Abstract
Background
Hypertrophic scars and keloids are postsurgery problems. Some studies showed that onion extract and aloe vera might be beneficial for postoperative scars. However, few of the randomized clinical trials were investigated.
Aims
To compare the efficacy of silicone gel containing onion extract and aloe vera (SGOA) to silicone gel sheets (SGS) to prevent postoperative hypertrophic scars and keloids.
Methods
The prospective randomized assessor‐blind controlled trial was conducted with 40 patients who had undergone surgery. The patients were divided into two groups: one treated with SGOA, the other with SGS. The patients were evaluated after 1, 2, and 3 months. The objective assessment was to determine the incidences of scarring, erythema, and melanin values using Mexameter, and pliability through Cutometer. The subjective assessment consisted of the patient and observer scar assessment scale (POSAS) and patient satisfaction.
Results
After the 12‐week follow‐up, there was no statistically significant difference in the scarring incidence rate of both groups. There were no statistical differences in the POSAS score, erythema, and melanin value between both groups. Using objective assessment, pliability in the SGOA group was statistically significantly higher compared to the SGS group. Pain and itchiness significantly decreased in both groups. No adverse effects were reported in either group.
Conclusion
Silicone gel containing onion extract and aloe vera is effective as SGS for postoperative scar prevention.
Keywords: aloe vera, hypertrophic scars, keloids, onion extract, silicone gel
1. INTRODUCTION
Hypertrophic scarring is a problem faced by postoperative patients, particularly individuals with darker skin complexions such as Asians. 1 Factors causing hypertrophic scars include race, injury type, and scar location. Studies revealed a 40%‐70% incidence of postoperative scars with an equal sex distribution. 2 Additionally, hypertrophic scars affect patients' physical and mental health such as anxiety, depression, and lack of self‐esteem. They can be asymptomatic, sometimes causing pain and itchiness. Therefore, hypertrophic scar prevention is highly important. The prevention guidelines that are currently in place are silicone‐based therapy, onion extract, and pressure garments. 3
Silicone gel sheets (SGS) are the current standard treatment for hypertrophic scars. Mustoe 4 discovered that when the stratum corneum is injured, transepidermal water loss increases, causing dehydration in keratinocytes. This alerts dermal fibroblast in the dermis to produce more collagen. Therefore, hydration from silicone products may be able to prevent hypertrophic scars. However, there are several restrictions. The sheet easily slips when applied, especially on the areas around the joints and sweaty skin. It is aesthetically displeasing when applied on the face. Tape used to prevent the sheets from slipping may cause an allergic rash. Therefore, the use of SGS is less desirable for some patients. Silicone‐based products have been developed in the form of gel.
In vitro studies on onion extract found that fibrinolytic activity decreased extracellular matrix deposition and fibroblast proliferation. 5 Quercetin in onion extract is found to reduce fibroblast function in the tissue formation process. It stimulates the function of matrix metalloproteinase‐1 (MMP‐1) which reduces collagen production. 5 Moreover, onion extract is also believed to have anti‐inflammatory and antimicrobial effects. 6 Some studies on the preventive and treatment effectiveness of onion extract on hypertrophic scars demonstrated its capability to improve scar appearance. 7 , 8 , 9
Aloe vera has been used in the medical field for ages. Apart from treating burns, aloe vera has wound‐healing effects. 10 , 11 It is also used to treat postoperative wounds.
Aloe vera has many active ingredients such as glucomannan which affects fibroblast growth factor. 12 It was found that aloe vera increases collagen content in scar tissue, changes the collagen composition, and increases degree of collagen‐crosslinks. It helps decrease collagen type III and increase collagen type I concentration during the remodeling phase of wound healing. Scar tissue becomes more organized, collagen fibers highly align, and free spaces between the fibers decrease. 10 These lead to the scar tissue size to decrease. It also contains magnesium lactate which reduces histamine production resulting in decreased itchiness and irritation. 13 Moreover, aloe vera has many anti‐inflammatory agents such as C‐glucosyl chromone, campesterol, β‐sisosterol, lupeol, auxins, gibberellins, and peptidase bradykinase. 14 , 15 , 16 In the study of Molazem et al, 17 aloe vera was used on patients who underwent cesarean section, discovering wounds healed faster. Eshghi et al 18 studied patients that underwent hemorrhoidectomy, finding that wounds healed faster and were less painful compared to a placebo.
Previous studies found that silicone gel, onion extract, and aloe vera were effective in treating scars with different working mechanisms. There has been no study on SGOA to compare with SGS. Therefore, the researchers are interested in studying the effectiveness of SGOA in postoperative scar prevention compared to SGS.
2. MATERIAL AND METHODS
2.1. Patients
A 12‐week, prospective, controlled, assessor‐blind, randomized clinical trial was conducted with 40 postoperative patients from Srinakharinwirot University Skin Center and the Rajavithi Hospital Surgery Department. The sample size was calculated using two independent means which were acquired from previous research. 19 The mean difference in the scar height (mm) of the patients treated with silicone gel vs a placebo and SGS vs a placebo (SD) was 1.17 (0.58) and 0.55 (0.73), respectively. The sample size of 36 patients was required, with 95% confidence and 80% power. With a dropout rate of 10%, 40 patients had to be recruited.
The enrolled patients were aged 18‐60 years old, underwent surgery within 2 weeks prior joining the research, and had wounds at least 1.5 cm long. The patients with the following conditions were excluded from the research. Patients with infections, rashes, or discharge producing wounds. Patients with underlying diseases such as diabetes mellitus, systemic lupus erythematosus, dermatomyositis, systemic sclerosis, and other autoimmune diseases. Patients who took systemic or topical corticosteroids 1 month prior to the study. Patients treated with immunosuppressive drugs, chemotherapy, radiation therapy, or diagnosed with cancer. Patients with allergies to silicone gel, onion extract, or aloe vera. All details of the research were explained to all of the patients. The patients who willingly participated in the research signed a consent form. Patients were gathered consecutively from November 2019 to March 2020 if they met this criteria.
Computerized block randomization with an allocation ratio of 1:1 was used to determine the treatment method for patients. Treatments were distributed to each patient, respectively, according to their numbers. The patients were divided into two groups: the first group received SGOA (Esensia Scar‐s gel®, Skin & Health Care Co.), and the second group received SGS (Actewound®). The patients received treatment 2 weeks after surgery or immediately after suture removal. To maximize the product efficacy, the first group applied a thin film of gel twice a day to ensure constant contact. The second group used SGS 24 hours per day. Both groups did not apply product during showers and had to continue the treatment every day for 12 consecutive weeks. Throughout the participation, the patients had to stop using systemic or topical corticosteroids and any other drugs affecting the wound. The patients' follow‐ups occurred in the 4th, 8th, and 12th weeks. All patients were required to log their compliance in the provided forms given to them throughout the study.
2.2. Assessment
Hypertrophic scars are defined as lesions that elevate and do not extend beyond the original wound. Keloids are lesions that extend beyond the original wound to the surrounding tissue. 20 Persons with a past or a family history of hypertrophic scars were categorized in the high risk group. The incidence assessment was conducted in the 12th week, which was the primary outcome of this research.
On the first day and every follow‐up (4th, 8th, 12th week), the patients' wounds would be assessed by objective scar measurement, including erythema and melanin values using Mexameter® MX18 (Courage and Khazaka Electronic GmbH). The pliability of the wound was assessed by Cutometer® dual MPA 580 (Courage and Khazaka Electronic GmbH), using the R0 parameter to measure distensibility which correlated to skin firmness. Subjective scar measurement was also conducted which included the patient and observer scar assessment scale (POSAS). This single‐blind assessment was performed by one physician throughout the research. The observer scale assessment included vascularity, pigmentation, thickness, relief, pliability, and surface area. The scores ranged from 1 (normal skin) to 10 (worst imaginable skin) in each category. Therefore, the total scores ranged from 1 to 60. The question items of the patients' self‐assessment included pain, pruritus, color, stiffness, thickness, and regularity. The scores of each question ranged from 1 to 10, so the total scores ranged from 1 to 60. Furthermore, the researcher would measure the satisfaction scores and adverse effects.
2.3. Statistical analysis
The categorical data were described using frequency and percentage. For the continuous data, mean ± standard deviation was used. An independent Student's t test was used to compare the mean for continuous data between the treatment groups. Chi‐square was used to analyze the differences in categorical variables. Linear mixed model analysis was used to compare means for continuous data at each visit and between the treatment groups. Multiple logistic regression analysis was used to find the association between the treatment groups and scar formation adjusted for scar locations. The data analysis was performed using Stata version 13, and the P‐value < .05 was considered statistically significant.
3. RESULTS
3.1. Demographic data
Over the 12‐week study, all 40 patients were divided into two groups; each group had 20 patients with no loss follow‐up (Figure 1). There were 36 females (90%) and four males (10%). The average age and body mass index (BMI) of the patients were 39.58 years old and 22.67 kg/m2, respectively. All patients were Asians with Fitzpatrick skin type III (25%) and type IV (75%). None of the patients altered the treatment methods. The demographic data are shown in Table 1.
FIGURE 1.
Flow diagram of the study
TABLE 1.
Baseline demographic characteristics
| Data | SGOA (n = 20) | SGS (n = 20) | P‐value |
|---|---|---|---|
| Female | 19 (95%) | 17 (85%) | .292 |
| Male | 1 (5%) | 3 (15%) | |
| Age | 35.95 ± 15.09 | 43.60 ± 13.01 | .094 |
| BMI (kg/m2) | 22.23 ± 0.60 | 23.12 ± 0.62 | .3147 |
| Fitzpatrick skin type | |||
| Type 3 | 5 (25%) | 5 (25%) | 1.000 |
| Type4 | 15 (75%) | 15 (75%) | |
| Risk group | |||
| Low risk | 8 (40%) | 14 (70%) | .057 |
| High risk | 12 (60%) | 6 (30%) | |
| Type of surgery | |||
| Benign breast mass excision | 12 (60%) | 14 (70%) | .783 |
| Thyroidectomy | 1 (5%) | 1 (5%) | |
| Subcutaneous nodule excision | 7 (35%) | 5 (25%) | |
| Location | |||
| Face | 5 (25%) | 2 (10%) | .373 |
| Trunk | 12 (60%) | 14 (70%) | |
| Extremities | 3 (15%) | 4 (20%) | |
| POSAS‐observer | 14.05 ± 6.11 | 14.1 ± 4.54 | .9767 |
| POSAS‐patient | 21.8 ± 8.83 | 21.1 ± 5.36 | .7635 |
| Erythema index | 402.67 ± 80.79 | 419.84 ± 63.97 | .4610 |
| Melanin index | 309.12 ± 146.51 | 310.67 ± 193.51 | .9772 |
| R0 parameter (Cutometer) | 0.2025 ± 0.16 | 0.1776 ± 0.09 | .5504 |
3.2. Outcome
It was found that six patients (30%) from the SGOA group and four patients (20%) from the SGS group had hypertrophic scars. However, there was no statistically significant difference (P = .465), RR = 1.5, 95% CI (0.4978, 4.5195). No keloids were found in either group. Subgroup analysis was conducted and categorized the patients into two groups: high risk and low risk. There was no statistically significant difference in both the high risk group (P = .289), RR = 2.5, 95% CI (0.370, 16.888) and the low risk group (P = .601), RR = 0.583, 95% CI (0.7216, 4.715) (Table 2). There was no statistically significant difference in scar formation in terms of location between the groups which were differentiated into three regions; face, trunk, and extremities (P = .286, P = .250, and P = .270, respectively) (Table 3).
TABLE 2.
Incidence of hypertrophic scars in the SGOA and SGS groups
| SGOA (n = 20) | SGS (n = 20) | P‐value | |
|---|---|---|---|
| No abnormal scar | 14 (70%) | 16 (80%) | .465 |
| Hypertrophic scar | 6 (30%) | 4 (20%) | |
| Subgroup analysis | |||
| High risk group | |||
| No scar formation | 7 (58.33%) | 5 (83.33%) | .289 |
| Hypertrophic scar | 5 (41.67%) | 1 (16.67%) | |
| Low risk group | |||
| No scar formation | 7 (87.5%) | 11 (78.57%) | .601 |
| Hypertrophic scar | 1 (12.5%) | 3 (21.43%) | |
TABLE 3.
Incidence of hypertrophic scars in the SGOA and SGS groups in various locations
| Location | SGOA (n = 20) | SGS (n = 20) | P‐value a | P‐value b |
|---|---|---|---|---|
| Face | 0 (0%) | 1 (50%) | .286 | .355 |
| Trunk | 4 (33.33%) | 2 (14.29%) | .250 | |
| Extremities | 2 (66.67%) | 1 (25%) | .270 |
P‐value compared scar formation of each location between the SGOA and SGS groups.
P‐value compared scar formation between the SGOA and SGS groups.
From the follow‐ups (4th, 8th, 12th weeks), it was revealed that the erythema, melanin values, and pliability improved. However, there were no differences in erythema and melanin values between the two groups (P = .863, P = .571, respectively). Both groups showed a significantly increased R0 parameter since the 4th week. However, the SGOA group had statistically significant greater pliability than the SGS group (P = .009). Pigmentation significantly decreased in the SGOA and SGS group in the 4th week and 12th week, respectively (Table 4). When conducting subjective scar assessment, each physician‐assessed POSAS of vascularity, pigmentation, thickness, pliability, relief, and surface area did not show a statistically significant difference between both groups (P = .125, P = .809, P = .108, P = .559, P = .731, and P = .353, respectively) (Figure 2). The patient‐assessed POSAS also did not show any statistically significant difference in terms of pain, itching, color, stiffness, thickness, and regularity (P = .459, P = .068, P = .681, P = .997, P = .569, P = .825 and P = .467, respectively) (Figure 3).
TABLE 4.
Mean values of objective parameters between the SGOA and SGS groups
| Week | SGOA (n = 20) | SGS (n = 20) | P‐value a | ||
|---|---|---|---|---|---|
| Mean (SD) | P‐value b | Mean (SD) | P‐value b | ||
| Erythema index (Mexameter) | |||||
| 0 | 402.67 (80.79) | 419.84 (63.97) | .863 | ||
| 4 | 420.25 (86.37) | .274 | 432.12 (82.46) | .339 | |
| 8 | 417.75 (88.59) | .348 | 408.72 (61.22) | .387 | |
| 12 | 411.68 (106.55) | .575 | 407.48 (97.78) | .336 | |
| Melanin index (Mexameter) | |||||
| 0 | 309.12 (146.51) | 310.67 (193.51) | .571 | ||
| 4 | 264.28 (115.49) | .042 | 303.73 (216.63) | .687 | |
| 8 | 249.67 (131.44) | .007 | 290.73 (183.78) | .247 | |
| 12 | 244.84 (123.02) | .004 | 274.33 (208.46) | .035 | |
| R0 parameter (Cutometer) | |||||
| 0 | 0.2025 (0.16) | 0.1776 (0.09) | .009 | ||
| 4 | 0.3184 (0.18) | <.001 | 0.2176 (0.09) | .042 | |
| 8 | 0.3518 (0.16) | <.001 | 0.2347 (0.07) | .004 | |
| 12 | 0.3824 (0.14) | <.001 | 0.2834 (0.08) | <.001 | |
Values are mean (SD), SD standard deviation.
P‐value compared between two treatment groups.
P‐value compared each follow‐up visit to the baseline.
FIGURE 2.
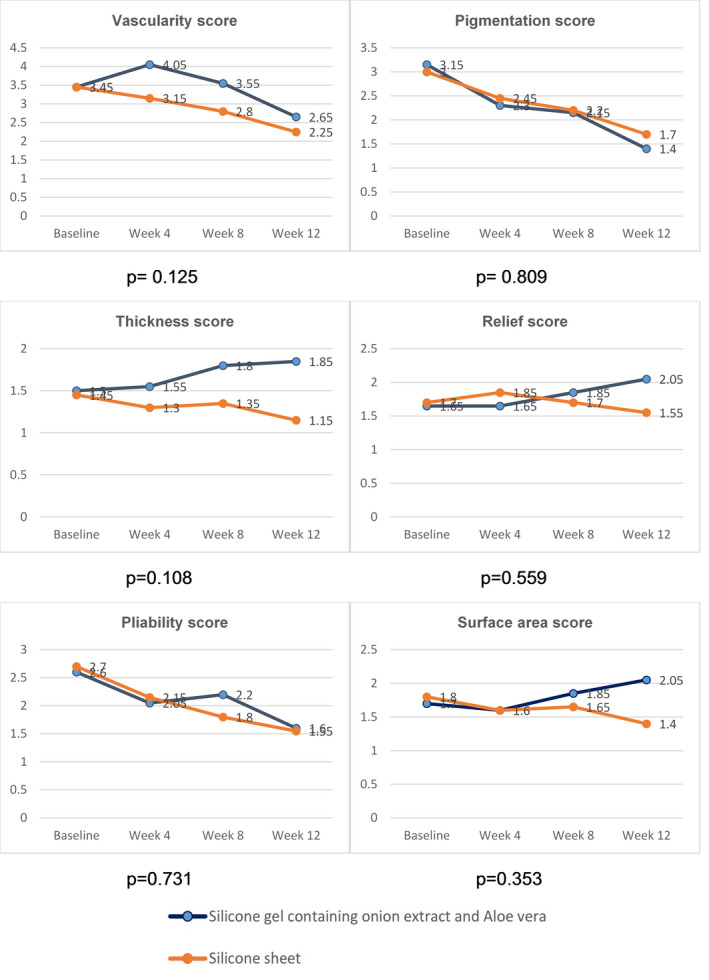
Mean observer‐assessed POSAS scores in the SGOA and SGS groups during each follow‐up
FIGURE 3.
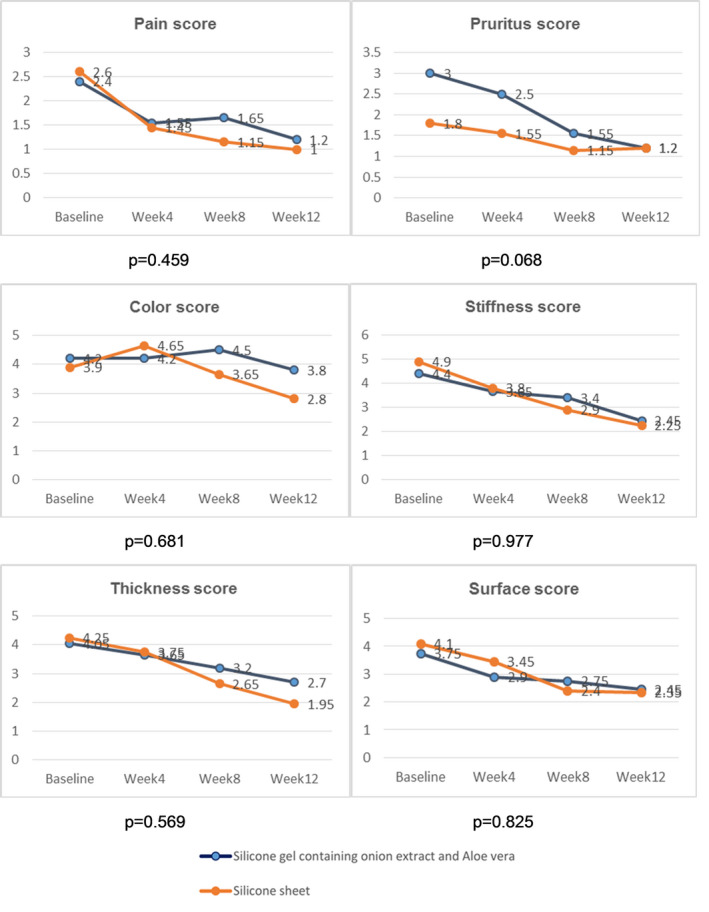
Mean patient‐assessed POSAS scores in the SGOA and SGS groups during each follow‐up
The patient's satisfaction in the SGOA and the SGS group were 4.50 ± 0.81 and 4.65 ± 0.57, respectively. No adverse effects were observed in either group.
Overall, 35 patients (87.5%) had excellent compliance, and they never forgot to apply their assigned product. Four patients (10%) had good compliance, and they sometimes forgot to apply their assigned product. One patient (2.5%) had poor compliance, and the patient mostly forgot to apply their assigned product.
4. DISCUSSION
The results showed that SGOA has a comparable effectiveness for preventing postoperative scars as SGS. This is the first research on SGOA for preventing postoperative scars.
Silicone's true mechanism for treating hypertrophic scars remains unknown. Many theories have tried to explain its mechanism, such as increased temperature, oxygen tension, blood flow, and pressure effects. 21 However, it is mostly believed that silicone may work through hydration and occlusion. 4 Kim et al 22 found that the efficiency of SGS and silicone gel in preventing hypertrophic scars was similar, although silicone gel was easier to use. Hosnuter et al 23 discovered that treating hypertrophic scars was most effective when combining SGS with onion extract. Jenwitheesuk et al 24 found that silicone gel containing onion extract was able to prevent postoperative hypertrophic scars compared to the placebo. The study showed that pigmentation was reduced significantly compared to the placebo but showed no difference in vascularity, pliability, and height using subjective assessment.
Our study demonstrated that SGOA has a comparable efficacy in preventing hypertrophic scars as SGS which is accounted as standard treatment. SGOA has aloe vera which has anti‐inflammatory and well‐organized collagen production effects. Therefore, we assumed that aloe vera might have synergistic effects with onion extract and silicone gel in preventing scar formation. The SGS group had lower scar incidence than the SGOA group (20% vs 30%). However, no statistically significant difference was found. This might be due to fewer high risk patients in the SGS group. None of the groups had keloids. When differentiating between body regions, we also found that there was no statistically significant difference of scar formation in different locations between both groups (P = .355). We also conducted both subjective and objective assessment to reduce bias. Pliability increased while pain and itchiness decreased in both groups. Pigmentation decreased significantly since the 4th week of the SGOA group and the 12th week of the SGS group. This might result from the anti‐inflammation effect of onion extract and aloe vera. Because of this, SGOA may be beneficial for some patients who might have cosmetic concerns of pigmentation. In addition, the SGS group had lower POSAS scores than the SGOA group; nonetheless, there was no statistically significant difference. Both groups reported no adverse effects. Thus, SGOA can prevent hypertrophic scars and keloids comparable to SGS. Moreover, a benefit of SGOA is that it is more convenient to use than SGS.
This study has some limitations. Our follow‐up period was 3 months. Though hypertrophic scar formation usually occurs within 4‐8 weeks postsurgery, some may occur 1 year following surgery. Hence, longer follow‐up is recommended in further studies. Another limitation is that only one physician assessed the wounds. Future studies should have more than one physician to evaluate the wounds. In addition, we found that the number of scars on each region was quite small. Therefore, more number of patients should be investigated.
5. CONCLUSION
Silicone gel containing onion extract and aloe vera is safe and effective in preventing hypertrophic scars.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Pangkanon W and Udompataikul M involved in conceptualization and validation. Pangkanon W and Kamanamool N involved in methodology and formal analysis. Pangkanon W, Yenbutra P, and Tannirandorn A involved in data curation and investigation. Pangkanon W contributed in writing—original draft preparation. Pangkanon W, Yenbutra P, Kamanamool N, Tannirandorn A, and Udompataikul M involved in writing—review and editing.
ETHICAL APPROVAL
The trial was reviewed and approved by the Institutional Review Board of Srinakharinwirot University (approval No. SWUEC‐309/2562F) and Institutional Review Board of Rajavithi Hospital (approval No. 62124). The trial was registered at the Thai Clinical Trials Registry (TCTR, http://www.clinicaltrials.in.th/), number TCTR20200806004. This study was conducted in accordance with good clinical practice and the Declaration of Helsinki guidelines. Written informed consent was obtained from all study patients at the time of enrollment.
ACKNOWLEDGMENTS
The authors would like to thank Dr Thitikorn Krisorakun, MD, for assisting with data collection. This study was funded by Skin & Health Care Co., Ltd. However, the sponsor had no influence in affecting the study design, data collection, data analysis, discussion, and conclusion.
Pangkanon W, Yenbutra P, Kamanamool N, Tannirandorn A, Udompataikul M. A comparison of the efficacy of silicone gel containing onion extract and aloe vera to silicone gel sheets to prevent postoperative hypertrophic scars and keloids. J Cosmet Dermatol.2021;20:1146–1153. 10.1111/jocd.13933
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Alster TS, Tanzi EL. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4(4):235‐243. [DOI] [PubMed] [Google Scholar]
- 2. Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1‐2):113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foo CW, Tristani‐Firouzi P. Topical modalities for treatment and prevention of postsurgical hypertrophic scars. Facial Plast Surg Clin North Am. 2011;19(3):551‐557. [DOI] [PubMed] [Google Scholar]
- 4. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32(1):82‐92. [DOI] [PubMed] [Google Scholar]
- 5. Cho JW, Cho SY, Lee SR, Lee KS. Onion extract and quercetin induce matrix metalloproteinase‐1 in vitro and in vivo. Int J Mol Med. 2010;25(3):347‐352. [PubMed] [Google Scholar]
- 6. Augusti KT. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol. 1996;34(7):634‐640. [PubMed] [Google Scholar]
- 7. Draelos ZD. The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J Cosmet Dermatol. 2008;7(2):101‐104. [DOI] [PubMed] [Google Scholar]
- 8. Beuth J, Hunzelmann N, Van Leendert R, Basten R, Noehle M, Schneider B. Safety and efficacy of local administration of contractubex to hypertrophic scars in comparison to corticosteroid treatment. Results of a multicenter, comparative epidemiological cohort study in Germany. In Vivo. 2006;20(2):277‐283. [PubMed] [Google Scholar]
- 9. Koc E, Arca E, Surucu B, Kurumlu Z. An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloids. Dermatol Surg. 2008;34(11):1507‐1514. [DOI] [PubMed] [Google Scholar]
- 10. Oryan A, Mohammadalipour A, Moshiri A, Tabandeh MR. Topical application of Aloe vera accelerated wound healing, modeling, and remodeling: an experimental study. Ann Plast Surg. 2016;77(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 11. Khorasani G, Ahmadi A, Jalal Hosseinimehr S, Ahmadi A, Taheri A, Fathi H. The effects of Aloe vera cream on split‐thickness skin graft donor site management: a randomized, blinded, placebo‐controlled study. Wounds. 2011;23(2):44‐48. [PubMed] [Google Scholar]
- 12. Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24(1):103‐154. [DOI] [PubMed] [Google Scholar]
- 13. Varaei S, Ardabili F, Irani P, Ranjbar H. The effect of Aloe vera gel and nitrofurazone on dressing related pain of superficial burn wounds. World J Plast Surg. 2017;6:254‐256. [PMC free article] [PubMed] [Google Scholar]
- 14. Surjushe A, Vasani R, Saple DG. Aloe vera: a short review. Indian J Dermatol. 2008;53(4):163‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta VK, Malhotra S. Pharmacological attribute of Aloe vera: revalidation through experimental and clinical studies. AYU. 2012;33(2):193‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bera T. Phytochemical constituents of Aloe Vera and their multifunctional properties: a comprehensive review. Int J Pharm Pharm Sci. 2018;9:1416‐1423. [Google Scholar]
- 17. Molazem Z, Mohseni F, Younesi M, Keshavarzi S. Aloe vera gel and cesarean wound healing; a randomized controlled clinical trial. Glob J Health Sci. 2014;7(1):203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eshghi F, Hosseinimehr SJ, Rahmani N, Khademloo M, Norozi MS, Hojati O. Effects of Aloe vera cream on posthemorrhoidectomy pain and wound healing: results of a randomized, blind, placebo‐control study. J Altern Complement Med. 2010;16(6):647‐650. [DOI] [PubMed] [Google Scholar]
- 19. Chernoff WG, Cramer H, Su‐Huang S. The efficacy of topical silicone gel elastomers in the treatment of hypertrophic scars, keloid scars, and post‐laser exfoliation erythema. Aesthetic Plast Surg. 2007;31(5):495‐500. [DOI] [PubMed] [Google Scholar]
- 20. Berman B, Maderal A, Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg. 2017;43(Suppl 1):S3‐S18. [DOI] [PubMed] [Google Scholar]
- 21. Bloemen MC, van der Veer WM, Ulrich MM, van Zuijlen PP, Niessen FB, Middelkoop E. Prevention and curative management of hypertrophic scar formation. Burns. 2009;35(4):463‐475. [DOI] [PubMed] [Google Scholar]
- 22. Kim S‐M, Choi J‐S, Lee J‐H, Kim Y‐J, Jun Y‐J. Prevention of postsurgical scars: comparsion of efficacy and convenience between silicone gel sheet and topical silicone gel. J Korean Med Sci. 2014;29(Suppl 3):S249‐S253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hosnuter M, Payasli C, Isikdemir A, Tekerekoglu B. The effects of onion extract on hypertrophic and keloid scars. J Wound Care. 2007;16(6):251‐254. [DOI] [PubMed] [Google Scholar]
- 24. Jenwitheesuk K, Surakunprapha P, Jenwitheesuk K, Kuptarnond C, Prathanee S, Intanoo W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int Wound J. 2012;9(4):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


