Abstract
Multiparameter flow cytometry plays an important role in the diagnosis, staging, and monitoring of patients with a suspected hematological malignancy. The ClearLLab 10C Panels consist of four reagent panels (B‐Lineage Tube, T‐Lineage Tube, and 2 Myeloid Lineage Tubes), each consisting of 10 color/10 antibody conjugates utilizing Beckman Coulters proprietary dry format optimized for investigating patients with suspected leukemia or lymphoma. A multicenter study was conducted to evaluate the performance of the ClearLLab 10C Panels for qualitative assessment of normal versus abnormal phenotype in peripheral blood, bone marrow, and lymph node samples with suspected hematological malignancies. ClearLLab 10C was compared to laboratory developed tests (LDTs) and final clinical diagnosis. Four clinical sites were used to enroll patient's spent specimens (n = 453); three laboratories in North America and one in Europe. Of the 453 specimens, 198 had no malignancy and 255 contained an abnormal population. The diagnostic accuracy of the ClearLLab 10C Panels was achieved with sensitivity of 96% and specificity of 95% with respect to patient final clinical diagnosis. The agreement of phenotyping between ClearLLab10C Panels and LDTs was 98%. Any differences noted between ClearLLab 10C and LDT were due to either the presence of populations below the level of detection, the lack of clinical information provided to the evaluators, or marker(s) not present in these panels. Overall, the ClearLLab 10C demonstrated excellent agreement to LDTs and diagnosis. These four reagent panels can be adopted by individual laboratories to assess the presence or absence of malignancy.
Keywords: flow cytometry, immunophenotyping, leukemia, lymphoma, multiparameter
1. INTRODUCTION
Flow cytometric analysis of blood, bone marrow, and tissue specimens with suspected hematologic malignancies has been shown to be an important complimentary laboratory test (Arber et al., 2016; Craig & Foon, 2008; Davis, Holden, & Mea, 2007; Orfao et al., 1999; Swerdlow et al., 2008; Swerdlow et al., 2016; Swerdlow et al., 2017). Final diagnosis requires a comprehensive assessment based on clinical features, morphology cytochemistry, karyotyping, FISH, and molecular genetic analyses.
In the past few years, a number of standardized in vitro diagnostic (IVD) panels for leukemia and lymphoma immunophenotyping by flow cytometry have allowed laboratories that may lack the necessary expertise to design and validate a similar panel to run more complex flow cytometry testing. The first of these FDA cleared reagents was the ClearLLab Reagents Panel which consists of five 5 color tubes designed to run on the Beckman Coulter FC500 (Hedley et al., 2018). Since the release of the FC500 in the early 2000s flow cytometry instrumentation has advanced with 10 and 12 color systems now routinely available allowing for more complex testing to be performed with even fewer tubes (Hedley, Keeney, Popma, & Chin‐Yee, 2015; Jacob et al., 2017; Johansson et al., 2014; Porwit & Rajab, 2015; Rajab, Axler, Leung, Wozniak, & Porwit, 2017; van Dongen et al., 2012; Wood, 2006). In light of this, the ClearLLab LS (Beckman Coulter Inc., Miami, FL) screening tube was developed to eliminate unnecessary testing in samples that did not contain abnormal lymphoid populations and identify those that would require additional immunophenotyping (Hedley et al., 2018). As with other screening tubes (e.g., BD's OneFlow LST; Moloney et al., 2019), there are limitations with the ClearLLab LS (Hedley et al., 2015; Moloney et al., 2019). As predominately a lymphoid screening tube it does not contain all the necessary antigens to completely characterize an abnormal population. Subsequent to the detection of an abnormal lymphoid population in most circumstances, the laboratory would require at least one subsequent tube following the initial screen to fully characterize the abnormal population. In this study, we evaluated the Beckman Coulter ClearLLab 10C system (Beckman Coulter), which consists of four reagent panels (B Cell Tube, T Cell Tube, M1 Cell Tube, and M2 Cell Tube), each consisting of 10 color/10 antibody conjugates in dry format (Table 1) as a way to more comprehensively characterize specimens sent for flow cytometric testing.
TABLE 1.
The ClearLLab 10C Panels consist of four reagent panels (B Cell Tube, T Cell Tube, M1 Cell Tube, and M2 Cell Tube), each consisting of 10 color/10 antibody conjugates utilizing DURA technology dry format
| Blue laser (488 nm) | Red laser (638 nm) | Violet laser (405 nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FITC | PE | ECD | PE–Cy5.5 | PE‐Cy7 | APC | APC‐A700 | APC‐A750 | PacB | KrO | |
| B Cell Tube | Kappa | Lambda | CD10 | CD5 | CD200 | CD34 | CD38 | CD20 | CD19 | CD45 |
| T Cell Tube | TCRγδ | CD4 | CD2 | CD56 | CD5 | CD34 | CD7 | CD8 | CD3 | CD45 |
| M1 Cell Tube | CD16 | CD7 | CD10 | CD13 | CD64 | CD34 | CD14 | HLA‐DR | CD11b | CD45 |
| M2 Cell Tube | CD15 | CD123 | CD117 | CD13 | CD33 | CD34 | CD38 | HLA‐DR | CD19 | CD45 |
The 4 ten color tubes were specifically designed to allow for versatile recognition of various cell populations. Selected antibody combinations were based on publications, Bethesda guidelines, and known reactivity with different cell populations (Davis et al., 2007; Swerdlow et al., 2016; Swerdlow et al., 2017; Wood, 2005). For example, the B‐cell tube was designed for identification of B‐cell maturation from the blast stage (CD19 + CD10 + CD34+, CD20−) to terminal differentiation as a plasma cell (CD19+, SIg−, and CD38 bright), as well as, to detect a monoclonal B‐cell population (CD19 and/or CD20 positive) with or without expression of CD5 and CD10, and with restricted light chain expression (Craig & Foon, 2008). The addition of CD200 is useful in discriminating chronic lymphocytic leukemia from Mantle cell lymphoma, with the former positive and the latter negative for this surface antigen (Palumbo et al., 2009). CD34 was present in all four tubes to allow detection of the majority of blast cell populations, as well as, providing a constant backbone (together with CD45 expression) to identify these populations across multiple tubes. The T cell tube defined all major subsets of T cells and NK cells with the additional advantage of containing an antibody directed to the γδ chain in T cells, useful in detecting T‐cell large granular lymphocyte populations. The two myeloid tubes were designed to cover myelomonocytic maturation (M1) and maturation of myeloid blasts (M2). The presence of multiple monocyte markers in M1 is important as both CD14 and CD16 are GPI linked and are negative in PNH clones (Sutherland et al., 2018). CD7 that is putatively a T cell marker may be expressed in early myelomonocytic cells and has been linked to a poorer prognosis when co‐expressed with CD14 (Del Poeta et al., 1995). The combination of CD15, CD34, CD123, CD117, and HLA‐DR in the M2 tube, combined with classic light scatter changes, is very useful for detecting acute promyelocytic leukemia, which requires both morphology and cytogenetics to confirm. Initial evaluation performed by the manufacturer, determined the appropriate fluorochromes for each cell surface marker which were optimized for signal to noise while minimizing fluorochrome overlap.
This multicenter research study used a standardized setup protocol, a fixed leukemia and lymphoma immunophenotyping panel and standardized analysis templates. The ClearLLab 10C system contains quality control material (ClearLLab Control Cells), which can be used as staining controls for individual laboratories or for a lot comparison to different sites. This complete system was designed for the Beckman Coulter Navios flow cytometer and has recently received FDA approval. As it was specifically designed for the Navios, evaluation using other flow cytometers was not assessed.
The goal of this study was to test the effectiveness of the ClearLLab 10C Panel to successfully identify and characterize normal and abnormal populations from specimens submitted for routine flow cytometry testing in the laboratory setting across four sites. Both normal and abnormal populations' phenotype and lineage was evaluated using all four tubes from the ClearLLab 10C panel thereby providing a comprehensive assessment of immunophenotype. While it may not be necessary to run all four tubes in routine practice on all samples, one of the aims of the study was to ensure that normal samples did not express aberrant staining with the combinations utilized and, to examine samples with the presence of nonlineage specific antigen expression (e.g., presence of CD13 or CD33 in B‐ALL; Drexler, Thiel, & Ludwig, 1991).
2. MATERIALS AND METHODS
2.1. Sites
Four clinical sites participated in this study to enroll patients in the evaluation of the ClearLLab 10C system; three laboratories in North America and one in Europe. Protection of human subjects was maintained according to national and international standards for the conduct of clinical studies including 21CFR Parts 50 and 56 and International Conference on Harmonization (ICH) E6—Good Clinical Practice Consolidated Guideline. The study was registered in the National Library of Medicine database for clinical trials (https://clinicaltrials.gov/) with the record number of NCT03413644. Ethical approval for this study was obtained at each of the clinical sites.
2.2. Specimens
A total of 453 specimens (198 without malignancy, 255 containing an abnormal population) were included in this study from subjects having, or suspected of having a hematological malignancy. For this study, residual sample was used after routine clinical workup had been performed and included specimens from patients with: chronic leukemia (n = 67, 26%), acute leukemia (n = 46, 18%), non‐Hodgkin lymphoma (NHL) (n = 98, 38%), plasma cell neoplasms (PCN) (n = 18, 7%), myelodysplastic syndromes (MDS) (n = 17, 7%), myeloproliferative neoplasms (MPN) (n = 4, 2%), and five cases (2%) classified as a hematological subtype outside of the categories above. Types of specimens included in this study were peripheral whole blood (PB), bone marrow (BM), and lymph node (LN) samples. PB and BM samples were collected in ACD, K2ETDA, or Heparin. The distribution of samples was PB (n = 214, 47%), BM (n = 182, 40%), and LN (n = 57, 13%).
2.3. Flow cytometers and quality control
Navios and Navios EX flow cytometers (Beckman Coulter) were used in this study and were maintained and quality controlled per the manufacturer's instructions. Flow Check Pro Fluorospheres, which monitor the C.V. for each parameter, and Flow Set Pro Fluorospheres fluorescent microspheres (Beckman Coulter) with set targets for each PMT for this application (provided by the manufacturer and applicable on either the Navios or Navios EX) were run daily to monitor the instrument performance. All control results were captured on Levy‐Jennings plots and reviewed daily. Samples were not run if any parameter fell outside of the established ranges provided by the manufacturer. As all sites used the same reagent combinations and either the Navios or Navios EX flow cytometer (the Navios EX being an update to the Navios) standardized settings could be used. In addition, a daily process control using ClearLLab Control Cells was performed to ensure appropriate sample preparation, staining, and acquisition.
This stabilized product mimics whole blood (contains both erythrocytes and leukocytes), is prepared, and acquired exactly as a patient specimen would be. Importantly, this product has very similar light scatter and antigen staining characteristics as those of patient specimens. There are two preparations of ClearLLab control cells; the first is a normal preparation replicating the characteristics of clinical specimens (e.g., lysing, light scatter, antigen expression, and antibody staining properties). The second is the normal preparation spiked with a defined percentage of CD34 positive cells. Analysis protocols, assay ranges for the control cells and Levy‐Jennings plots were all supplied with the product. For QC to pass (allowing study specimens to be run) both control preparations had to be within the assay assigned ranges for the lots of ClearLLab control cells. The quality control package with Kaluza C software contained all the limits for each of the populations assessed and is supplied with each lot of control material for both normal and abnormal control cells.
2.4. Compensation of the ClearLLab 10C panels
Target channels were provided by Beckman Coulter and distributed to each laboratory, they were selected to achieve optimal signal for both dim and bright antigens. Once theses target channels were achieved using FlowSet Pro beads the voltages remained unchanged for 1 month and monitored daily (with tolerances for acceptability). In this study, the voltages were manually adjusted to achieve the desired target channels for each detector.
Using ClearLLab Compensation Beads, 10‐color compensation was performed with a 10 color dried compensation kit (ClearLLab Compensation Kit, Beckman Coulter). The dried compensation kit came with 10 individual single color tubes (CD4 FITC, CD4 PE, CD3 ECD, CD4 PE‐Cy5.5, CD4 PECy7, CD4 APC, CD4 APC‐Alexa700, CD4 APC‐Alexa750, CD4 PacB, and CD8 KrO) for determining the compensation matrix. In brief, one drop of positive bead and one drop of negative bead were added directly to each tube in the compensation kit, vortexed thoroughly, and incubated for 15 min in the dark. The Antibody Capture Positive Beads contains beads coated with an IgG‐binding agent that will bind mouse isotypes, whereas the Antibody Capture Negative Beads acts as a negative control and do not bind fluorochrome‐conjugated antibodies. Post incubation each tube was washed once with 0.3% bovine serum albumin (BSA) in phosphate buffered saline. After centrifugation, the supernatant was decanted and samples re‐suspended in 1 mL of phosphate buffered saline (PBS) with 0.02% sodium azide and 0.2% BSA. Compensation tubes were run with the fixed voltages, established previously by running FlowSet Pro beads to attain target channel (described above).Verification of the compensation matrix was performed by analyzing a sample stained with all 4 ClearLLab 10C tubes. If required any small adjustment was made to the matrix for each tube individually, this was then saved as the final compensation matrix for that tube. Compensation was monitored daily using the ClearLLab control cells as the reagents provide extremely stable antigen expression on both negative and positive populations. Compensation was checked daily by visual examination of these populations and also by confirming the relative percentages as captured in the Levy Jennings plots. In addition, each patients sample was assessed for appropriate staining of expected negative and positive populations. Full compensation was performed monthly as defined by the study parameters. During the study, should there have been any major changes to instrument hardware or optics, full compensation would have been performed; however, this was not the case.
2.5. ClearLLab 10C panels application
Briefly, 100 μL of specimen (2–20 × 109 cells/L) was prewashed to avoid plasma/serum protein interferences prior to sample staining with all 4 ClearLLab 10C panels according to the manufacturer's recommendations. All specimens were evaluated for cell concentration and viability, diluted or concentrated if needed to be within the range specified by the manufacturer. Samples were prewashed three times in PBS with 2% heat inactivated fetal calf serum (FCS) then stained using the ClearLLab 10C Panels, lysed with 1x IOTest3 lysing solution containing 1x IOTest3 fixative solution followed by a final wash step in PBS with 2% heat inactivated FCS. Stained samples were acquired on the Navios or Navios EX cytometers using acquisition protocols provided by the manufacturer and analysis was further conducted using Kaluza C software Version 1.0 with defined templates provided by the manufacturer (Beckman Coulter.). As this study aimed to evaluate the reactivity of all reagents on all cellular populations, with the exception of lymph nodes with limited sample volume which only had the B and T tube run, all other specimen types were tested with all four antibody tubes.
2.6. Laboratory developed tests: Setup and performance
Each site in this study routinely performed quality control, monitored instrument performance, and participated in quality assurance programs according to local/regional/national guidelines. Each site in this study used their own antigen/antibody combinations to determine the presence or absence of an abnormal population with any patient sample. One site used five color instrumentation and the other three sites used 10 color instrumentation. Each laboratory followed their own process for the order in which the panels were run. A summary of these can be found in the Supplementary Data S1.
2.7. Phenotype assessment of the ClearLLab 10C panels
Qualitative phenotype assessment (presence or absence of abnormal populations) using the ClearLLab 10C Panels was evaluated by the principal investigator (PI) on each site independently with no additional information provided [such as the morphology, laboratory developed test (LDT) result, molecular or genetic testing results]. The detection limit was arbitrarily set at 1% for this study with 50,000 CD45 positive events collected per ClearLLab tube. Acquisition of more events may have led to detection of smaller “abnormal” populations; however, this was not an objective of this study.
2.8. Patient final diagnosis
Review of cytometry files from LDTs was performed as routine in each lab and final classification retrieved from Pathology was based on the integrated approach of WHO 2016 classification and the Bethesda recommendations (Arber et al., 2016; Davis et al., 2007; Swerdlow et al., 2016; Swerdlow et al., 2017). Of note, the final diagnosis was not always expected to agree with the flow cytometric findings. For example, a patient with a final diagnosis of lymphoma who did not have bone marrow involvement would produce a “nonmalignant” flow result but final classification would be malignant.
2.9. Statistical analysis
Clinical diagnostic accuracy was evaluated by calculating Sensitivity (% of patients with malignancy correctly identified) and Specificity (% of patients with no malignancy correctly identified). In addition, Positive Predictive Value (PPV) (probability of malignant patients with abnormal phenotype) and Negative Predictive Value (NPV) (probability of nonmalignant patients with normal phenotype) were calculated. The 95% confidence intervals (two‐sided) were calculated using the Score approach.
Agreement of ClearLLab 10C Panels and LDTs was assessed by calculating agreements statistics, including positive percent agreement (PPA), negative percent agreement (NPA) as well as overall agreement (OPA).
3. RESULTS
Analysis of listmode data from all specimens was broken into two sections; Section 1 was the comparison of the ClearLLab LS to the patient final diagnosis (4 sites, 453 Specimens), and Section 2 was comparison of the ClearLLab 10C Panel to the LDTs (4 sites, 451 specimens). Examples of the analysis for the ClearLLab 10C panels can be seen in Figures 1 and 2.
FIGURE 1.
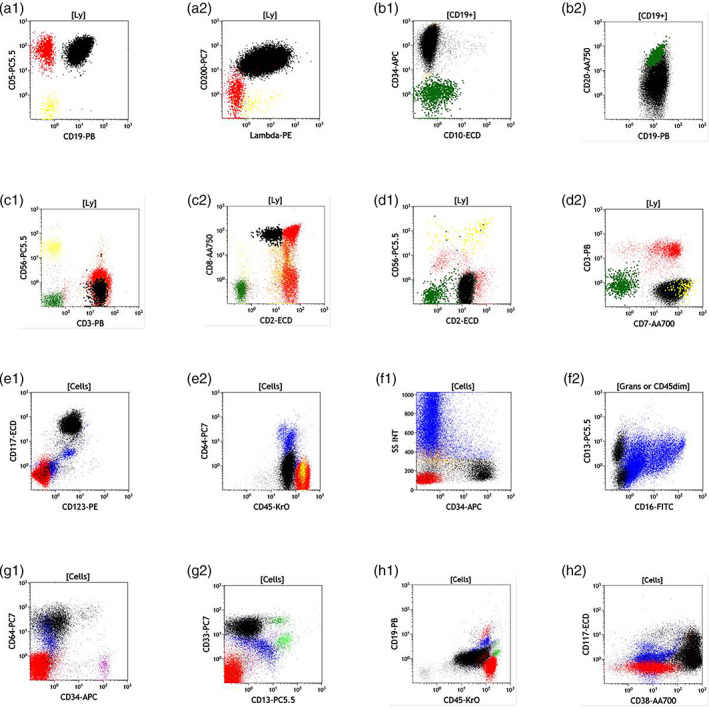
Color Key; T cells are red, normal B cells are green, NK cells are yellow, granulocytes are blue, monocytes are light green, and all abnormal populations are colored in black. Panels (a1) and (a2) show a B‐cell chronic lymphocytic leukemia that is CD19 + CD5 + CD200 + Lambda+. B1 and B2 show an example of a ClearLLab staining of a CD10 negative B‐cell acute lymphoblastic leukemia that expresses CD34, CD19 and partial CD20. C1 and C2 show an example of T cell large granular lymphocyte population that is CD3+ and expressed dim CD2 and CD8 (confirmed by T‐cell gene re‐arrangement). D1 and D2 show an example of a T‐cell leukemia that is surface CD3 negative but expresses CD7 and CD2. E1:E2 are an example of acute myeloid leukemia that expressing bright CD117 and CD123 but does not express CD64 and is dimCD45 positive. F1:F2 is an example of patient sample with myelodysplastic syndrome 2 with 11% blasts. G1:G2 is an example of acute monocytic leukemia that does not express CD34 or CD13 but expresses CD33 and CD64, in this example, the normal CD34 expressing blasts are colored pink. H1:H2 An example of a sample containing myeloma with 55% plasma cells that are bright CD38, dim CD45, partial CD117, weak CD19 and does not express CD34
FIGURE 2.
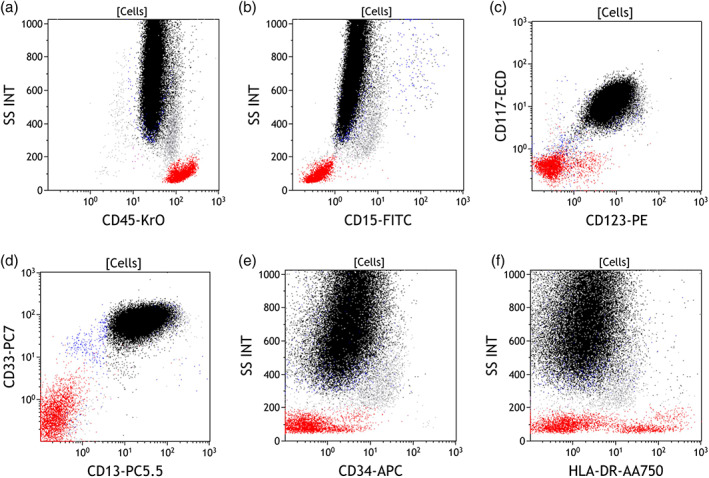
A specimen sample from a patient with confirmed acute promyelocytic leukemia. Promyeolocytes colored in black and are identified by expression pattern with negative CD34, CD15, and HLA‐DR while positive for CD13, CD33, CD117, and CD123
3.1. Section 1: Comparison of the ClearLLab 10C panels to the patients final diagnosis
The results were analyzed using 2 × 2 table for all specimens combined with sensitivity and specificity being calculated (Table 2). The diagnostic accuracy performance of the ClearLLab 10C Panels (presence or absence of abnormal population in flow immunophenotyping) was compared to the clinical diagnosis of the subject (hematologically malignant or hematologically nonmalignant). The qualitative assessment indicated 94% agreement in detecting the presence of an abnormal phenotype (237 out of 253 hematological malignancies) and agreed 96% to the clinical diagnosis in excluding the presence of an abnormal phenotype (190 out of 198 hematological nonmalignancies).
TABLE 2.
Comparison of ClearLLab 10C to final patient diagnosis
| Final patient diagnosis | |||
|---|---|---|---|
| ClearLLab 10C result | Hematologically malignant | Hematologically nonmalignant | Sum |
| Presence of abnormal phenotype | 237 | 8 | 245 |
| Absence of abnormal phenotype | 18 | 190 | 208 |
| Sum | 255 | 198 | 453 |
3.2. Section 2: Comparison of the ClearLLab 10C panels to LDTs
The qualitative immunophenotyping assessment (presence or absence of abnormal population in flow immunophenotyping) was compared between LDTs and the ClearLLab 10C Panels (see Table 3). It should be noted that LDT results for two samples could not be obtained (n = 451). The results showed that 95% agreement for detecting presence of abnormal populations (243 out of 255 seen in LDTs) and agreed 98% in excluding the presence of an abnormal phenotype (193 out of 196 no abnormal in LDTs). A list of the discordant results of the ClearLLab 10C to LDT can be seen in Tables 4 and 5. An overall agreement was 96% between LDTs and the qualitative phenotype results from the ClearLLab 10C Panels. A list of the lineage agreement and maturity of the abnormal populations of the ClearLLab 10C to LDT can be seen in Tables 6 and 7. Overall agreement for lineage was 98% and for maturity 88% for the abnormal populations.
TABLE 3.
Comparison of ClearLLab 10C to LDT
| LDT result | Sum | ||
|---|---|---|---|
| ClearLLab 10C result | Presence of Abnormal phenotype | Absence of Abnormal phenotype | |
| Presence of abnormal phenotype | 243 | 3 | 246 |
| Absence of abnormal phenotype | 12 | 193 | 205 |
| Sum | 255 | 196 | 451 |
Abbreviation: LDT, laboratory developed test.
TABLE 4.
False negative (FN) discordant results of ClearLLab to LDT
| Specimen type | ClearLLab 10C result | LDT result | Comments |
|---|---|---|---|
| PB | No abnormal phenotype | B‐cell—non‐Hodgkin lymphoma, B‐cell type, NOS | Small B cell population CD5‐CD10‐with kappa light chain excess |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Abnormal population < 1% |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Abnormal population < 1% |
| PB | No abnormal phenotype | B‐cell—plasma cell myeloma | Poor viability sample. Surface light chain negative, intracellular positive |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Lack of marker(s) in ClearLLab 10C panel |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Abnormal population < 1% |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Abnormal population < 1% |
| BM | No abnormal phenotype | B‐cell—plasma cell myeloma | Small plasma cell population surface light chain negative, intracellular positive |
| BM | No abnormal phenotype | Other—other interpretation | Unable to discern malignancy: Abnormal phenotype (1.6%): Dim45+ 34+ 33+ 117+ 56± 7± |
| PB | No abnormal phenotype | T/NK‐cell—adult T‐cell leukemia/lymphoma | Unable to discern disease state without more clinical information |
| BM | No abnormal phenotype | T/NK‐cell—T‐cell large granular lymphocytic leukemia | Unable to discern disease state without more clinical information |
| PB | No abnormal phenotype | T/NK‐cell—T‐cell large granular lymphocytic leukemia | Unable to discern disease state without more clinical information |
Abbreviation: BM, bone marrow; LDT, laboratory developed test; PB, peripheral whole blood.
TABLE 5.
False positive (FP) discordant results of ClearLLab to LDT
| Specimen type | ClearLLab 10C result | LDT result |
|---|---|---|
| PB | 1% abnormal B cells: CD45+ CD19+ CD20+ CD5 + bright CD10− Kappa− Lambda + dim CD200+ CD38− CD34− | No malignancy |
| BM | 1% abnormal B cells: CD45+ CD19+ CD20± CD5 + bright CD10− Kappa+ Lambda− CD200+ CD38− CD34− | No malignancy |
| PB | 1% abnormal blast cells: CD45± CD13+ CD34+ DR+ CD11b + CD16− CD7− CD10− CD64− CD14− | No malignancy |
Abbreviation: BM, bone marrow; LDT, laboratory developed test; PB, peripheral whole blood.
TABLE 6.
Comparison of lineage assignment of ClearLLab 10C to institutional laboratory developed tests
| Clinical diagnostic outcome | Sum | ||||
|---|---|---|---|---|---|
| ClearLLab 10C panels | B lineage | T lineage | Myeloid lineage | Unknown | |
| B lineage | 162 | 0 | 1 | 0 | 163 |
| T lineage | 0 | 13 | 0 | 0 | 13 |
| Myeloid lineage | 2 | 0 | 54 | 0 | 56 |
| Unknown | 3 | 0 | 0 | 0 | 3 |
| Sum | 167 | 13 | 55 | 0 | 235 |
TABLE 7.
Comparison of maturity assignment of ClearLLab 10C to institutional laboratory developed tests
| Clinical diagnostic outcome | Sum | |||
|---|---|---|---|---|
| ClearLLab 10C panels | Immature | Mature | Unknown | |
| Immature | 43 | 9 | 0 | 52 |
| Mature | 8 | 163 | 0 | 171 |
| Unknown | 9 | 3 | 0 | 12 |
| Sum | 60 | 175 | 0 | 235 |
4. DISCUSSION
The heterogenous nature and varied clinical characteristics of hematologic malignancies may limit the detection of abnormal populations by flow cytometric analysis. Certain patients with a malignancy may present with normal peripheral blood counts and normal flow immunophenotyping in a given specimen type. For example, a patient diagnosed with non‐Hodgkin's lymphoma in a lymph node may present with no other detectable disease as the malignant cells may be confined. Specimens may be sent for flow cytometric analysis for very specialized testing such as the detection of very small abnormal populations (e.g., assessment of minimal residual disease in myeloma or B cell ALL (Borowitz et al., 2008; Borowitz et al., 2015; Munshi et al., 2017; Rawstron et al., 2013; Stetler‐Stevenson et al., 2016)). Incidental “abnormal” phenotype(s) may be seen in flow cytometry analysis in specimens of patients with increased lymphocytes as a response to infections (viral and/or bacterial), systemic inflammation, or incidentally identified small clonal B‐lymphocyte populations seen in patients with concurrent conditions such as myelodysplasia, post chemotherapy, or infection (Chen, Asplund, McKenna, & Kroft, 2004).
A study requirement for specimen enrolment was that all QC had to pass daily, this included the preparation, staining, acquisition and analysis of both type of control cells. There were no failures of the control cell QC during this study across four sites. Each site had multiple operators and multiple different lots of control material were used (data not shown). The use of this type of control material, where all the processing and analysis steps are the same as patient samples, was seen to minimize variations and maximize consistency of results from the ClearLLab 10C panels.
Principal investigator evaluation of the ClearLLab 10C panels was performed solely on pdfs of the analyzed listmode data without access to the laboratory results (clinical samples assigned a study identification number), any clinical information, morphology, or cytogenetics. As noted above, the detection limit was set at 1% for this study, therefore samples with abnormal populations below this level were not considered positive. This process of evaluation allows for the objective comparison of the ClearLLab panels to the final patient diagnosis. In this study, 18 patients had a malignancy that were not identified in the ClearLLab 10C but did not include any acute leukemia's or MPNs which were all correctly identified. Of the 18 patients; seven patients had a plasma cell neoplasm (PCN), three patients NHL, three low grade MDS, three chronic leukemia, and two other malignancy. The two other samples were patients with Hodgkin’s lymphoma for which flow cytometry is of limited value. Of the seven patients with PCN, four samples contained abnormal populations lower than the set limit of detection with the ClearLLab 10C of less than 1%. The other three had small populations, less than 5%, and all were surface light chain negative but cytoplasmic positive. It should be noted that the ClearLLab 10C does contain CD38, CD56, and CD117 all which may be abnormally expressed on PCN (see Figure 1: H1, H2 for PCN with partial CD117 expression) aiding the detection of abnormal plasma cells. Of the three patients with a final diagnosis of NHL, two specimens were PB and did not contain any abnormal population and the third specimen contained a small population with clinical significance undetermined without clinical and morphological examinations. The three patients with low grade MDS (less than 5% blasts detected by flow cytometry) and three with abnormal T‐cell population (all less than 5%) required morphological confirmation, or the clinical context to determine whether the patient had a malignancy or to differentiate abnormal from reactive.
Accuracy determination of the ClearLLab 10C performance in each specimen type (PB, BM, and LN) cannot be assessed in this study as the study was not powered to ask this question; however, the data in this study suggest a high degree of agreement in all specimen types. For a number of samples with an abnormal population detected, further testing would have to be performed to determine a complete phenotype. Definitive confirmation of lineage in acute leukemia requires a minimum of intracellular staining with myeloperoxidase (myeloid), cCD3 (T cell), and CD19, with one or two of the following (depending on strong or weak expression of CD19) CD79a, cCD22, and CD10 (Swerdlow et al., 2017). Overall, the ClearLLab 10C system did detect 98% of the abnormal populations when compared to the LDTs (see Tables 3 and 4) and would have excluded 96% of the negative samples from further testing. Of the three samples that were false positive samples (Table 5), an abnormal population detected in the ClearLLab 10C and not in the LDTs, all three were populations of 1% reported within the study with unknown significance without clinical correlation. Upon further independent review of the analyzed files of the three false positive specimens, all three had populations that were below the reportable percentage, which highlights the need to educate users of this system about the limit of detection and reportable populations. Although two sites did show slightly higher discrepancy numbers that the other two (both less than 5%) this was not significant. There was a single case where a small B‐cell population with Kappa excess (<2% of total leukocytes) was not gated in the ClearLLab system. The rest of these discrepancies were resolved with; clinical information, the use additional markers (such as cytoplasmic or markers of immaturity), and strictly applying the limit of detection for this system (Figure 2).
As discussed above, the presence of CD200 in the B‐cell tube allows the rapid detection of chronic lymphocytic cases which comprised 53 out of the 453 cases without the requirement for further testing in most cases (Alapat et al., 2012; Challagundla, Medeiros, Kanagal‐Shamanna, Miranda, & Jorgensen, 2014). Having TCR γδ in the T tube is useful for isolating this population of T cells which are an area of interest in hematological and solid cancers (Handgretinger & Schilbach, 2018; Rei, Pennington, & Silva‐Santos, 2015). The design of the two myeloid tubes is such that the maturation sequence of myelomoncytic cells can be mapped.
Currently, the majority of laboratories in North America rely on in‐house LDTs for their routine testing of specimens investigated for leukemia and lymphoma. Numerous studies have shown that preparation of liquid panels within a laboratory is potentially error prone and time consuming to validate (Moloney et al., 2019; Rajab et al., 2017). Furthermore, the expertise and cost required to develop an effective LDT may not be present in smaller laboratories; therefore, it is reasonable that given these issues as well as the development and validation costs, the use of a premade stable flow cytometry product would largely reduce laboratory indirect costs and improve efficiency and accuracy. Additionally a stable, dried, product that can be stored at room temperature for an extended period of time (>1 year) is an additional bonus as storage of large amounts of liquid reagent may not be possible on site. The use of the standardized reagent panels in the ClearLLab 10C system used in this study also offers the potential to facilitate inter‐laboratory comparisons and centralized interpretations of flow cytometric data.
In addition to the advantages of standardized panels, removal of the burden of LDT creation and thereafter, individual titration, pipetting, cocktail creation, lot validation, lot stability testing, and documentation duties should allow for significant time savings for laboratories while eliminating multiple sources of error (Braylan, Orfao, Borowitz, & Davis, 2001; Correia et al., 2018; Moloney et al., 2019). While this study used all four tubes for each sample with the exception of lymph nodes, laboratories with access to clinical and other laboratory information may decide to process only lymphoid or even only a B‐cell tube. For large reference laboratories with limited patient information, running the full panel of four tubes may be a more attractive option. The use of the FDA cleared ClearLLab 10C panels has the potential to significantly standardize panels and increase productivity in the investigation of hematolymphoid neoplasms, by eliminating the need for further testing on specimens and providing clear direction for follow‐up testing.
CONFLICT OF INTEREST
B.H. and M. K. are consultants for Beckman Coulter and G. C., J. R., R. O., A. S., K. L., R. M., and L. T. are employees of Beckman Coulter.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
The authors thank the subjects who participated in the study and all site personnel involved in generating the data for this study. The study was funded by and all materials for this project were supplied by Beckman Coulter Inc.
Hedley BD, Cheng G, Keeney M, et al. A multicenter study evaluation of the ClearLLab 10C panels. Cytometry. 2021;100:225–234. 10.1002/cyto.b.21935
REFERENCES
- Alapat, D. , Coviello‐Malle, J. , Owens, R. , Qu, P. , Barlogie, B. , Shaughnessy, J. D. , & Lorsbach, R. B. (2012). Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. American Journal of Clinical Pathology, 137, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber, D. A. , Orazi, A. , Hasserjian, R. , Thiele, J. , Borowitz, M. J. , Le Beau, M. M. , … Vardiman, J. W. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood, 127, 2391–2405. [DOI] [PubMed] [Google Scholar]
- Borowitz, M. J. , Devidas, M. , Hunger, S. P. , Bowman, W. P. , Carroll, A. J. , Carroll, W. L. , … Children’s Oncology, G. (2008). Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children's Oncology Group study. Blood, 111, 5477–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz, M. J. , Wood, B. L. , Devidas, M. , Loh, M. L. , Raetz, E. A. , Salzer, W. L. , … Larsen, E. (2015). Prognostic significance of minimal residual disease in high risk B‐ALL: A report from Children's Oncology Group study AALL0232. Blood, 126, 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braylan, R. C. , Orfao, A. , Borowitz, M. J. , & Davis, B. H. (2001). Optimal number of reagents required to evaluate hematolymphoid neoplasias: Results of an international consensus meeting. Cytometry, 46, 23–27. [DOI] [PubMed] [Google Scholar]
- Challagundla, P. , Medeiros, L. J. , Kanagal‐Shamanna, R. , Miranda, R. N. , & Jorgensen, J. L. (2014). Differential expression of CD200 in B‐cell neoplasms by flow cytometry can assist in diagnosis, subclassification, and bone marrow staging. American Journal of Clinical Pathology, 142, 837–844. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Asplund, S. L. , McKenna, R. W. , & Kroft, S. H. (2004). Characterization of incidentally identified minute clonal B‐lymphocyte populations in peripheral blood and bone marrow. American Journal of Clinical Pathology, 122, 588–595. [DOI] [PubMed] [Google Scholar]
- Correia, R. P. , Rajab, A. , Bento, L. C. , Alexandre, A. M. , Vaz, A. C. , Schimidell, D. , … Bacal, N. S. (2018). A ten‐color tube with dried antibody reagents for the screening of hematological malignancies. International Journal of Laboratory Hematology, 40, 136–143. [DOI] [PubMed] [Google Scholar]
- Craig, F. E. , & Foon, K. A. (2008). Flow cytometric immunophenotyping for hematologic neoplasms. Blood, 111, 3941–3967. [DOI] [PubMed] [Google Scholar]
- Davis, B. , Holden, J. , & Mea, B. (2007). 2006 Bethesda international Concsensus recommendations on the flow Cytometric Immunophenotypic analysis of Hematolymphoid Neoplasia: Medical indications. Cytometry Part B (Clinical Cytometry), 72B, S5–S13. [DOI] [PubMed] [Google Scholar]
- Del Poeta, G. , Stasi, R. , Venditti, A. , Cox, C. , Aronica, G. , Masi, M. , … Papa, G. (1995). CD7 expression in acute myeloid leukemia. Leukemia & Lymphoma, 17, 111–119. [DOI] [PubMed] [Google Scholar]
- Drexler, H. G. , Thiel, E. , & Ludwig, W. D. (1991). Review of the incidence and clinical relevance of myeloid antigen‐positive acute lymphoblastic leukemia. Leukemia, 5, 637–645. [PubMed] [Google Scholar]
- Handgretinger, R. , & Schilbach, K. (2018). The potential role of gammadelta T cells after allogeneic HCT for leukemia. Blood, 131, 1063–1072. [DOI] [PubMed] [Google Scholar]
- Hedley, B. D. , Cheng, G. , Luider, J. , Kern, W. , Lozanski, G. , Chin‐Yee, I. , … Tejidor, L. (2018). Initial flow cytometric evaluation of the Clearllab lymphoid screen. Cytometry. Part B, Clinical Cytometry, 94, 707–713. [DOI] [PubMed] [Google Scholar]
- Hedley, B. D. , Keeney, M. , Popma, J. , & Chin‐Yee, I. (2015). Novel lymphocyte screening tube using dried monoclonal antibody reagents. Cytometry. Part B, Clinical Cytometry, 88, 361–370. [DOI] [PubMed] [Google Scholar]
- Jacob, M. C. , Souvignet, A. , Pont, J. , Solly, F. , Mondet, J. , Kesr, S. , … Cesbron, J. Y. (2017). One tube with eight antibodies for 14‐part bone marrow leukocyte differential using flow cytometry. Cytometry Part B, Clinical Cytometry, 92, 299–309. [DOI] [PubMed] [Google Scholar]
- Johansson, U. , Bloxham, D. , Couzens, S. , Jesson, J. , Morilla, R. , Erber, W. , & Macey, M. (2014). British Committee for Standards in H. Guidelines on the use of multicolour flow cytometry in the diagnosis of haematological neoplasms. British Committee for Standards in Haematology. British Journal of Haematology, 165, 455–488. [DOI] [PubMed] [Google Scholar]
- Moloney, E. , Watson, H. , Barge, D. , Allen, A. J. , Carey, P. , Hislop, J. , … Greystoke, B. (2019). Efficiency and health economic evaluations of BD OneFlow flow Cytometry reagents for diagnosing chronic lymphoid leukemia. Cytometry Part B, Clinical Cytometry, 96, 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi, N. C. , Avet‐Loiseau, H. , Rawstron, A. C. , Owen, R. G. , Child, J. A. , Thakurta, A. , … Gregory, W. M. (2017). Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: A meta‐analysis. JAMA Oncology, 3, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfao, A. , Schmitz, G. , Brando, B. , Ruiz‐Arguelles, A. , Basso, G. , Braylan, R. , … San Miguel, J. F. (1999). Clinically useful information provided by the flow cytometric immunophenotyping of hematological malignancies: Current status and future directions. Clinical Chemistry, 45, 1708–1717. [PubMed] [Google Scholar]
- Palumbo, G. A. , Parrinello, N. , Fargione, G. , Cardillo, K. , Chiarenza, A. , Berretta, S. , … Di Raimondo, F. (2009). CD200 expression may help in differential diagnosis between mantle cell lymphoma and B‐cell chronic lymphocytic leukemia. Leukemia Research, 33, 1212–1216. [DOI] [PubMed] [Google Scholar]
- Porwit, A. , & Rajab, A. (2015). Flow cytometry immunophenotyping in integrated diagnostics of patients with newly diagnosed cytopenia: One tube 10‐color 14‐antibody screening panel and 3‐tube extensive panel for detection of MDS‐related features. International Journal of Laboratory Hematology, 37(Suppl 1), 133–143. [DOI] [PubMed] [Google Scholar]
- Rajab, A. , Axler, O. , Leung, J. , Wozniak, M. , & Porwit, A. (2017). Ten‐color 15‐antibody flow cytometry panel for immunophenotyping of lymphocyte population. International Journal of Laboratory Hematology, 39(Suppl 1), 76–85. [DOI] [PubMed] [Google Scholar]
- Rawstron, A. C. , Bottcher, S. , Letestu, R. , Villamor, N. , Fazi, C. , Kartsios, H. , … Ghia, P. (2013). European research initiative in CLL. Improving efficiency and sensitivity: European research initiative in CLL (ERIC) update on the international harmonised approach for flow cytometric residual disease monitoring in CLL. Leukemia, 27, 142–149. [DOI] [PubMed] [Google Scholar]
- Rei, M. , Pennington, D. J. , & Silva‐Santos, B. (2015). The emerging Protumor role of gammadelta T lymphocytes: Implications for cancer immunotherapy. Cancer Research, 75, 798–802. [DOI] [PubMed] [Google Scholar]
- Stetler‐Stevenson, M. , Paiva, B. , Stoolman, L. , Lin, P. , Jorgensen, J. L. , Orfao, A. , … Rawstron, A. C. (2016). Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry Part B, Clinical Cytometry, 90, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, D. R. , Illingworth, A. , Marinov, I. , Ortiz, F. , Andreasen, J. , Payne, D. , … Keeney, M. (2018). ICCS/ESCCA consensus guidelines to detect GPI‐deficient cells in paroxysmal nocturnal hemoglobinuria (PNH) and related disorders part 2 ‐ reagent selection and assay optimization for high‐sensitivity testing. Cytometry Part B, Clinical Cytometry, 94, 23–48. [DOI] [PubMed] [Google Scholar]
- Swerdlow, S. , Campo, E. , Harris, N. , Jaffe, E. , Pileri, S. , Stein, H. , … & Vardiman, J. (2008). WHO classification of tumours of Haematopoietic and lymphoid tissues (4th ed.). Lyon: International Agency for Research on Cancer. [Google Scholar]
- Swerdlow, S. H. , Campo, I. , Pileri, S. A. , Harris, N. L. , Stein, H. , Siebert, R. , … & Jaffe, E. S. (2017). WHO classification of tumours of haematopoietic and lymphoid tissues (4th ed.). Lyon: International Agency for Research on Cancer. [Google Scholar]
- Swerdlow, S. H. , Campo, E. , Pileri, S. A. , Harris, N. L. , Stein, H. , Siebert, R. , … Jaffe, E. S. (2016). The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 127, 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen, J. J. , Lhermitte, L. , Bottcher, S. , Almeida, J. , van der Velden, V. H. , Flores‐Montero, J. , … EuroFlow, C. (2012). EuroFlow antibody panels for standardized n‐dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia, 26, 1908–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, B. (2006). 9‐color and 10‐color flow cytometry in the clinical laboratory. Archives of Pathology & Laboratory Medicine, 130, 680–690. [DOI] [PubMed] [Google Scholar]
- Wood, B. L. (2005). Ten‐color Immunophenotyping of hematopoietic cells. Current Protocols in Cytometry, 33, 6.21.1–6.21.11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


