Abstract
Aim
To better understand the healthcare burden of people with type 2 diabetes (T2D) and estimated glomerular filtration rate (eGFR) < 90 mL/min/1.73 m2 in Ontario, Canada.
Materials and Methods
We used administrative data to evaluate the prevalence of T2D, eGFR < 90 mL/min/1.73 m2 and adverse cardiovascular co‐morbidities in individuals aged ≥ 30 years living in Ontario, Canada. We also examined incremental healthcare costs and healthcare resource utilization (HCRU) for these patients with specific incident cardiovascular and renal outcomes, in comparison with controls without these outcomes.
Results
While the prevalence of T2D in the general population aged ≥ 30 years in Ontario increased by 1.8% over a 5‐year period (2011‐2012 to 2015‐2016), the prevalence of eGFR < 90 mL/min/1.73 m2 among people with T2D increased by 35%. In comparison with corresponding controls without these outcomes, the per patient average total costs (Canadian dollars) over a 2‐year analysis period were higher for patients with cardiovascular disease/chronic kidney disease related death ($69 827; n = 32 407), doubling of serum creatinine ($52 260; n = 22 825), those who started dialysis ($150 627; n = 3499) or received a kidney transplant ($50 664; n = 651). Similarly, HCRU was significantly greater for patients with these incident outcomes.
Conclusions
This real‐world retrospective study highlights an increasing prevalence of T2D, eGFR < 90 mL/min/1.73 m2, and the substantially higher healthcare costs and HCRU when these patients have adverse cardiovascular and renal outcomes. The existence of such a large economic burden underpins the importance of preventing these diabetes‐related complications.
Keywords: cardiovascular disease, diabetes complications, diabetic nephropathy, health economics, population study, type 2 diabetes
1. INTRODUCTION
In 2019, diabetes affected an estimated 463 million individuals worldwide, with a global economic burden of $760 billion (US dollars). 1 An estimated 3.4 million (9.3% of the population) Canadians were living with diabetes in 2015, which is predicted to reach 5 million (12.1%) by 2025. 2 A Public Health Agency of Canada report estimated the cost of diabetes in 2000 to be $2.5 billion (Canadian dollars); however, this estimate may be conservative as it excluded costs associated with diabetes complications. 3
People with diabetes are at an increased risk of developing kidney disease, with ~40% developing abnormal albuminuria or low estimated glomerular filtration rate (eGFR) during their lifetime. 4 , 5 , 6 The onset of abnormal albuminuria is associated with a greater risk of cardiovascular disease (CVD) and progressive kidney function loss 5 , 7 and can result in the need for chronic renal replacement (dialysis or kidney transplantation), contributing to high healthcare resource utilization (HCRU) and healthcare costs. 8 , 9
Two recent Canadian studies, using administrative healthcare databases, evaluated outcomes in relation to renal function among people with diabetes. An Alberta study described the prevalence of CVD and chronic kidney disease (CKD) among patients with type 2 diabetes (T2D), while an Ontario study investigated the HCRU of older adults living with both type 1 diabetes (T1D) and T2D across the spectrum of eGFR values. 10 , 11 There are insufficient data on the prevalence and burden of illness (BOI) associated with T2D, eGFR < 90 mL/min/1.73 m2 and related adverse outcomes in Canada. To determine the potential benefits of early prevention strategies, there is a need to understand the associated HCRU and healthcare costs for people with T2D and mild decrease in eGFR, in addition to patients with T2D and CKD. 12 Ontario, Canada's largest province with 38.3% of its population, is ethnically diverse with 67.8% people of European origin, 2.8% people of indigenous origins and 29.3% people from racialized groups including South Asian origin (8.7%), Chinese origin (5.7%), and African origin (4.7%), making it a unique source to study a globally relevant population. 13 , 14 , 15
This real‐world retrospective population study was undertaken to understand the healthcare burden of select adverse cardiovascular and renal events in people with T2D and eGFR < 90 mL/min/1.73 m2 in Ontario, Canada (Figure 1). We estimated the prevalence of T2D among adults aged ≥ 30 years old and quantified the proportion with eGFR < 90 mL/min/1.73 m2 and associated CVD co‐morbidities. We also evaluated the BOI of these patients by examining incident T2D‐related outcomes including CVD/CKD related death, doubling of serum creatinine, kidney dialysis and kidney transplantation.
FIGURE 1.
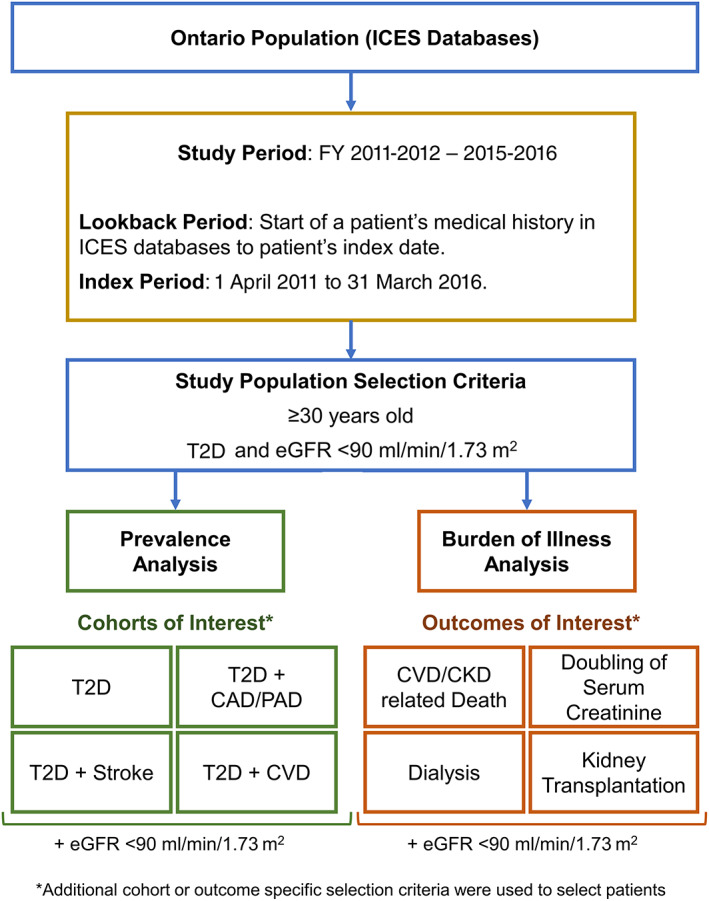
Study schematic of prevalence and burden of illness analyses of type 2 diabetes (T2D) with estimated glomerular filtration rate (eGFR) < 90 mL/min/1.73 m2 in Ontario, Canada. Abbreviations: CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cardiovascular disease; FY, fiscal years; ICES, Institute for Clinical Evaluative Sciences; PAD, peripheral artery disease
2. MATERIALS AND METHODS
2.1. Study design and setting
We conducted a population‐based retrospective observational study using administrative data to evaluate the prevalence, healthcare costs and HCRU of people living with T2D, eGFR < 90 mL/min/1.73 m2 and related co‐morbidities in Ontario, Canada. The study was approved by the institutional review board at Advarra Canada (approval number Pro00035931). As this was a retrospective study using administrative data, informed patient consent was not required.
2.2. Data sources
This study utilized administrative databases from the Institute for Clinical Evaluative Sciences (ICES) that contain publicly funded health services records for the Ontario population. These de‐identified record‐level databases include information such as physician claims submitted to the Ontario Health Insurance Plan (OHIP), medical drug claims submitted to the Ontario Drug Benefit Program, discharge summaries of hospital stay and emergency department visits, and laboratory values from the Ontario Laboratories Information System. To identify diabetes patients, we used the ICES Ontario Diabetes Database (ODD), which is a highly representative database that identifies diabetes patients through a clinically validated algorithm. 16 , 17
2.3. Study population
The study population included people, aged ≥ 30 years old, with T2D and at least two eGFR test values < 90 mL/min/1.73 m2 (Figure 1). The ODD was used to identify diabetes patients aged ≥ 30 years old at index. Patients first diagnosed with diabetes at < 19 years of age or those diagnosed with T1D were excluded. eGFR values were derived from serum creatinine laboratory values using the modification of diet in renal disease (MDRD) equation. 18 A lookback period, spanning the start of a patient's medical history in ICES databases to their index date, was used to identify patients meeting the selection criteria. Eligible patients were indexed to specific cohorts or outcomes from 1 April 2011 to 31 March 2016 (index period) on the first date (index date) they satisfied the cohort or outcome‐specific selection criteria described below.
2.3.1. Prevalence cohorts
We estimated the prevalence of T2D overall, T2D with eGFR < 90 mL/min/1.73 m2 and associated subgroups of CVD co‐morbidities. CVD was defined to include coronary artery disease (CAD), peripheral artery disease (PAD) and cerebrovascular disease/stroke, as identified through corresponding diagnosis/billing codes. People with T2D and eGFR < 90 mL/min/1.73 m2 were grouped into the following four cohorts: (a) T2D, (b) T2D + CAD/PAD, (c) T2D + stroke and (d) T2D + CVD. Patients could be indexed to multiple cohorts on meeting the cohort‐specific criteria. Patients remained in the cohort until the end of the index period, a death record, or loss of OHIP coverage (for ≥3 consecutive quarters), whichever occurred first.
2.3.2. BOI outcomes
The BOI of patients with T2D and eGFR < 90 mL/min/1.73 m2 was evaluated using a case‐control study. For this analysis, patients were eligible if they were recorded as alive at least 2 years post index and were OHIP eligible within 2 years prior to and post index. We indexed cases for the following four incident outcomes: (a) CVD/CKD related death, (b) doubling of serum creatinine, (c) dialysis and (d) kidney transplantation. For each outcome, controls included people with T2D and eGFR < 90 mL/min/1.73 m2, without the specific outcome during the lookback, index and analysis periods. Cases were exactly matched to controls based on age (±3 years), sex, ODD entry year, ICES co‐morbidity score range, geographical location based on the Local Health Integration Network and the neighbourhood income quintile. The ICES co‐morbidity score was based on a patient's hospital diagnoses. ICD‐9 and ICD‐10 diagnosis codes for chronic diseases were used to assign weights, depending on the severity, and these weights were summed to determine the score. Any cases without a matched control were excluded.
The CVD/CKD related death cases included patients who had a CVD or CKD diagnosis or event ≤ 60 days prior to a death record. 19 Eligible patients were indexed on the date exactly 2 years prior to their death record. The doubling of serum creatinine cases included patients who had at least two blood serum creatinine laboratory test values, where the second value was ≥2 times the value of the first, and the two laboratory values were > 90 days and ≤ 2 years apart. 20 These patients were indexed on the date of the second test. The doubling of serum creatinine was selected as an outcome of interest because it is used as a marker of declining renal function in nephrology trials and is associated with a higher risk of cardiovascular incidents in diabetes patients. 20 The dialysis and kidney transplantation cases included patients who had an incident procedure code for kidney dialysis or kidney transplant, respectively. Patients were indexed on their first procedure code date of kidney dialysis or kidney transplant, respectively. Controls were assigned the index date of their matched case. The outcomes were not mutually exclusive; it was possible for a patient to index to more than one outcome if they satisfied individual outcome‐specific selection criteria.
2.4. Measures and outcomes
2.4.1. Prevalence
The yearly patient counts and percentage prevalence for each patient cohort were determined for 5 fiscal years (2011‐2012 to 2015‐2016). For each year, the percentage prevalence of each cohort in the general population and among people with T2D was calculated by dividing the patient counts by the respective Ontario general population (from Statistics Canada) or number of people with T2D (aged ≥30 years), respectively.
2.4.2. Burden of illness
The healthcare costs and HCRU for each year in a 2‐year analysis period, following the index date, were calculated for patients in each of the outcomes, except for CVD/CKD related death, where burden was analysed in the 2 years leading up to their death record. The patients’ baseline characteristics were assessed prior to index. Costs for multiple healthcare encounters such as physician visits (general practitioner/family medicine [GP/FM] and specialist), hospital costs (e.g. emergency department, inpatient hospitalization), public drug plans and dialysis clinics were measured. For hospital costs, we used the resource intensity weight methodology from the Canadian MIS Database, which attributes a hospital‐specific cost to the resource intensity of each visit. 21 , 22 The per patient average costs are reported here; all costs are reported in Canadian dollars (2018). For HCRU analysis, counts of all healthcare encounters such as physician visits, dialysis clinic visits, emergency department visits and inpatient hospitalizations were measured. The average counts per patient are reported here.
2.5. Statistical analyses
No imputation was performed for missing values, and all data management and data analysis were performed using SAS version 9.3 or higher. Mean and standard deviation (SD) as well as median and interquartile range (IQR) for continuous measures, and frequencies and percentages for categorical variables, are reported. Because healthcare cost data are not normally distributed, an unadjusted gamma model was used to determine variance, and compare cases and controls within each year; the associated P‐value is reported. Because HCRU count data are not normally distributed, an unadjusted Poisson model or an unadjusted negative binomial model was used, where appropriate, to compare cases and controls. The associated P‐value is reported for each year.
3. RESULTS
3.1. Prevalence of T2D with eGFR < 90 mL/min/1.73 m2
The prevalence of T2D in the general population aged ≥ 30 years in Ontario increased by 1.8% over a 5‐year period from 2011‐2012 to 2015‐2016 (11.50% to 13.25%) (Figure 2). The prevalence of T2D with eGFR < 90 mL/min/1.73 m2 and CVD co‐morbidities increased in the general population as well as among people with T2D. Among people with T2D aged ≥ 30 years in Ontario, the prevalence of eGFR < 90 mL/min/1.73 m2 and CVD co‐morbidities increased by 16.5% for the T2D cohort (46.81% to 63.27%), by 4.9% for the T2D + CAD/PAD cohort (28.23% to 33.13%), by 0.4% for the T2D + stroke cohort (10.37% to 10.74%), and by 5.5% for the T2D + CVD cohort (30.27% to 35.77%).
FIGURE 2.
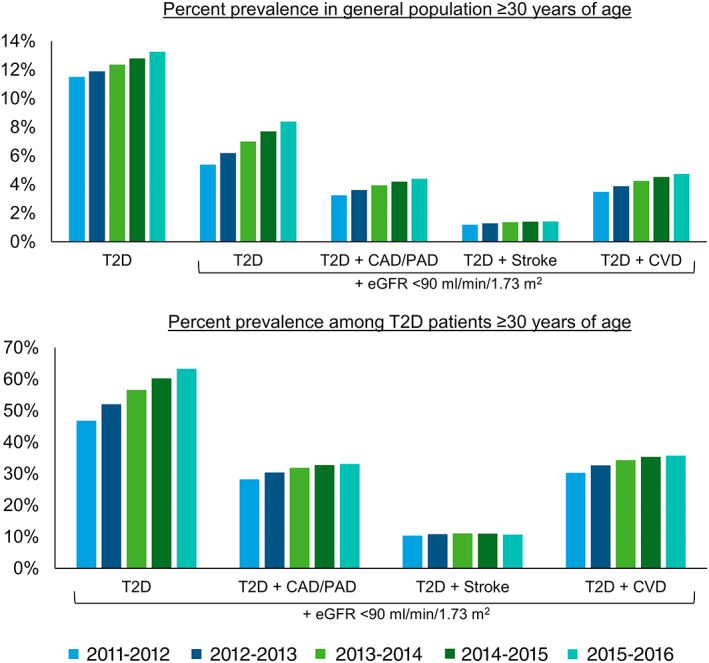
Percentage prevalence of type 2 diabetes (T2D), estimated glomerular filtration rate (eGFR) < 90 mL/min/1.73 m2 and cardiovascular disease subgroups in individuals aged ≥ 30 years in Ontario, Canada. Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; PAD, peripheral artery disease
3.2. BOI analyses of T2D with eGFR < 90 mL/min/1.73 m2
We are reporting BOI of T2D with eGFR < 90 mL/min/1.73 m2 and incident T2D‐related outcomes, specifically CVD/CKD related death, doubling of serum creatinine, dialysis and kidney transplantation (Figure 1). The cases included patients with a specific incident outcome, while the corresponding matched controls included patients who did not have the specific outcome.
3.2.1. Baseline characteristics
Over 5 years, 2011‐2012 to 2015‐2016, the total unique number of people with T2D, eGFR < 90 mL/min/1.73 m2, and who were aged ≥ 30 years in Ontario, was 811 794 (Table S2). Of these people, there were 32 407 (4.0%) with CVD/CKD related death, 22 825 (2.8%) who had a doubling of serum creatinine, 3499 (0.4%) who started dialysis and 651 (0.1%) who received a kidney transplant. As multiple criteria were used to match patients in the outcome cases and corresponding controls, many of the baseline characteristics were similar and are summarized in Table 1 and Table S4.
TABLE 1.
Baseline characteristics of people with T2D and eGFR < 90 mL/min/1.73 m2 (aged ≥30 years) for burden of illness analyses
| Outcome | CVD/CKD related death | Doubling of serum creatinine | Dialysis | Kidney transplantation | ||||
|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | Case | Control | |
| Number, n | 32 407 | 98 680 | 22 825 | 73 017 | 3499 | 11 004 | 651 | 2129 |
| Age, years | ||||||||
| Age, mean ± SD | 78.63 ± 9.28 | 77.29 ± 8.92 | 71.35 ± 10.82 | 71.15 ± 10.32 | 68.94 ± 10.48 | 69.27 ± 10.02 | 66.12 ± 10.46 | 66.74 ± 9.93 |
| Age, median (IQR) | 80 (73‐86) | 78 (72‐84) | 72 (64‐80) | 72 (64‐79) | 70 (62‐77) | 70 (63‐77) | 66 (60‐74) | 67 (60‐74) |
| Age group, n (%) | ||||||||
| 30‐44 | 37 (0.1%) | 83 (0.1%) | 234 (1.0%) | 549 (0.8%) | 57 (1.6%) | 141 (1.3%) | 20 (3.1%) | 42 (2.0%) |
| 45‐64 | 2648 (8.2%) | 8927 (9.0%) | 5729 (25.1%) | 18 186 (24.9%) | 1072 (30.6%) | 3203 (29.1%) | 265 (40.7%) | 813 (38.2%) |
| 65‐84 | 20 146 (62.2%) | 67 950 (68.9%) | 14 347 (62.9%) | 47 382 (64.9%) | 2180 (62.3%) | 7117 (64.7%) | 347 (53.3%) | 1221 (57.4%) |
| 84+ | 9576 (29.5%) | 21 720 (22.0%) | 2515 (11.0%) | 6900 (9.4%) | 190 (5.4%) | 543 (4.9%) | 19 (2.9%) | 53 (2.5%) |
| Sex | ||||||||
| Female, n (%) | 14 723 (45.4%) | 45 087 (45.7%) | 11 512 (50.4%) | 36 960 (50.6%) | 1253 (35.8%) | 3929 (35.7%) | 227 (34.9%) | 769 (36.1%) |
| Income quintiles, n (%) | ||||||||
| 1 ‐ lowest | 8226 (25.4%) | 25 209 (25.5%) | 5831 (25.5%) | 18 711 (25.6%) | 973 (27.8%) | 3040 (27.6%) | 163 (25.0%) | 536 (25.2%) |
| 2 | 7413 (22.9%) | 22 692 (23.0%) | 5224 (22.9%) | 16 877 (23.1%) | 821 (23.5%) | 2612 (23.7%) | 146 (22.4%) | 495 (23.3%) |
| 3 | 6420 (19.8%) | 19 641 (19.9%) | 4560 (20.0%) | 14 485 (19.8%) | 688 (19.7%) | 2146 (19.5%) | 127 (19.5%) | 417 (19.6%) |
| 4 | 5552 (17.1%) | 16 823 (17.0%) | 3922 (17.2%) | 12 533 (17.2%) | 531 (15.2%) | 1695 (15.4%) | 127 (19.5%) | 400 (18.8%) |
| 5 ‐ highest | 4796 (14.8%) | 14 315 (14.5%) | 3288 (14.4%) | 10 411 (14.3%) | 486 (13.9%) | 1511 (13.7%) | 88 (13.5%) | 281 (13.2%) |
| ICES co‐morbidity score, n (%) | ||||||||
| Missing | 14 744 (45.5%) | 51 270 (52.0%) | 7557 (33.1%) | 28 984 (39.7%) | 1060 (30.3%) | 4073 (37.0%) | 138 (21.2%) | 522 (24.5%) |
| 0 | 3805 (11.7%) | 11 472 (11.6%) | 2852 (12.5%) | 9632 (13.2%) | 336 (9.6%) | 1113 (10.1%) | 84 (12.9%) | 304 (14.3%) |
| 1‐2 | 7688 (23.7%) | 23 894 (24.2%) | 7153 (31.3%) | 23 784 (32.6%) | 1035 (29.6%) | 3499 (31.8%) | 233 (35.8%) | 828 (38.9%) |
| 3‐4 | 4909 (15.1%) | 10 240 (10.4%) | 4317 (18.9%) | 9276 (12.7%) | 827 (23.6%) | 1934 (17.6%) | 157 (24.1%) | 403 (18.9%) |
| 5+ | 1261 (3.9%) | 1804 (1.8%) | 946 (4.1%) | 1341 (1.8%) | 241 (6.9%) | 385 (3.5%) | 39 (6.0%) | 72 (3.4%) |
| ODD entry year, n (%) | ||||||||
| Prevalent in 1994 | 3066 (9.5%) | 8311 (8.4%) | 1700 (7.4%) | 4878 (6.7%) | 379 (10.8%) | 1109 (10.1%) | 45 (6.9%) | 132 (6.2%) |
| 1994‐1999 | 5485 (16.9%) | 15 270 (15.5%) | 3555 (15.6%) | 10 319 (14.1%) | 668 (19.1%) | 1857 (16.9%) | 81 (12.4%) | 233 (10.9%) |
| 2000‐2005 | 10 581 (32.7%) | 32 877 (33.3%) | 7246 (31.7%) | 23 702 (32.5%) | 1096 (31.3%) | 3557 (32.3%) | 198 (30.4%) | 673 (31.6%) |
| 2006‐2011 | 10 995 (33.9%) | 35 113 (35.6%) | 7685 (33.7%) | 25 796 (35.3%) | 1015 (29.0%) | 3401 (30.9%) | 240 (36.9%) | 814 (38.2%) |
| 2012‐2015 | 2280 (7.0%) | 7109 (7.2%) | 2639 (11.6%) | 8322 (11.4%) | 341 (9.7%) | 1080 (9.8%) | 87 (13.4%) | 277 (13.0%) |
| HbA1c | ||||||||
| HbA1c, n (%) | 24 583 (75.9%) | 78 399 (79.4%) | 18 842 (82.5%) | 59 381 (81.3%) | 2824 (80.7%) | 9098 (82.7%) | 488 (75.0%) | 1752 (82.3%) |
| HbA1c (%), mean ± SD | 6.88 ± 1.78 | 6.84 ± 1.59 | 7.03 ± 2.14 | 6.91 ± 1.46 | 6.77 ± 1.47 | 7.02 ± 1.47 | 6.83 ± 3.12 | 7.07 ± 2.69 |
| HbA1c (%), median (IQR) | 6.6 (6.1‐7.3) | 6.6 (6.1‐7.2) | 6.7 (6.1‐7.5) | 6.6 (6.2‐7.3) | 6.5 (5.9‐7.2) | 6.7 (6.2‐7.5) | 6.4 (5.9‐7.2) | 6.6 (6.1‐7.4) |
| HbA1c category, n (%) | ||||||||
| Missing | 7824 (24.1%) | 20 281 (20.6%) | 3983 (17.5%) | 13 636 (18.7%) | 675 (19.3%) | 1906 (17.3%) | 163 (25.0%) | 377 (17.7%) |
| ≤ 6.5 | 12 032 (37.1%) | 37 766 (38.3%) | 8564 (37.5%) | 27 092 (37.1%) | 1530 (43.7%) | 3829 (34.8%) | 273 (41.9%) | 795 (37.3%) |
| 6.5 < HbA1c ≤ 7 | 4765 (14.7%) | 17 059 (17.3%) | 3507 (15.4%) | 12 769 (17.5%) | 455 (13.0%) | 1905 (17.3%) | 81 (12.4%) | 341 (16.0%) |
| 7 < HbA1c ≤ 8.5 | 5767 (17.8%) | 18 632 (18.9%) | 4753 (20.8%) | 14 814 (20.3%) | 592 (16.9%) | 2447 (22.2%) | 101 (15.5%) | 446 (20.9%) |
| > 8.5 | 2019 (6.2%) | 4942 (5.0%) | 2018 (8.8%) | 4706 (6.4%) | 247 (7.1%) | 917 (8.3%) | 33 (5.1%) | 170 (8.0%) |
| Serum creatinine | ||||||||
| Serum creatinine, n (%) | 28 756 (88.7%) | 86 646 (87.8%) | 21 863 (95.8%) | 65 056 (89.1%) | 3307 (94.5%) | 9803 (89.1%) | 599 (92.0%) | 1919 (90.1%) |
| Serum creatinine (μmol/L), mean ± SD | 123.69 ± 97.22 | 95.26 ± 43.65 | 134.71 ± 105.08 | 89.29 ± 34.18 | 417.87 ± 304.08 | 94.38 ± 35.14 | 362.86 ± 340.05 | 98.94 ± 67.59 |
| Serum creatinine (μmol/L), median (IQR) | 99 (80‐132) | 87 (74‐105) | 107 (83‐147) | 83 (71‐99) | 386 (144‐583) | 87 (75‐103) | 172 (95‐571) | 87 (74‐103) |
| UACR | ||||||||
| UACR, n (%) | 13 606 (42.0%) | 46 294 (46.9%) | 11 269 (49.4%) | 35 372 (48.4%) | 2084 (59.6%) | 5736 (52.1%) | 237 (36.4%) | 1071 (50.3%) |
| UACR (mg/mmol), mean ± SD | 33.10 ± 94.54 | 9.90 ± 38.98 | 60.56 ± 145.54 | 8.62 ± 33.25 | 182.29 ± 235.80 | 11.39 ± 40.81 | 67.45 ± 152.97 | 13.68 ± 52.29 |
| UACR (mg/mmol), median (IQR) | 3.7 (1.1‐16.8) | 1.9 (0.3‐4.5) | 4.2 (1.1‐27.8) | 1.5 (0.1‐3.6) | 62 (4.1‐300.3) | 1.9 (0.3‐4.7) | 10.5 (2.8‐47) | 1.8 (0.3‐4.4) |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ICES, Institute for Clinical Evaluative Sciences; IQR, interquartile range; ODD, Ontario diabetes database; SD, standard deviation; T2D, type 2 diabetes; UACR, urinary albumin to creatinine ratio.
eGFR values were calculated using the Modification of diet in renal disease (MDRD) formula: (GFR [mL/min per 1.73 m2] = 186.3 × serum creatinine [mg/dL]−1.154 × age [years]−0.203 × 0.742 [if female]).
3.2.2. Healthcare costs
Patients with each of the outcomes had higher average annual costs than the respective controls (Table 2 and Table S5). In comparison with respective controls, the average total costs over 2 years were $69 827 (276%) higher for patients with CVD/CKD related death, $52 260 (249%) higher for patients with doubling of serum creatinine, $150 627 (648%) higher for patients needing dialysis, and $50 664 (188%) higher for patients receiving a kidney transplant. Across all outcomes, inpatient hospitalization costs were the major driver of the higher costs for cases.
TABLE 2.
Healthcare costs of people with T2D and eGFR < 90 mL/min/1.73 m2 (aged ≥ 30 years) in Ontario, Canada
| Outcome | Year 1 | Year 2 | Year 1 + year 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case vs. control | Case | Control | Case vs control | Case vs. control | ||||
| Mean (SD) | Mean (SD) | Percentage increase | P‐value | Mean (SD) | Mean (SD) | Percentage increase | P‐value | Difference | Percentage increase | |
| CVD/CKD related death | ||||||||||
| Physician costs ‐ GP/FM | $605 (907) | $408 (1041) | 48% | <.001 | $1399 (2961) | $379 (824) | 269% | <.001 | $1218 | 155% |
| Inpatient hospitalization costs | $6883 (21 290) | $2595 (10 704) | 165% | $32 580 (50 406) | $2434 (10 486) | 1239% | $34 433 | 685% | ||
| Long‐term care costs | $4250 (13 081) | $2267 (9532) | 87% | $6177 (15 411) | $2351 (9752) | 163% | $5808 | 126% | ||
| Dialysis clinic costs | $2046 (12 838) | $193 (4005) | 960% | $2550 (13 998) | $233 (4401) | 994% | $4170 | 979% | ||
| Public drug plan costs | $3404 (5827) | $2229 (3896) | 53% | <.001 | $3837 (7118) | $2244 (4270) | 71% | <.001 | $2768 | 62% |
| Outpatient clinic costs | $967 (1552) | $573 (1110) | 69% | <.001 | $2013 (2314) | $530 (1031) | 280% | <.001 | $1877 | 170% |
| Physician costs ‐ specialist | $3109 (3641) | $1860 (2128) | 67% | <.001 | $6783 (6541) | $1733 (2079) | 291% | <.001 | $6298 | 175% |
| Total costs | $27 563 (40 298) | $12 802 (22 792) | 115% | <.001 | $67 590 (66 732) | $12 524 (22 849) | 440% | <.001 | $69 827 | 276% |
| Doubling of serum creatinine | ||||||||||
| Physician costs ‐ GP/FM | $819 (2767) | $385 (708) | 113% | <.001 | $614 (1037) | $361 (729) | 70% | <.001 | $688 | 92% |
| Inpatient hospitalization costs | $16 217 (39 679) | $2293 (11 896) | 607% | $7478 (26 214) | $2161 (10 277) | 246% | $19 242 | 432% | ||
| Long‐term care costs | $2634 (10 406) | $1255 (7153) | 110% | $3366 (11 822) | $1296 (7325) | 160% | $3449 | 135% | ||
| Dialysis clinic costs | $3390 (15 409) | $87 (2617) | 3797% | $4739 (19 189) | $88 (2634) | 5285% | $7955 | 4545% | ||
| Public drug plan costs | $3583 (8601) | $1984 (3672) | 81% | <.001 | $3804 (8241) | $2026 (4049) | 88% | <.001 | $3377 | 84% |
| Outpatient clinic costs | $1694 (1964) | $577 (1115) | 194% | <.001 | $1173 (1683) | $522 (1032) | 125% | <.001 | $1768 | 161% |
| Physician costs ‐ specialist | $5155 (5210) | $1822 (2135) | 183% | <.001 | $3639 (4246) | $1690 (2052) | 115% | <.001 | $5282 | 150% |
| Total costs | $42 027 (58 302) | $10 683 (21 547) | 293% | <.001 | $31 200 (47 588) | $10 284 (20 479) | 203% | <.001 | $52 260 | 249% |
| Dialysis | ||||||||||
| Physician costs ‐ GP/FM | $853 (1176) | $412 (805) | 107% | <.001 | $585 (1180) | $402 (3360) | 46% | <.001 | $624 | 77% |
| Inpatient hospitalization costs | $41 702 (76 295) | $3109 (12 139) | 1241% | $11 844 (36 719) | $2710 (11 741) | 337% | $47 727 | 820% | ||
| Long‐term care costs | $960 (6009) | $1025 (6505) | −6% | $1602 (8428) | $1055 (6564) | 52% | $482 | 23% | ||
| Dialysis clinic costs | $32 834 (38 809) | $8 (390) | 410 325% | $32 246 (41 878) | $43 (1571) | 74 891% | $65 029 | 127 508% | ||
| Public drug plan costs | $3212 (14 631) | $2103 (4201) | 53% | <.001 | $3700 (12 483) | $2116 (4533) | 75% | <.001 | $2692 | 64% |
| Outpatient clinic costs | $2220 (2204) | $663 (1228) | 235% | <.001 | $1475 (1899) | $581 (1114) | 154% | <.001 | $2451 | 197% |
| Physician costs ‐ specialist | $12 353 (7292) | $1997 (2477) | 519% | <.001 | $7792 (6201) | $1810 (2323) | 330% | <.001 | $16 338 | 429% |
| Total costs | $106 637 (95 149) | $12 051 (23 717) | 785% | <.001 | $67 223 (70 818) | $11 182 (23 029) | 501% | <.001 | $150 627 | 648% |
| Kidney transplantation | ||||||||||
| Physician costs ‐ GP/FM | $434 (559) | $433 (1034) | 0% | 0.9855 | $369 (580) | $393 (862) | −6% | 0.3977 | −$24 | −3% |
| Inpatient hospitalization costs | $25 604 (28 158) | $4225 (23 567) | 506% | $4550 (14 337) | $3043 (12 471) | 50% | $22 885 | 315% | ||
| Long‐term care costs | $182 (2526) | $1004 (6512) | −82% | $295 (3479) | $1045 (6872) | −72% | −$1573 | −77% | ||
| Dialysis clinic costs | $3553 (15 976) | $722 (7724) | 392% | $3168 (15 759) | $731 (7918) | 333% | $5269 | 363% | ||
| Public drug plan costs | $7362 (17 696) | $2236 (5082) | 229% | <.001 | $5134 (7000) | $2050 (4455) | 150% | <.001 | $8210 | 192% |
| Outpatient clinic costs | $3967 (3059) | $756 (1419) | 425% | <.001 | $1850 (1823) | $667 (1298) | 177% | <.001 | $4393 | 309% |
| Physician costs ‐ specialist | $9371 (5305) | $2282 (3071) | 311% | <.001 | $3618 (3687) | $1945 (2502) | 86% | <.001 | $8762 | 207% |
| Total costs | $55 381 (48 244) | $14 442 (34 031) | 283% | <.001 | $22 167 (30 184) | $12 442 (26 886) | 78% | <.001 | $50 664 | 188% |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GP/FM, general practitioner/family medicine; SD, standard deviation; T2D, type 2 diabetes.
CVD/CKD related death outcome
Patients with CVD/CKD related death were indexed exactly 2 years before their death record. Their average total annual costs were significantly greater than their controls at $27 563 versus $12 802 in year 1 and $67 590 versus $12 524 in year 2 (P < .001). Year 2 was immediately before the death event and much higher costs were observed in comparison with year 1. Over 2 years, the total public healthcare costs for 32 407 patients were $3.08 billion. The main drivers for these costs were inpatient hospitalization, long‐term care, public drug plan and specialist physician visit costs.
Doubling of serum creatinine outcome
Patients with doubling of serum creatinine had significantly higher average annual total costs than their controls at $42 027 versus $10 683 in year 1 and $31 200 versus $10 284 in year 2 (P < .001). Over 2 years, the total public healthcare costs for 22 825 patients were $1.67 billion. The major drivers of these high costs were inpatient hospitalization, followed by specialist physician visits, public drug plan and dialysis clinic costs.
Dialysis outcome
The average total costs over 2 years were highest for patients requiring dialysis. They were also significantly higher than matched controls at $106 637 versus $12 051 in year 1 and $67 223 versus $11 182 in year 2 (P < .001). Over 2 years, the total public healthcare costs for 3499 patients were $608.34 million. The main drivers of these high costs were inpatient hospitalization and dialysis clinic costs.
Kidney transplantation outcome
Patients who received a kidney transplant had significantly higher average annual total costs than their matched controls at $55 381 versus $14 442 in year 1 and $22 167 versus $12 442 in year 2 (P < .001). Over 2 years, the total public healthcare costs for 651 patients were $50.48 million. The higher average annual total cost in year 1 was mainly driven by inpatient hospitalization costs, potentially explained by the costs associated with the transplantation procedure. Other drivers of these high costs were specialist physician visits, public drug plan and outpatient clinic costs.
3.2.3. Healthcare resource utilization
For patients in each of the case outcomes, the HCRU was significantly higher than the controls for both years of analysis and are summarized in Table 3 and Table S6. One of the highest touchpoints across all cases was visits to specialist physicians; the average total visits over 2 years were higher by 63.6 visits for patients with CVD/CKD related death, 48.0 visits for patients with doubling of serum creatinine, 128.1 visits for patients requiring dialysis, and 49.6 visits for patients who received a kidney transplant. Other healthcare resources highly utilized across all cases included visits to GP/FM, dialysis clinics and outpatient visits. Additionally, the average total length of stay in the hospital for cases in most outcomes was also significantly longer, with the highest total length of stay in the hospital for dialysis patients (27.7 more days over 2 years).
TABLE 3.
Healthcare resource utilization of people with T2D and eGFR < 90 mL/min/1.73 m2 (aged ≥30 years) in Ontario, Canada
| Outcome | Year 1 | Year 2 | ||||
|---|---|---|---|---|---|---|
| Case | Control | P‐value | Case | Control | P‐value | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| CVD/CKD related death | ||||||
| Number of patients, n | 32 407 | 98 680 | 32 407 | 98 680 | ||
| Dialysis clinic visits | 3.54 (22.22) | 0.33 (6.89) | <.001 | 4.37 (23.91) | 0.40 (7.50) | <.001 |
| Physician visits ‐ GP/FM | 11.02 (14.86) | 7.59 (10.08) | <.001 | 21.16 (22.42) | 7.14 (10.00) | <.001 |
| Inpatient hospitalization visits | 0.56 (1.02) | 0.23 (0.63) | 1.87 (1.53) | 0.23 (0.62) | <.001 | |
| Long‐term care visits | 0.66 (1.94) | 0.35 (1.41) | <.001 | 1.04 (2.41) | 0.36 (1.42) | <.001 |
| Outpatient clinic visits | 2.71 (4.34) | 1.61 (3.11) | <.001 | 5.91 (6.78) | 1.54 (3.01) | <.001 |
| Physician visits ‐ specialist | 31.05 (38.86) | 17.20 (24.13) | <.001 | 64.93 (62.10) | 15.21 (20.81) | <.001 |
| Inpatient hospitalizations | ||||||
| Number of patients, n (%) | 10 762 (33.2%) | 16 419 (16.6%) | 28 341 (87.5%) | 15 601 (15.8%) | ||
| Total length of stay (days) | 20.11 (42.35) | 14.89 (35.73) | <.001 | 27.88 (37.07) | 14.49 (33.56) | <.001 |
| Doubling of serum creatinine | ||||||
| Number of patients, n | 22 825 | 73 017 | 22 825 | 73 017 | ||
| Dialysis clinics visits | 5.86 (26.63) | 0.15 (4.55) | <.001 | 8.13 (32.74) | 0.15 (4.49) | <.001 |
| Physician visits ‐ GP/FM | 14.38 (17.98) | 7.40 (9.45) | <.001 | 10.88 (15.39) | 7.01 (9.34) | <.001 |
| Inpatient hospitalization visits | 1.06 (1.28) | 0.21 (0.59) | <.001 | 0.57 (1.11) | 0.20 (0.59) | <.001 |
| Long‐term care visits | 0.43 (1.64) | 0.20 (1.07) | <.001 | 0.52 (1.74) | 0.20 (1.08) | <.001 |
| Outpatient clinic visits | 4.77 (5.53) | 1.63 (3.15) | <.001 | 3.43 (4.92) | 1.53 (3.04) | <.001 |
| Physician visits ‐ specialist | 46.21 (49.54) | 15.63 (21.12) | <.001 | 31.70 (38.79) | 14.25 (18.92) | <.001 |
| Inpatient hospitalizations | ||||||
| Number of patients, n (%) | 13 775 (60.4%) | 10 719 (14.7%) | 7311 (32.0%) | 10 073 (13.8%) | ||
| Total length of stay (days) | 22.42 (49.64) | 13.49 (30.72) | <.001 | 21.94 (61.82) | 13.55 (30.73) | <.001 |
| Dialysis | ||||||
| Number of patients, n | 3499 | 11 004 | 3499 | 11 004 | ||
| Physician visits ‐ GP/FM | 14.30 (18.30) | 7.72 (10.27) | <.001 | 9.73 (15.32) | 7.08 (9.76) | <.001 |
| Inpatient hospitalization visits | 1.35 (1.24) | 0.27 (0.73) | <.001 | 0.70 (1.21) | 0.23 (0.68) | <.001 |
| Long‐term care visits | 0.17 (0.97) | 0.17 (1.01) | .8383 | 0.24 (1.19) | 0.17 (1.01) | .0005 |
| Outpatient clinic visits | 6.20 (6.20) | 1.85 (3.44) | <.001 | 4.29 (5.54) | 1.69 (3.24) | <.001 |
| Physician visits ‐ specialist | 98.72 (73.97) | 17.24 (24.45) | <.001 | 61.94 (53.20) | 15.35 (21.60) | <.001 |
| Inpatient hospitalizations | ||||||
| Number of patients, n (%) | 2739 (78.3%) | 1346 (12.2%) | 1903 (54.4%) | 1680 (15.3%) | ||
| Total length of stay (days) | 33.52 (57.80) | 15.10 (31.97) | <.001 | 24.94 (68.67) | 15.65 (34.64) | <.001 |
| Kidney transplantation | ||||||
| Number of patients, n | 651 | 2129 | 651 | 2129 | ||
| Dialysis clinics visits | 6.16 (27.20) | 1.28 (13.62) | <.001 | 5.40 (26.66) | 1.25 (13.48) | <.001 |
| Physician visits ‐ GP/FM | 8.12 (8.86) | 7.67 (9.64) | .2570 | 7.02 (8.27) | 7.18 (10.77) | .6870 |
| Inpatient hospitalization visits | 1.72 (1.24) | 0.29 (0.78) | <.001 | 0.45 (0.88) | 0.26 (0.72) | <.001 |
| Long‐term care visits | 0.03 (0.39) | 0.15 (0.94) | .0011 | 0.05 (0.55) | 0.15 (0.94) | .0133 |
| Outpatient clinic visits | 11.09 (8.57) | 2.12 (3.99) | <.001 | 5.38 (5.27) | 1.95 (3.83) | <.001 |
| Physician visits ‐ specialist | 56.27 (40.43) | 18.83 (26.86) | <.001 | 28.35 (31.26) | 16.19 (22.81) | <.001 |
| Inpatient hospitalizations | ||||||
| Number of patients, n (%) | 648 (99.5%) | 395 (18.6%) | 180 (27.6%) | 352 (16.5%) | ||
| Total length of stay (days) | 13.74 (15.67) | 18.56 (45.74) | .0230 | 11.78 (14.88) | 17.13 (44.30) | .0236 |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GP/FM, general practitioner/family medicine; SD, standard deviation; T2D, type 2 diabetes.
4. DISCUSSION
4.1. Main findings
This is the first large and comprehensive population‐based study to assess the prevalence and BOI of T2D with eGFR < 90 mL/min/1.73 m2 in Ontario, Canada. This study captures the increasing prevalence of eGFR < 90 mL/min/1.73 m2 as well as subgroups of CVD co‐morbidities among people with T2D from 2011‐2012 to 2015‐2016. The substantially higher healthcare costs and HCRU of patients with T2D, eGFR < 90 mL/min/1.73 m2 and adverse cardiovascular and renal incident outcomes including CVD/CKD related death, doubling of serum creatinine, dialysis and kidney transplantation, are evident from this study. This analysis highlights not only the real‐world burden of T2D with eGFR < 90 mL/min/1.73 m2 and adverse outcomes on the healthcare system, but also the importance of avoiding disease complications and preventing disease progression.
Over 2 years, the total public healthcare costs for patients with CVD/CKD related death, doubling of serum creatinine, dialysis and kidney transplantation outcomes were $3.08 billion, $1.67 billion, $608.34 million and $50.48 million, respectively. In comparison with respective controls, the per patient average total costs over 2 years were $69 827 (276%) higher for patients with CVD/CKD related death, $52 260 (249%) higher for patients with doubling of serum creatinine, $150 627 (648%) higher for patients needing dialysis and $50 664 (188%) higher for patients receiving a kidney transplant. With the only distinction between cases and controls being the specific T2D‐related outcome, our results suggest that the higher healthcare costs and HCRU are a direct consequence of the specific outcome. In addition to indicating a burden on the healthcare system, the significantly greater number of visits to physicians and dialysis clinics and longer total stays in the hospital would also impact the quality of life for patients by taking time away from work, family and other personal activities. 23
Any measures that could prevent these T2D‐related adverse cardiovascular and renal outcomes, such as public health awareness campaigns, early screening of kidney disease, glycaemic and blood pressure control, healthy living initiatives or earlier initiation of more efficacious therapies, could result in substantial cost and resource savings for the healthcare system. Annual urinary albumin to creatinine ratio (UACR) measurements are recommended for CKD screening in people with T2D. 12 However, in the year prior to index, UACR values were reported for only 36% to 60% of BOI analysis patients. This reflects a gap in documentation and/or screening that could lead to delays in preventing disease progression. Importantly, early improvements in UACR have been associated with positive long‐term renal and cardiovascular outcomes. 24 For people with diabetes and CKD, treatment with renin‐angiotensin‐aldosterone system (RAAS) blockers such as angiotensin‐converting enzyme (ACE) inhibitors or angiotensin‐receptor blockers (ARB) is recommended for blood pressure control. 12 Additionally, based on the renal and cardiovascular protective effects of sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors, recent guidelines recommend the use of metformin and SGLT2 inhibitors as first‐line antihyperglycaemic therapies in patients with T2D and CKD. 12 , 25
4.2. Comparison with previous literature
This study is the first large and comprehensive evaluation of the prevalence and BOI of T2D, an eGFR < 90 mL/min/1.73 m2 and associated co‐morbidities in Ontario, Canada. An Alberta study reported the prevalence of CKD among people with T2D aged ≥18 years as 33%, based on patients’ most recent eGFR value and albuminuria diagnosis. 10 Our study reports a higher prevalence of eGFR < 90 mL/min/1.73 m2 among people with T2D aged ≥ 30 years in Ontario (63% for 2015‐2016). While our study selection criteria did not include albuminuria values because UACR laboratory results were not uniformly reported, we included patients with two eGFR test values < 90 mL/min/1.73 m2 and identified people with T2D and mild loss of kidney function as well. Koye et al. highlight the global variation in the prevalence of CKD among diabetes patients. 26 Within Europe, the age‐ and sex‐adjusted values varied between 15.4% in the Netherlands and 41.5% in Germany. 27 In the US National Health and Nutrition Examination Survey (NHANES), while the CKD prevalence among adult diabetes patients was 26.2% (2009‐2014), 28 the prevalence of mildly decreased eGFR (60‐89 mL/min/1.73 m2) was 44.6% (1999‐2012). 29
A recent study described the HCRU in older diabetes patients (aged >50 years), including those on dialysis, in Ontario, Canada. 11 They found high HCRU for these patients, including a higher number of physician visits, hospitalizations and diabetes‐related complications, similar to our results. Studies from Alberta have reported the total direct cost of dialysis treatment for patients with end‐stage renal disease to be $74 315 per year (2000 Canadian dollars) and for a kidney transplant to be $75 000 ‐ $79 000 (year 1) and $20 000 ‐ $22 000 (year 2) (2008 Canadian dollars). 30 , 31 In comparison, we found the average total annual costs to be higher for dialysis cases ($106 637 in year 1 and $67 223 in year 2) and lower for kidney transplantation cases ($55 381 in year 1 and $22 167 in year 2). These differences could be explained by differing management practices, costs and reimbursement status among Canadian provinces, the difference in dollar values in these years, specific cost calculation algorithms, and additional diabetes‐related health complications in our patient population.
4.3. Strengths and limitations
This study has several important strengths, including its large size, population‐based design, and analysis of both prevalence and BOI. We utilized the validated ICES administrative and laboratory databases that are completely integrated and provide end to end patient interactions within the Ontario healthcare system. With nearly 40% of Canada's population, not only is Ontario a good representation of the country, it also provides a unique opportunity to study a globally relevant population because of the large ethnic diversity within Ontario. 13 , 14 , 15 To reflect the real‐world T2D patient population, we only used eGFR values as a selection criteria because a majority of patients may not have UACR assessments. We provide a comprehensive analysis of the healthcare costs and HCRU segmented by patients with incident T2D‐related outcomes.
This study also has limitations that should be considered when interpreting results. This study is subject to measurement errors common to administrative databases such as possible inaccuracy of diagnostic and procedural codes. Additionally, as ICES databases lack visibility into privately reimbursed expenses, the analysis may be missing costs associated with privately covered drugs and health services. Moreover, this study assessed the direct healthcare costs of people with T2D, eGFR < 90 mL/min/1.73 m2 and adverse outcomes; the total non‐healthcare–related costs and impact to quality of life were not estimated. To identify diabetes patients, we used the ODD, which is not specific to T1D or T2D. Although we used additional criteria to exclude T1D patients, it is possible that our data may include a small number of T1D patients and exclude some T2D patients. The eGFR calculation, using the MDRD equation, was not corrected for race or ethnicity; this may have affected eGFR values for those of African descent. Because we required at least two eGFR test values < 90 mL/min/1.73 m2, we may have missed patients who experienced a rapid decrease in kidney function or mortality, underestimating the prevalence of that group. Some patients with acute kidney injury may still have been included in the dialysis cases and contributed to the BOI. The analysis period for the CVD/CKD related death outcome was the 2‐year period prior to the death event while it was 2 years following the index event for the other three outcomes, and thus are not directly comparable. Hospital costs were calculated using a weighted average methodology that could potentially underestimate outliers. 21 , 22 Finally, the results from this study may not be generalizable globally because the burden of disease, management practices or reimbursement status may vary among different countries.
4.4. Conclusion
In conclusion, this retrospective observational study provides a comprehensive evaluation of the prevalence and BOI of patients ≥ 30 years old with T2D, eGFR < 90 mL/min/1.73 m2 and associated co‐morbidities in Ontario, Canada. We found that the prevalence of eGFR < 90 mL/min/1.73 m2, as well as co‐morbidities such as CVD with or without stroke, has increased over time. The healthcare costs and HCRU are significantly higher for those patients with adverse cardiovascular or renal outcomes (CKD or CVD related death, doubling of serum creatinine, dialysis and kidney transplantation) compared with controls. These findings illustrate the substantial economic burden of T2D and eGFR < 90 mL/min/1.73 m2 on the healthcare system and indicate the importance of preventing disease progression. This real‐world assessment provides insights for clinicians, researchers and policymakers to better understand the outcomes for these patients as well as the need to implement more effective strategies to curtail the clinical and economic burden of T2D, eGFR < 90 mL/min/1.73 m2 and related cardiovascular and renal outcomes.
CONFLICT OF INTEREST
The authors declare the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: WR, DZ, MT and JBR are full‐time employees of Janssen Canada, Inc. VM, SG, PG, AT and BM are full‐time employees of IQVIA. IQVIA is a consulting company that has received consulting fees from Janssen Canada, Inc. SWT did not receive payment for any aspect of the manuscript submission; he participates on a steering committee for Bayer Fidelio/Figaro studies, and a speaker's bureaux with CHEP+, a not‐for‐profit CPD organization that provides Canadian‐accredited continuing education.
AUTHOR CONTRIBUTIONS
DZ and JBR contributed to the study concept, design and plan development. DZ, JBR, WR, AT, VM, SG and BM contributed to the study design and protocol and their respective reviews. DZ, WR, AT, VM, SG and BM contributed to the data collection. WR, DZ, MT, AT, VM, SG, PG, BM, SWT and JBR contributed to the analysis and interpretation of data. WR, DZ, MT, AT, VM, SG, PG, BM, SWT and JBR contributed to the intellectual content during manuscript drafting or revision, and approved the final draft of the manuscript for submission.
Supporting information
Table S1a. Study population counts from patient selection criteria for prevalence analysis
Table S1b. Study population counts from patient selection criteria for burden of illness analysis
Table S2. Patient Counts for Prevalence of T2D and eGFR <90 mL/min/1.73 m2 in individuals aged ≥30 years in Ontario, Canada
Table S3. Percent Prevalence of T2D, eGFR <90 mL/min/1.73 m2, and cardiovascular disease subgroups in individuals aged ≥30 years in Ontario, Canada
Table S4. Baseline Geography (LHIN) of people with T2D and eGFR <90 mL/min/1.73 m2 (aged ≥30 years) for Burden of Illness Analyses
Table S5. Additional Healthcare Costs of people with T2D and eGFR <90 mL/min/1.73 m2 (aged ≥30 years) in Ontario, Canada
Table S6. Additional Healthcare Resource Utilization of people with T2D and eGFR<90 mL/min/1.73 m2 (aged ≥30 years) in Ontario, Canada
ACKNOWLEDGEMENTS
Sha Kang (Janssen), Aren Fischer (IQVIA) and Dorian Murariu (IQVIA) were involved with the study concept, design and protocol. This work was funded by Janssen Inc. Sponsorship and article‐processing charges for this study were provided by Janssen Inc.
Rapattoni W, Zante D, Tomas M, et al. A retrospective observational population‐based study to assess the prevalence and burden of illness of type 2 diabetes with an estimated glomerular filtration rate < 90 mL/min/1.73 m2 in Ontario, Canada. Diabetes Obes Metab. 2021;23:916‐928. 10.1111/dom.14294
Funding information This work was funded by Janssen Inc.
DATA AVAILABILITY STATEMENT
The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access.
REFERENCES
- 1. International Diabetes Federation . IDF Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019. [Google Scholar]
- 2. Diabetes Canada Clinical Practice Guidelines Expert Committee , Houlden RL. Introduction. Can J Diabetes. 2018;42(Suppl 1):S1‐S5. [DOI] [PubMed] [Google Scholar]
- 3. Public Health Agency of Canada . Diabetes in Canada: Facts and figures from a public health perspective . Ottawa, Canada: Public Health Agency of Canada, 2011. [Google Scholar]
- 4. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gansevoort RT, Correa‐Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339‐352. [DOI] [PubMed] [Google Scholar]
- 6. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73‐81. [DOI] [PubMed] [Google Scholar]
- 7. Radbill B, Murphy B, LeRoith D. Rationale and strategies for early detection and management of diabetic kidney disease. Mayo Clin Proc. 2008;83(12):1373‐1381. [DOI] [PubMed] [Google Scholar]
- 8. Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258‐1270. [DOI] [PubMed] [Google Scholar]
- 9. Doshi SM, Friedman AN. Diagnosis and Management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(8):1366‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tonelli M, Wiebe N, Richard JF, Klarenbach SW, Hemmelgarn BR. Characteristics of adults with type 2 diabetes mellitus by category of chronic kidney disease and presence of cardiovascular disease in Alberta, Canada: a cross‐sectional study. Can J Kidney Health Dis. 2019;6:2054358119854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemens KK, Ouedraogo A, Nash DM, Garg AX, Shariff SZ. The health and health Care of Adults with Type 1 and 2 diabetes across the Spectrum of estimated glomerular filtration rates. Can J Diabetes. 2019;43(2):105‐114;e104. [DOI] [PubMed] [Google Scholar]
- 12. Diabetes Canada Clinical Practice Guidelines Expert Committee , McFarlane P, Cherney D, Gilbert RE, Senior P. Chronic kidney disease in diabetes. Can J Diabetes. 2018;42(Suppl 1):S201‐S209. [DOI] [PubMed] [Google Scholar]
- 13. 2016 Census ‐ Immigration and Ethnocultural Diversity Highlight Tables . Statistics Canada, 2020. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/imm/Table.cfm?Lang=E&T=41&Geo=00&SP=1&vismin=1&age=1&sex=1. Accessed November 12, 2020.
- 14. Census Profile . 2016. Census. Statistics Canada. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. Accessed November 12, 2020.
- 15. Census Highlights . 2020. Ontario Ministry of Finance. https://www.fin.gov.on.ca/en/economy/demographics/census/. Accessed November 12, 2020.
- 16. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512‐516. [DOI] [PubMed] [Google Scholar]
- 17. Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population‐based validation study. BMC Health Serv Res. 2018;18(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247‐254. [DOI] [PubMed] [Google Scholar]
- 19. Silver SA, Harel Z, McArthur E, et al. Causes of death after a hospitalization with AKI. J Am Soc Nephrol. 2018;29(3):1001‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider C, Coll B, Jick SS, Meier CR. Doubling of serum creatinine and the risk of cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes mellitus: a cohort study. Clin Epidemiol. 2016;8:177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manitoba Centre for Health Policy . 2020. Concept: Calculating Hospital Costs Using Cost Per Weighted Case (CPWC)/Cost of a Standard Hospital Stay (CSHS) values. http://mchp‐appserv.cpe.umanitoba.ca/viewConcept.php?printer=Y&conceptID=1100. Accessed March 12, 2020.
- 22. Canadian Institutes for Health Information . 2020. Patient Cost Estimator. https://www.cihi.ca/en/patient-cost-estimator. Accessed March 12, 2020.
- 23. Evans RW, Manninen DL, Garrison LP Jr, et al. The quality of life of patients with end‐stage renal disease. N Engl J Med. 1985;312(9):553‐559. [DOI] [PubMed] [Google Scholar]
- 24. Oshima M, Neuen BL, Li J, et al. Early change in albuminuria with Canagliflozin predicts kidney and cardiovascular outcomes: a post hoc analysis from the CREDENCE trial. J Am Soc Nephrol. 2020;31:2925‐2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence‐based advances in monitoring and treatment. Kidney Int. 2020;98(4):839‐848. [DOI] [PubMed] [Google Scholar]
- 26. Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bruck K, Stel VS, Gambaro G, et al. CKD prevalence varies across the European general population. J Am Soc Nephrol. 2016;27(7):2135‐2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988‐2014. JAMA. 2016;316(6):602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving global outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end‐stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40(3):611‐622. [DOI] [PubMed] [Google Scholar]
- 31. Barnieh L, Manns BJ, Klarenbach S, McLaughlin K, Yilmaz S, Hemmelgarn BR. A description of the costs of living and standard criteria deceased donor kidney transplantation. Am J Transplant. 2011;11(3):478‐488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1a. Study population counts from patient selection criteria for prevalence analysis
Table S1b. Study population counts from patient selection criteria for burden of illness analysis
Table S2. Patient Counts for Prevalence of T2D and eGFR <90 mL/min/1.73 m2 in individuals aged ≥30 years in Ontario, Canada
Table S3. Percent Prevalence of T2D, eGFR <90 mL/min/1.73 m2, and cardiovascular disease subgroups in individuals aged ≥30 years in Ontario, Canada
Table S4. Baseline Geography (LHIN) of people with T2D and eGFR <90 mL/min/1.73 m2 (aged ≥30 years) for Burden of Illness Analyses
Table S5. Additional Healthcare Costs of people with T2D and eGFR <90 mL/min/1.73 m2 (aged ≥30 years) in Ontario, Canada
Table S6. Additional Healthcare Resource Utilization of people with T2D and eGFR<90 mL/min/1.73 m2 (aged ≥30 years) in Ontario, Canada
Data Availability Statement
The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access.


