in Health Technology Assessments: a systematic review of the literature from 2008 to 2013. Curr Med Res Opin. 2016;32:207‐218. [DOI] [PubMed] [Google Scholar]
- 22. Clarke P, Gray A, Briggs A, Farmer A, Fenn P, Stevens R, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47:1747‐1759. [DOI] [PubMed] [Google Scholar]
- 23. ZIN . Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg, 2016. Available at https://www.zorginstituutnederland.nl/over‐ons/publicaties/publicatie/2016/02/29/richtlijn‐voor‐het‐uitvoeren‐van‐economische‐evaluaties‐in‐de‐gezondheidszorg Last accessed 1 May 2020. [Google Scholar]
- 24. Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, Forciea MA, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569‐576. [DOI] [PubMed] [Google Scholar]
- 25. Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E, et al. Durability and tolerability of dapagliflozin over 52 weeks as add‐on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015;17:1075‐1084. [DOI] [PubMed] [Google Scholar]
- 26. Hertroijs DF, Elissen AM, Brouwers MC, Schaper NC, Köhler S, Popa MC, et al. A risk score including body mass index, glycated haemoglobin and triglycerides predicts future glycaemic control in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:681‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52‐week randomized trial. Diabetes Care. 2013;36:2508‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. University College London . Health Survey for England, 2003. Available at 10.5255/UKDA-SN-5098-1 Last accessed 1 May 2020. [DOI] [Google Scholar]
- 29. ZIN . GIPdatabank, 2016. Available at https://www.gipdatabank.nl/ Last accessed 1 May 2020. [Google Scholar]
- 30. Statistics Netherlands . Consumentenprijzen; prijsindex, 2018. Available at https://opendata.cbs.nl/statline/#/CBS/nl/ Last accessed 1 May 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


