Abstract
Background
Treatment‐resistant depression (TRD) is a debilitating chronic mental illness that confers increased morbidity and mortality, decreases the quality of life, impairs occupational, social, and offspring development, and translates into increased costs on the healthcare system. The goal of this study is to reach an agreement on the concept, definition, staging model, and assessment of TRD.
Methods
This study involved a review of the literature and a modified Delphi process for consensus agreement. The Appraisal of Guidelines for Research & Evaluation II guidelines were followed for the literature appraisal. Literature was assessed for quality and strength of evidence using the grading, assessment, development, and evaluations system. Canadian national experts in depression were invited for the modified Delphi process based on their prior clinical and research expertize. Survey items were considered to have reached a consensus if 80% or more of the experts supported the statement.
Results
Fourteen Canadian experts were recruited for three rounds of surveys to reach a consensus on a total of 27 items. Experts agreed that a dimensional definition for treatment resistance was a useful concept to describe the heterogeneity of this illness. The use of staging models and clinical scales was recommended in evaluating depression. Risk factors and comorbidities were identified as potential predictors for treatment resistance.
Conclusions
TRD is a meaningful concept both for clinical practice and research. An operational definition for TRD will allow for opportunities to improve the validity of predictors and therapeutic options for these patients.
Keywords: Canada, comorbidity, consensus, depression, depressive disorder, major depressive disorder, risk factors, treatment resistant
1. INTRODUCTION
Depressive disorders constitute one of the most common disabling diseases worldwide with an estimated prevalence of 264 million globally (Disease, and Injury Incidence and Prevalence Collaborators, 2018). In the Canadian population, depression has a lifetime prevalence of 9.9% (Patten et al., 2015) with an estimated economic cost of 12 billion dollars a year (Tanner et al., 2020). Among those who receive first‐line treatment for depression, approximately 50% do not achieve remission and about two‐thirds require further sequential treatment trials to achieve remission (Ferrari et al., 2013; Rush, Trivedi, Wisniewski, Stewart, et al., 2006). This disease burden confers increased morbidity and mortality, decreases the quality of life (Alonso et al., 2011), impairs occupational, social, and offspring development (Greenberg et al., 2015; Ishak et al., 2013; Lim et al., 2008), and translates into increased costs on the health‐care system (Coiro et al., 2012; Weissman et al., 2014). For both patients living with depression that does not remit despite multiple treatment trials, and clinicians providing treatment, consensus on the definition, and treatment protocols for treatment‐resistant depression (TRD) are needed.
There is a lack of consensus on the definition of TRD. Broadly conceptualized as partial or no response to adequate evidence‐based trials of treatment, TRD has a prevalence of up to 60% of the depression cohort (Berman et al., 1997). Treatment resistance may be conceptualized to include characteristics, such as chronic and persistent symptoms of low mood, repeated depressive episodes, or poor response to medications or other therapies. The challenges of defining TRD include the heterogeneity of the syndrome, lack of standardization of criteria for an “adequate trial,” and whether to include nonpharmacological treatments, resulting in a lack of consensus and validity of definition. The most utilized definition in the last 2 decades with support from the largest naturalistic trial (sequenced treatment alternatives to relieve depression [STAR*D]) is the lack of response after two antidepressant treatment trials (Berman et al., 1997). Several countries have suggested an internationally accepted TRD definition (Vaccarino & Kennedy, 2020), but unanimity has been elusive (Fava, 2003).
Having a consensus for the definition of TRD could improve and standardize care by indicating when a treatment resistance algorithm should start. No current definition provides sufficient detail for the accurate triaging and staging of patients in clinical settings or proper assessment for the selection of research participants. Therefore, since the late 1990s, researchers have proposed more detailed staging models intended to serve both clinical and research needs. The lack of a universally accepted staging model or a consensus definition for TRD creates ongoing barriers for patients to receive the appropriate care based on the severity, chronicity, and treatment response.
The goal of this study is to reach an agreement on the concept, definition, staging model, and assessment of TRD. Creating an optimal definition for TRD will help improve research to address patient needs, better define this heterogeneous population, and allow clinicians to communicate using the same terminology. Operationally, this would allow more personalized care to match patients with the right treatment in a timely manner. Continued work on a more refined TRD definition and criteria can improve the comparability of research studies, enable international collaborations on the subject, and improve treatment outcomes in major depressive disorder (MDD).
2. METHODS
This study involved two phases: (1) a literature review and (2) a modified Delphi process to achieve expert consensus.
Phase 1 involved an evidence‐based literature review of TRD by a working group from Western University. This provided the groundwork for the development of initial questions and statements for sharing with expert panel members. The Appraisal of Guidelines for Research & Evaluation II guidelines were followed throughout the literature appraisal process (Hoffmann‐Esser et al., 2017). An experienced information specialist assisted in the development of the literature search strategy. Initially, a literature search was conducted for English‐language materials published between January 1, 1998 and March 31, 2020 using MedLine, PsycINFO, and Cochrane databases. Keywords included treatment‐resistant depression, refractory depression, difficult to treat depression, treatment, therapeutics, risk factor, and diagnostic tools. Due to a large number of publications, the search was subsequently limited to publications since January 1, 2013, as systematic reviews and meta‐analyses have been synthesized regarding TRD before that date (Greden et al., 2011; Kasper & Montgomery, 2013).
Study selection of the most relevant sources was conducted separately by two reviewers to reduce the possibility of omission of relevant publications (Yuri E. Rybak and Ka S. P. Lai). This review was focused and selective rather than a systematic review, and the authors decided to include or exclude papers based on the pertinence of the information it contained. Literature was combined based on themes relevant to treatment resistance including definition, risk factors, and diagnostic tools (Supporting Information 1). These reviewers then independently assessed each study for the quality and strength of evidence using the grading, assessment, development, and evaluation (GRADE) system (Guyatt et al., 2011). The GRADE system provides a systematic approach to review literature based on a guideline developed by researchers worldwide. The GRADE system rates evidence based on five criteria: risk of bias; publication bias; imprecision; inconsistency; and indirectness. Each criterion can be rated as having an unlikely risk of bias to a high risk of bias. The process of quality of evidence assessment and risk of bias is provided in Supporting Information 2. On the basis of five criteria, an overall rating for the quality of evidence was assigned.
GRADE suggests that randomized controlled trials start as high‐quality evidence and observational studies as low‐quality evidence, and in accordance with the GRADE criteria, each article is rated as providing a weak, moderate, or high certainty of evidence (Table 1) (Burns et al., 2011; Schünemann et al., 2013). The GRADE system is a widely accepted tool used in the process of building recommendations and answering clinical questions with recommendations. A third reviewer (Amer M. Burhan) resolved disagreements in ratings between the two reviewers to assist in reaching a consensus.
Table 1.
Level and grades of evidence based on the grading, assessment, development, and evaluation system (Burns et al., 2011; Schünemann et al., 2013)
| Grade | Definition |
| High | We are very confident that the true effect is close to that of the estimate of the effect |
| Moderate | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different |
| Low | Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect |
| Very Low | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect |
Once the Phase 1 literature review was completed, the recruitment of experts for Phase 2 began. Experts were approached via recruitment emails. Experts were selected based on the following criteria: currently, a consultant psychiatrist in a Canadian academic center as an expert in mood disorders, currently an academic expert contributing in the field of mood disorders, and agreement to participate in the Delphi method. To be considered as an academic expert, the individual must have met at least one of the following criteria: involvement in one or more international TRD organizations, authorship on one or more articles in mood disorders in a peer‐reviewed journal during the preceding 2 years, or involved in responsibilities related to the provision of teaching in mood disorders for over 1 year.
Overall, 18 national experts from seven Canadian universities (University of British Columbia, University of Calgary, McGill University, University of Ottawa, Queen's University, University of Toronto, and Western University) were selected. Of the 18 experts contacted, 14 agreed to participate in the consensus‐building process. Two initial meetings were held to discuss the need for a definition of TRD, the current gaps in the literature, and the consensus process. We used the modified Delphi methodology to generate consensus via an iterative process until the agreement was reached (Figure 1). In contrast to the traditional Delphi methodology, a modified Delphi methodology was adopted allowing for the creation of initial questions and statements based on current knowledge and literature. This facilitated the more efficient development of subsequent statements and recommendations.
Figure 1.
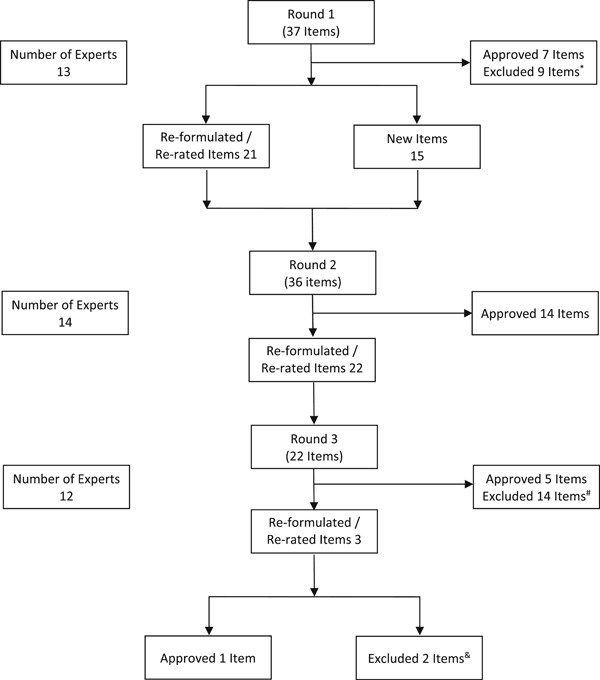
Modified Delphi methodology. Three rounds of surveys were conducted to reach a consensus on a total of 27 items. Items that were reformulated may have been summarized into a single item or reworded as multiple items for improved clarity. *Grip strength, neuroticism, personality traits, social inhibition, body mass index, age, height, and biomarkers questions were excluded given low approval ratings by experts and lack of current literature support. HAM‐D‐24 was excluded given preference for the HAM‐D‐17. #Items #2–4 (melancholic features, atypical features, number of stressful life events), #6–14 (various depression and comorbidity scales), #18 (having both dichotomous and dimensional definitions), and #22 (definition to include psychotherapy) were excluded given low approval ratings by experts. &Items #2 and #3 (recommendation of a clinical staging tool) were excluded given low approval ratings by experts. HAM‐D, Hamilton depression rating scale
Experts communicated their findings via Survey Monkey (https://surveymonkey.com/). Please see Supporting Information 3 for full surveys distributed. Items were considered to have reached a consensus if 80% or more of the experts rated the item as “agree” or “strongly agree.” Statements that reached consensus in one round were not represented in the subsequent round. Where survey items achieved less than 80% of the agreement as “agree” or “strongly agree,” these items were either rephrased for the next round or omitted depending on the comments from the experts. Data were ultimately synthesized for narrative review.
3. RESULTS/DISCUSSION
Results are presented as a logical sequence of essential questions and answers to assist with clinical and research utility. Each item contains a body of evidence‐based literature review and the experts’ consensus opinions. The consensus results are complemented by the experts’ comments. Table 2 presents the summary of the 27 items which reached consensus from this group of experts.
Table 2.
Summary of statements from the modified Delphi methodology
| Statement | Level of Agreement |
|---|---|
| Treatment‐resistant depression (TRD) as a categorical or dimensional definition | |
| A definition for TRD is currently being used in clinical practice by the expert. | 85% |
| TRD is a meaningful concept in clinical practice. | 92% |
| An operational definition for TRD is needed for clinicians and researchers. | 100% |
| TRD should be defined in a dimensional sense only | 92% |
| Definition of the initial stage of TRD | |
| TRD should be defined by the failure of two adequately dosed and evidence supported trials of antidepressant medications. | 83% |
| Nonresponse in TRD should be defined as less than 50% reduction in symptom severity | 83% |
| The minimum length of the antidepressant trial should be 4–6 weeks | 83% |
| Evaluation | |
| HAM‐D‐17 should be used in the ongoing assessment of depressive symptoms | 83% |
| Risk factors and comorbidities | |
| Anxiety comorbidity | 93% |
| Psychotic features | 93% |
| A higher number of lifetime depressive episodes | 100% |
| Partial remission | 92% |
| Number of lifetime episodes | 93% |
| Number of previous antidepressant trials | 92% |
| Number of previous augmentation agents | 86% |
| Previously failed psychotherapy | 100% |
| Previous failed ECT | 93% |
| The long duration of illness | 85% |
| Symptom severity of the current episode | 100% |
| Greater number of hospitalizations | 92% |
| Prevalence of comorbidities (psychiatric and medical) | 92% |
| Comorbid personality disorder | 92% |
| Comorbid medical illness | 86% |
| Comorbid substance use | 100% |
| Bipolarity features | 92% |
| Current psychosocial stressors | 93% |
| Assessment/staging models | |
| TRD staging models have clinical relevance in both clinical practice and research | 93% |
| The DM‐TRD is recommended as a clinical staging tool in TRD | 72% |
| The Maudsley staging model is recommended as a clinical staging tool in TRD | 45% |
Note: Statements were considered to have reached consensus if 80% or more of the experts rated the item as “agree” or “strongly agree.”
Abbreviations: DM‐TRD, Dutch measure for quantification of treatment resistance in depression; ECT, electroconvulsive therapy; HAM‐D‐17, Hamilton depression rating scale (17‐items).
3.1. Is TRD a useful concept?
The concept of TRD captures clinical reality since it reflects a large clinical population with a common clinical outcome. It is not a new concept as the term has been utilized by the mental health community since the 1970s. Historically, it has survived over more than 4 decades and has periodically attracted more attention every time a new potentially effective treatment emerges.
As with any concept, however, TRD does not perfectly fit the clinical variability of real‐life cases. Each of the two major components of the concept, response and treatment, has inherent limitations when it comes to individual responses. For instance, the line between response and nonresponse is clouded by partial responders, fully refractory cases and those with very different sets of symptoms and heterogeneity of treatment resistance (Malhi & Byrow, 2016). The treatment component is often characterized as treatment adequacy and may be difficult to assess due to the variability of treatment options, the match between treatment and sets of symptoms, or the presence of comorbid conditions and psychosocial factors.
Despite the imprecision of the concept of TRD, the expert panel reiterated its usefulness both for clinical practice and research. In the first round of the survey, 92% felt that TRD is a meaningful concept and 85% of respondents use a TRD definition in their clinical practice. All (100%) respondents indicated that an operational definition of TRD was needed. A definition for TRD helps clinicians to become aware of the persistence of depression in individual cases in a timely fashion and to foresee potential treatment challenges and consider more effective interventions.
3.2. A dichotomous versus dimensional approach to TRD?
Although TRD has been historically defined as a dichotomy (i.e., categorically), this definition has been criticized as not representing clinical reality, as was shown in antidepressant treatment switch trials including STAR*D. (Rush, Kraemer, et al., 2006; Rush, Trivedi, Wisniewski, Stewart, et al., 2006) Nevertheless, the dichotomous definition aids in identifying and differentiating responders from those with less severe forms of treatment resistance. In this capacity, it has been used in research and practice guidelines for several decades (Malhi & Byrow, 2016; Murphy et al., 2017; Pérez‐Wehbe et al., 2014). Panelists’ indicated that the dichotomous definition may be more suited to an initial stage of TRD. Experts favored using only the dimensional concept to delineate the progression or severity of TRD (92%).
The continuous approach (referred to from here on as dimensional) utilizes a staging model as a tool. It was proposed as a more refined transitional diagnostic approach with the potential to improve the logic and timing of intervention (McGorry & Hickie, 2019). The opinion was that with advancing treatment resistance, it becomes increasingly obvious that treatment is not a one‐size‐fits‐all concept. On the basis of unanimous expert opinion, TRD staging models have relevance in both clinical practice and research. A scoring system can define clarify heterogeneity within the very diverse TRD population. The experts accommodated both concepts favoring the dichotomy concept to define initial treatment resistance and the dimensional concept to evaluate the stages of progression in TRD.
3.3. What is the operational definition for initial treatment resistance?
On the basis of the most recent systematic review, no agreed‐upon dichotomous definition exists for TRD (GRADE‐high) (Gaynes et al., 2018). Although experts often agree on a requirement for two treatment failures, they do not agree on the definition for the adequacy of either dose or duration or outcome measures. Definitions do not regularly incorporate failure with regard to augmentation agents (Vaccarino & Kennedy, 2020). Besides differences in criteria, none of the definitions of TRD have been systematically examined for reliability and predictive value utility (GRADE‐high; Gaynes et al., 2018; Souery et al., 2006). In reviewing the STAR*D reports, one can clearly see that the crucial point at which the risk of treatment resistance markedly increased was after two treatment failures (Rush, Trivedi, Wisniewski, Nierenberg, et al., 2006).
Recent reviews usually define failure to respond as “lack of decrease in depressive severity of at least 50%” (GRADE‐high) (Gaynes et al., 2018). The report from the European Group for the Study of Resistant Depression identifies resistance as less than 50% symptom reduction (Bartova et al., 2019). Although reports do not consistently agree on a definition of adequate dose and duration, the minimum duration cited is typically 4 weeks (Gaynes et al., 2018), and the range of 4–12 weeks (Ng et al., 2019). This expert panel proposes the following criteria for initial treatment resistance (Table 3).
Table 3.
Operational definition suggested for the initial stage of treatment‐resistant depression (TRD)
| Operational criteria | Quality of evidence | Level of agreement |
|---|---|---|
| TRD should be defined by the failure to achieve response to two adequately dosed and evidence supported trials of antidepressant medications | GRADE‐high | 83%, 10/12 |
| Nonresponse should be defined as less than 50% symptom reduction | GRADE‐high | 83%, 10/12 |
| The minimal length of antidepressants trial should be defined as 4–6 weeks | GRADE‐high | 83%, 10/12 |
Abbreviation: GRADE, grading, assessment, development, and evaluation.
The treatment failure component of the definition of TRD revealed a diversity of opinions among experts based on conflicting data on the effectiveness of switching antidepressants and the benefits of using antidepressants from different groups in managing TRD. There exists mixed evidence regarding the advantages of prescribing medications from the same class or different classes and therefore this study deliberately did not explore this (Bschor et al., 2018; Carvalho et al., 2014). The other area of consideration is how the failure of an antidepressant trial is defined. On one hand, failure of two antidepressants can mean nonresponse during the initiation of treatment, but the failure of an antidepressant may also occur during the maintenance phase of treatment with the relapse of a depressive episode following a remission despite being on adequate doses of medication. The mechanism of tachyphylaxis remains unclear and continues to be under investigation (Targum, 2014). Given the multifactorial etiology for medication failures, these considerations further warrant a dimensional approach to define TRD and clarify the heterogeneity of this population.
3.4. Should other treatments such as psychotherapy be included in the initial definition of TRD?
Historically, definitions of TRD do not incorporate psychotherapy. Even though there is robust evidence for treatments such as cognitive‐behavioral therapy and interpersonal therapy in mild‐to‐moderate depression, definitions of TRD have primarily a pharmacological basis and (Cuijpers et al., 2016; Parikh et al., 2016; Ruhe et al., 2012) this omission is repeatedly highlighted as significant (Conway et al., 2017; Gronemann et al., 2018).
Evidence‐based psychological treatments are widely accepted as essential components of initial treatment for depression in most reputable national guidelines, such as the Canadian Mood and Anxiety Treatment (Lam et al., 2016), National Institute for Health and Care Excellence (National Collaborating Centre for Mental Health, 2010), American Psychological Association (Gelenberg et al., 2010), and the Royal Australian and New Zealand College of Psychiatrists (Malhi et al., 2015). In the most recently published Health Quality Ontario Standards, after initial diagnosis is made, treatment requires the following: people with major depression have timely access to either antidepressant medication or evidence‐based psychotherapy based on their preference (Health Quality Ontario, 2018). People with severe or persistent depression should be offered a combination of both treatments. Failure to respond to psychotherapy has been included in TRD definitions in the past (Wijeratne & Sachdev, 2008) and one of the more recent proposed definitions of TRD (Conway et al., 2017).
Despite this, only 54% of experts supported the statement “TRD should be defined by failure of two adequately dosed and evidence supported trials of antidepressant medications and one course of evidence‐based psychotherapy.” However, failure to respond to psychotherapy was strongly recommended as a risk factor and indicator for the severity of treatment resistance (100%). The importance of psychotherapy at the early stage of depression was not disputed, but psychotherapy failure was not included mainly for practicality and feasibility. One of the main reasons outlined by the experts was difficulty standardizing this form of treatment. Although the standard has been well developed in research trials, in practice many factors, such as therapist training, expertize, therapeutic rapport, and patients’ readiness for change may be difficult to capture. The other barrier is accessibility as psychotherapy is not always available. Overall, this study emphasizes the importance of the role of psychotherapy in the comprehensive assessment of treatment resistance.
3.5. How should an evaluation of advanced resistance and dimensional definition be conducted?
The principle of clinical staging for depression was first proposed by Sackeim et al. (1990) who introduced the antidepressant treatment history form which was further developed by Sackeim et al. (1990) and Thase and Rush (1997). Since initial development, a number of scales have been developed including the Antidepressant Treatment Response Questionnaire, a user‐friendly tool for assessment of prior antidepressant trials (Chandler et al., 2010; Desseilles et al., 2011). More recent staging models such as the Maudsley staging model (MSM) and the Dutch measure for quantification of treatment resistance in depression (DM‐TRD) are among the most evidence‐based and comprehensive in 2020. Both staging models are promising clinician‐rated prediction tools for the expected course of depression in a broad population of patients (GRADE‐moderate) (van Belkum et al., 2018; Fekadu et al., 2009).
There are many practical benefits to using staging models as it leads to more refined, personalized, and extended diagnoses to outline an accurate treatment plan (Ruhe et al., 2012). Once completed, a staging model serves as a concise source of valuable longitudinal information that assists busy clinicians to make better‐informed choices of the next treatment option. Thus, the inclusion of staging models has the potential to improve communication among physicians during the referral process and help match the correct treatment for the patient, whether it be antidepressant medication, augmentation, psychotherapy, ketamine, therapeutic brain stimulation, or other treatments.
In the second round of the Delphi process, 93% of the expert panelists feel that TRD staging models have relevance in both clinical practice and research. The DM‐TRD is more comprehensive and includes ratings for functional impairment, psychosocial stressors, and comorbidities. The DM‐TRD has been found to outperform the MSM in its ability to predict future depressive symptomatology (GRADE‐high; van Dijk et al., 2019; Peeters et al., 2016). On the basis of a naturalistic study, the DM‐TRD has also predicted the Beck depression inventory (BDI) scores, the severity of future depressive symptoms, and the degree of remission at week 16 (GRADE‐high) (Peeters et al., 2016).
Both staging models were supported by the panel with preference given to DM‐TRD (75%) over MSM (50%). Apart from higher predictive capacity, the inclusion of psychotherapy trial failure was another advantage of the DM‐TRD that the panel valued. This was a logical step, given unanimous approval of previously failed psychotherapy trials as an important risk factor for TRD. Furthermore, the inclusion of functional disability and comorbidities are additional benefits for a comprehensive evaluation. The expert panelists expressed concerns that the DM‐TRD takes more time to complete, although when completed during history taking or for a familiar patient, it may take only 5 min. Thus, the use of an objective staging tool is recommended to rating the severity and extent of treatment resistance.
3.6. What clinical risk factors predispose an individual to TRD?
Evidence about what risk factors are associated with a TRD diagnosis is remarkably limited (GRADE‐high) (Bennabi et al., 2015; Gaynes et al., 2018). Clinical factors have been found to predict increasing odds of developing TRD including comorbidities such as generalized anxiety disorder, panic disorder, and psychosis (GRADE‐moderate; Kautzky et al., 2019). Comorbid anxiety disorders have been replicated as one of the most prominent risk factors for TRD (GRADE‐moderate; Cepeda et al., 2018; Kautzky et al., 2019; Souery et al., 2006) and associations for a poorer response, although no clear association on remission rates (GRADE‐moderate; De Carlo et al., 2016). Consistent with the literature, this panel almost unanimously agreed with the increased risk of TRD in the presence of both anxiety (93%) and psychotic features (93%) (Table 4).
Table 4.
Risk factors for the development of treatment‐resistant depression
| Clinical risk factor | Quality of evidence | Level of agreement |
|---|---|---|
| Symptom severity of the current episode | GRADE‐high | 100%, 14/14 |
| Frequent and recurrent depressive episode | GRADE‐high | 100%, 14/14 |
| The long duration of illness | GRADE‐moderate | 85%, 11/13 |
| Current psychosocial stressors | GRADE‐moderate | 93%, 13/14 |
| Number of lifetime episodes | GRADE‐moderate | 93%, 13/14 |
| Bipolarity features (e.g., family history) | GRADE‐moderate | 92%, 12/13 |
| Current psychosocial stressors | GRADE‐moderate | 93%, 13/14 |
| Stressful life events or trauma | GRADE‐moderate | 85%, 11/13 |
| Comorbidity | Level of evidence | Level of agreement |
| Anxiety | GRADE‐moderate | 93%, 13/14 |
| Psychotic features | GRADE‐moderate | 93%, 13/14 |
| Personality disorders | GRADE‐moderate | 92%, 12/13 |
| Substance use disorders | GRADE‐moderate | 100%, 14/14 |
| Comorbid medical illness | GRADE‐moderate | 86%, 12/14 |
| Treatment factors | Level of evidence | Level of agreement |
| Number of previous antidepressant trials | GRADE‐moderate | 92%, 12/13 |
| Number of previous augmentation agents | GRADE‐moderate | 86%, 12/14 |
| Failed psychotherapy trials | GRADE‐moderate | 100%, 11/11 |
| Previously failed trial of electroconvulsive therapy | GRADE‐moderate | 93%, 13/14 |
| Greater number of hospitalizations | GRADE‐moderate | 92%, 12/13 |
Note: Considerations include clinical risk factors, comorbidities, and treatment risk factors.
Abbreviation: GRADE, grading, assessment, development, and evaluation.
Inconsistent evidence has been found for subtypes of major depression (GRADE‐moderate; De Carlo et al., 2016; Gronemann et al., 2018). Melancholic features have been found in the literature to increase the likelihood of both nonresponse and non‐remission (GRADE‐moderate; De Carlo et al., 2016; Souery et al., 2006). Expert panelists have commented that subtypes should remain as part of an assessment for TRD as melancholic features may predict TRD and atypical features may suggest bipolarity. However, the expert panelists did not reach a consensus in finding certain subtypes of depression to be risk factors in the development of TRD (50% for melancholic and 42% for atypical features). Experts have suggested that despite the evidence, patients with these subtypes may present with multiple subtypes together (e.g., have both melancholic and atypical features), and patients may not rate as severely depressed using objective scales such as the Hamilton depression rating scale.
Convincing evidence emerged on the following group of factors reflecting the severity of current depressive symptoms. Higher baseline symptom severity is one of the strongest predictors of poor response (Gronemann et al., 2018; Nuñez et al., 2018; Peeters et al., 2016) and lower remission rates (GRADE‐high; Bartova et al., 2019; De Carlo et al., 2016). The longer duration of the current episode has also been found to increase the risk of TRD (GRADE‐moderate; Kautzky et al., 2019). The expert panel reached consensus with at least 85% on all of the above factors: Higher baseline severity, lower remission rates, and longer duration of the current episode.
When there is clinical suspicion for bipolarity in unipolar depression, literature has suggested the possibility of a more severe course of illness with higher symptom severity, suicidality, and poor response to antidepressants (Rybakowski, 2012; Serretti & Fabbri, 2013; Souery et al., 2012). There is evidence that patients with TRD have high rates of undiagnosed or hidden bipolar disorder (Correa et al., 2010). Therefore, clinicians are encouraged to be vigilant in screening for bipolarity using validated rating scales and to obtain a collateral history from informants. Features of bipolarity (e.g., family history of bipolar disorder) as a risk factor received approval by the panel at 92% level.
Evidence has been found for the group of factors reflecting the course of depression. A higher number of lifetime depressive episodes was replicated as one of the most prominent risk factors for TRD (GRADE‐moderate; Kautzky et al., 2019; Souery et al., 2006). A greater number of prior antidepressant trials also increased the risk of TRD (GRADE‐moderate; Kautzky et al., 2019). A greater number of hospitalizations was associated with a lower response rate (De Carlo et al., 2016). Even if the patient had more than one hospitalization, there were associations with poorer outcomes and nonresponse to at least two antidepressants (Souery et al., 2006). These factors are consistent with other clinical factors such as the longer duration of a current depressive episode. In addition, the number of previous augmentation trials as well as electroconvulsive therapy (ECT) failure is recommended to be included in the assessment of more advanced stages of resistance. However, clinicians must be mindful that it is possible for a patient to be falsely considered “treatment resistant” to ECT as there may have been the poor quality of ECT or inadequate electrical charge in relation to a climbing seizure threshold. Failure of evidence‐based psychotherapy trials as a risk factor was unanimously approved by the panel. The expert panel reached a consensus with at least 85% approval on all of the above factors.
Comorbid personality disorders have also been associated with decreased response rates in some studies (Fava et al., 1996; Takahashi et al., 2013) and decreased remission rates (GRADE‐moderate). Substance abuse has been associated with a poorer response to antidepressant treatment (Bennabi et al., 2015; van Dijk et al., 2019). As personality and substance use disorders may often masquerade as depression, a careful longitudinal history is important to establish accurate psychiatric diagnoses. The expert panel recommended that personality and substance use disorders should be strongly considered as risk factors (consensus was above 90%).
Unexpectedly, medical comorbidities as a risk factor showed no clear evidence that it is associated with decreased response rates either (De Carlo et al., 2016; Fava et al., 1996). Nevertheless, medical comorbidities may play a significant role in poor recovery and certainly should be considered individually (Bennabi et al., 2015). Thus, it was endorsed by the panel as a significant risk factor (86%).
Often omitted in TRD literature, stressful life events and traumatic experiences are now gaining more attention. Childhood adversity has recently been claimed the single biggest contributor to psychiatric disorders (Lippard & Nemeroff, 2020), but evidence of its role in TRD is notably still lacking. In one of the most recent studies, the inclusion of childhood adversity as a factor did not improve a model's predictive value (van Dijk et al., 2019). The statement was formulated for the experts using a general term as “stressful life events or trauma” which received strong support among the experts as a risk factor for the development of TRD (85%). Current stressful life events as a risk factor for the development of TRD was also approved by 93% of the panel. Notably, these two and medical comorbidities are the only risk factors that we have decided to include in the consensus process despite conflicting research evidence.
3.7. Are there any evidence‐based clinical scales for the assessment of depressive symptoms in TRD?
Clinical scales have been recommended to objectively track treatment response. The most commonly used measures of symptom severity for depression are the Hamilton depression rating scale (HAM‐D) (Hamilton, 1960) and Montgomery–Asberg depression rating scale (MADRS) (Montgomery & Asberg, 1979), and the preferred outcome measures in TRD are the HAM‐D and clinical global impressions (GRADE‐high; Gaynes et al., 2018). Overall, the HAM‐D is the most frequently used tool by research, with the HAM‐D‐17 the most commonly applied. Regardless of the instrument used, the preferred outcome measure in studies is remission of depressive symptoms using a standardized and validated instrument.
Among this group of experts, as a clinician‐rated tool, the HAM‐D‐17 is recommended as a scale for ongoing assessment of TRD (91%). Experts find that the HAM‐D‐17 is reliable for comparisons with previous studies and most practical. As a self‐report scale, the patient health questionnaire‐9 is simple and easy to administer in clinical practice, although maybe best used as a screening tool (Kroenke et al., 2001). Other scales such as the MADRS or BDI‐II may not be as familiar to clinicians (Beck et al., 1996). It was agreed that regardless of the scale used, it is important to have a measure of symptom severity.
3.8. Are there any evidence‐based clinical scales for the assessment of psychiatric comorbidities in the setting of TRD?
Few researchers have investigated scales to specifically assess psychiatric comorbidities in individuals with MDD. As such, there is no consensus regarding which screening instruments to use in the setting of TRD to assess for comorbidities, but rather to rely on clinical judgment. As with any screening instrument, concerns are related to the length of the instrument and thus practicality. Overall, the literature suggests that the Mini International Neuropsychiatric Interview remains a thorough tool to review diagnostic considerations in depression such as bipolar disorder, anxiety disorders, and posttraumatic stress disorder while being shorter and less tedious than the structured clinical interview for the DSM (First et al., 1997; Sheehan et al., 1998). However, only 58% of the experts endorsed using this tool in the initial assessment of TRD.
One can look to specific disorders to find a screening tool that best fits that diagnosis. At this time, there are no specific assessment tools that are specifically recommended by this group of experts to be used in TRD. However, as with any disorder, the use of an objective instrument can be valuable as a measure of symptom severity if that specific comorbid diagnosis is present. Thus, we would recommend using an objective instrument to track symptom severity of comorbid psychiatric disorders in the setting of TRD.
Of note, rephrasing the statement for endorsement was done between Rounds 2 and 3. Experts were more amenable to the endorsement of the scale if it were to be used as part of the assessment for TRD. In Round 3, it was asked whether the scale would be useful as part of an “initial” assessment and experts were less favorable in terms of recommending its use. Thus, these factors may reflect general concerns regarding the length of time to administer these scales. It was felt that scales are useful in objective tracking of symptomatology only if the comorbid diagnosis is present and the clinician can select the specific scales which are relevant in that patient.
4. CONCLUSION AND FUTURE DIRECTIONS
TRD is a meaningful concept both for clinical practice and research. However, the lack of consistency in the definition and assessment of TRD are major barriers to progress in clinical research and treatment of resistant depression. Consensus‐based concepts, definitions, and evaluations are needed to standardize the operational definition of TRD. Only with an operational definition and classification of TRD, the severity will patients be able to be appropriately triaged for appropriate treatment strategies.
There are many opportunities to utilize the concept of TRD and improve the validity of predictors and therapeutic options for TRD. Biological markers may help to integrate the overlapping areas of treatment resistance, chronicity, duration of illness, and illness progression. As the access to novel neurostimulation strategies is made available, these treatment modalities can be considered as part of the staging and prognostication of TRD. For example, at the time of this study, access to repetitive transcranial magnetic stimulation (rTMS) is currently restricted in Canada, as only 3 (Alberta, Quebec, and Saskatchewan) out of 10 provinces have publicly funded rTMS availability.
Future empirical studies should address the inconsistencies between current evidence‐ and expert‐based guidelines in TRD. For example, the current literature suggests that a history of trauma may not necessarily be predictive of TRD, although empirically one can hypothesize that severe trauma could predispose a patient to treatment resistance. Therefore, studies may wish to study the role of patient resiliency as a protective factor or explore how trauma‐informed interventions may help patients process these life experiences.
As novel treatment modalities are studied for TRD, this group of experts agreed that ongoing objective clinical scales should be used in tracking symptoms of depression and their comorbidities. With advances in mobile health technologies and applications, patient self‐reported outcome scales may be useful in tracking symptom change in real‐time to offer valuable information about the effectiveness of treatment strategies. It is hoped that having an operational definition for TRD will allow researchers to use common clinical terminology and allow for new treatments to be studied in the future for these patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supplementary information.
Supplementary information.
Supplementary information.
ACKNOWLEDGMENTS
The authors would like to thank Elizabeth Russel and Allison Fairbairn, information specialists, for their support in designing and performing the literature search for this study. They would also like to thank Dr. Rickinder Sethi for his assistance in the literature review and his assistance in the study. At last, they would like to thank Zafiris Jeffrey Daskalaskis for the participation in the modified Delphi process.
Rybak YE, Lai KSP, Ramasubbu R, et al. Treatment resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depression and Anxiety. 2021;38:456–467. 10.1002/da.23135
[Correction added on 25 February 2021, after first online publication: Dr. Fidel Vila‐Rodriguez was added as an author with the approval of all coauthors.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alonso, J. , Petukhova, M. , Vilagut, G. , Chatterji, S. , Heeringa, S. , Üstün, T. B. , Alhamzawi, A. O. , Viana, M. C. , Angermeyer, M. , Bromet, E. , Bruffaerts, R. , de Girolamo, G. , Florescu, S. , Gureje, O. , Haro, J. M. , Hinkov, H. , Hu, C. , Karam, E. G. , Kovess, V. , … Kessler, R. C. (2011). Days out of role due to common physical and mental conditions: Results from the WHO world mental health surveys. Molecular Psychiatry, 16(12), 1234–1246. 10.1038/mp.2010.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartova, L. , Dold, M. , Kautzky, A. , Fabbri, C. , Spies, M. , Serretti, A. , Souery, D. , Mendlewicz, J. , Zohar, J. , Montgomery, S. , Schosser, A. , & Kasper, S. (2019). Results of the European group for the study of resistant depression (GSRD)—Basis for further research and clinical practice. World Journal of Biological Psychiatry, 20(6), 427–448. 10.1080/15622975.2019.1635270 [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. (1996). Beck depression inventory–II, Psychological assessment. Psychological Corporation. [Google Scholar]
- van Belkum, S. M. , Geugies, H. , Lysen, T. S. , Cleare, A. J. , Peeters, F. P. M. L. , Penninx, B. W. J. H. , Schoevers, R. A. , & Ruhe, H. G. (2018). Validity of the Maudsley staging method in predicting treatment‐resistant depression outcome using the Netherlands study of depression and anxiety. Journal of Clinical Psychiatry, 79(1), 17m11475. 10.4088/JCP.17m11475 [DOI] [PubMed] [Google Scholar]
- Bennabi, D. , Aouizerate, B. , El‐Hage, W. , Doumy, O. , Moliere, F. , Courtet, P. , Nieto, I. , Bellivier, F. , Bubrovsky, M. , Vaiva, G. , Holztmann, J. , Bougerol, T. , Richieri, R. , Lancon, C. , Camus, V. , Saba, G. , Haesbaert, F. , d'Amato, T. , Charpeaud, T. , … Haffen, E. (2015). Risk factors for treatment resistance in unipolar depression: A systematic review. Journal of Affective Disorders, 171, 137–141. 10.1016/j.jad.2014.09.020 [DOI] [PubMed] [Google Scholar]
- Berman, R. M. , Narasimhan, M. , & Charney, D. S. (1997). Treatment‐refractory depression: Definitions and characteristics. Depression and Anxiety, 5(4), 154–164. [DOI] [PubMed] [Google Scholar]
- Bschor, T. , Kern, H. , Henssler, J. , & Baethge, C. (2018). Switching the antidepressant after nonresponse in adults with major depression: A systematic literature search and meta‐analysis. Journal of Clinical Psychiatry, 79(1), 11–18. 10.4088/JCP.16r10749 [DOI] [PubMed] [Google Scholar]
- Burns, P. B. , Rohrich, R. J. , & Chung, K. C. (2011). The levels of evidence and their role in evidence‐based medicine. Plastic and Reconstructive Surgery, 128(1), 305–310. 10.1097/PRS.0b013e318219c171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho, A. F. , Berk, M. , Hyphantis, T. N. , & McIntyre, R. S. (2014). The integrative management of treatment‐resistant depression: A comprehensive review and perspectives. Psychotherapy and Psychosomatics, 83(2), 70–88. 10.1159/000357500 [DOI] [PubMed] [Google Scholar]
- Cepeda, M. S. , Reps, J. , & Ryan, P. (2018). Finding factors that predict treatment‐resistant depression: Results of a cohort study. Depression and Anxiety, 35(7), 668–673. 10.1002/da.22774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, G. M. , Iosifescu, D. V. , Pollack, M. H. , Targum, S. D. , & Fava, M. (2010). RESEARCH: Validation of the Massachusetts General Hospital antidepressant treatment history questionnaire (ATRQ). CNS Neuroscience & Therapeutics, 16(5), 322–325. 10.1111/j.1755-5949.2009.00102.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiro, M. J. , Riley, A. , Broitman, M. , & Miranda, J. (2012). Effects on children of treating their mothers' depression: Results of a 12‐month follow‐up. Psychiatric Services, 63(4), 357–363. 10.1176/appi.ps.201100126 [DOI] [PubMed] [Google Scholar]
- Conway, C. R. , George, M. S. , & Sackeim, H. A. (2017). Toward an evidence‐based, operational definition of treatment‐resistant depression: When enough is enough. JAMA Psychiatry, 74(1), 9–10. 10.1001/jamapsychiatry.2016.2586 [DOI] [PubMed] [Google Scholar]
- Correa, R. , Akiskal, H. , Gilmer, W. , Nierenberg, A. A. , Trivedi, M. , & Zisook, S. (2010). Is unrecognized bipolar disorder a frequent contributor to apparent treatment resistant depression? Journal of Affective Disorders, 127(1‐3), 10–18. 10.1016/j.jad.2010.06.036 [DOI] [PubMed] [Google Scholar]
- Cuijpers, P. , Donker, T. , Weissman, M. M. , Ravitz, P. , & Cristea, I. A. (2016). Interpersonal psychotherapy for mental health problems: A comprehensive meta‐analysis. American Journal of Psychiatry, 173(7), 680–687. 10.1176/appi.ajp.2015.15091141 [DOI] [PubMed] [Google Scholar]
- De Carlo, V. , Calati, R. , & Serretti, A. (2016). Socio‐demographic and clinical predictors of non‐response/non‐remission in treatment resistant depressed patients: A systematic review. Psychiatry Research, 240, 421–430. 10.1016/j.psychres.2016.04.034 [DOI] [PubMed] [Google Scholar]
- Desseilles, M. , Witte, J. , Chang, T. E. , Iovieno, N. , Dording, C. M. , Ashih, H. , Nyer, M. , Freeman, M. P. , Fava, M. , & Mischoulon, D. (2011). Assessing the adequacy of past antidepressant trials: A clinician's guide to the antidepressant treatment response questionnaire. Journal of Clinical Psychiatry, 72(8), 1152–1154. 10.4088/JCP.11ac07225 [DOI] [PubMed] [Google Scholar]
- van Dijk, D. A. , van den Boogaard, Th M. , Deen, M. L. , Spijker, J. , Ruhe, H. G. , & Peeters, F. (2019). Predicting clinical course in major depressive disorder: The association between DM‐TRD score and symptom severity over time in 1115 outpatients. Depression and Anxiety, 36(4), 345–352. 10.1002/da.22865 [DOI] [PubMed] [Google Scholar]
- Disease and Injury Incidence and Prevalence Collaborators . (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: A systematic analysis for the global burden of disease study 2017. Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava, M. (2003). Diagnosis and definition of treatment‐resistant depression. Biological Psychiatry, 53(8), 649–659. 10.1016/s0006-3223(03)00231-2 [DOI] [PubMed] [Google Scholar]
- Fava, M. , Alpert, J. E. , Borus, J. S. , Nierenberg, A. A. , Pava, J. A. , & Rosenbaum, J. F. (1996). Patterns of personality disorder comorbidity in early‐onset versus late‐onset major depression. American Journal of Psychiatry, 153(10), 1308–1312. 10.1176/ajp.153.10.1308 [DOI] [PubMed] [Google Scholar]
- Fekadu, A. , Wooderson, S. , Donaldson, C. , Markopoulou, K. , Masterson, B. , Poon, L. , & Cleare, A. J. (2009). A multidimensional tool to quantify treatment resistance in depression: The Maudsley staging method. Journal of Clinical Psychiatry, 70(2), 177–184. 10.4088/jcp.08m04309 [DOI] [PubMed] [Google Scholar]
- Ferrari, A. J. , Charlson, F. J. , Norman, R. E. , Patten, S. B. , Freedman, G. , Murray, C. J. L. , Vos, T. , & Whiteford, H. A. (2013). Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Medicine, 10(11), e1001547. 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. , Gibbon, M. , Spitzer, R. L. , Benjamin, L. S. , & Williams, J. B. W. (1997). Structured clinical interview for DSM‐IV® axis II personality disorders SCID‐II. American Psychiatric Publishing. [Google Scholar]
- Gaynes, B. N. , Asher, G. , Gartlehner, G. , Hoffman, V. , Green, J. , Boland, E. , & Lohr, K. N. (2018). Definition of treatment‐resistant depression in the Medicare population. Agency for Healthcare Research and Quality: Rockville, MD. [PubMed] [Google Scholar]
- Gelenberg, A. J. , Freeman, M. P. , Markowitz, J. C. , Rosenbaum, J. F. , Thase, M. E. , Trivedi, M. H. , & Silbersweig, D. A. (2010). American Psychiatric Association practice guideline for the treatment of patients with major depressive disorder. American Journal of Psychiatry, 167(10), 1. [Google Scholar]
- Greden, J. F. , Riba, M. B. , & McInnis, M. G. (2011). Treatment resistant depression: A roadmap for effective care. American Psychiatric Publishing. [Google Scholar]
- Greenberg, P. E. , Fournier, A. A. , Sisitsky, T. , Pike, C. T. , & Kessler, R. C. (2015). The economic burden of adults with major depressive disorder in the United States (2005 and 2010). Journal of Clinical Psychiatry, 76(2), 155–162. 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- Gronemann, F. H. , Jorgensen, M. B. , Nordentoft, M. , Andersen, P. K. , & Osler, M. (2018). Incidence of, risk factors for, and changes over time in treatment‐resistant depression in Denmark: A register‐based cohort study. Journal of Clinical Psychiatry, 79(4), 17m11845. 10.4088/JCP.17m11845 [DOI] [PubMed] [Google Scholar]
- Guyatt, G. , Oxman, A. D. , Akl, E. A. , Kunz, R. , Vist, G. , Brozek, J. , Norris, S. , Falck‐Ytter, Y. , Glasziou, P. , & deBeer, H. (2011). GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology, 64(4), 383–394. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann‐Esser, W. , Siering, U. , Neugebauer, E. A. , Brockhaus, A. C. , Lampert, U. , & Eikermann, M. (2017). Guideline appraisal with AGREE II: Systematic review of the current evidence on how users handle the 2 overall assessments. PLoS One, 12(3), e0174831. 10.1371/journal.pone.0174831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak, W. W. , Balayan, K. , Bresee, C. , Greenberg, J. M. , Fakhry, H. , Christensen, S. , & Rapaport, M. H. (2013). A descriptive analysis of quality of life using patient‐reported measures in major depressive disorder in a naturalistic outpatient setting. Quality of Life Research, 22(3), 585–596. 10.1007/s11136-012-0187-6 [DOI] [PubMed] [Google Scholar]
- Kasper, S. , & Montgomery, S. A. (2013). Treatment‐resistant depression. Wiley‐Blackwell. [Google Scholar]
- Kautzky, A. , Dold, M. , Bartova, L. , Spies, M. , Kranz, G. S. , Souery, D. , Montgomery, S. , Mendlewicz, J. , Zohar, J. , Fabbri, C. , Serretti, A. , Lanzenberger, R. , Dikeos, D. , Rujescu, D. , & Kasper, S. (2019). Clinical factors predicting treatment resistant depression: Affirmative results from the European multicenter study. Acta Psychiatrica Scandinavica, 139(1), 78–88. 10.1111/acps.12959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. L. , & Williams, J. B. (2001). The PHQ‐9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, R. W. , Kennedy, S. H. , Parikh, S. V. , MacQueen, G. M. , Milev, R. V. , Ravindran, A. V. , & Group, C. D. W. (2016). Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Introduction and methods. Canadian Journal of Psychiatry, 61(9), 506–509. 10.1177/0706743716659061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, K. L. , Jacobs, P. , Ohinmaa, A. , Schopflocher, D. , & Dewa, C. S. (2008). A new population‐based measure of the economic burden of mental illness in Canada. Chronic Diseases in Canada, 28(3), 92–98. [PubMed] [Google Scholar]
- Lippard, E. T. C. , & Nemeroff, C. B. (2020). The devastating clinical consequences of child abuse and neglect: Increased disease vulnerability and poor treatment response in mood disorders. American Journal of Psychiatry, 177(1), 20–36. 10.1176/appi.ajp.2019.19010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi, G. S. , Bassett, D. , Boyce, P. , Bryant, R. , Fitzgerald, P. B. , Fritz, K. , Hopwood, M. , Lyndon, B. , Mulder, R. , Murray, G. , Porter, R. , & Singh, A. B. (2015). Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Australian & New Zealand Journal of Psychiatry, 49(12), 1087–1206. [DOI] [PubMed] [Google Scholar]
- Malhi, G. S. , & Byrow, Y. (2016). Is treatment‐resistant depression a useful concept? Evidence‐Based Mental Health, 19(1), 1–3. 10.1136/eb-2015-102299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery, S. A. , & Asberg, M. (1979). A new depression scale designed to be sensitive to change. British Journal of Psychiatry, 134, 382–389. 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- McGorry, P. D. , & Hickie, I. B. (Eds.). (2019). Clinical staging in psychiatry: Making diagnosis work for research and treatment. Cambridge University Press. [Google Scholar]
- Murphy, J. A. , Sarris, J. , & Byrne, G. J. (2017). A review of the conceptualisation and risk factors associated with treatment‐resistant depression. Depression Research and Treatment, 2017, 4176825. 10.1155/2017/4176825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health . (2010). Depression: The treatment and management of depression in adults (updated edition). [PubMed] [Google Scholar]
- Ng, C. H. , Kato, T. , Han, C. , Wang, G. , Trivedi, M. , Ramesh, V. , Shao, D. , Gala, S. , Narayanan, S. , Tan, W. , Feng, Y. , & Kasper, S. (2019). Definition of treatment‐resistant depression—Asia Pacific perspectives. Journal of Affective Disorders, 245, 626–636. 10.1016/j.jad.2018.11.038 [DOI] [PubMed] [Google Scholar]
- Nuñez, N. A. , Comai, S. , Dumitrescu, E. , Ghabrash, M. F. , Tabaka, J. , Saint‐Laurent, M. , Vida, S. , Kolivakis, T. , Fielding, A. , Low, N. , Cervantes, P. , Booij, L. , & Gobbi, G. (2018). Psychopathological and sociodemographic features in treatment‐resistant unipolar depression versus bipolar depression: A comparative study. BMC Psychiatry, 18(1), 68. 10.1186/s12888-018-1641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Quality Ontario . (2018). Major depression care for adults and adolescents . https://www.hqontario.ca/portals/0/documents/evidence/quality-standards/qs-depression-clinical-guide-1609-en.pdf
- Parikh, S. V. , Quilty, L. C. , Ravitz, P. , Rosenbluth, M. , Pavlova, B. , Grigoriadis, S. , Velyvis, V. , Kennedy, S. H. , Lam, R. W. , MacQueen, G. M. , Milev, R. V. , Ravindran, A. V. , & Uher, R. (2016). Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: Section 2. Psychological treatments. Canadian Journal of Psychiatry, 61(9), 524–539. 10.1177/0706743716659418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten, S. B. , Williams, J. V. , Lavorato, D. H. , Wang, J. L. , McDonald, K. , & Bulloch, A. G. (2015). Descriptive epidemiology of major depressive disorder in Canada in 2012. Canadian Journal of Psychiatry, 60(1), 23–30. 10.1177/070674371506000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, F. P. M. L. , Ruhe, H. G. , Wichers, M. , Abidi, L. , Kaub, K. , van der Lande, H. J. , Spijker, J. , Huibers, M. J. H. , & Schene, A. H. (2016). The Dutch measure for quantification of treatment resistance in depression (DM‐TRD): An extension of the Maudsley staging method. Journal of Affective Disorders, 205, 365–371. 10.1016/j.jad.2016.08.019 [DOI] [PubMed] [Google Scholar]
- Pérez‐Wehbe, A. I. , Perestelo‐Pérez, L. , Bethencourt‐Pérez, J. M. , Cuéllar‐Pompa, L. , & Peñate‐Castro, W. (2014). Treatment‐resistant depression: A systematic review of systematic reviews. International Journal of Clinical and Health Psychology, 14(2), 145–153. [Google Scholar]
- Ruhe, H. G. , van Rooijen, G. , Spijker, J. , Peeters, F. P. , & Schene, A. H. (2012). Staging methods for treatment resistant depression. A systematic review. Journal of Affective Disorders, 137(1‐3), 35–45. 10.1016/j.jad.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Rush, A. J. , Kraemer, H. C. , Sackeim, H. A. , Fava, M. , Trivedi, M. H. , Frank, E. , Ninan, P. T. , Thase, M. E. , Gelenberg, A. J. , Kupfer, D. J. , Regier, D. A. , Rosenbaum, J. F. , Ray, O. , & Schatzberg, A. F. (2006). Report by the ACNP task force on response and remission in major depressive disorder. Neuropsychopharmacology, 31(9), 1841–1853. 10.1038/sj.npp.1301131 [DOI] [PubMed] [Google Scholar]
- Rush, A. J. , Trivedi, M. H. , Wisniewski, S. R. , Nierenberg, A. A. , Stewart, J. W. , Warden, D. , Niederehe, G. , Thase, M. E. , Lavori, P. W. , Lebowitz, B. D. , McGrath, P. J. , Rosenbaum, J. F. , Sackeim, H. A. , Kupfer, D. J. , Luther, J. , & Fava, M. (2006). Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry, 163(11), 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Rush, A. J. , Trivedi, M. H. , Wisniewski, S. R. , Stewart, J. W. , Nierenberg, A. A. , Thase, M. E. , Ritz, L. , Biggs, M. M. , Warden, D. , Luther, J. F. , Shores‐Wilson, K. , Niederehe, G. , & Fava, M. (2006). Bupropion‐SR, sertraline, or venlafaxine‐XR after failure of SSRIs for depression. New England Journal of Medicine, 354(12), 1231–1242. 10.1056/NEJMoa052963 [DOI] [PubMed] [Google Scholar]
- Rybakowski, J. K. (2012). Bipolarity and inadequate response to antidepressant drugs: clinical and psychopharmacological perspective. Journal of Affective Disorders, 136(1‐2), e13–e19. 10.1016/j.jad.2011.05.005 [DOI] [PubMed] [Google Scholar]
- Sackeim, H. A. , Prudic, J. , Devanand, D. P. , Decina, P. , Kerr, B. , & Malitz, S. (1990). The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. Journal of Clinical Psychopharmacology, 10(2), 96–104. 10.1097/00004714-199004000-00004 [DOI] [PubMed] [Google Scholar]
- Schünemann, H. , Brożek, J. , Guyatt, G. , & Oxman, A. (2013). GRADE handbook for grading quality of evidence and strength of recommendations: Chapter 5 .
- Serretti, A. , & Fabbri, C. (2013). Shared genetics among major psychiatric disorders. Lancet, 381(9875), 1339–1341. 10.1016/S0140-6736(13)60223-8 [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V. , Lecrubier, Y. , Sheehan, K. H. , Amorim, P. , Janavs, J. , Weiller, E. , & Dunbar, G. C. (1998). The Mini‐International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. The Journal of Clinical Psychiatry, 59(Suppl 20), 22–33. [PubMed] [Google Scholar]
- Souery, D. , Papakostas, G. I. , & Trivedi, M. H. (2006). Treatment‐resistant depression. Journal of Clinical Psychiatry, 67(Suppl 6), 16–22. [PubMed] [Google Scholar]
- Souery, D. , Zaninotto, L. , Calati, R. , Linotte, S. , Mendlewicz, J. , Sentissi, O. , & Serretti, A. (2012). Depression across mood disorders: Review and analysis in a clinical sample. Comprehensive Psychiatry, 53(1), 24–38. 10.1016/j.comppsych.2011.01.010 [DOI] [PubMed] [Google Scholar]
- Takahashi, M. , Shirayama, Y. , Muneoka, K. , Suzuki, M. , Sato, K. , & Hashimoto, K. (2013). Personality traits as risk factors for treatment‐resistant depression. PLoS One, 8(5), e63756. 10.1371/journal.pone.0063756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner, J. A. , Hensel, J. , Davies, P. E. , Brown, L. C. , Dechairo, B. M. , & Mulsant, B. H. (2020). Economic burden of depression and associated resource use in Manitoba, Canada. Canadian Journal of Psychiatry, 65(5), 338–346. 10.1177/0706743719895342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum, S. D. (2014). Identification and treatment of antidepressant tachyphylaxis. Innovations in Clinical Neuroscience, 11(3‐4), 24–28. [PMC free article] [PubMed] [Google Scholar]
- Thase, M. E. , & Rush, A. J. (1997). When at first you don't succeed: Sequential strategies for antidepressant nonresponders. Journal of Clinical Psychiatry, 58(Suppl 13), 23–29. [PubMed] [Google Scholar]
- Vaccarino, S. R. , & Kennedy, S. H. (2020). Treatment resistant depression. In Vasquez G. H., Zarate C. A., & Brietzke E. (Eds.), Ketamine for treatment resistant depression. (pp. 33–84). Elsevier Academic Press. [Google Scholar]
- Weissman, M. M. , Wickramaratne, P. , Pilowsky, D. J. , Poh, E. , Hernandez, M. , Batten, L. A. , Flament, M. F. , Stewart, J. W. , & Blier, P. (2014). The effects on children of depressed mothers' remission and relapse over 9 months. Psychological Medicine, 44(13), 2811–2824. 10.1017/S003329171400021X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeratne, C. , & Sachdev, P. (2008). Treatment‐resistant depression: Critique of current approaches. Australian and New Zealand Journal of Psychiatry, 42(9), 751–762. 10.1080/00048670802277206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Supplementary information.
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


