Abstract
Aims
Coronavirus disease 2019 (COVID‐19) is caused by a novel severe acute respiratory syndrome coronavirus 2. It can lead to multiorgan failure, including respiratory and cardiovascular decompensation, and kidney injury, with significant associated morbidity and mortality, particularly in patients with underlying metabolic, cardiovascular, respiratory or kidney disease. Dapagliflozin, a sodium‐glucose cotransporter‐2 inhibitor, has shown significant cardio‐ and renoprotective benefits in patients with type 2 diabetes (with and without atherosclerotic cardiovascular disease), heart failure and chronic kidney disease, and may provide similar organ protection in high‐risk patients with COVID‐19.
Materials and methods
DARE‐19 (NCT04350593) is an investigator‐initiated, collaborative, international, multicentre, randomized, double‐blind, placebo‐controlled study testing the dual hypotheses that dapagliflozin can reduce the incidence of cardiovascular, kidney and/or respiratory complications or all‐cause mortality, or improve clinical recovery, in adult patients hospitalized with COVID‐19 but not critically ill on admission. Eligible patients will have ≥1 cardiometabolic risk factor for COVID‐19 complications. Patients will be randomized 1:1 to dapagliflozin 10 mg or placebo. Primary efficacy endpoints are time to development of new or worsened organ dysfunction during index hospitalization, or all‐cause mortality, and the hierarchical composite endpoint of change in clinical status through day 30 of treatment. Safety of dapagliflozin in individuals with COVID‐19 will be assessed.
Conclusions
DARE‐19 will evaluate whether dapagliflozin can prevent COVID‐19‐related complications and all‐cause mortality, or improve clinical recovery, and assess the safety profile of dapagliflozin in this patient population. Currently, DARE‐19 is the first large randomized controlled trial investigating use of sodium‐glucose cotransporter 2 inhibitors in patients with COVID‐19.
Keywords: clinical trial, dapagliflozin, phase III study, randomized trial, SGLT2 inhibitor
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first identified in Wuhan, China, in December 2019 as the cause of an outbreak of coronavirus disease 2019 (COVID‐19), a type of viral pneumonia. SARS‐CoV‐2 has 89% nucleotide identity with bat SARS‐like‐CoV‐2 and 82% nucleotide identity with human SARS‐CoV. 1 Early epidemiological data indicate that ~12% of SARS‐CoV‐2‐positive patients develop symptoms that are significant enough to require hospitalization, and of these, nearly 24% may need to be treated in the intensive care unit. 2 , 3 Hospital admission occurs at a median time of day 7 from the onset of symptoms, and many recover with supportive care. 4 In others, progressive deterioration is seen. Although respiratory failure is a key manifestation in most patients with COVID‐19, it is increasingly being recognized that COVID‐19 is a systemic disease, in which cardiovascular and kidney complications are common, and may be key drivers of poor outcomes, including death. 5 , 6 , 7 , 8 , 9
Risk factors associated with COVID‐19 disease progression include older age and presence of cardiometabolic comorbidities such as type 2 diabetes (T2D), atherosclerotic cardiovascular disease (ASCVD), hypertension, heart failure (HF) and chronic kidney disease (CKD). 2 , 9 , 10 Observational studies have shown that age, male sex, obesity, ethnicity, chronic heart disease and reduced kidney function are independent risk predictors for COVID‐related death, 11 whereas the data on prognostic importance of hypertension have been less clear, with different studies producing mixed results. 12 Individuals that have a combination of such underlying health conditions, and with evidence of acute myocardial injury, kidney injury and/or HF specifically, have a high risk of death because of COVID‐19. 5 , 13 , 14 , 15 , 16 The underlying mechanisms behind this greater risk are not yet fully understood, but compromised baseline organ function and metabolism combined with greater susceptibility to endothelial injury, inflammatory insults and tissue hypoxia probably play a role. 6 , 7 , 17 , 18
Over the past several months, the unpredictable nature of the evolving global pandemic and the change in available standard of care (including medications) for treatment of COVID‐19 have resulted in substantially lower rates of complications and death than what was observed early on, and studies suggest that mortality from COVID‐19 is decreasing even after accounting for secular change in patient characteristics. 19 Consequently, faster and more complete recovery has now become an important treatment goal, on par with prevention of complications and death in patients hospitalized with COVID‐19.
Although the primary action of sodium‐glucose cotransporter‐2 (SGLT2) inhibitors is on glucose and sodium reabsorption in the proximal convoluted tubule of the kidney, they have been shown to provide substantial cardiorenal protection in patient populations similar to those at risk for COVID‐19 complications, namely, individuals with T2D, ASCVD, HF and CKD. SGLT2 inhibitors reduced the risk of cardiovascular and kidney events in patients with T2D in four large outcome trials investigating empagliflozin, canagliflozin and dapagliflozin. 20 , 21 , 22 , 23 In the DAPA‐HF trial, dapagliflozin reduced the risk of death and worsening of HF by 26% in patients with HF with reduced ejection fraction, with identical effects in patients with and without T2D 24 , 25 ; these benefits were observed within days of randomization and were significant by day 28 of treatment. 26 SGLT2 inhibitors also consistently reduce the risk of kidney disease progression and acute kidney injury (AKI). 20 , 21 , 22 , 23 Recently, the DAPA‐CKD trial showed statistically significant and clinically meaningful effects of SGLT2 inhibitors on the primary endpoint of a composite of worsening of renal function or risk of death in adult patients with CKD; the study also met all its secondary endpoints in patients with CKD, with and without T2D. 27 , 28 An important, and somewhat unexpected finding in the DAPA‐CKD trial was a large, 31% relative reduction in all‐cause mortality in patients treated with dapagliflozin versus placebo, which was an effect driven largely by lower risk of death from non‐cardiovascular causes (including death from infectious disease and other aetiologies). These findings suggest that SGLT2 inhibitors may even have protective properties in clinical scenarios beyond chronic conditions such as T2D, cardiovascular and kidney disease. 28
The mechanisms that could explain the various protective effects of SGLT2 inhibitors overlap substantially with those triggered in COVID‐19 (Figure 1). SGLT2 inhibitors decrease glucose and insulin levels, and shift energy metabolism to an increased reliance on lipid oxidation, with a reduced reliance on glucose, and inhibition of glycolysis. 29 This mechanism may be particularly important in COVID‐19, as SARS‐CoV‐2 may depend on the glycolytic pathway for its replication, stimulating lipogenesis, which appears to be one of the key drivers of cellular damage. 30 , 31 SGLT2 inhibitors improve endothelial function within 48 h of treatment, probably because of reduced oxidative stress. 32 SGLT2 inhibitors have significant anti‐inflammatory effects, reducing levels of C‐reactive protein and interleukin‐6 33 ; experimental studies have shown reduced activation of the NLRP3 inflammasome, 34 and protection against septic AKI on SGLT2 inhibitor treatment. 35 SGLT2 inhibitors increase erythropoiesis resulting in increased haematocrit, 36 , 37 and together with improved endothelial function 32 may improve oxygen delivery to tissues. Moreover, SGLT2 inhibitors result in reduced extracellular volume in patients with fluid overload, such as those with CKD and HF, 38 , 39 and appear to reduce pulmonary artery pressure in patients with HF rapidly, 40 leading to haemodynamic decongestion. Thus, SGLT2 inhibitors may favourably affect multiple processes, including but not limited to energy metabolism, endothelial function, oxidative stress, inflammation and autophagy, which are dysregulated during a major acute illness such as COVID‐19. 34 , 41 , 42 , 43 , 44 , 45 Such metabolic restoration may help prevent multiorgan damage in the setting of COVID‐19, and could provide critical and complementary efficacy to other therapeutic approaches across various stages of the disease course, 18 including in patients hospitalized with COVID‐19 but not yet critically ill. Importantly, SGLT2 inhibitors may be most effective in patients who are at the highest risk of COVID‐19 complications, that is, those with cardiometabolic comorbidities (Figure 1). Early data from observational studies, although limited by potential residual confounding and small numbers, also suggests that background SGLT inhibitor therapy in patients with T2D that are hospitalized with COVID‐19 is associated with a lower risk of in‐hospital death and complications, as compared with other glucose‐lowering drugs. 46
FIGURE 1.
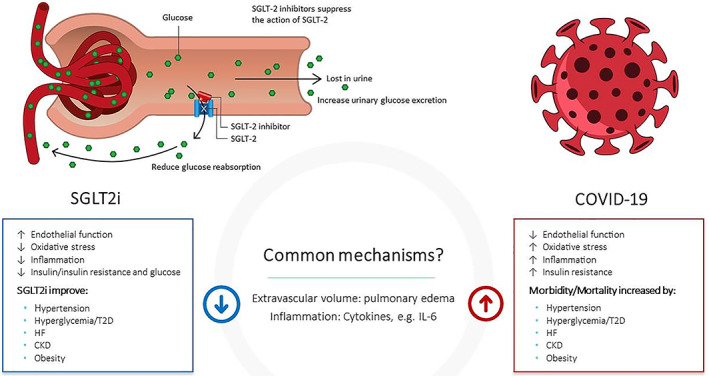
Primary action of dapagliflozin is on the SGLT2s expressed on the proximal tubules of the nephrons of the kidney. SGLT2 inhibition results in an immediate adaptive response to minimize loss of water and sodium. Loss of glucose is balanced by increased endogenous glucose production. 41 , 45 The new homeostasis may be responsible for the favourable effects of dapagliflozin on the cardiovascular, renal and immune functions. The effects on glucose control, including insulin demand, endothelial function, oxidative stress, oxygen delivery capacity, congestion and inflammation 32 , 33 , 34 , 35 , 36 , 42 , 43 are probably most important to protect from worsening of organ function in hospitalized patients with COVID‐19 and medical history with risk factors, including hypertension, T2D, HF, CKD and obesity. CKD, chronic kidney disease; HF, heart failure; SGLT2i, sodium‐glucose cotransporter 2 inhibitor; T2D, type 2 diabetes
Thus, we hypothesize that dapagliflozin, a potent, highly‐selective and orally‐active SGLT2 inhibitor, has the potential to impart end‐organ protection against SARS‐CoV‐2 by lowering the risks of cardiovascular and kidney complications, and possibly by preventing worsening of respiratory failure, thus reducing the risk of disease progression and death and improving clinical recovery in patients with COVID‐19.
2. MATERIALS AND METHODS
2.1. Study design and population
The dapagliflozin in respiratory failure in patients with COVID‐19 study (DARE‐19; NCT04350593) is a randomized, multicentre, double‐blind, placebo‐controlled, parallel‐group, international Phase III trial in countries with a high prevalence of COVID‐19. It includes patients with history of the following cardiometabolic risk factors; hypertension, T2D, ASCVD, HF (regardless of ejection fraction) and/or CKD (stages 3‐4, defined as estimated glomerular filtration rate (eGFR) between 25 and 60 mL/min/1.73 m2); these conditions are known to increase the risk of COVID‐19 complications, and occur in large proportions of patients hospitalized with COVID‐19. 9 , 10 , 15 , 47 Notably, SGLT2 inhibitors have already shown benefits in these patient populations. 20 , 21 , 22 , 23 , 24 , 48 , 49 Therefore, while patients in the DARE‐19 study will all be hospitalized with COVID‐19, it is not expected that the risk factor profile of the DARE‐19 study population will differ substantially from those already studied with SGLT2 inhibitors in general, and dapagliflozin specifically.
The study plans to enrol approximately 1200 adult patients hospitalized with COVID‐19 across several countries in North America (United States, Canada, Mexico), Europe (United Kingdom), Latin America (Brazil and Argentina) and Asia (India) with a high prevalence of COVID‐19. Study inclusion and exclusion criteria are detailed in Table 1. Eligible individuals will include adults hospitalized with COVID‐19 (oxygen saturation ≥94% on ≤5 L of supplemental oxygen) with chest radiography findings consistent with COVID‐19 (as judged by the local investigator), and at least one of the cardiometabolic risk factors (hypertension, T2D, ASCVD, HF and/or CKD [eGFR between 25 and 60 mL/min/1.73 m2]). Key exclusion criteria are evidence of critical illness, severe CKD or acute renal failure on presentation, type 1 diabetes and previous history of diabetic ketoacidosis (DKA).
TABLE 1.
Eligibility criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
Abbreviations: BiPAP, Bilevel positive airway pressure; BP, blood pressure; CKD, chronic kidney disease; COVID‐19, coronavirus disease 2019; CPAP, Continuous positive airway pressure; CT, computed tomography; eGFR, estimated glomerular ejection fraction; LVEF, left ventricular ejection fraction; SARS‐CoV‐2, severe acute respiratory syndrome‐related coronavirus 2; SGLT2i, sodium‐glucose cotransporter 2 inhibitor; T2D, type 2 diabetes.
Proportion of patients randomized without a confirmed SARS‐CoV‐2‐positive test will be closely monitored, and may be capped if it becomes greater than anticipated.
Use of rescue therapies, including immune modulators, monoclonal antibody therapies, antiviral therapies and other agents that are approved or being used through open‐label compassionate/expanded use programmes or in accordance with the local standard of care is permitted during the study.
Study design allows two attempts to meet the randomization criteria after enrolment.
Eligible individuals providing informed consent will enter a screening period of no more than 2 days. Randomization will be stratified by country. Individuals will be randomized 1:1 to dapagliflozin 10 mg or matched placebo once daily for 30 days; under most circumstances patients will be starting investigational product on the day of randomization. After the last dose of investigational product on day 30, patients will be followed for an observational period of an additional 60 days (Figure 2).
FIGURE 2.

Study flow chart
Treatment with dapagliflozin or matching placebo will be continued even if mechanical ventilation becomes necessary during COVID‐19 treatment. In this case, the tablets will be crushed and administered via the gastric tube. All individuals will be treated according to local guidelines and standard of care treatment for patients hospitalized with COVID‐19 (including open‐label rescue therapies) at the participating hospitals.
2.2. Study governance
DARE‐19 is an investigator‐initiated collaborative study, with the study design and protocol developed, and study procedures operationalized through close collaboration between Saint Luke's Mid America Heart Institute (Global Sponsor) and AstraZeneca (Funding Source). As a key collaborator, AstraZeneca participates in the conduct of the study and statistical analyses, which will be independently verified by the statistical group at Saint Luke's Mid America Heart Institute. This study is being directed by the Executive Committee, comprising academic leaders and non‐voting representatives from AstraZeneca. The Executive Committee will plan and direct trial‐related academic publications. Safety is being closely monitored by an Independent Data and Safety Monitoring Committee (IDSMC). The trial will be reviewed by the regulatory authorities and ethics committees in each country/institution, as applicable. Informed consent will be obtained from all participating individuals before initiation of study procedures.
2.3. Procedures
The goals of this study are to prevent COVID‐19‐related organ dysfunction during the index hospitalization (which is the most acute setting in individuals with COVID‐19) or mortality through day 30, and to improve clinical recovery. During this study, if an individual is discharged from the index hospitalization without an event of organ dysfunction (Figure 1, Table 2), then the individual will be assumed to have made a partial or full recovery from COVID‐19 and therefore no longer be at high risk for developing COVID‐19‐related organ dysfunction. Therefore, for the assessment of primary efficacy endpoints only vital status information will be used after hospital discharge (for example, in the time‐to‐event analysis patients discharged from hospital without an event and alive on day 30 will be censored at day 30). After all individuals complete the 30‐day treatment period, and collection of the required data for this treatment period is completed, the study database will be locked, and the efficacy and safety report will be generated. An observational follow‐up will continue for an additional 60 days, during which vital status and serious adverse events will continue to be assessed.
TABLE 2.
Secondary objectives
| Time to hospital discharge a |
| Total number of days alive and free from respiratory decompensation a requiring initiation of mechanical ventilation (includes invasive or non‐invasive ventilation, CPAP, or BiPAP) from randomization through day 30 |
| Total number of days alive, not in the ICU, and free from respiratory decompensation a requiring initiation of mechanical ventilation (includes invasive or non‐invasive ventilation, CPAP, or BiPAP) from randomization through day 30 |
| Time to composite of acute kidney injury b or initiation of renal replacement therapy c , or death from any cause through day 30 |
| Time to death from any cause |
Abbreviations: BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ICU, intensive care unit; SAE, serious adverse event.
Refers to index hospitalization only.
Acute kidney injury defined as: an episode of doubling serum creatinine compared with baseline during index hospitalization, or an SAE with preferred term of acute kidney injury following discharge and through day 30.
Renal replacement therapy defined as: initiation of renal replacement therapy during index hospitalization or an SAE with a preferred term for renal replacement therapy (i.e. haemodialysis, haemofiltration, continuous haemodiafiltration, dialysis, peritoneal dialysis, dialysis device insertion, renal replacement therapy or artificial kidney device user) following discharge and through day 30.
2.4. Study objectives and endpoints
The primary objective of the study is to determine whether dapagliflozin 10 mg is superior to placebo in reducing the incidence of complications and all‐cause death, or in improving clinical recovery, in patients hospitalized with COVID‐19. The dual primary endpoints are the following.
Time to first occurrence of COVID‐19‐related new or worsened cardiovascular/renal or respiratory organ dysfunction during the index hospitalization, or time to death from any cause at any time during the 30‐day treatment period (Figure 3A).
Hierarchical composite endpoint (HCE) of change in clinical status during the 30 days of treatment (Figure 3B).
FIGURE 3.
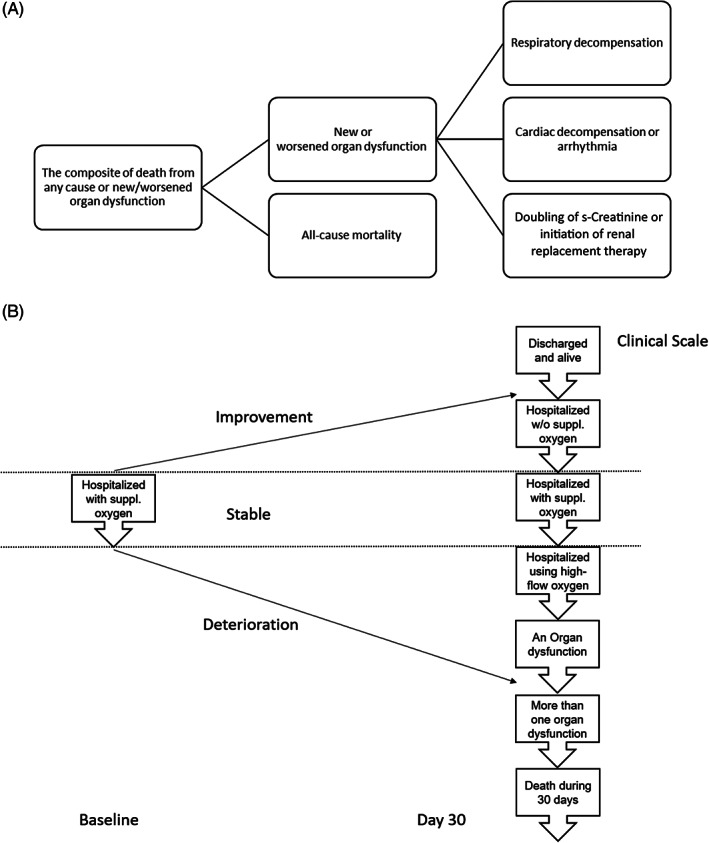
Primary endpoints and components. A, First primary endpoint is a composite of new/worsened organ dysfunction during the index hospitalization or death from any cause at any time during the 30‐day treatment period. New/worsened organ dysfunction is defined as at least one of the following: respiratory decompensation requiring initiation of mechanical ventilation (includes invasive or non‐invasive ventilation, continuous positive airway pressure or bilevel positive airway pressure), and/or initiation of ECMO; new or worsening congestive heart failure (HF); requirement for vasopressor therapy and/or inotropic or mechanical circulatory support; ventricular tachycardia or fibrillation lasting at least 30 s and/or associated with haemodynamic instability or pulseless electrical activity, or resuscitated cardiac arrest; doubling of serum creatinine or initiation of renal replacement therapy. Congestive HF defined as at least one of the following: initiation of new intravenous therapy for HF; reinstitution of previous intravenous therapy for HF; increase in current intravenous therapy for HF. This is based on modification of a previous definition of in‐hospital worsening HF. B, Second primary endpoint is the hierarchical composite outcome measure (by increasing severity): 1. Death from any cause through day 30; 2. New/worsened organ dysfunction (as defined above); 3. Clinical status at day 30 for patients still hospitalized and without any worsening organ dysfunction (hospitalized, on high flow oxygen devices; hospitalized, requiring supplemental oxygen; hospitalized, not requiring supplemental oxygen); 4. Hospital discharge before day 30 and alive at day 30
Therefore, our dual primary objectives are aligned to capture both critical components of treatment benefit (prevention of complications and improving clinical recovery). Therefore, finding a therapeutic effect for either or both dual primary endpoints would be considered a significant and clinically meaningful benefit for patients hospitalized with COVID‐19.
Figure 3 shows the definition of the primary endpoints and their components. The HCE combines clinical deterioration (e.g. organ dysfunction as defined in the prevention composite endpoint) with clinical improvement (e.g. change in clinical status and hospital discharge) into a single metric. This endpoint is designed to capture the treatment effect on the change in clinical status (recovery) of patients hospitalized for COVID‐19 after 30 days of treatment. It provides a more nuanced measure to detect improvement during the 30 days of treatment, compared with the first primary endpoint (prevention), by recognizing the various severities of different events that patients may experience.
The secondary endpoints and their definitions are listed in Table 2. These include time to hospital discharge; total number of days alive and free from mechanical ventilation; total number of days alive, not in the ICU and free from mechanical ventilation; time to a composite kidney endpoint; and time to death from any cause.
Exploratory analyses are listed in Table S1 and will include assessment of various biomarkers and laboratory parameters of disease severity (all collected at local laboratories at each site), and composite scores of clinical status.
Outcome events are reported by the local investigators and will not be adjudicated.
2.5. Safety objectives
In addition to evaluating the efficacy endpoints, one of the key objectives of the DARE‐19 trial is to assess the safety profile of dapagliflozin in patients hospitalized with COVID‐19. Several measures have been incorporated in the protocol to minimize potential risks to participants. Patients with type 1 diabetes and those with any history of DKA are excluded from the study. Careful monitoring of acid‐base balance and kidney function during hospitalization is mandated; in the absence of monitoring, dapagliflozin treatment will be temporarily interrupted. In the event of an abnormal increase in anion gap and/or reduction in bicarbonate levels, measurement of blood ketones, lactate levels and pH analysis will be performed to rule out the possibility of DKA (the diagnosis of which is based on strict protocol‐defined criteria, see Table S2). The IDSMC will continuously review the safety data.
3. STATISTICAL CONSIDERATIONS
3.1. Sample size and power calculations
It is estimated that a sample size of approximately 1200 patients will provide adequate power to detect the treatment effect on prevention or recovery, when the dual endpoints are tested with alpha split between these endpoints. Since the original protocol was designed, the change in standard of care for treatment of COVID‐19 resulted in lower event rates. Consequently, faster and more complete recovery has now become an important treatment goal on par with the prevention of complications and death in patients hospitalized with COVID‐19, prompting the addition of ‘recovery’ to the primary objectives.
It is now estimated that about 10%‐20% of patients will develop COVID‐19‐related complications during the index hospitalization or will experience death during the 30‐day treatment period, while 80%‐90% of patients will recover without experiencing death or early complications. Therefore, the sample size of approximately 1200 patients will provide approximately 100‐250 events for the first dual primary endpoint (prevention).
To control the type I error for the dual primary endpoints, the allocated alpha of 5% will be split between them. For example, in the case of an even split, 100 events of the prevention endpoint will detect a hazard ratio of 0.54 with about 80% power at the type I control level of 2.5%, while the minimal detectable hazard ratio in this case is about 0.64. For the second dual primary endpoint (recovery) the sample size of 1200 patients will detect a Win Ratio of 1.23 with at least 80% power for a hypothetical alpha of 2.5%.
3.2. Planned statistical approach
A strong control of the type 1 error rate will be applied in testing the dual primary and secondary efficacy endpoints. For the dual primary endpoints, alpha will be allocated to each test of hypothesis, such that success on one of the endpoints will be sufficient to support a conclusion of effectiveness. 50 No interim efficacy analysis is planned.
All primary and secondary efficacy endpoints will be analysed based on the intent‐to‐treat principle. The time‐to‐event endpoints (i.e. the first primary endpoint, time to hospital discharge, time to death) will be analysed using the Cox proportional hazards regression, stratified by country, including a factor for treatment group, and will be adjusted for age and sex. Analysis for time to composite kidney endpoint will be done in the same way but will be adjusted only for baseline eGFR value. The HCE evaluating the clinical status change will be analysed using Win Ratio analysis based on the order (ranking) described in Table 2. 51 , 52 , 53
Full details of all analyses, including the multiple testing strategy, will be provided in a statistical analysis plan completed before the end of the trial and unblinding of the results.
3.3. Pre‐specified subgroups
For the dual primary endpoints, subgroup analyses have been preplanned based on population demographics and clinical characteristics. Subgroups include age (≥60 versus <60 years), sex, race, country, the five cardiometabolic risk factors of the inclusion criteria (hypertension, ASCVD, HF, T2D and baseline eGFR with categories ≥60 versus <60 mL/min/1.73 m2), 54 as well as subgroups defined by baseline laboratory measurements above or below median; including N‐terminal pro B‐type natriuretic peptide, high‐sensitivity troponin, high‐sensitivity C‐reactive protein and D‐dimer.
3.4. Current status
The first patient was enrolled in the DARE‐19 study on 22 April 2020 in the United States. The study is currently enrolling patients across multiple sites in the United States, Brazil, Mexico, Argentina, India, Canada and the United Kingdom.
4. DISCUSSION
The incidence of COVID‐19 continues to increase globally, with a marked negative impact on health care systems and economies. The human toll of COVID‐19 continues to grow exponentially, with morbidity and mortality remaining stubbornly high and with few efficacious therapies available. Thus, there is a pressing need for additional effective and safe therapeutic interventions to treat this disease and its complications.
When dealing with a condition such as COVID‐19 that remains incompletely understood, it is important that the search for effective treatments is expansive enough to encompass a wide variety of agents with different modes of action, and that the methodological rigour of randomized controlled trials is maintained, so that clinicians and regulators can have accurate estimates of treatment benefits and risks. Accordingly, various options are being explored for the treatment of COVID‐19. Numerous clinical trials evaluating antiviral and anti‐inflammatory therapies, immune modulators, targeted monoclonal antibodies and vaccines are now under way. 55 , 56 Important data are being reported on the use of antiviral interventions such as remdesivir, predominantly in less severely ill patients, 49 , 57 and corticosteroids such as dexamethasone in critically ill patients. 58 These agents have shown tangible improvements in outcomes, while other treatments showed no significant benefit. 56 , 59 , 60 Although implementation of treatment options with proven efficacy (such as remdesivir and dexamethasone) is critically important, they offer incremental improvement over supportive care. Some of these proven treatments may not be widely available, highlighting the need for additional therapeutic options with a favourable efficacy and safety balance. If found to be effective and safe in COVID‐19, repurposing of the agents already on the market for other indications is a particularly attractive option, as many of these may be immediately available to patients, including in resource‐constrained regions.
Based on clinical experience, it is clear that COVID‐19 is a systemic disease, affecting multiple organ systems. Patients with COVID‐19 and cardiometabolic risk factors have the highest risk for disease progression, associated complications and death. 2 , 9 , 10 Although the reasons behind this remain unclear, presence of cardiometabolic comorbidities and its underlying milieu of obesity, insulin resistance, inflammation and endothelial dysfunction at baseline, combined with already impaired target organ function, could increase susceptibility to further impairments in endothelial function, oxidative stress, inflammation and metabolic derangements, all seen in this patient population (Figure 1). Impaired T‐cell responses, including to viral pathogens are also well described in obese, insulin‐resistant individuals. 61
Against this proinflammatory and pro‐thrombotic setting, SGLT2 inhibitors have the potential to affect many of these imbalances favourably. SGLT2 inhibitors inhibit glycolysis and stimulate lipolysis; this may be critically important, as respiratory viruses appear to hijack the glycolytic pathway to replicate, causing lipogenesis that may cause cell injury. 30 SGLT2 inhibitors improve endothelial function, reduce insulin resistance and positively influence oxidative stress and background inflammation. 29 , 32 , 33 Therefore, SGLT2 inhibitors may halt or reverse the pathophysiological cycle leading to organ failure, thus providing organ protection to patients with COVID‐19. Although the short duration of dapagliflozin therapy in this study may be questioned with respect to its probable impact, results from the DAPA‐HF study suggest that at least some benefits of dapagliflozin are seen rapidly; specifically, reductions in cardiovascular death and HF hospitalization were seen within days. 26 Dapagliflozin has also shown to improve endothelial function within 48 h, 32 and reduce pulmonary artery pressure in HF within 2 days. 40 SGLT2 inhibitors have been shown to improve mitochondrial function, 62 and insulin sensitivity while increasing fatty acid oxidation, 29 all of which may enhance the ability of organs to resist physiological stress.
Of even greater importance than the mechanistic underpinnings, SGLT2 inhibitors have already shown robust cardiorenal protection in patients with cardiometabolic comorbidities similar to COVID‐19 (hypertension, T2D, ASCVD, HF, CKD) that increase the risk of complications and death 20 , 21 , 22 , 23 , 24 (although these studies were predominantly undertaken in ambulatory settings and not during acute hospitalization). Nevertheless, data from two recent trials of patients hospitalized with decompensated HF 63 , 64 suggest that SGLT2 inhibitors may extend these benefits to individuals that have conditions that are more acute, potentially including those with COVID‐19, without additional safety concerns. Therefore, SGLT2 inhibitors should be considered as a possible therapeutic option to provide organ protection, reduce the risk of complications and death, and hasten recovery in COVID‐19. Recent editorials by other academic groups reiterate these potential benefits of SGLT2 inhibitors in COVID‐19 and support testing this hypothesis in a randomized controlled clinical trial setting. 65 , 66 Patients who are hospitalized, but not yet critically ill, with COVID‐19 were regarded as the ideal population to investigate the benefit‐risk of dapagliflozin therapy. 64
Recent commentaries have noted risks of DKA and volume depletion in patients with COVID‐19 and T2D, 67 and theoretical potential for increased risk of DKA with SGLT2 inhibitors in this patient group. Limited data from observational case series of patients presenting with DKA in the setting of COVID‐19 show that less than 6% of these patients were on a background of SGLT2 inhibitor therapy. 68 Large outcome trials show that while the risk of DKA is higher with SGLT2 inhibitors, these events are rare. 20 , 21 , 22 , 24 Another study investigating the effects of SGLT2 inhibitors in acutely hospitalized patients found no events of DKA in patients treated with empagliflozin, with one occurrence of DKA in the placebo group; there was no imbalance in volume depletion events between the two treatment groups. 64 Outcome trials have also showed consistent and robust benefits of SGLT2 inhibitors on kidney outcomes with no increase in the risk of AKI, 22 , 69 and experimental studies suggest that the effect of dapagliflozin on fluid status may also be favourable, thereby reducing the risk of precipitating acute renal impairment. 38 , 70 Nevertheless, we have implemented robust measures in the DARE‐19 study to minimize possible risks, such as exclusion of patients at higher risk of DKA (type 1 diabetes or history of DKA), daily measurements of acid‐base balance and kidney function during hospitalization, and frequent independent assessment of safety events by the IDSMC. These measures will permit appropriate assessment of the benefit‐risk balance of dapagliflozin in this setting.
In conclusion, SGLT2 inhibitors may be promising agents to protect target organs, reduce the risk of complications and death, and improve clinical recovery in patients hospitalized with COVID‐19. DARE‐19 is a randomized, placebo‐controlled, international study evaluating the potential benefits, potential risks, and balance of benefit and risk in this vulnerable patient group. The results of this study have the potential to inform international guidelines and clinical practice.
CONFLICT OF INTEREST
M.K. has received a research grant for the conduct of this study from AstraZeneca. He has also received grant and research support from AstraZeneca outside the submitted work. He has received a grant and honoraria from Boehringer Ingelheim, and honoraria from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes), Janssen, Bayer, Novartis, Applied Therapeutics, Amarin, Eli Lilly, and Vifor Pharma outside the submitted work. O.B. reports grants from AstraZeneca, Novartis, Bayer, Amgen, Boehringer‐Ingelheim and Pfizer, outside the submitted work. G.G.K. is the Principal Investigator of a biostatistics grant from AstraZeneca outside the submitted work. He is also the Principal Investigator for biostatistics grants from other biopharmaceutical sponsors that have no relationship to the submitted work. F.M. reports receiving honoraria from AstraZeneca for serving on the executive committee during the conduct of the study. O.M. has nothing to disclose. S.V. reports receiving grants, speaker honoraria and consulting fees from Boehringer‐Ingelheim, AstraZeneca, and Janssen during the conduct of this study. He has received speaker honoraria and consulting fees from Eli Lilly, and speaker honoraria from EOCI Pharmacomm Ltd, Sun Pharmaceuticals, and Toronto Knowledge Translation Working Group during the conduct of this study. He has also received grants and consulting fees from Amgen; grants, speaker honoraria and consulting fees from Bayer, and from Merck; grants from Bristol‐Myers Squibb; speaker honoraria and consulting fees from HLS Therapeutics, Novo Nordisk, and Sanofi; and speaker honoraria from Novartis outside the submitted work. V.C. declares no competing interests. A.J. received research support for this study from AstraZeneca. He has stock options in DexCom, and has a pending patent for fusion protein nanodiscs for the treatment of HF. P.A., S.B.G., J.B., C.D.S., A.M.L., J.O. and R.E. are employees and shareholders of AstraZeneca.
AUTHOR CONTRIBUTIONS
M.K., S.B.G., J.O., P.A. and R.E. drafted the manuscript. O.M, O.B., G.G.K., F.M., V.C., S.V., C.D.S., A.M.L., J.B., and A.J. critically reviewed the manuscript.
Supporting information
Table S1. Exploratory measures
Table S2. Diagnostic criteria for DKA
ACKNOWLEDGMENTS
Support in formatting and submitting the manuscript was provided by Parita Sheth (inScience Communications, Springer Healthcare Ltd, UK), and funded by AstraZeneca. The DARE‐19 study is sponsored by Saint Luke's Mid America Heart Institute with AstraZeneca as the funding source.
Kosiborod M, Berwanger O, Koch GG, et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure because of COVID‐19: Design and rationale for the DARE‐19 study. Diabetes Obes Metab. 2021;23:886–896. 10.1111/dom.14296
DATA AVAILABILITY STATEMENT
Data sharing not applicable, as this article is a description of the study design
REFERENCES
- 1. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ronco C, Reis T, Husain‐Syed F. Management of acute kidney injury in patients with COVID‐19. Lancet Respir Med. 2020;8:738‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831‐840. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA. 2020;323:1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care. 2020;43:1382‐1391. [DOI] [PubMed] [Google Scholar]
- 13. Vrsalovic M, Vrsalovic PA. Cardiac troponins predict mortality in patients with COVID‐19: a meta‐analysis of adjusted risk estimates. J Infect. 2020;8:e99‐e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shi S, Qin M, Shen B, et al. Association of Cardiac Injury with Mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pranata R, Huang I, Lukito AA, Raharjo SB. Elevated N‐terminal pro‐brain natriuretic peptide is associated with increased mortality in patients with COVID‐19: systematic review and meta‐analysis. Postgrad Med J. 2020;96:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID‐19. Heart. 2020;106:1132‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ayres JS. A metabolic handbook for the COVID‐19 pandemic. Nat Metab. 2020;2:572‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horwitz LI, Jones SA, Cerfolio RJ, et al. Trends in COVID‐19 risk‐adjusted mortality rates. J Hosp Med. 2020. 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 21. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 22. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347‐357. [DOI] [PubMed] [Google Scholar]
- 23. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995‐2008. [DOI] [PubMed] [Google Scholar]
- 25. Petrie MC, Verma S, Docherty KF, et al. Effect of Dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sabatine MS, DeMets DK, Inzucchi SE, et al. Timing of onset of clinical benefit with Dapagliflozin in patients with heart failure: an analysis from the Dapagliflozin and prevention of adverse‐outcomes in heart failure trial (DAPA‐HF). Circulation. 2019;140:E973‐E974. [Google Scholar]
- 27. McMurray JJV, Wheeler DC, Stefansson BV, et al. Effect of dapagliflozin on clinical outcomes in patients with chronic kidney disease, with and without cardiovascular disease. Circulation. 2020. 10.1161/CIRCULATIONAHA.120.051675. [DOI] [PubMed] [Google Scholar]
- 28. Heerspink HJL, Stefansson BV, Correa‐Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436‐1446. [DOI] [PubMed] [Google Scholar]
- 29. Daniele G, Xiong J, Solis‐Herrera C, et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care. 2016;39:2036‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS‐CoV‐2 infection and monocyte response through a HIF‐1α/glycolysis‐dependent Axis. Cell Metab. 2020;32:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Icard P, Lincet H, Wu Z, et al. The key role of Warburg effect in SARS‐CoV‐2 replication and associated inflammatory response. Biochimie. 2020;180:169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low‐grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457‐464. [DOI] [PubMed] [Google Scholar]
- 34. Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maayah ZH, Ferdaoussi M, Takahara S, Soni S, Dyck JRB. Empagliflozin suppresses inflammation and protects against acute septic renal injury. Inflammopharmacology. 2020. 10.1007/s10787-020-00732-4. [DOI] [PubMed] [Google Scholar]
- 36. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghanim H, Abuaysheh S, Hejna J, et al. Dapagliflozin suppresses Hepcidin and increases erythropoiesis. J Clin Endocrinol Metab. 2020;105:dgaa057. [DOI] [PubMed] [Google Scholar]
- 38. Ohara K, Masuda T, Morinari M, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr. 2020;12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffin M, Rao VS, Ivey‐Miranda J, et al. Empagliflozin in heart failure: diuretic and cardio‐renal effects. Circulation. 2020;142:1028‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mullens W, Martens P, Forouzan O, et al. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. 2020;7:2071–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferrannini E. Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27‐38. [DOI] [PubMed] [Google Scholar]
- 42. Aragon‐Herrera A, Feijoo‐Bandin S, Otero Santiago M, et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170:113677. [DOI] [PubMed] [Google Scholar]
- 43. Tanaka S, Sugiura Y, Saito H, et al. Sodium‐glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94:912‐925. [DOI] [PubMed] [Google Scholar]
- 44. Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium‐glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;22:618‐628. [DOI] [PubMed] [Google Scholar]
- 45. Esterline RL, Vaag A, Oscarsson J, Vora J. Mechanisms in endocrinology: SGLT2 inhibitors: clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. 2018;178:R113‐R125. [DOI] [PubMed] [Google Scholar]
- 46. Perez‐Belmonte LM, Torres‐Pena JD, Lopez‐Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID‐19 in association with glucose‐lowering drugs: a nationwide cohort study. BMC Med. 2020;18:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in new York City. N Engl J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 ‐ final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. US Food & Drug Administration . Multiple endpoints in clinical trials guidance for industry. 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/multiple-endpoints-clinical-trials-guidance-industry. Accessed December 2020.
- 51. Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur Heart J. 2012;33:176‐182. [DOI] [PubMed] [Google Scholar]
- 52. Koch GG, Tangen CM, Jung JW, Amara IA. Issues for covariance analysis of dichotomous and ordered categorical data from randomized clinical trials and non‐parametric strategies for addressing them. Stat Med. 1998;17:1863‐1892. [DOI] [PubMed] [Google Scholar]
- 53. Gasparyan SB, Folkvaljon F, Bengtsson O, Buenconsejo J, Koch GG. Adjusted win ratio with stratification: calculation methods and interpretation. Stat Methods Med Res. 2020. 10.1177/0962280220942558. [DOI] [PubMed] [Google Scholar]
- 54. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hossein‐Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID‐19. J Mol Med. 2020;98:789‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. National Institute of Health . Coronavirus disease 2019 (COVID‐19) treatment guidelines. 2020. https://www.covid19treatmentguidelines.nih.gov/. Accessed December 2020. [PubMed]
- 57. WHO solidarity trial consortium . Repurposed antiviral drugs for COVID‐19–interim WHO SOLIDARITY trial results. medRxiv. 2020. 10.1101/2020.1110.1115.20209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Horby P, Lim WS, Emberson J , et al. Effect of dexamethasone in hospitalized patients with COVID‐19: preliminary report. 2020; 10.1101/2020.06.22.20137273v1 [DOI]
- 59. Horby P, Mafham M, Linsell L , et al. Effect of hydroxychloroquine in hospitalized patients with COVID‐19: preliminary results from a multi‐centre, randomized, controlled trial. 2020. 10.1101/2020.07.15.20151852v1 [DOI]
- 60. Trials R. Statement from the Chief Investigators: no Clinical Benefit from Use of Hydroxychloroquine in Hospitalised Patients with COVID‐19. 2020.
- 61. Costanzo AE, Taylor KR, Dutt S, Han PP, Fujioka K, Jameson JM. Obesity impairs gammadelta T cell homeostasis and antiviral function in humans. PLoS One. 2015;10:e0120918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maejima Y. SGLT2 inhibitors play a salutary role in heart failure via modulation of the mitochondrial function. Front Cardiovasc Med. 2019;6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020. 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 64. Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail. 2020;22:713‐722. [DOI] [PubMed] [Google Scholar]
- 65. Scheen AJ. SGLT2 inhibition during the COVID‐19 epidemic: friend or foe? Diabetes Metab. 2020;46:343–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fernandez‐Fernandez B, D'Marco L, Gorriz JL, et al. Exploring sodium glucose co‐Transporter‐2 (SGLT2) inhibitors for organ protection in COVID‐19. J Clin Med. 2020;9:E2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Katulanda P, Dissanayake HA, Ranathunga I, et al. Prevention and management of COVID‐19 among patients with diabetes: an appraisal of the literature. Diabetologia. 2020;63:1440‐1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Armeni E, Aziz U, Qamar S, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID‐19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. AstraZeneca . Farxiga met all primary and secondary endpoints in groundbreaking Phase III DAPA‐CKD trial for the treatment of patients with chronic kidney disease. 2020.
- 70. Ohara K, Masuda T, Murakami T, et al. Effects of the sodium‐glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: a comparison study with furosemide and tolvaptan. Nephrology. 2019;24:904‐911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Exploratory measures
Table S2. Diagnostic criteria for DKA
Data Availability Statement
Data sharing not applicable, as this article is a description of the study design


