Abstract
Introduction
Fetal growth restriction is associated with adverse perinatal outcome and the clinical management of these pregnancies is a challenge. The aim of this study was to investigate the potential of cerebroplacental ratio (CPR) to predict adverse perinatal outcome in high‐risk pregnancies in the third trimester. Another aim was to study whether the CPR has better predictive value than its components, middle cerebral artery (MCA) pulsatility index (PI) and umbilical artery (UA) PI.
Material and methods
The study was a retrospective cohort study including 1573 singleton high‐risk pregnancies with Doppler examinations performed at 32+0 to 40+6 gestational weeks at Lund University Hospital and the University Hospital of Malmö between 29 December 1994 and 31 December 2017. Receiver operating characteristics (ROC) curves were used to investigate the predictive value of the gestational age‐specific z‐scores for CPR, UA PI and MCA PI, respectively, for the primary outcome “perinatal asphyxia/mortality” and the secondary outcomes “birthweight small for gestational age (SGA)” and two composite outcomes: “appropriate for gestational age/large for gestational age liveborn infants with neonatal morbidity” and “SGA liveborn infants with neonatal morbidity.”
Results
The performance in predicting perinatal asphyxia/mortality was poor for all three variables and did not differ significantly. The ROC area under curve (AUC) was 0.56, 0.55 and 0.53 for CPR, UA PI and MCA PI z‐scores, respectively. The ROC AUC for CPR z‐scores to predict SGA was 0.73, significantly higher than that for either UA PI or MCA PI (P < .001). The ability of CPR and the MCA PI to predict appropriate for gestational age/large for gestational age infant morbidity and SGA infant morbidity was similar and significantly better than UA PI (P < .001).
Conclusions
In the present study, none of the three Doppler measures proved to be useful in predicting perinatal asphyxia and mortality. CPR and MCA PI were equally good in predicting neonatal morbidity, especially in SGA pregnancies, and both were significantly better predictors than the UA PI. CPR had a high predictive value for SGA at birth, better than that of its two components, UA PI and MCA PI.
Keywords: adverse perinatal outcome, asphyxia, cerebroplacental ratio, Doppler ultrasound, fetal growth restriction, fetus, small for gestational age
Abbreviations
- AGA
appropriate for gestational age
- AUC
area under curve
- CPR
cerebroplacental ratio
- FGR
fetal growth restriction
- GW
gestational weeks
- LGA
large for gestational age
- MCA
middle cerebral artery
- PI
pulsatility index
- ROC
receiver operating characteristics
- SGA
small for gestational age
- UA
umbilical artery
Key message.
All three Doppler measures had poor predictive value for perinatal asphyxia/mortality. Cerebroplacental ratio had high predictive capacity for small for gestational age at birth.
1. INTRODUCTION
Fetal growth restriction (FGR) is associated with increased risk of adverse perinatal outcome as well as long‐term morbidity. 1 , 2 The clinical management, including timing and mode of delivery, of pregnancies complicated by FGR varies between perinatal centers and is a challenge. 3 Another matter of concern is fetuses appropriate for gestational age (AGA) that show signs of blood flow redistribution. 4 The use of Doppler measurements of pulsatility index (PI) in the umbilical artery (UA) in surveillance of high‐risk pregnancies is associated with a decrease in perinatal mortality and in the rates of cesarean section. 5 In recent years, the cerebroplacental ratio (CPR) has re‐emerged as a potential predictor of adverse perinatal outcome, particularly in pregnancies with suspected FGR. The ratio between the PI in the middle cerebral artery (MCA) and PI in UA reflects both the preferential supply to the brain of the fetus and the increase in placental resistance. 6 Studies on the clinical usefulness of CPR have reported divergent results, and the integration of CPR in clinical practice is under debate. 7 , 8 , 9 , 10 , 11 Different thresholds for CPR have been used and no consensus exists regarding whether a fixed cutoff level is superior to a cutoff related to either estimated fetal weight or gestational age. 12 , 13 , 14
The aim of this retrospective registry study was to investigate the ability of CPR to predict adverse perinatal outcome in moderate, late preterm and term high‐risk pregnancies. Another aim was to study whether the CPR has better predictive value than its components, MCA PI and UA PI.
2. MATERIAL AND METHODS
2.1. Data collection
The study is a retrospective cohort study. Data were collected from a database including all obstetric Doppler measurements performed at Lund University Hospital since 1994, and at the University Hospital of Malmö since 1990. The results of clinically indicated Doppler examinations of pregnancies were prospectively stored in the database. The background data of the study comprised 6049 Doppler examinations performed at 32+0 to 40+6 gestational weeks (GW) with records of both UA PI and MCA PI between 29 December 1994 and 31 December 2017.
Records from multiple pregnancies and pregnancies with major fetal malformations, chromosomal abnormalities, fetal arrhythmias or isoimmunization were excluded (n = 3334). When there was more than one Doppler examination during pregnancy, the last one before delivery was used in the analysis; pregnancies with the last examination ≥14 days before delivery were excluded as well as those delivered at ≥42+0 weeks. Pregnancies with absent or reversed end‐diastolic (ARED) flow in umbilical artery were also excluded because that Doppler finding is an established indication for delivery after 32 GW (n = 23). Figure 1 presents a flowchart of the study. Two subgroup analyses were performed:
FIGURE 1.
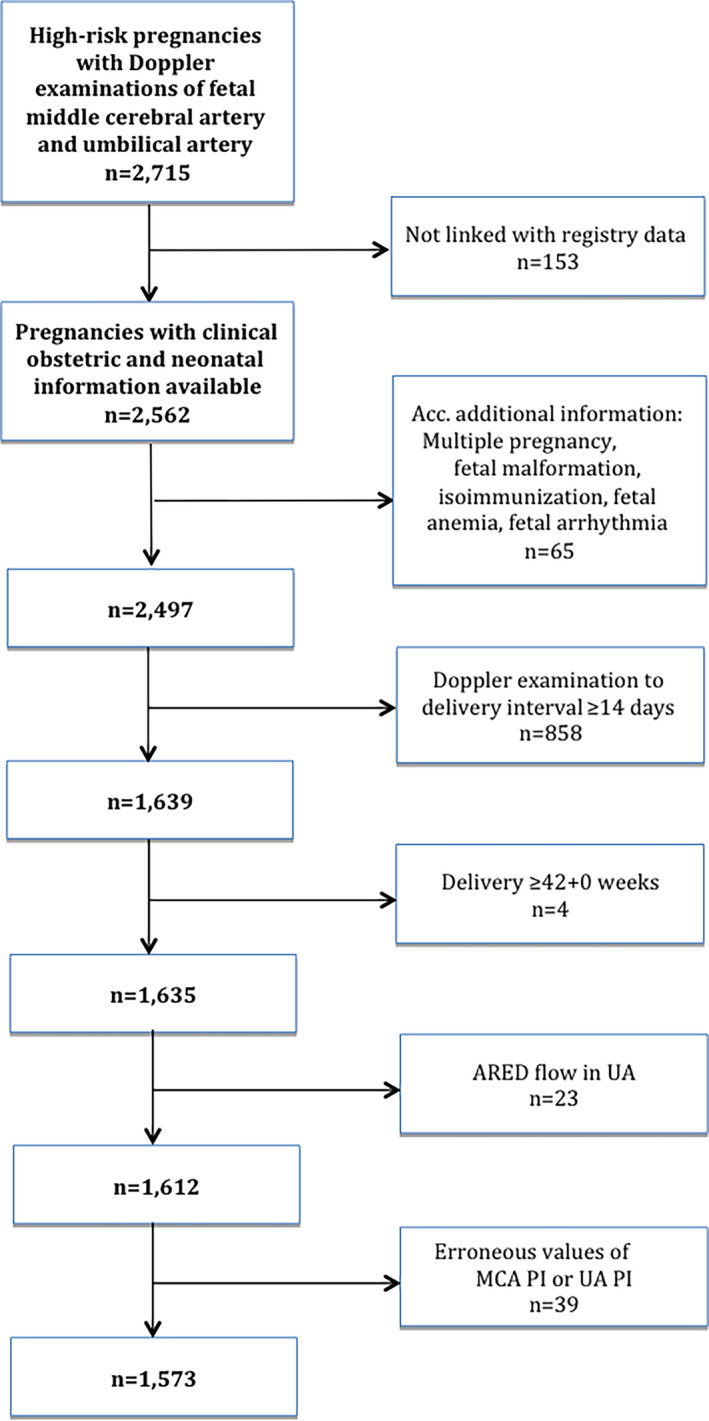
Flowchart of the study. ARED, absent or reversed end‐diastolic flow; MCA, middle cerebral artery; PI, pulsatility index; UA, umbilical artery [Color figure can be viewed at wileyonlinelibrary.com]
Subgroup A (n = 1241): Pregnancies after exclusion of cesarean sections before onset of labor. Subgroup B (n = 814): Pregnancies with spontaneous onset of labor (prelabor cesarean sections and inductions of labor excluded).
The indications for Doppler examinations were suspected FGR, maternal hypertension or preeclampsia either in the present or previous pregnancy, pregestational diabetes, oligohydramnios or polyhydramnios, decreased fetal movements, and concomitant maternal diseases, for example, autoimmune diseases. Only records with available neonatal and maternal clinical information obtained from a regional perinatal quality register (Perinatal Revision South; PRS) were included. 15 PRS contains data from all obstetric and neonatal units in the Southern Healthcare Region of Sweden since 1995. Maternal background information, such as body mass index (BMI) and smoking, refers to records from women’s first visit to the antenatal care clinic. Gestational age (GA) was based on routine ultrasound dating in the first half of pregnancy. Medical conditions and diagnoses were based on International Classification of Diseases (ICD) codes (ICD‐9, ICD‐10). Infants were defined as small for gestational age (SGA) or large for gestational age (LGA) when weighing respectively >2 standard deviations (SD) below or >2 SD above the mean birthweight for GA and gender according to the Swedish intrauterine growth curve. 16
2.2. Doppler ultrasound examination
The Doppler examinations were performed using ultrasound systems that belonged to the top class scanners at the time of examinations (Acuson, ATL, Philips, GE). All examinations were done in one of two ultrasound laboratories specialized in obstetric Doppler by just a few experienced sonographers, guaranteeing uniform and good quality examinations.
Doppler signals from UA were obtained from the free‐floating portion of the umbilical cord with the insonation angle kept below 30°. Sample volume was located to cover the vessel lumen, carefully avoiding the nearby umbilical vein. The fetal MCA artery was located in a transverse view of fetal skull and the Doppler signals were recorded from the MCA closest to the transducer. The sample volume of the spectral Doppler was located in the proximal third of the MCA, close to its origin in the circle of Willis. The insonation angle was kept as close to 0° as possible. Undue pressure by the ultrasound transducer was avoided.
A sequence of Doppler signals corresponding on average to 10 heart beats with uniform waveform appearance were recorded from the two vessels of interest during maternal voluntary apnea and during periods of fetal quiescence, that is, without fetal general and/or breathing movements, and periods with no uterine contractions. With very few exceptions, the thermal index was kept below 1.0, never exceeding 1.5. The tracing of maximum blood flow velocity (envelope of the Doppler spectrum) and calculation of PI was done automatically by the ultrasound machine and the resulting PI was stored in the clinical database of the Doppler laboratory. The results of MCA Doppler examinations were not used clinically. A hard copy of the frozen image of the Doppler signals accepted for calculation was stored. The individual CPRs were calculated post hoc for the purposes of the present study.
2.3. Outcome measures
The primary outcome measure was the composite adverse outcome “perinatal asphyxia/mortality”, defined as one or more stillbirths, Apgar score <7 at 5 minutes, death during neonatal hospitalization, seizures and hypoxic ischemic encephalopathy grades II and III. The secondary measures were the SGA birthweight among liveborn infants with no asphyxia/mortality, and two composite adverse outcomes: “AGA/LGA liveborn infants with neonatal morbidity” and “SGA live‐born infants with neonatal morbidity”. Neonatal morbidity was defined as admission to neonatal care unit, mild respiratory disturbance, meconium aspiration syndrome, respiratory distress syndrome, bronchopulmonary dysplasia, persistent pulmonary hypertension of the newborn, supplemental oxygen at discharge, intraventricular hemorrhage grades 3 and 4, periventricular leukomalacia and seizures.
2.4. Data processing and statistical methods
We used the reference curves of Ebbing et al to transform the actual Doppler measures (UA PI, MCA PI and CPR) to GA‐specific z‐scores. 12
Receiver operating characteristic (ROC) curves were used to investigate the predictive value for the four outcomes of the GA‐specific z‐scores for CPR, UA PI and MCA PI, respectively. The method proposed by DeLong et al was used to compute the ROC area under curve (AUC), the variance of each ROC AUC, and the variance of the difference between two dependent ROC AUCs. 17
2.5. Ethical approval
The study was approved by the Research Ethics Committee of Lund University, Sweden, 26 February 2015 (reference number 2015/82).
3. RESULTS
After exclusions, the study included 1573 singleton pregnancies (Figure 1). The population characteristics data and the perinatal outcome are shown in Table 1. Of the 1573 fetuses, 46.7% were SGA and 3.3% died in utero or during neonatal hospitalization, or had postnatal signs of asphyxia (see above). Of the liveborn AGA or LGA infants, 250 (16.0%) suffered from neonatal morbidity. The corresponding number for SGA liveborn infants was 27.6% (Table 2). For population characteristics data and the perinatal outcome of the two subgroup analyses see Tables S1 and S2.
TABLE 1.
Characteristics of the study cohort, n = 1573. The data are presented as median [range] or n (%)
| Maternal age, years | 30.0 | [14‐46] |
| <20 | 43 | (2.7) |
| ≥35 | 322 | (20.5) |
| Maternal BMI, kg/m2 | 23.2 | [15.4‐50.0] |
| <18.5 | 56/1342 | (4.2) |
| ≥30.0 | 166/1342 | (12.4) |
| Maternal smoking | 222/1431 | (15.5) |
| Nullipara | 944 | (60.0) |
| Male sex | 785 | (49.9) |
| Gestational age at delivery, weeks + days | 37+6 | [32+0‐41+6] |
| 32‐33 | 140 | (8.9) |
| 34‐36 | 385 | (24.5) |
| 37‐39 | 851 | (54.1) |
| 40‐41 | 197 | (12.5) |
| Delivery start spontaneous | 814 | (51.8) |
| Induction of labor | 427 | (27.2) |
| CS before delivery start | 332 | (21.1) |
| Mode of delivery spontaneous vaginal | 755 | (48.6) |
| Operative vaginal | 49 | (3.2) |
| Emergency CS | 572 | (36.8) |
| Elective CS | 177 | (11.4) |
| Missing | 20 | |
| Interval Doppler examination to delivery, days | 3 | [0‐13] |
| Apgar score at 5 min | 10 | [0‐10] |
| 0‐3 | 13 | (0.8) |
| 4‐6 | 31 | (2.0) |
| 7‐10 | 1515 | (97.2) |
| Missing | 14 | |
| Birthweight, g | 2495 | [1025‐5242] |
Abbreviations: BMI, body mass index; CS, cesarean section.
TABLE 2.
Perinatal outcome
| n | % | |
|---|---|---|
| Total | 1573 | 100 |
| Stillbirth | 9 | 0.6 |
| Apgar score 5 min <7 | 44 | 2.8 |
| SGA | 734 | 46.7 |
| Admission to neonatal care unit | 681 | 43.3 |
| BPD |
26 |
1.7 |
| RDS | 26 | 1.7 |
| Mild respiratory disturbance/ transient tachypnea of newborn | 34 | 2.2 |
| PPHN | 2 | 0.13 |
| Meconium aspiration syndrome | 0 | 0 |
| IVH grade 3‐4, PVL | 2 | 0.13 |
| Seizures | 7 | 0.4 |
| HIE II‐III | 1 | 0.06 |
| Death during neonatal hospitalization | 6 | 0.4 |
| Composite adverse outcome | ||
| Perinatal asphyxia/mortality a | 52 | 3.3 |
| AGA/LGA liveborn infants with neonatal morbidity b | 250/1564 | 16.0 |
| SGA liveborn infants with neonatal morbidity b | 431/1564 | 27.6 |
Abbreviations: AGA, birthweight appropriate for gestational age; BPD, bronchopulmnonary dysplasia; HIE, hypoxic ischemic encephalopathy; IVH, intraventricular hemorrhage; LGA, birthweight large for gestational age; PPHN, persistent pulmonary hypertension of newborn; PVL, periventricular leukomalacia; RDS, respiratory distress syndrome; SGA, small for gestational age (>2 SD below the expected mean according to the Swedish intrauterine growth curve for gestational and sex 16 ).
Perinatal asphyxia/mortality: Stillbirth, death during neonatal hospitalization, Apgar score <7 at 5 min, seizures, HIE II‐III.
Neonatal morbidity; At least one of the following: Admission to neonatal care unit, BPD, meconium aspiration syndrome, RDS, mild respiratory disturbance/transient tachypnea of newborn, PPHN, IVH grade 3‐4, PVL, seizures.
Figure 2 shows the ROC curves for the z‐scores of the three Doppler variables as predictors of the four outcome measures. The performance in predicting perinatal asphyxia/mortality was poor for all three variables and did not significantly differ (ROC AUC was 0.56, 0.55 and 0.53 for CPR, UA PI and MCA PI z‐scores, respectively) (Table 3). In the Subgroup A (prelabor cesarean sections excluded), the corresponding ROC AUC for predicting perinatal asphyxia/mortality was 0.57, 0.60 and 0.51 for CPR, UA PI and MCA PI z‐scores, respectively. The P‐value for the difference between the ROC AUC for CPR and MCA was .012 (Table S3). Among pregnancies with spontaneous onset of labor (Subgroup B), UA PI performed significantly better than CPR and MCA PI; the ROC AUC was 0.54, 0.60 and 0.46 for CPR, UA PI and MCA PI, respectively (Table S3).
FIGURE 2.
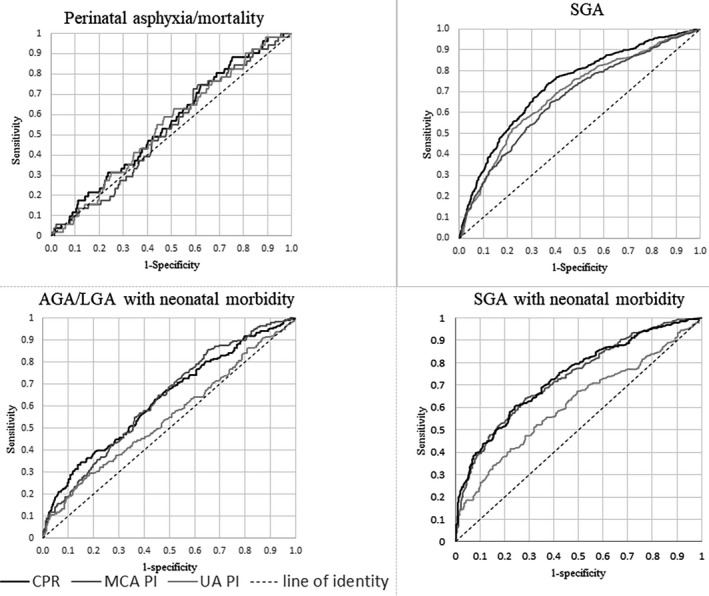
Receiver operating characteristic curves of the gestational age‐specific z‐scores for cerebroplacental ratio (CPR), umbilical artery pulsatility index (UA PI) and middle cerebral artery pulsatility index (MCA PI), respectively, for the four outcomes perinatal asphyxia/mortality, small for gestational age (SGA), appropriate for gestational age/large for gestational age (AGA/LGA) liveborn infants with neonatal morbidity and SGA liveborn infants with neonatal morbidity
TABLE 3.
Area under the curve (AUC) for receiver operating characteristic (ROC) curves according to perinatal outcome and Doppler measures
| UA PI z‐score | MCA PI z‐score | CPR z‐score | Significance of difference, P‐value | |||||
|---|---|---|---|---|---|---|---|---|
| ROC AUC | 95% CI | ROC AUC | 95% CI | ROC AUC | 95% CI | CPR ‐PI UA | CPR ‐PI MCA | |
| Perinatal asphyxia/mortality a | 0.55 | 0.48‐0.63 | 0.53 | 0.45‐0.60 | 0.56 | 0.48‐0.64 | .38 | .082 |
| SGA (no asphyxia/mortality) | 0.69 | 0.66‐0.72 | 0.67 | 0.64‐0.70 | 0.73 | 0.70‐0.76 | <.001 | <.001 |
| AGA/LGA liveborn infants with neonatal morbidity b | 0.55 | 0.50‐0.59 | 0.63 | 0.59‐0.67 | 0.63 | 0.59‐0.67 | <.001 | .44 |
|
SGA liveborn infants with neonatal morbidity b |
0.62 | 0.58‐0.66 | 0.73 | 0.70‐0.77 | 0.74 | 0.70‐0.78 | <.001 | .48 |
Abbreviations: AGA, appropriate for gestational age; CI, confidence interval; CPR, cerebroplacental ratio; LGA, large for gestational age; MCA, middle cerebral artery; PI, pulsatility index; SGA, small for gestational age according to the Swedish standard; 16 UA, umbilical artery.
Perinatal asphyxia/mortality: Stillbirth, neonatal death, Apgar score <7 at 5 min, seizures, HIE II‐III.
Neonatal morbidity; At least one of the following: Admission to neonatal care unit, BPD, meconium aspiration syndrome, RDS, mild respiratory disturbance/transient tachypnea of newborn, PPHN, IVH grade 3‐4, PVL, seizures.
The ROC AUC for CPR z‐scores to predict SGA was 0.73, which was significantly higher than that for either UA PI (0.69) or MCA PI (0.67). The ability of CPR and the MCA PI z‐scores to predict AGA/LGA infant morbidity was similar (ROC AUC 0.63 for both) and significantly better than that of UA PI (ROC AUC 0.55). The predictive capacity of CPR for SGA infant morbidity (ROC AUC 0.74) was similar to that for MCA PI (ROC AUC 0.73). Both were significantly better than UA PI ROC AUC (0.62) (Figure 2, Table 3). The results of the two subgroup analyses for the outcomes SGA, AGA/LGA and SGA infant morbidity showed results similar to those of the whole study population (Table S3).
4. DISCUSSION
In the present study on a high‐risk population of moderate preterm, late preterm and term singleton fetuses with positive end‐diastolic velocity in umbilical artery, all three Doppler variables had ROC AUC close to the line of identity for perinatal asphyxia or mortality, suggesting that none of the three measures possesses any predictive value in this regard.
CPR showed a relatively high predictive capacity for SGA birthweight and performed better as a predictor of SGA than its components UA PI and MCA PI. However, although statistically significant, the differences between the absolute values of ROC AUC were small, and it is uncertain whether the differences are of any clinical importance. This study has no data on other methods, such as estimated fetal weight, as predictors of SGA. Randomized controlled trials showed that fetal weight estimated with ultrasound in the third trimester is a better predictor of SGA than reported for the Doppler variables in this study. 18 For neonatal morbidity, CPR and MCA PI had better predictive ability among infants born SGA than among infants born AGA/LGA, and these Doppler measures were significantly better than UA PI.
In the search for an indicator of fetal hypoxia, CPR has been proposed as suitable Doppler parameter in early 1990s. 6 The rational for using CPR was the animal studies on experimental hypoxia that showed redistribution of fetal blood flow with preferential supply to the brain. 19 , 20 Of Doppler parameters, the CPR was shown to be best at following the acute pO2 changes in sheep fetus. 21 The early, usually small, human studies on the predictive performance of CPR with regard to the adverse outcome in fetuses at risk for FGR were promising. 6 , 8 , 22 They raised hopes that CPR might be the indicator of imminent fetal hypoxia, especially in the late third trimester, when UA PI was not found to perform satisfactorily. 23 An explanation for this generally accepted view of a worse performance of UA PI might be the large compensatory capacity of the placenta; animal experiments have shown that almost two‐thirds of the fetal placental vascular tree has to be blocked to induce changes in UA hemodynamics. 24 In humans, CPR was proposed to reflect the redistribution of fetal blood flow due to hypoxia even if the UA PI is within normal range. 25
Several investigators have reported a robust ability of CPR to predict perinatal outcome in terms of emergency cesarean section indicated by fetal distress and of admission to neonatal care unit. 26 , 27 It should be kept in mind that the association between Doppler measures and emergency cesarean section might in many studies have been confounded by cognizance of the Doppler results by clinicians. Some recent studies promoted the combined use of CPR and mean uterine artery PI, either with or without fetal biometry as an additional parameter, for prediction of stillbirth and perinatal loss at term. 28 , 29 However, an increasing number of studies could not confirm the potential clinical value of CPR and no randomized trial has been performed so far. 14 , 30 Some authors have found a good predictive value for CPR and adverse pregnancy outcome before, but not after, 34 GW, 31 and several meta‐analyses pointed out the heterogeneity of data sources, various cutoff values and the risk of publication bias. 11 , 32
Two large screening studies at 30‐34 GW (n = 30 780) and 35‐37 weeks (n = 6178) found a significant association between CPR and SGA; 30 , 33 however, both studies found the CPR to have poor predictive performance regarding the adverse pregnancy outcome. This is in accord with our results in a cohort of pregnancies with complications mainly due to uteroplacental causes; the predictive power of CPR was good regarding SGA birthweight and neonatal morbidity but very poor with regard to perinatal asphyxia and mortality. This may reflect the fact that Doppler velocimetry is aimed at predicting chronic hypoxia rather than acute severe hypoxia after obstetric events such as placental abruption and umbilical cord events. Mode of delivery can affect the pregnancy outcome, since the situation for fetuses subjected to the stress of labor contractions and fetuses delivered with cesarean section before the onset of labor is different. It has been reported that unfavorable cervix status at start of labor induction of SGA fetuses can affect perinatal outcome. 34 The current study does not have any information on cervix score. However, we performed subgroup analyses after exclusion of prelabor cesarean sections and labor inductions. The results were similar to those in the total cohort, but UA PI performed better than MCA PI and CPR when prelabor cesarean section was excluded and among pregnancies with spontaneous onset of labor in predicting perinatal asphyxia/mortality.
A strength of the current study is the relatively large material and the fact that the Doppler examinations were all performed by a few, highly specialized and experienced sonographers. The latter aspect is important in view of the fact that Doppler examinations are open to methodological error, something not always recognized by examiners. An important feature of our study is that the Doppler variable MCA PI was collected prospectively; however, it was not used in the clinical decision‐making during the whole study period. In addition, the interval between examination and birth was short (median 3 days). Another advantage of the present study is that we excluded all cases with ARED flow, which is a generally accepted indication for delivery after 32 GW. The information on neonatal outcome was retrieved using a population‐based, high‐quality clinical register, keeping lack of information and drop‐out rate to a minimum. Furthermore, the study investigated the performance of various Doppler measures by comparing the ROC AUCs, thereby evaluating the total predictive ability, not only the predictive capacity using arbitrarily selected cutoff levels. Only a few published studies have compared the performance of CPR with that of UA PI and MCA PI, respectively. 13 , 35
A limitation of our study is that it is retrospective and that the indications for Doppler examinations varied. We attempted to circumvent this disadvantage by excluding all pregnancies with diagnoses of conditions that might have influenced fetal circulation in themselves. Thus, the study group comprised mainly pregnancies with complications of uteroplacental origin. Unfortunately, the subgroup of pregnancies with the composite outcome variable perinatal asphyxia/mortality was rather small. Another drawback was that the gestational age at examination varied from 32+0 to 40+6 gestational weeks, which could introduce serious confounding, as all Doppler measures evaluated are known to be dependent on gestational age. Therefore, we used z‐scores of investigated measures based on the published gestational age‐specific reference values. 12 The two composite adverse outcomes – “AGA/LGA live‐born infants with neonatal morbidity” and “SGA live‐born infants with neonatal morbidity” – included diagnoses such as bronchopulmonary dysplasia, persistent pulmonary hypertension of the newborn and intraventricular hemorrhage. The results might be affected by the fact that preterm infants born after 32+0 gestational weeks and days, were included. Even though we excluded all cases with ARED flow, the fact that the UA PI was available to the clinicians and potentially used in the clinical decision‐making could introduce an intervention bias.
5. CONCLUSION
In a cohort of high‐risk pregnancies after 32 GW, none of the three Doppler measures proved to be useful in predicting perinatal asphyxia or mortality. CPR and MCA PI were equally good in predicting neonatal morbidity, especially in SGA pregnancies, and both were significantly better predictors than the UA PI. CPR was highly predictive of SGA at birth. Its performance was better than that of its two components, UA PI and MCA PI. The question of possible clinical usefulness of CPR in the late third trimester still remains open, awaiting randomized controlled trials.
CONFLICT OF INTEREST
None.
Supporting information
Table S1
Table S2
Table S3
Bonnevier A, Maršál K, Brodszki J, Thuring A, Källén K. Cerebroplacental ratio as predictor of adverse perinatal outcome in the third trimester. Acta Obstet Gynecol Scand.2021;100:497–503. 10.1111/aogs.14031
Funding informationThe Department of Research and Development of Region Skane financially supported this study.
REFERENCES
- 1. Kramer MS, Olivier M, McLean FH, Willis DM, Usher RH. Impact of intrauterine growth retardation and body proportionality on fetal and neonatal outcome. Pediatrics. 1990;86:707–713. [PubMed] [Google Scholar]
- 2. Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. [DOI] [PubMed] [Google Scholar]
- 3. Maršál K. Obstetric management of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23:857–870. [DOI] [PubMed] [Google Scholar]
- 4. Morales‐Rosello J, Khalil A, Morlando M, Papageorghiou A, Bhide A, Thilaganathan B. Changes in fetal Doppler indices as a marker of failure to reach growth potential at term. Ultrasound Obstet Gynecol. 2014;43:303–310. [DOI] [PubMed] [Google Scholar]
- 5. Westergaard HB, Langhoff‐Roos J, Lingman G, Maršál K, Kreiner S. A critical appraisal of the use of umbilical artery Doppler ultrasound in high risk pregnancies: use of meta‐analyses in evidence‐based obstetrics. Ultrasound Obstet Gynecol. 2001;17:466–476. [DOI] [PubMed] [Google Scholar]
- 6. Gramellini D, Folli MC, Raboni S, Vadora E, Merialdi A. Cerebral‐umbilical Doppler ratio as a predictor of adverse perinatal outcome. Obstet Gynecol. 1992;79:416–420. [DOI] [PubMed] [Google Scholar]
- 7. Alfirevic Z, Stampalija T, Dowswell T. Fetal and umbilical Doppler ultrasound in high‐risk pregnancies. Cochrane Database Syst Rev. 2017;(6):CD007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baschat AA, Gembruch U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet Gynecol. 2003;21:124–127. [DOI] [PubMed] [Google Scholar]
- 9. DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well‐being in SGA and AGA fetuses. Am J Obstet Gynecol. 2015;213:5–15. [DOI] [PubMed] [Google Scholar]
- 10. Vollgraff Heidweiller‐Schreurs CA, De Boer MA, Heymans MW, et al. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: systematic review and meta‐analyses. Ultrasound Obstet Gynecol. 2018;51:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conde‐Agudelo A, Villar J, Kennedy SH, Papageorghiou AT. Predictive accuracy of cerebroplacental ratio for adverse perinatal and neurodevelopmental outcomes in suspected fetal growth restriction: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;52:430–441. [DOI] [PubMed] [Google Scholar]
- 12. Ebbing C, Rasmussen S, Kiserud T. Middle cerebral artery blood flow velocities and pulsatility index and cerebroplacental pulsatility ratio: longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet Gynecol. 2007;30:287–296. [DOI] [PubMed] [Google Scholar]
- 13. Twomey S, Flatley C, Kumar S. The association between a low cerebro‐umbilical ratio at 30–34 weeks gestation, increased intrapartum operative intervention and adverse perinatal outcomes. Eur J Obstet Gynecol Reprod Biol. 2016;203:89–93. [DOI] [PubMed] [Google Scholar]
- 14. Sirico A, Diemert A, Glosemeyer P, Hecher K. Prediction of adverse perinatal outcome by cerebroplacental ratio adjusted for estimated fetal weight. Ultrasound Obstet Gynecol. 2018;51:381–386. [DOI] [PubMed] [Google Scholar]
- 15. Molin J. A regional perinatal database in southern Sweden – a basis for quality assurance in obstetrics and neonatology. Acta Obstet Gynecol Scand Suppl. 1997;164:37–39. [PubMed] [Google Scholar]
- 16. Maršál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–848. [DOI] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 18. Skråstad RB, Eik‐Nes SH, Sviggum O, et al. A randomized controlled trial of third‐trimester routine ultrasound in a non‐selected population. Acta Obstet Gynecol Scand. 2013;92:1353–1360. [DOI] [PubMed] [Google Scholar]
- 19. Jensen A, Hohmann M, Künzel W. Dynamic changes in organ blood flow and oxygen consumption during acute asphyxia in fetal sheep. J Dev Physiol. 1987;9:543–559. [PubMed] [Google Scholar]
- 20. Malcus P, Hökegård K‐H, Kjellmer I, Lingman G, Maršál K, Rosén K‐G. The relationship between arterial blood velocity waveforms and acid‐base status in the fetal lamb during acute experimental asphyxia. J Matern Fetal Investig. 1991;1:29–34. [Google Scholar]
- 21. Arbeille P, Maulik D, Fignon A, et al. Assessment of the fetal pO2 changes by cerebral and umbilical Doppler on lamb fetuses during acute hypoxia. Ultrasound Med Biol. 1995;21:861–870. [DOI] [PubMed] [Google Scholar]
- 22. Chang TC, Robson SC, Spencer JA, Gallivan S. Prediction of perinatal morbidity at term in small fetuses: comparison of fetal growth and Doppler ultrasound. Br J Obstet Gynaecol. 1994;101:422–427. [DOI] [PubMed] [Google Scholar]
- 23. Figueras F, Caradeux J, Crispi F, Eixarch E, Peguero A, Gratacos E. Diagnosis and surveillance of late‐onset fetal growth restriction. Am J Obstet Gynecol. 2018;218:S790–S802. [DOI] [PubMed] [Google Scholar]
- 24. Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. Am J Obstet Gynecol. 1989;161:1055–1060. [DOI] [PubMed] [Google Scholar]
- 25. Cruz‐Martinez R, Figueras F, Hernandez‐Andrade E, Oros D, Gratacos E. Fetal brain Doppler predict cesarean delivery for nonreassuring fetal status in term small‐for‐gestational‐age fetuses. Obstet Gynecol. 2011;117:618–626. [DOI] [PubMed] [Google Scholar]
- 26. Bligh LN, Alsolai A. Cerebroplacental ratio thresholds measured within 2 weeks before birth and risk of Cesarean section for intrapartum fetal compromise and adverse neonatal outcome. Ultrasound Obstet Gynecol. 2018;52:340–346. [DOI] [PubMed] [Google Scholar]
- 27. Khalil A, Morales‐Rosello J, Morlando M, Hannan H, et al. Is fetal cerebroplacental ratio an independent predictor of intrapartum fetal compromise and neonatal unit admission? Am J Obstet Gynecol. 2015;45:156–161. [DOI] [PubMed] [Google Scholar]
- 28. Khalil A, Morales‐Roselló J, Townsend R, et al. Value of third‐trimester cerebroplacental ratio and uterine artery Doppler indices as predictors of stillbirth and perinatal loss. Ultrasound Obstet Gynecol. 2016;47:74–80. [DOI] [PubMed] [Google Scholar]
- 29. Monaghan C, Binder J, Thilaganathan B, Morales‐Roselló J, Khalil A. Perinatal loss at term: role of uteroplacental and fetal Doppler assessment. Ultrasound Obstet Gynecol. 2018;52:72–77. [DOI] [PubMed] [Google Scholar]
- 30. Bakalis S, Akolekar R, Gallo DM, Poon LC, Nicolaides KH. Umbilical and fetal middle cerebral artery Doppler at 30–34 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2015;45:409–420. [DOI] [PubMed] [Google Scholar]
- 31. Bahado‐Singh RO, Kovanci E, Jeffres A, Oz U, Deren O, Copel J, et al. The Doppler cerebroplacental ratio and perinatal outcome in intrauterine growth restriction. Am J Obstet Gynecol. 1999;180:750–756. [DOI] [PubMed] [Google Scholar]
- 32. Vollgraff Heidweiller‐Schreurs CA, Korevaar DA, Mol BWJ, et al. Publication bias may exist among prognostic accuracy studies of middle cerebral artery Doppler ultrasound. J Clin Epidemiol. 2019;116:1–8. [DOI] [PubMed] [Google Scholar]
- 33. Akolekar R, Syngelaki A, Gallo DM, Poon LC, Nicolaides KH. Umbilical and fetal middle cerebral artery Doppler at 35–37 weeks' gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet Gynecol. 2015;46:82–92. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Simon R, Figueras F, Savchev S, Fabre E, Gratacos E, Oros D. Cervical condition and fetal cerebral Doppler as determinants of adverse perinatal outcome after labor induction for late‐onset small‐for‐gestational‐age fetuses. Ultrasound Obstet Gynecol. 2015;46:713–717. [DOI] [PubMed] [Google Scholar]
- 35. Vergani P, Andreotti C, Roncaglia N, et al. Doppler predictors of adverse neonatal outcome in the growth restricted fetus at 34 weeks' gestation or beyond. Am J Obstet Gynecol. 2003;189:1007–1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


