Abstract
Background
The effect of calcium channel blockers (CCB) on mortality and ischaemic stroke risk in dementia patients is understudied.
Objectives
To calculate the risk of death and ischaemic stroke in dementia patients treated with CCBs, considering individual agents and dose response.
Methods
Longitudinal cohort study with 18 906 hypertensive dementia patients from the Swedish Dementia Registry (SveDem), 2008–2014. Other Swedish national registries contributed information on comorbidities, dispensed medication and outcomes. Individual CCB agents and cumulative defined daily doses (cDDD) were considered.
Results
In patients with hypertension and dementia, nifedipine was associated with increased mortality risk (aHR 1.32; CI 1.01–1.73; P < 0.05) compared to non‐CCB users. Patients diagnosed with Alzheimer’s dementia (AD) or dementia with Lewy bodies/Parkinson’s disease dementia (DLB‐PDD) taking amlodipine had lower mortality risk (aHR, 0.89; CI, 0.80–0.98; P < 0.05 and aHR 0.58; CI, 0.38–0.86; P < 0.01, respectively), than those taking other CCBs. Amlodipine was associated with lower stroke risk in patients with Alzheimer’s dementia compared to other CCBs (aHR 0.63; CI, 0.44–0.89; P < 0.05).
Sensitivity analyses with propensity score‐matched cohorts repeated the results for nifedipine (aHR 1.35; 95% CI, 1.02–1.78; P < 0.05) and amlodipine in AD (aHR, 0.87; CI, 0.78–0.97; P < 0.05) and DLB‐PDD (aHR, 0.56, 95%CI, 0.37–0.85; P < 0.05).
Conclusion
Amlodipine was associated with reduced mortality risk in dementia patients diagnosed with AD and DLB‐PDD. AD patients using amlodipine had a lower risk of ischaemic stroke compared to other CCB users.
Keywords: dementia, antihypertensive drugs, stroke, mortality, calcium, alzheimer’s disease
Introduction
Mortality and the risk of ischaemic stroke are higher in dementia patients compared to their cognitively intact peers [1, 2, 3, 4]. Modifiable risk factors, such as hypertension, diabetes, hyperlipidaemia, obesity and smoking, are frequent in patients with dementia and contribute to dementia risk [5].
Calcium channel blockers (CCB) are classified based on their pharmacological properties in three groups, phenylalkylamines, benzothiazepines and dihydropyridines (DHP). The three groups have different vascular selectivity, with dihydropyridines displaying high affinity towards calcium channels in vascular smooth muscles compared to phenylalkylamines and benzothiazepines high selectivity in myocardium [6]. Phenylalkylamines and benzothiazepines are often referred to as non‐dihydropyridines (non‐DHP). Calcium channel blockers (CCB) are commonly used in clinical practice for management of hypertension and angina pectoris. In the non‐DHP group, therapeutic indications extend to the treatment of supraventricular arrhythmias and atrial fibrillation [7]. One classification, based on individual pharmacological properties, duration of action and severity and frequency of adverse effects, divides CCBs in three generations, with new highly lipophilic agents sometimes being regarded as the fourth‐generation CCBs [6, 8].
The role of calcium is widely recognized in multiple cellular mechanisms, serving as an important intracellular second messenger [9]. This opens a possible therapeutic role of CCB treatment beyond the cardiovascular system in a broad spectrum of neurological disorders [7]. The calcium hypothesis of Alzheimer’s disease postulates that an alteration in calcium signalling affects neuronal susceptibility to apoptotic cell mechanisms and interferes with proximal pathways of the amyloid cascade by disrupting amyloid precursor protein metabolism and Tau phosphorylation [10, 11]. A disruption in calcium homeostasis could be a common pathway in neurodegenerative diseases [11].
Studies on the effects of CCBs on cognition and dementia risk have reported contradictory results, [12, 13, 14, 15, 16, 17, 18, 19, 20] and there are limited data focusing on the mortality or stroke risk once dementia is established [17, 18]. A Cochrane systematic review provided no convincing evidence for a protective effect of nimodipine use in AD and vascular dementia (VaD), which was followed by the American Heart Association (AHA) scientific statement published in 2016, concluding there were still insufficient data to make evidence‐based recommendations in the general population [21, 22]. The Systolic hypertension in Europe trial (SYST‐EUR) is the only clinical trial to‐date focusing on CCB use and cognition. The SYST‐EUR reported a 50% reduction in incident AD and VaD in patients aged 60 years or older using nitrendipine [23].
In dementia patients, CCBs could reduce mortality risk not only through their systemic antihypertensive effect, but also through a putative effect on neurodegeneration. Prior studies did not have sufficient sample size to conduct detailed analyses of individual agents or stratify by age, sex and dementia type. For this reason, the aim of our study was to use the Swedish Dementia Registry to investigate the association of CCBs with stroke and mortality risk in dementia patients, considering CCB classes, individual agents, cumulative drug exposure and conducting stratified analyses by age, sex and dementia type.
Methods
Study population
This registry‐based longitudinal cohort study was conducted on 18 906 dementia patients from the Swedish Dementia Registry (SveDem) from 2008–2014. The Swedish Stroke Registry (RiksStroke), Swedish Prescribed Drug Register (PDR), Swedish Total Population Register (TPR) and Swedish National Patient Register (NPR) were merged using the personal identification number to contribute comorbidities and outcomes. SveDem is a national quality registry on incident dementia disorders, established in 2007, and previously described in depth [1, 24]. SveDem aims to achieve full coverage for all dementia diagnoses in Sweden and currently includes 100% of memory clinics and around 75% of primary care. In 2018, the estimated coverage of SveDem was 27 to 46% but information is lacking on how many patients actually receive a dementia diagnosis each year in Sweden [1, 24, 25, 26, 27, 28]. SveDem provided demographic information for the cohort, including age, sex, cognitive evaluation assessed with the Mini‐Mental State Examination (MMSE), body mass index and type of dementia diagnosis at baseline (Fig. 1, Figure S1). Patients with missing data on diagnosis or MMSE score at baseline and those without hypertension were excluded (Fig. 1).
Fig. 1.
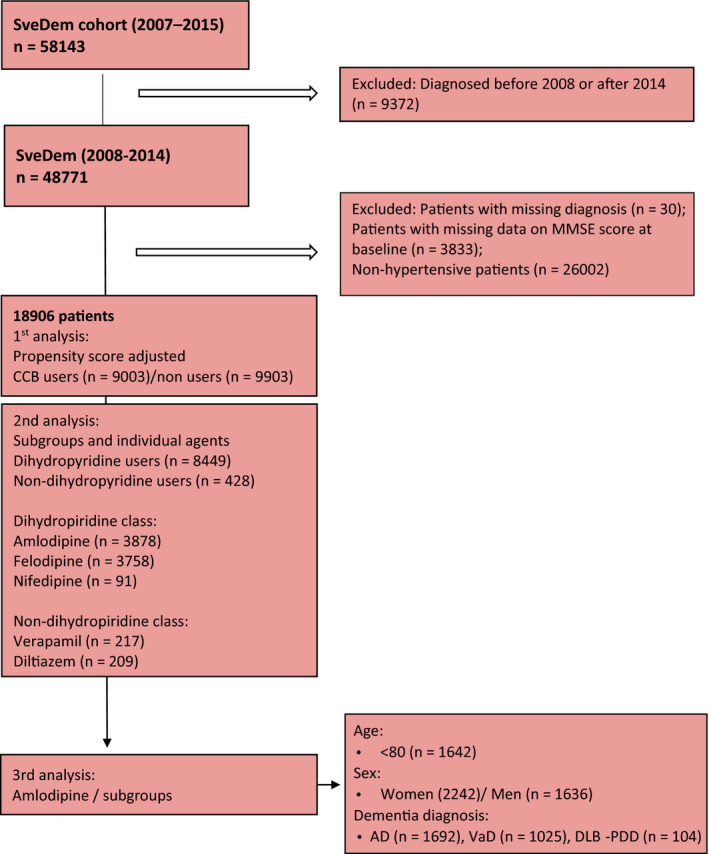
Patient selection. SveDem, Swedish Dementia Registry; n, number of patients; MMSE, Mini‐Mental State Examination; baseline, date at the time of dementia diagnosis; CCBs, Calcium Channel Blockers; AD, Alzheimer’s disease; VaD, Vascular dementia; DLB‐PDD, Dementia with Lewy Bodies‐Parkinson’s disease dementia; FTD, Frontotemporal dementia.
Comorbidities
Comorbidities were collected from the NPR, which covers> 99% of all inpatient discharges and specialized outpatient visits [29]. Diagnoses are classified according to the International Statistical Classification of Diseases, 10th revision (ICD‐10) [30, 31]. Data were gathered on hypertension (ICD‐10 codes I10‐I15), hypertension with organ damage (H35.0, I11.0, I11.9, I12.0, I12.9, I13.0, I13.1, I13.9, I67.4), diabetes (E10‐E14), arrhythmia (I49.x), atrial fibrillation (I48.x), alcohol‐related diseases (E24.4, F10, G31.2, G62.1, G72.1, I42.6, K29.2, K70, K86.0, O35.4, P04.3, Q86.0, T51, Y90, Y91, Z50.2, Z71.4), angina pectoris (I20.x), renal disease (I12.0, I13.1, N03.2‐N03.7, N05.2‐N05.7, N18.x, N19.x, N25.0, Z49.0, Z49.1, Z49.2, Z94.0, Z99.2), heart failure (I50.0, I50.1, I50.9), myocardial infarction (MI) (I21.x, I22.x, I25.2), respiratory disease (J40‐47, J60‐67, J68.4, J70.1, J70.3) and ischaemic stroke from RiksStroke.
Exposures
Exposure to CCBs was defined as at least one prescription dispensed in a Swedish pharmacy in the three years prior to baseline, that is date of dementia diagnosis. Data on dispensed medication were extracted from the PDR, which holds information on all prescriptions dispensed by pharmacies, with> 99% coverage throughout Sweden from 2005 [31]. Medication is classified based on the Anatomical Therapeutic Chemical classification(ATC) [32]. CCBs (C08) were extracted and classified following the ATC system [32]. CCBs may be classified based on their pharmacological properties in three groups, phenylalkylamines, benzothiazepines and dihydropyridines (DHP). Following the ATC, CCBs are subdivided into selective CCBs with mainly vascular effects (with DHPs within this group – C08CA) vs selective CCBs with direct cardiac effects (non‐DHPs, including phenylalkylamine and benzothiazepine derivatives‐C08D) [32]. The DHP class comprised amlodipine (C08CA01), felodipine (C08CA02), isradipine (C08CA03), nifedipine (C08CA05), nimodipine (C08CA06) and lercanidipine (C08CA13). Non‐DHP class included verapamil (C08DA01) and diltiazem (C08DB01). Short‐acting agents, nifedipine, diltiazem and verapamil are regarded as a first‐generation CCBs and felodipine, isradipine, nimodipine and first‐generation agents in sustained release (SR) form, as second‐generation CCBs. The third‐generation CCBs are the new, long‐lasting DHPs, amlodipine and lercanidipine, with more gradual onset, fewer side effects and better tolerance. (Fig. 1) [8]. Doses were expressed as defined daily dose (DDD) [33]. DDD is by definition the assumed average maintenance dose per day for a drug used for its main indication in adults (Table S1) [33]. By translating the cumulative prescribed dose within a time period into cumulative DDD, different medications which are normally prescribed at different dosages can be compared. Cumulative DDD per person was used within the three‐year period prior to dementia to investigate a dose–response effect.
Information was obtained on other prescribed medication, including diuretics (C03), β‐blockers (C07), angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II antagonists (ARB) (C09), lipid‐modifying agents (C10), antithrombotics (B01) and nonsteroidal anti‐inflammatory drugs (N02BA01, M01A). The total number of medications which the patient took at the time of dementia diagnosis was included as a control variable.
Outcomes
RiksStroke provided data on the occurrence of the first ischaemic stroke (ICD‐10 I63) after dementia diagnosis [34]. Information on all‐cause death was obtained from the TPR [35].
Statistical analysis
Baseline characteristics between CCB users and nonusers are presented by number of cases and percentages for categorical variables and as mean ± SD or median (IQR) for continuous variables. To assess differences between groups, Pearson’s chi‐square was used for categorical variables and Student’s t‐test or Mann–Whitney U‐test was used for continuous variables, as appropriate.
Incidence rates with 95% confidence intervals (95% CI) were calculated using the exact method. We estimated hazard ratios (HR) and 95% confidence intervals (CI), using Cox proportional hazard models, to determine the association between CCBs and risk of death and ischaemic stroke. The baseline entry point was considered the time of dementia diagnosis for a total of 55 045 person‐years of observation. Patients were censored at the date of first ischaemic stroke, death or the end of the follow‐up (31 December 2014), for the outcome of stroke. For the outcome of death, patients were censored at the time of death or the end of follow‐up (29 February 2016). Two models are presented: adjusted for propensity score (PS) and propensity score matched for CCB use at baseline. Inclusion of MMSE score was used to reflect severity of dementia at baseline. Cubic splines were used for age and MMSE (knots set at age 65, 75 and 85 and MMSE 10, 20 and 25). Potential confounders based on predicted multicollinearity were excluded. Covariates included in the final propensity score were age, sex, dementia type, comorbidities (diabetes, arrhythmia, atrial fibrillation, heart failure, renal disease, alcohol‐related diseases, angina pectoris, previous myocardial infarction (MI), previous cerebral stroke‐only used in survival analysis), medication (β‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor (ARB) blockers, other antihypertensives), statins, antithrombotics, acetylsalicylic acid, nonsteroidal anti‐inflammatory drugs (NSAIDs)). Multiple different interactions between the above factors were also included. Sensitivity analyses controlling for body mass index were also performed.
Analyses were done by using all‐CCB, DHP and non‐DHP class, individual agent and dose effects. Schoenfeld residuals were used to assess the proportional hazards assumption. Piecewise linear splines were used to graphically model the associations between cumulative defined daily dose (cDDD) over the three years prior to dementia diagnosis and study outcomes. Spline knots were set with fixed values of DDD distribution (550, 1095, 1645 and 2190), and a DDD 0 was used as the reference value. The cohort was stratified based on dementia type, age (two groups with the mean as a cut‐point) and sex. The dementia types Alzheimer’s disease (AD), vascular dementia (VaD), mixed dementia, dementia with Lewy bodies (DLB), Parkinson’s disease with dementia (PDD) and frontotemporal dementia (FTD) were considered. AD and mixed dementia were merged into one group (abbreviated AD). Patients diagnosed with DLB and PDD were merged into one group as they can be considered two syndromes within the same spectrum (abbreviated DLB‐PDD) [36]. Multiplicative interactions between diagnosis type, age, sex and CCB users in predicting the outcomes were also explored. Sensitivity analyses were conducted, using 1:1 PS matching for the outcome of death and stroke, and Fine–Gray models taking death as a competing event, for the analysis of stroke.
All statistical tests were two‐sided, with P < 0.05 considered as significant. IBM SPSS Statistics for Windows software, version 25.0 (IBM Corp, Armonk, NY, USA), SAS, version 9.4 (SAS Institute, Cary, NC, USA) and Stata version 15.0 (StataCorp, College Station, TX, USA) were used.
Ethical considerations and participant consent
The regional ethics review board in Stockholm, Sweden, approved this study (dnr 2015/743–31/4). This study complies with the Helsinki Declaration. Patients and caregivers were informed of participation in SveDem upon registration and could refuse or withdraw consent. Data were anonymized before analysis.
Results
A total of 18 906 dementia patients were included in the cohort. In the three years prior to baseline, 9003 (47.6%) patients obtained at least one CCB prescription. CCB users had more comorbidities and took more medication compared to nonusers (Table 1). From baseline to the end of follow‐up, 8182 (43.2%) patients died and 749 (3.9%) suffered an ischaemic stroke (Table 1). Amlodipine (3878; 43.1%) was the most commonly prescribed individual agent, followed by felodipine, prescribed to 3758 (41.7%) patients. (Fig. 1; Figure S1).
Table 1.
Descriptive characteristics of CCB users and nonusers in the dementia cohort
| Svedem | CCB nonusers | % | CCB users | % | P value |
|---|---|---|---|---|---|
| Number | 9903 | 52.4 | 9003 | 47.6 | |
| Women | 5863 | 59.2 | 5266 | 58.5 | 0.320 |
| Age, median (IQR | 81.0 (76.0–86.0) | 81.0 (76.0–86.0) | 0.069 | ||
| Diagnosis | |||||
| AD | 4520 | 45.6 | 3856 | 42.8 | <0.001 |
| VaD | 2424 | 24.5 | 2467 | 27.4 | |
| DLB‐PDD | 294 | 3.0 | 249 | 2.8 | |
| FTD | 96 | 1.0 | 84 | 0.9 | |
| Unspecified and other | 2569 | 25.9 | 2347 | 26.1 | |
| Body mass index, median (IQR) | 24.9 (22.3–28.1) | 24.9 (22.4–28.3) | 0.430 | ||
| MMSE score at baseline, median (IQR) | 21.0 (18.0–24.0) | 21.0 (18.0–24.0) | 0.260 | ||
| Comorbidities, % | |||||
| Hypertension with organ damage | 537 | 5.4 | 578 | 6.4 | 0.004 |
| Diabetes | 2137 | 21.6 | 2299 | 25.5 | <0.001 |
| Renal disease | 447 | 4.5 | 573 | 6.4 | <0.001 |
| Respiratory disease | 1175 | 11.9 | 1024 | 11.4 | 0.290 |
| Alcohol‐related diseases | 354 | 3.6 | 294 | 3.3 | 0.240 |
| Cerebral stroke | 2190 | 22.1 | 2228 | 24.7 | <0.001 |
| Atrial fibrillation | 2685 | 27.1 | 2244 | 24.9 | <0.001 |
| Other arrhythmias | 622 | 6.3 | 579 | 6.4 | 0.670 |
| Heart failure | 2016 | 20.4 | 1425 | 15.8 | <0.001 |
| Congestive heart failure | 657 | 6.6 | 369 | 4.1 | <0.001 |
| Left ventricular heart failure | 286 | 2.9 | 169 | 1.9 | <0.001 |
| Heart failure unspecified | 1798 | 18.2 | 1251 | 13.9 | <0.001 |
| Angina pectoris | 2271 | 22.9 | 2203 | 24.5 | 0.013 |
| Myocardial infarction | 1828 | 18.5 | 1635 | 18.2 | 0.600 |
| Medications (baseline), % | |||||
| Antithrombotics | 7460 | 75.3 | 6980 | 77.5 | <0.001 |
| AAS | 6980 | 70.5 | 6587 | 73.2 | <0.001 |
| NSAID | 2797 | 28.2 | 2654 | 29.5 | 0.061 |
| Hypolipemics | 4884 | 49.3 | 5340 | 54.8 | <0.001 |
| Non‐CCB antihypertensives | 9409 | 95.0 | 5010 | 55.6 | <0.001 |
| ACE inhibitors/ARB | 6086 | 61.5 | 8382 | 93.1 | <0.001 |
| β‐blockers | 6539 | 66.0 | 5831 | 64.8 | <0.001 |
| Diuretics | 5532 | 55.9 | 6123 | 68.0 | 0.004 |
| Total number of drugs, median (IQR) | 6 (3–8) | 6 (4–9) | <0.001 | ||
| Outcome events | |||||
| Ischaemic stroke after dementia | 390 | 3.9 | 359 | 4.0 | 0.860 |
| Death (baseline‐end of follow‐up) | 4323 | 43.7 | 3859 | 42.9 | 0.280 |
CCBs, Calcium Channel Blockers; AD, Alzheimer’s disease; VaD, Vascular dementia; DLB‐PDD, Lewy Bodies‐Parkinson's Disease Dementia; FTD, Frontotemporal dementia; Unspecified and other: unspecified dementia and other miscellaneous types, such as corticobasal syndrome or alcohol‐related dementia; MMSE, Mini‐Mental State Examination, AAS, acetylsalicylic acid, NSAID, nonsteroidal anti‐inflammatory drugs; ACE inhibitors, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; baseline, date at the time of dementia diagnosis. Body mass index: missing n = 6424.
Categorical variables presented as numbers and percentages (%); P values were obtained by using chi‐square test. For results presented as median and interquartile range (IQR), P values were obtained by using Mann–Whitney U‐test.
There was no association between CCB use and death (adjusted hazard ratio (aHR), 1.02; 95% CI, 0.97–1.06) or ischaemic stroke (aHR, 1.03; 95% CI, 0.89–1.19) compared to nonusers (Fig. 2, Table 2). Nifedipine (aHR, 1.31; 95% CI, 1.00–1.72) and felodipine (aHR, 1.06; 95% CI, 1.00–1.12) were associated with higher risk of death compared to non‐CCB users(Fig. 2, Table 2).
Fig. 2.
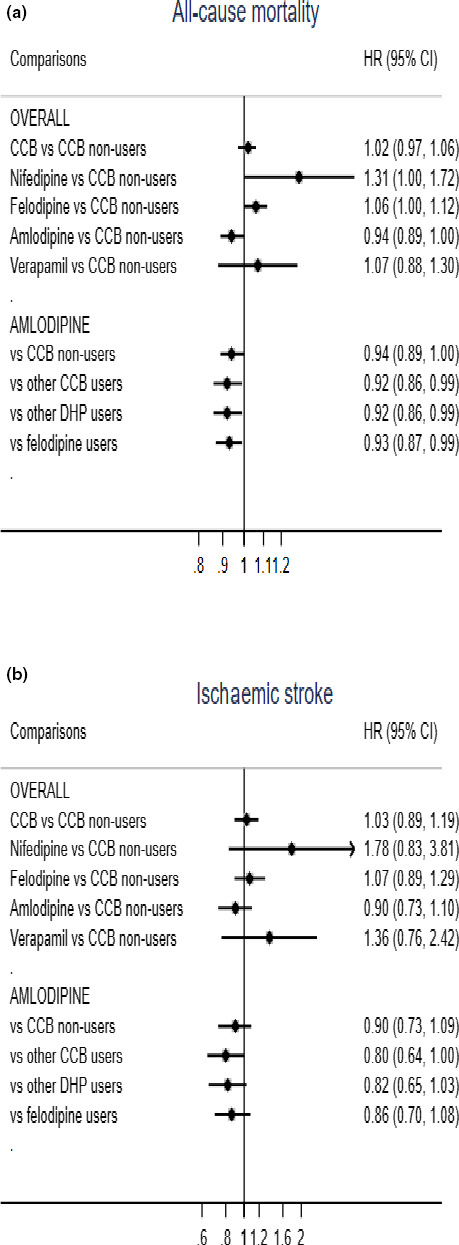
Association between CCB use and (a) all‐cause mortality and (b) ischaemic stroke risk in dementia patients, adjusted for propensity score of CCB treatment. CCBs, Calcium Channel Blockers; DHPs, dihydropyridines; baseline, date at the time of dementia diagnosis. Propensity score (PS) included age, sex, dementia type, Mini‐mental state examination score (MMSE) at baseline, comorbidities (hypertension with organ damage, diabetes, arrhythmia, atrial fibrillation, heart failure [congestive heart failure, left ventricular heart failure and heart failure unspecified], renal disease, alcohol‐related diseases, angina pectoris, previous myocardial infarction (MI), previous cerebral stroke – only used in survival analysis), medication (β‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor (ARB) blockers, other antihypertensives), statins, diuretics, antithrombotics, acetylsalicylic acid, nonsteroidal anti‐inflammatory drugs (NSAIDs)). Multiple different interactions between the above factors were also included. For the analysis of ischaemic stroke, we performed Fine–Gray models taking death due to stroke causes as a competing event.1 Reference: 1. Austin PC, Fine JP, Practical recommendations for reporting Fine–Gray model analyses for competing risk data. Stat Med. 2017 Nov 30;36(27):4391‐4400.
Table 2.
Associations of CCB medications and risk of death and ischaemic stroke in Cox proportional hazard models
| Survival | Ischaemic stroke | |||||
|---|---|---|---|---|---|---|
| No. (events) | HR | (95% CI) | No. (events) | Sub HR | (95% CI) | |
| Adjusted for propensity score | ||||||
| CCB nonusers | 9903 (4323) | Ref. | 9903 (390) | Ref. | ||
| CCB vs CCB nonusers | ||||||
| CCB vs CCB nonusers | 9003 (3859) | 1.02 | 0.97–1.06 | 9003 (359) | 1.03 | 0.89–1.19 |
| Nifedipine vs CCB nonusers | 91 (53) | 1.31 * | 1.00–1.72 | 91 (7) | 1.78 | 0.83–3.81 |
| Felodipine vs CCB nonusers | 3758 (1761) | 1.06 * | 1.00–1.12 | 3758 (164) | 1.07 | 0.89–1.29 |
| Amlodipine vs CCB nonusers | 3878 (1457) | 0.94 | 0.89–1.00 | 3878 (127) | 0.90 | 0.73–1.10 |
| Verapamil vs CCB nonusers | 217 (103) | 1.07 | 0.88–1.30 | 217 (12) | 1.36 | 0.76–2.42 |
| Amlodipine vs other CCBs | ||||||
| Other CCBs | 4376 (2082) | Ref. | 4376 (204) | Ref. | ||
| Amlodipine | 3878 (1457) | 0.92 * | 0.86–0.99 | 3878 (127) | 0.80 | 0.64–1.00 |
| other DHPs | 3948 (1864) | ref | 3948 (180) | |||
| Amlodipine | 3878 (1457) | 0.92 * | 0.86–0.99 | 3878 (127) | 0.82 | 0.65–1.03 |
| Felodipine | 3758 (1761) | ref | 3758 (164) | |||
| Amlodipine | 3878 (1457) | 0.93 * | 0.87–0.99 | 3878 (127) | 0.86 | 0.70–1.08 |
| Sensitivity analysis using 1:1 propensity score matching | ||||||
| 1. PS matching 1:1 CCB/CCB nonusers | ||||||
| CCB nonusers | 8113 (3490) | Ref. | 8168 (328) | Ref. | ||
| CCB users | 8113 (3465) | 1.01 | 0.96–1.06 | 8168 (322) | 1.00 | 0.85–1.16 |
| Nifedipine users | 82 (49) | 1.35 * | 1.02–1.78 | 84 (7) | 1.87 | 0.87–4.00 |
| Felodipine users | 3404 (1592) | 1.06* | 1.00–1.13 | 3433 (150) | 1.06 | 0.87–1.27 |
| Amlodipine users | 3500 (1298) | 0.93 * | 0.87–0.99 | 3504 (110) | 0.84 | 0.68–1.05 |
| Verapamil users | 200 (94) | 1.10 | 0.90–1.35 | 201 (11) | 1.33 | 0.72–2.42 |
| 2. PS matching 1:1 Amlodipine/other CCB users | ||||||
| Other CCB users | 3642 (1705) | Ref. | 3635 (162) | Ref. | ||
| Amlodipine | 3642 (1375) | 0.90 ** | 0.84–0.97 | 3635 (119) | 0.82 | 0.65–1.04 |
| AD | ||||||
| Amlodipine | 1575 (560) | 0.87 * | 0.78–0.97 | 1579 (44) | 0.61 ** | 0.42–0.87 |
| VaD | ||||||
| Amlodipine | 978 (405) | 1.01 | 0.88–1.15 | 968 (38) | 1.07 | 0.68–1.67 |
| DLB‐PDD | ||||||
| Amlodipine | 98 (35) | 0.56 ** | 0.37–0.85 | 95 (6) | 1.37 | 0.47–4.00 |
AD, Alzheimer’s disease; CCBs, Calcium Channel Blockers; CI, confidence intervals; DHP, dihydropyridines; DLB‐PDD, Dementia with Lewy bodies‐Parkinson’s disease dementia; FTD, Frontotemporal dementia.
Propensity score (PS) included age, sex, dementia type, Mini‐mental state examination score (MMSE) at baseline, comorbidities (hypertension with organ damage, diabetes, arrhythmia, atrial fibrillation, heart failure [congestive heart failure, left ventricular heart failure and heart failure unspecified], renal disease, alcohol‐related diseases, angina pectoris, previous myocardial infarction (MI), previous cerebral stroke – only used in survival analysis), medication (β‐blockers, angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor (ARB) blockers, other antihypertensives), statins, diuretics, antithrombotics, acetylsalicylic acid, nonsteroidal anti‐inflammatory drugs (NSAIDs)). Multiple different interactions between the above factors were also included.
1. For the PS‐matched analyses: The first matching was performed with a PS calculated on CCB use vs nonuse. 2.The second matching was performed with a PS calculated on amlodipine vs other CCB usersHR, hazard ratios; n, number of patients; non‐DHP, non‐dihydropyridines; VaD, Vascular dementia.
For the analysis of ischaemic stroke, we performed Fine–Gray models taking death due to stroke causes as a competing event.1
Reference: 1. Austin PC, Fine JP, Practical recommendations for reporting Fine‐Gray model analyses for competing risk data. Stat Med. 2017 Nov 30;36(27):4391‐4400.
P < 0.05, **P < 0.01, ***P < 0.001.
Bold indicates statistically significant values.
Amlodipine was associated with significantly lower mortality risk compared to other CCBs (aHR, 0.92; 95% CI, 0.86–0.99), other DHPs (aHR, 0.92; 95% CI, 0.86–0.99) or felodipine (aHR, 0.93; CI, 0.87–0.99). Sensitivity analysis, using 1:1 propensity score matching, confirmed a significant association with higher mortality risk for nifedipine when compared to other CCBs(HR 1.35; 95% CI, 1.02–1.78;) (Fig. 2, Table 2).
In AD patients, increasing cumulative doses of amlodipine were associated with reduced risk of death, up to the dose consistent with taking one DDD (5 mg daily) for three years (Fig. 3). Statistical significance was lost at higher doses, possibly due to the smaller sample size (Fig. 3c).
Fig. 3.
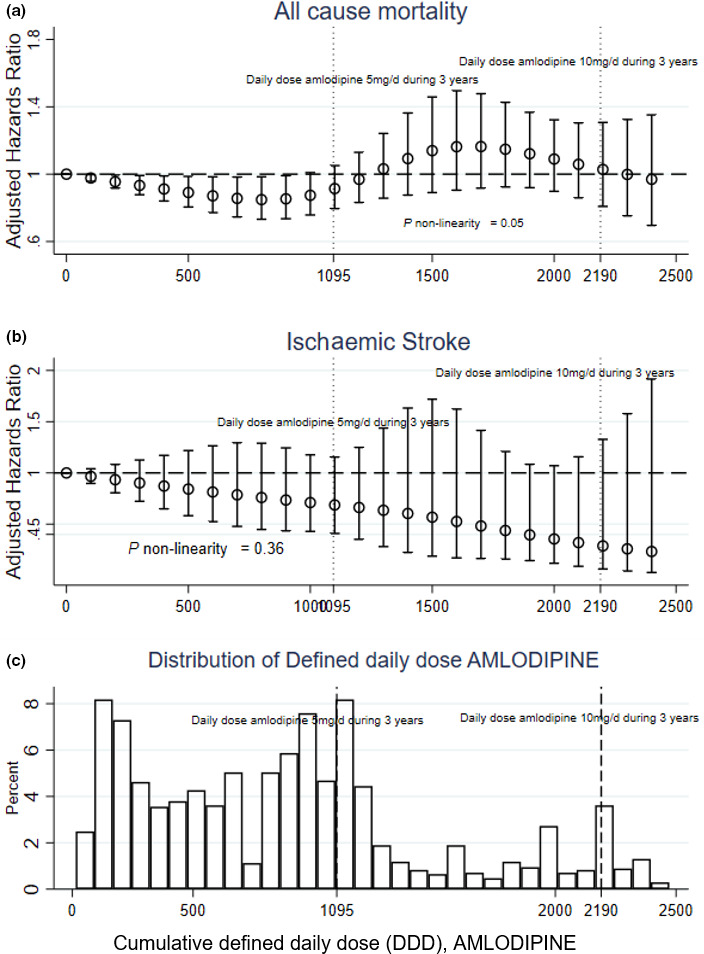
Amlodipine dose‐dependent hazard for death and ischaemic stroke calculated using Cox hazard regression models in AD patients for (a) mortality, (b) ischaemic stroke, and (c) histogram representing proportion of patients prescribed each cumulative defined daily dose. Hazard ratios (HR) and 95% confidence intervals (CI) for death and ischaemic stroke associated with cumulative defined daily dose (cDDD) of amlodipine. The defined daily dose (DDD) of a medication is defined by the World Health Organization and is the assumed average maintenance dose per day for a drug used for its main indication in adults. In the case of amlodipine, 1 DDD corresponds to 5mg per day. A patient taking 5mg per day of amlodipine during 1 year would have a cDDD of 365, and 1095 cDDD if this same dose was continued for 3 years. Vertical dotted lines mark the equivalent DDD resulting from 5mg of amlodipine during 3 years and from 10mg amlodipine during 3 years. Model adjusted for propensity score. The risk associated to amlodipine was modelled using linear splines with knots at fixed values of cDDD distribution (550, 1095, 1645, 2190). Estimates were adjusted for propensity score and Mini‐Mental State Examination measurements at baseline. cDDD = 0 was used as reference. (a) All‐cause mortality, (b) ischaemic stroke, (c) distribution of patients taking each cDDD during 3 years. A dose effect is evident at increasing cDDD of amlodipine up to the equivalent of 1DDD per day during 3 years. At higher doses, the effect is lost, possibly due to reduced sample size as is evident in the histogram (c).
In stratified propensity score‐adjusted analyses, amlodipine was associated with significantly lower risk of death in patients diagnosed with AD (aHR, 0.89; 95% CI, 0.80–0.98) or DLB‐PDD (aHR, 0.58; 95% CI, 0.38–0.86), but no association was found in patients diagnosed with VaD (aHR, 1.02; 95% CI, 0.90–1.16) compared to other CCB users (Table S2). Amlodipine was further associated with significantly lower risk of ischaemic stroke in patients with AD, compared to other CCB users (aHR 0.63; CI, 0.44–0.89; P < 0.05). Sensitivity analysis with 1:1 propensity score matching cohort produced similar results (Table 2). Sensitivity analyses controlling for BMI and number of prescribed drugs were not substantially different (results not presented).
Discussion
In this large Swedish longitudinal open‐cohort study, CCB use in the three years prior to dementia diagnosis was not associated with mortality, probably due to the fact that different individual agents presented different associations with mortality. Amlodipine was associated with reduced mortality risk, compared to users of other CCBs, other DHPs and felodipine. In stratified analyses, this association appeared in patients diagnosed with AD and DLB‐PDD and this finding was repeated in sensitivity analyses with propensity score matching. Furthermore, amlodipine was associated with lower risk of ischaemic stroke amongst the AD subgroup (Table 2).
Nifedipine was associated with higher risk of death compared to nonusers. The results in sensitivity analyses with propensity score‐matched cohorts were not statistically significant for ischaemic stroke due to a smaller sample size but pointed at increased risk with HR 1.87; 95% CI, 0.87–4.00. This implies that any result between a 13% stroke risk reduction to a 4 times higher stroke risk is reasonably compatible with the data.
Dose–response effects showed the lowest mortality risk in patients using the equivalent cumulative dose of one prescribed DDD of amlodipine (equivalent to 5mg) for all three years prior to dementia diagnosis. This dose response was not present at higher doses, but the sample of patients taking higher doses of amlodipine was also smaller.
Previous studies have explored the relationship between CCBs and the risk of stroke and mortality in nondementia cohorts, but few considered that different agents within the group could have differential effects on mortality. Even less information exists specifically in patients with dementia. Many studies grouped drugs without reporting results for individual agents, making their results hard to interpret in the context of the present study. For instance, the Baltimore Longitudinal Study of ageing (BLSA) did not report any association with mortality in DHP and non‐DHP users compared to nonusers [17]. Our results associating non‐DHPs with higher risk of death, especially in verapamil users, differ from these findings, but the BLSA did not distinguish between individual agents of the non‐DHP class, thus making it impossible to assess the origin of the reported effects.
There have been concerns connected to the use of short‐acting CCBs and occurrence of reflex tachycardia and higher mortality risk. The potential negative effects of nifedipine, one of the oldest CCBs, have been widely discussed in the past, with a meta‐analysis of clinical trials concluding that higher doses of short‐acting nifedipine increased mortality in patients with coronary disease [37]. These findings were later refuted by other publications [38] including the randomized controlled ACTION trial (HR 1.07; 95% CI 0.91–1.25, P = 0.41) [39]. A 2011 study on hypertensive older patients reported an association between short‐acting nifedipine and increased risk of stroke [40]. The Canadian Study of Aging primarily investigated the associations between CCBs and cognition, but also reported a higher mortality risk in nifedipine users [18]. Short‐acting CCBs are known for their negative inotropic properties that could worsen systolic heart failure, especially in patients with reduced left ventricular ejection fraction [7, 38]. Reflex sympathetic activation may also be a problem with long‐acting CCBs, including felodipine and amlodipine [41], although a more gradual onset of action might attenuate the negative consequences of these effects.
In our study, nifedipine users had significantly higher mortality risk. An increase in ischaemic stroke is also probable, although significance was lost in the propensity score‐matched cohort, possibly due to lower sample size. However, our data do not distinguish between short‐ and long‐acting nifedipine and the reasons for prescription could be different than for amlodipine. The clinical trial ALLHAT, with more than 30 000 hypertensive patients, reported the effects of antihypertensives on all‐cause mortality, stroke and cardiovascular events. Amlodipine was chosen as a representative agent for the CCB group. There was no effect of amlodipine use and all‐cause mortality, stroke or major cardiovascular events, when compared to diuretics [42]. In contrast, the PREVENT trial showed that amlodipine use slowed down the progression of carotid artery atherosclerosis [43]. Another randomized controlled trial, with 19 257 patients, ASCOT‐BPLA, reported lower risk of stroke, all‐cause mortality and incidence of diabetes in amlodipine compared to atenolol [44]. However, there are limited data on amlodipine use and the risk of stroke and mortality in dementia patients. Our results in stratified analyses based on dementia type revealed an interesting association between amlodipine and lower risk of death in AD and DLB‐PDD patients. Furthermore, AD patients using amlodipine had a significantly lower risk of ischaemic stroke. To our knowledge, no studies reported amlodipine use and mortality in different dementia types and no study to this date reported a significantly lower risk of ischaemic stroke in dementia patients using amlodipine. The superiority of amlodipine compared to other CCBs in our cohort could lie in its unique pharmacological properties. Amlodipine is a slow‐release agent with a longer half‐life than other CCBs (35–40h), making it favourable for patient compliance with once‐per‐day dosing [45]. Gradual onset of action with little inotropic activity in standard therapeutic dosing lowers adverse effects described in short‐acting CCBs, such as a reflex increase in sympathetic activity and fluctuations in blood pressure, that could lead to a possible plaque rupture and higher risk of stroke and mortality [37].
Calcium targets different channels in the central nervous system, classified into subtypes L, N, P/Q, R and T [46]. Presynaptic N‐type channels are assumed to be involved in neurotransmitter release whilst postsynaptic voltage‐gated L‐type channels are assumed to be involved in regulation of neuronal excitability [8, 47]. A possible explanation of our results in the AD and DLB‐PDD subgroup could lie in amlodipine’s potential to block L‐type and N‐type calcium channels with similar affinity, [7] expanding its possible underlying neuroprotective mechanism beyond the vascular effect. The significant association with amlodipine and lower mortality risk in AD and DLB‐PDD, but not VaD, supports this hypothesis. However, confounding by indication is a concern, if patients with less problems with autonomic dysfunction are more likely to initiate and tolerate treatment with CCBs [36]. Since autonomic dysfunction is such a strong prognostic factor in DLB‐PDD, our results should be treated with caution. Amlodipine’s permeability through the blood–brain barrier (BBB) is questionable, compared to other reported lipophilic DHPs [48]. However, there is growing evidence of an increase in BBB permeability in ageing, neurodegeneration, hypertension and atherosclerosis [49]. It is possible that the concentration of amlodipine in the brains of older hypertensive patients and those with dementia is underestimated.
This study has several strengths and limitations. One strength is the power of a large cohort and long follow‐up, including 18 906 patients. This allowed examination of specific therapeutic agents in different dementia types, which was limited in previous studies.
Confounding by indication has already been mentioned as a concern. The patients prescribed medication to prevent cardiovascular disease might be those expected to live long enough to benefit from treatment. However, these patients also have higher comorbidity burden and are at greater risk of death and cardiovascular events. As mentioned above, the inotropic properties of non‐DHP and short‐acting CCBs are known to worsen heart failure in patients with reduced systolic left ventricular function, which could lead to lower prescription in this patient group.
A reverse epidemiology phenomenon has previously been observed with cardiovascular risk factors, including hypertension, in dementia, consisting of a paradoxical protective role of these risk factors in dementia patients [50, 51]. It is possible that hypertension improves cognition by aiding brain perfusion and is thus associated with less cognitive impairment and better prognosis. Although we attempted to define hypertension with organ damage as a measure of hypertensive severity, we did not have information on blood pressure as a continuous variable, which is an important factor. Although ICD‐10 coding allows for relatively high level of diagnostic precision, actual coding in the NPR tends to be less precise, with a large number of patients receiving ‘unspecified’ diagnoses, as can be seen with the example of heart failure in the descriptive tables. We did not have information on ejection fraction. A greater level of detail would have been valuable also for the angina pectoris diagnosis (since microvascular and/or vasospastic angina may be confounders). For these reasons, we included analyses comparing different types of CCBs which would reduce this bias by restricting analysis to patients with an indication for treatment. However, different CCBs have different indications (e.g. non‐DHP such as verapamil have an indication for rate control in atrial fibrillation) which could confound the results.
Although several indices of potential confounding were included, our database does not contain ICD‐10 coding information gathered outside hospital and outpatient specialist units. The prevalence of some comorbidities could be underestimated, thus presenting a residual confounding effect. In addition, we performed PS‐matched sensitivity analysis thus increasing the comparability between groups. Another epidemiological bias is the prevalent‐user design since data on date of treatment initiation were not available: nonusers could be former users who did not tolerate treatment, and thus not completely comparable to the treated group.
Because dementia is a gradually progressing condition, different subjects can be diagnosed at different time‐points, leading to bias from mixing incident and prevalent cases. We did not have a measure of frailty, so this is a further limitation. Exposure‐based cohorts sometimes suffer from immortal time bias when they define study entry at the time of medication exposure. To avoid these problems, we defined exposure in the three years leading up to dementia diagnosis and controlled for MMSE from that point.
Conclusions
Appropriate control of cardiovascular risk factors is important in patients with dementia. Amlodipine was associated with lower mortality risk in dementia patients diagnosed with AD and DLB‐PDD. AD patients using amlodipine had lower risk of ischaemic stroke compared to other CCB users. Confounding by indication is often a problem in cohort‐based studies on medication, such as this one. However, based on its favourable safety profile and antihypertensive properties demonstrated in clinical trials in the general population, amlodipine could be a good choice for the treatment of hypertensive patients with dementia, particularly those with AD, PDD or DLB.
Author contribution
Irena Kalar: Conceptualization (equal); Data curation (equal); Formal analysis (supporting); Investigation (equal); Validation (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Hong Xu: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Juraj Secnik: Methodology (supporting); Writing‐review & editing (supporting). Emilia Schwertner: Methodology (supporting); Writing‐review & editing (supporting). Milica G. Kramberger: Conceptualization (supporting); Writing‐review & editing (supporting). Bengt Winblad: Conceptualization (supporting); Funding acquisition (equal); Writing‐review & editing (equal). Mia von Euler: Conceptualization (supporting); Data curation (supporting); Writing‐review & editing (supporting). Maria Eriksdotter: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Writing‐review & editing (equal). Sara Garcia‐Ptacek: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
Conflict of Interest Statement
The authors report no conflicts of interest.
Supporting information
Figure S1. Study design.
Figure S2. Dose‐response association for cumulative doses of Calcium Channel Blockers with mortality and stroke risk calculated using Cox hazards regressions.
Table S1. Defined daily dose of main drugs discussed in this study.
Table S2. Stratified analyses of risk of death and ischaemic stroke using Cox–proportional hazard models.
Table S3. Descriptive characteristics of Amlodipine users and Other CCB users in the dementia cohort.
Table S4. (a) Descriptive characteristics of CCB users and CCB non‐users in the PS 1:1 matched cohort (death). (b) Descriptive characteristics of CCB users and CCB non‐users in the PS 1:1 matched cohort (stroke). (c) Descriptive characteristics of Amlodipine users and Other CCB users in the PS 1:1 matched cohort (death). (d) Descriptive characteristics of Amlodipine users and Other CCB users in the PS 1:1 matched cohort (stroke).
Acknowledgements
The authors of this study thank all SveDem and RiksStroke patients, caregivers, reporting units, coordinators and the steering committees in both registers. This study was financially supported by Forte Swedish Research Council for Health, Working Life and Welfare (Garcia‐Ptacek n. 2017‐01646), Johanniterorden i Sverige/Swedish Order of St John, the Swedish Society for Medical Research, Loo and Hans Osterman’s Medical Research Foundation, KI Foundation for Diseases of Aging, the Swedish Stroke Association, Stohne’s Foundation, the Foundation to the Memory of Sigurd and Elsa Golje’s, ALF (von Euler n. 20180302), The Swedish Research Council (B Winblad n. 2018‐02843), Margaretha af Ugglas Foundation (B Winblad) and the Swedish Dementia Foundation (I Kalar). Garcia‐Ptacek is supported by a postdoctoral fellowship from the Swedish Society for Medical Research. The funders played no role in study design or interpretation of results.
Kalar I, Xu H, Secnik J, Schwertner E, Kramberger MG, Winblad B, von Euler M, Eriksdotter M, Garcia‐Ptacek S (Karolinska Institutet, Stockholm, Sweden; University Medical Centre Ljubljana, Ljubljana; University of Ljubljana, Ljubljana, Slovenia; Karolinska Institutet, Stockholm; Karolinska University Hospital, Stockholm; Karolinska Institutet, Stockholm; Södersjukhuset, Stockholm, Sweden). Calcium channel blockers, survival and ischaemic stroke in patients with dementia: a Swedish registry study. J Intern Med 2021; 289: 508–522. 10.1111/joim.13170
References
- 1. Garcia‐Ptacek S, Farahmand B, Kareholt I, Religa D, Cuadrado ML, Eriksdotter M. Mortality risk after dementia diagnosis by dementia type and underlying factors: a cohort of 15,209 patients based on the Swedish Dementia Registry. J Alzheimers Dis 2021; 41: 467–77. [DOI] [PubMed] [Google Scholar]
- 2. Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci 2005; 230: 43–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhu L, Fratiglioni L, Guo Z, Winblad B, Viitanen M. Incidence of stroke in relation to cognitive function and dementia in the Kungsholmen Project. Neurology 2000; 54: 2103–7. [DOI] [PubMed] [Google Scholar]
- 4. Subic A, Cermakova P, Norrving B et al. Management of acute ischaemic stroke in patients with dementia. J Intern Med 2017; 281: 348–64. [DOI] [PubMed] [Google Scholar]
- 5. Winblad B, Amouyel P, Andrieu S et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol 2016; 15: 455–532. [DOI] [PubMed] [Google Scholar]
- 6. Lüscher TF, Cosentino F. The classification of calcium antagonists and their selection in the treatment of hypertension. Drugs 1998; 55: 509–17. [DOI] [PubMed] [Google Scholar]
- 7. Godfraind T. Discovery and Development of Calcium Channel Blockers. Front Pharmacol 2017; 8: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozawa Y, Hayashi K, Kobori H. New generation calcium channel blockers in hypertensive treatment. Curr Hypertens Rev 2006; 2: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pchitskaya E, Popugaeva E, Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 2018; 70: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khachaturian ZS. Calcium hypothesis of Alzheimer's disease and brain aging. Ann N Y Acad Sci 1994; 747: 1–11. [DOI] [PubMed] [Google Scholar]
- 11. Alzheimer's Association Calcium Hypothesis Workgroup . Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 2017; 13: 178‐82 e17. [DOI] [PubMed] [Google Scholar]
- 12. van Middelaar T, van Vught LA, Moll van Charante EP et al. Lower dementia risk with different classes of antihypertensive medication in older patients. J Hypertens 2017; 35: 2095–101. [DOI] [PubMed] [Google Scholar]
- 13. Feldman L, Vinker S, Efrati S et al. Amlodipine treatment of hypertension associates with a decreased dementia risk. Clin Exp Hypertens 2016; 38: 545–9. [DOI] [PubMed] [Google Scholar]
- 14. Tully PJ, Dartigues JF, Debette S, Helmer C, Artero S, Tzourio C. Dementia risk with antihypertensive use and blood pressure variability: A cohort study. Neurology 2016; 87: 601–8. [DOI] [PubMed] [Google Scholar]
- 15. Wu CL, Wen SH. A 10‐year follow‐up study of the association between calcium channel blocker use and the risk of dementia in elderly hypertensive patients. Medicine (Baltimore) 2016; 95: e4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khachaturian AS, Zandi PP, Lyketsos CG et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol 2006; 63: 686–92. [DOI] [PubMed] [Google Scholar]
- 17. Yasar S, Corrada M, Brookmeyer R, Kawas C. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging. Neurobiol Aging 2005; 26: 157–63. [DOI] [PubMed] [Google Scholar]
- 18. Maxwell CJ, Hogan DB, Ebly EM. Calcium‐channel blockers and cognitive function in elderly people: results from the Canadian Study of Health and Aging. CMAJ 1999; 161: 501–6. [PMC free article] [PubMed] [Google Scholar]
- 19. Gelber RP, Ross GW, Petrovitch H, Masaki KH, Launer LJ, White LR. Antihypertensive medication use and risk of cognitive impairment: the Honolulu‐Asia Aging Study. Neurology 2013; 81: 888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters R, Booth A, Peters J. A systematic review of calcium channel blocker use and cognitive decline/dementia in the elderly. J Hypertens 2014; 32: 1945–57; discussion 57–8. [DOI] [PubMed] [Google Scholar]
- 21. Lopez‐Arrieta JM, Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev 2000; CD000147. 10.1002/14651858.CD000147 [DOI] [PubMed] [Google Scholar]
- 22. Iadecola C, Yaffe K, Biller J et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension 2016; 68: e67–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Forette F, Seux ML, Staessen JA et al. Prevention of dementia in randomised double‐blind placebo‐controlled Systolic Hypertension in Europe (Syst‐Eur) trial. Lancet 1998; 352: 1347–51. [DOI] [PubMed] [Google Scholar]
- 24. Religa D, Fereshtehnejad SM, Cermakova P et al. SveDem, the Swedish Dementia Registry ‐ a tool for improving the quality of diagnostics, treatment and care of dementia patients in clinical practice. PLoS One 2015; 10: e0116538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swedish Dementia Registry‐yearly report . Svenska Demensregister SveDem årsrapport 2018. Huddinge. 2018; 2019: 9–10. [Google Scholar]
- 26. Religa D, Spangberg K, Wimo A, Edlund AK, Winblad B, Eriksdotter‐Jonhagen M. Dementia diagnosis differs in men and women and depends on age and dementia severity: data from SveDem, the Swedish Dementia Quality Registry. Dement Geriatr Cogn Disord 2012; 33: 90–5. [DOI] [PubMed] [Google Scholar]
- 27. Johnell K, Religa D, Eriksdotter M. Differences in drug therapy between dementia disorders in the Swedish dementia registry: a nationwide study of over 7,000 patients. Dement Geriatr Cogn Disord 2013; 35: 239–48. [DOI] [PubMed] [Google Scholar]
- 28. Garcia‐Ptacek S, Modeer IN, Kareholt I et al. Differences in diagnostic process, treatment and social Support for Alzheimer's dementia between primary and specialist care: results from the Swedish Dementia Registry. Age Ageing 2017; 46: 314–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ludvigsson JF, Andersson E, Ekbom A et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bramer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q 1988; 41: 32–6. [PubMed] [Google Scholar]
- 31. Wettermark B, Hammar N, Fored CM et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–35. [DOI] [PubMed] [Google Scholar]
- 32. Tzourio C, Anderson C, Chapman N et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med 2003; 163: 1069–75. [DOI] [PubMed] [Google Scholar]
- 33. WHO Collaborating Centre for Drug Statistics Methodology, ATC classification index with DDDs, 2020. Oslo, Norway 2019.
- 34. Asplund K, Hulter Asberg K, Appelros P et al. The Riks‐Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke 2011; 6: 99–108. [DOI] [PubMed] [Google Scholar]
- 35. Ludvigsson JF, Almqvist C, Bonamy AK et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016; 31: 125–36. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Ptacek S, Kramberger MG. Parkinson Disease and Dementia. J Geriatr Psychiatry Neurol 2016; 29: 261–70. [DOI] [PubMed] [Google Scholar]
- 37. Furberg CD, Psaty BM, Nifedipine Meyer JV. Dose‐related increase in mortality in patients with coronary heart disease. Circulation 1995; 92: 1326–31. [DOI] [PubMed] [Google Scholar]
- 38. Opie LH, Yusuf S, Kubler W. Current status of safety and efficacy of calcium channel blockers in cardiovascular diseases: a critical analysis based on 100 studies. Prog Cardiovasc Dis 2000; 43: 171–96. [DOI] [PubMed] [Google Scholar]
- 39. Poole‐Wilson PA, Lubsen J, Kirwan BA et al. Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004; 364: 849–57. [DOI] [PubMed] [Google Scholar]
- 40. Jung SY, Choi NK, Kim JY et al. Short‐acting nifedipine and risk of stroke in elderly hypertensive patients. Neurology 2011; 77: 1229–34. [DOI] [PubMed] [Google Scholar]
- 41. Lindqvist M, Kahan T, Melcher A, Ekholm M, Hjemdahl P. Long‐term calcium antagonist treatment of human hypertension with mibefradil or amlodipine increases sympathetic nerve activity. J Hypertens 2007; 25: 169–75. [DOI] [PubMed] [Google Scholar]
- 42. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288: 2981–97. [DOI] [PubMed] [Google Scholar]
- 43. Pitt B, Byington RP, Furberg CD et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation 2000; 102: 1503–10. [DOI] [PubMed] [Google Scholar]
- 44. Dahlof B, Sever PS, Poulter NR et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomised controlled trial. Lancet 2005; 366: 895–906. [DOI] [PubMed] [Google Scholar]
- 45. Fares H, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Amlodipine in hypertension: a first‐line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 2016; 3: e000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simms BA, Zamponi GW. Neuronal voltage‐gated calcium channels: structure, function, and dysfunction. Neuron 2014; 82: 24–45. [DOI] [PubMed] [Google Scholar]
- 47. Yagami T, Kohma H, Yamamoto Y. L‐type voltage‐dependent calcium channels as therapeutic targets for neurodegenerative diseases. Curr Med Chem 2012; 19: 4816–27. [DOI] [PubMed] [Google Scholar]
- 48. Bachmeier C, Beaulieu‐Abdelahad D, Mullan M, Paris D. Selective dihydropyiridine compounds facilitate the clearance of beta‐amyloid across the blood‐brain barrier. Eur J Pharmacol 2011; 659: 124–9. [DOI] [PubMed] [Google Scholar]
- 49. Montagne A, Barnes SR, Sweeney MD et al. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Levin NW, Handelman GJ, Coresh J, Port FK, Kaysen GA. Reverse epidemiology: a confusing, confounding, and inaccurate term. Semin Dialysis 2007; 20: 586–92. [DOI] [PubMed] [Google Scholar]
- 51. Garcia‐Ptacek S, Kareholt I, Farahmand B, Cuadrado ML, Religa D, Eriksdotter M. Body‐mass index and mortality in incident dementia: a cohort study on 11,398 patients from SveDem, the Swedish Dementia Registry. J Am Med Dir Assoc 2014; 15: 447.e1–e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design.
Figure S2. Dose‐response association for cumulative doses of Calcium Channel Blockers with mortality and stroke risk calculated using Cox hazards regressions.
Table S1. Defined daily dose of main drugs discussed in this study.
Table S2. Stratified analyses of risk of death and ischaemic stroke using Cox–proportional hazard models.
Table S3. Descriptive characteristics of Amlodipine users and Other CCB users in the dementia cohort.
Table S4. (a) Descriptive characteristics of CCB users and CCB non‐users in the PS 1:1 matched cohort (death). (b) Descriptive characteristics of CCB users and CCB non‐users in the PS 1:1 matched cohort (stroke). (c) Descriptive characteristics of Amlodipine users and Other CCB users in the PS 1:1 matched cohort (death). (d) Descriptive characteristics of Amlodipine users and Other CCB users in the PS 1:1 matched cohort (stroke).


