Summary
Non-human animals steeply discount the future, showing a preference for small, immediate over large, delayed rewards [1–5]. Currently unclear is whether discounting functions depend on context. Here we examine the effects of spatial context on discounting in cotton-top tamarins (Saguinus oedipus) and common marmosets (Callithrix jacchus), species known to differ in temporal discounting [5]. We presented subjects with a choice between small, nearby rewards and large, distant rewards. Tamarins traveled farther for the large reward than marmosets, attending to the ratio of reward differences rather than their absolute values. This species difference contrasts with performance on a temporal task in which marmosets waited longer than tamarins for the large reward. These comparative data indicate that context influences choice behavior, with the strongest effect seen in marmosets who discounted more steeply over space than over time. These findings parallel details of each species’ feeding ecology. Tamarins range over large distances and feed primarily on insects, which requires using quick, impulsive action. Marmosets range over shorter distances than tamarins and feed primarily on tree exudates, a clumped resource that requires patience to wait for sap to exude [6–9]. These results show that discounting functions are context-specific, shaped by a history of ecological pressures.
Keywords: discounting, foraging, context, tamarins, marmosets
Results and Discussion
Tradeoffs between smaller, immediate gains and larger, delayed rewards are ubiquitous for both humans and non-human animals (hereafter, animals) [10, 11]. Many animal species highly discount the future, devaluing rewards by 50% in the first few seconds of delay [1–3, 5, 12, 13]. Animal discounting stands in stark contrast to human discounting where subjects wait for weeks, months, and years [10, 14]. In these experiments, however, subjects often chose between hypothetical monetary rewards over hypothetical timeframes (e.g., “Would you prefer to receive $50 now or $2000 in three years?”). Experiments that more closely mimic the animal foraging tasks by offering real monetary rewards and making subjects wait for real time delays show much more impulsive choices in humans [15]. This implies that the experimental context in which discounting choices are framed can directly influence decision making.
Few studies have examined the effect of context on discounting behavior in animals (but see [4, 16–19]). Here we examine the role of context by comparing choice preferences in different types of discounting tasks: temporal and spatial.
In previous research on temporal discounting, we offered cotton-top tamarins and common marmosets choices between a small food reward available immediately and a larger reward available after a time delay [5]. Results showed that marmosets waited significantly longer for the larger reward, suggesting that they discounted the temporal delay less steeply than tamarins. In the current task, we assessed these species’ spatial discounting levels by presenting subjects with a choice between a smaller, closer reward and a larger, more distant reward. This choice maps onto natural foraging decisions frequently faced by animals: consume the few remaining food items nearby or travel to locate an untapped patch replete with food [20, 21]. We placed the close reward 35 cm from the starting position and placed the distant reward at one of seven distances, ranging from 35 to 245 cm away (Figure 1). With this design, we characterized how both species devalue food rewards as a function of travel distance. If context does not affect discounting, then we should find the same pattern observed in the temporal discounting experiment. Because the time to receiving the reward is proportional to the distance traveled, the more patient marmosets should also prefer to travel farther. If, however, context does influence discounting in these primates (as it can in humans [15, 22–24]), spatial discounting preferences may differ from temporal discounting preferences.
Figure 1: Experimental apparatus.
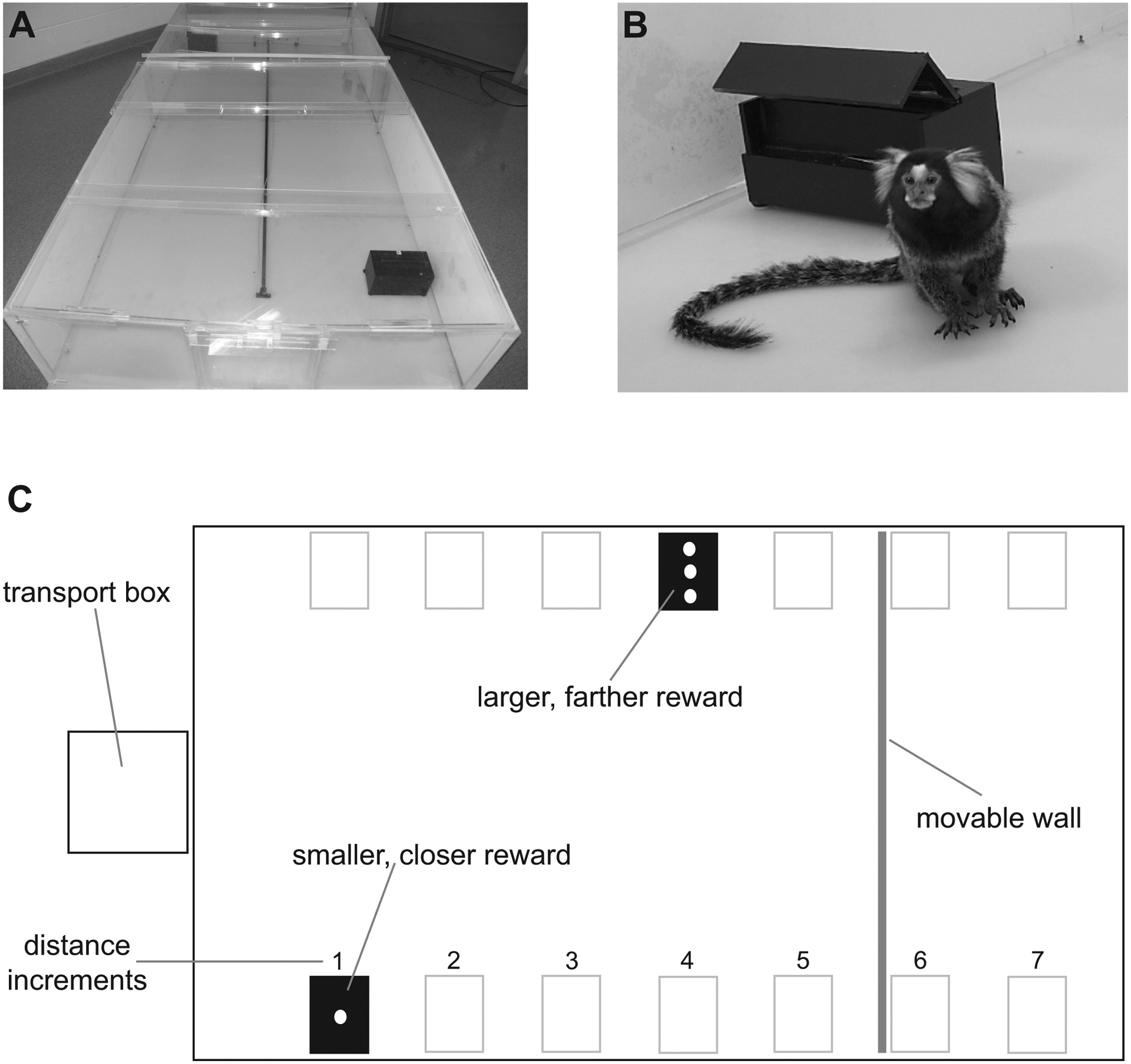
(A) Both tamarins and marmosets traveled to receive their rewards in a Plexiglas enclosure.
(B) The food rewards were lined up in an array on a ledge in the box, each piece approximately 1 cm apart.
(C) Food boxes were placed at one of seven distances (35–245 cm) from the front of the enclosure. A wall was placed behind the far box.
The magnitude of the reward also influences discounting decisions. Models predict that the ratio between reward values, and not the absolute magnitude of those rewards, should determine discounting patterns [1, 25, 26]. Discounting studies in pigeons and rats support these predictions: varying the magnitude of rewards does not influence discounting levels [3, 27, 28] (but see [29, 30] for possible exceptions). In contrast, humans seem to discount small rewards more highly than large rewards [14, 31, 32]. We tested for magnitude effects by offering our subjects two sets of numerical contrasts. Subjects chose between either one close and three far food pellets in one condition or two close and six far pellets in another condition. Therefore, we maintained the 1:3 ratio of the reward amounts but varied their absolute magnitudes, allowing us to assess whether these monkeys ignore magnitude as demonstrated by other animals or discount differently over different magnitudes like humans.
When we presented both rewards at the shortest distance (increment 1), subjects (pooled over species) chose the large reward in 96.1% ± 1.5% (mean ± SE) of the trials but only chose it in 68.8% ± 8.0% of the trials when we placed the large reward at the farthest distance (increment 7). The distance to the large reward significantly affected the subject’s probability of choosing the large reward (repeated-measures ANOVA: F5,32 = 5.35, p < 0.01). Subjects reduced their preferences for the large reward when placed farther away from them. However, a species difference appears to drive this distance effect. The two species tended to differ in their overall preference for the large reward, although this difference did not reach statistical significance (F1,6 = 4.42, p = 0.08). There was, however, a significant interaction between species and distance (F5,33 = 3.43, p = 0.01). Marmosets selected the larger reward less at the farthest distances (increments 6 and 7) relative to the closest distance (increment 1), but tamarins were equally likely to choose the larger reward at all distances (Bonferroni post-hoc tests, p < 0.05). Thus, marmosets selected the larger reward less frequently as a function of increasing distance, whereas tamarins maintained their preferences for the large reward independently of distance (Figure 2). Further analyses and a follow-up experiment suggest that neither satiation nor visual discrimination differences can account for this pattern (see Supplementary Materials).
Figure 2: Effect of species and distance on choice.
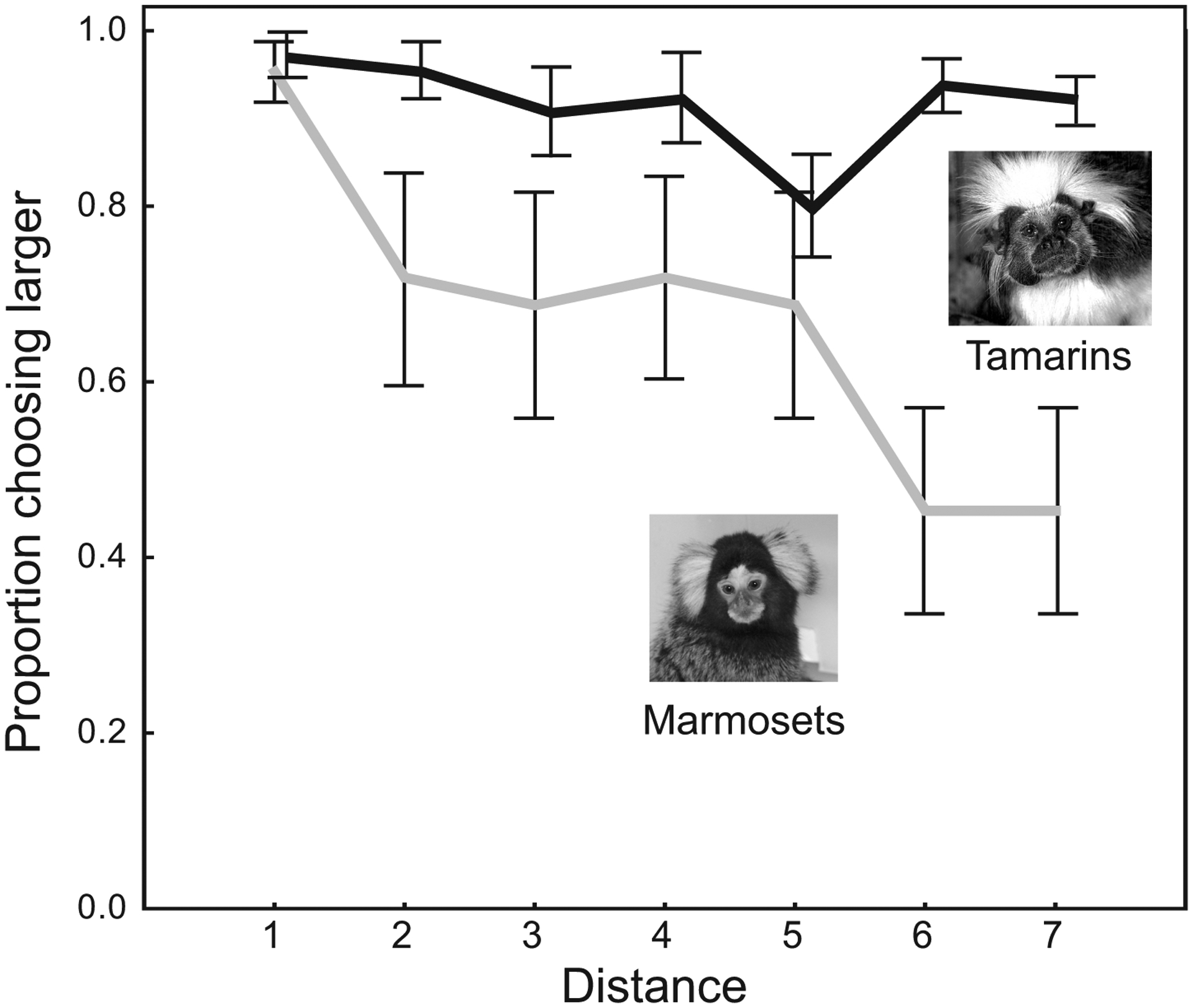
Tamarins maintained their preference for the large reward across all distances, whereas marmosets reduced their preference for large as the distance to large increased. Error bars represent standard error of the mean.
The marmosets’ relative preference for near contrasts with their ability to wait longer than tamarins for the large reward in the temporal discounting task [5]. This reversal could occur because tamarins travel faster than marmosets, therefore requiring less time to receive the large reward. To investigate this possibility, we measured the time required to travel to the closest and farthest rewards in follow-up sessions (see Supplementary Material). Overall, tamarins ran to the boxes in less time than the marmosets (F1,6 = 10.38, p = 0.02), and this difference depended on distance (F1,6 = 15.93, p < 0.01): tamarins traveled to the farthest rewards faster than the marmosets (planned comparison, F1,6 = 15.15, p < 0.01; Figure 3). Although marmosets did take longer than tamarins to reach the farthest reward, their travel times were nonetheless much shorter than the intervals that marmosets waited in the temporal discounting task. For temporal discounting, tamarins waited an average of 7.9 sec while marmosets waited an average of 14.4 sec for six food pellets [5]. To more quantitatively assess whether temporal discounting can account for the species difference in preferences, we used the hyperbolic discounting equation (where V = subjective value of a reward, A = reward amount, k = discount factor, and t = time delay to receiving the reward [33]) to estimate a discounting factor for each species using the data from the temporal discounting experiment (see Supplementary Materials). When we analyzed these discounting factors along with the travel times in the spatial discounting experiment, we found that these temporal discounting factors predicted complete preference for the more distant reward. Thus, we conclude that factors beyond those imposed by temporal discounting influenced the spatial discounting of marmosets. Though the marmoset results are inconsistent with temporal discounting alone, we cannot rule out an exclusive effect of temporal discounting on tamarins preferences. Further data are needed to clarify the role of time in tamarin spatial preferences.
Figure 3: Effect of species and distance on running time.
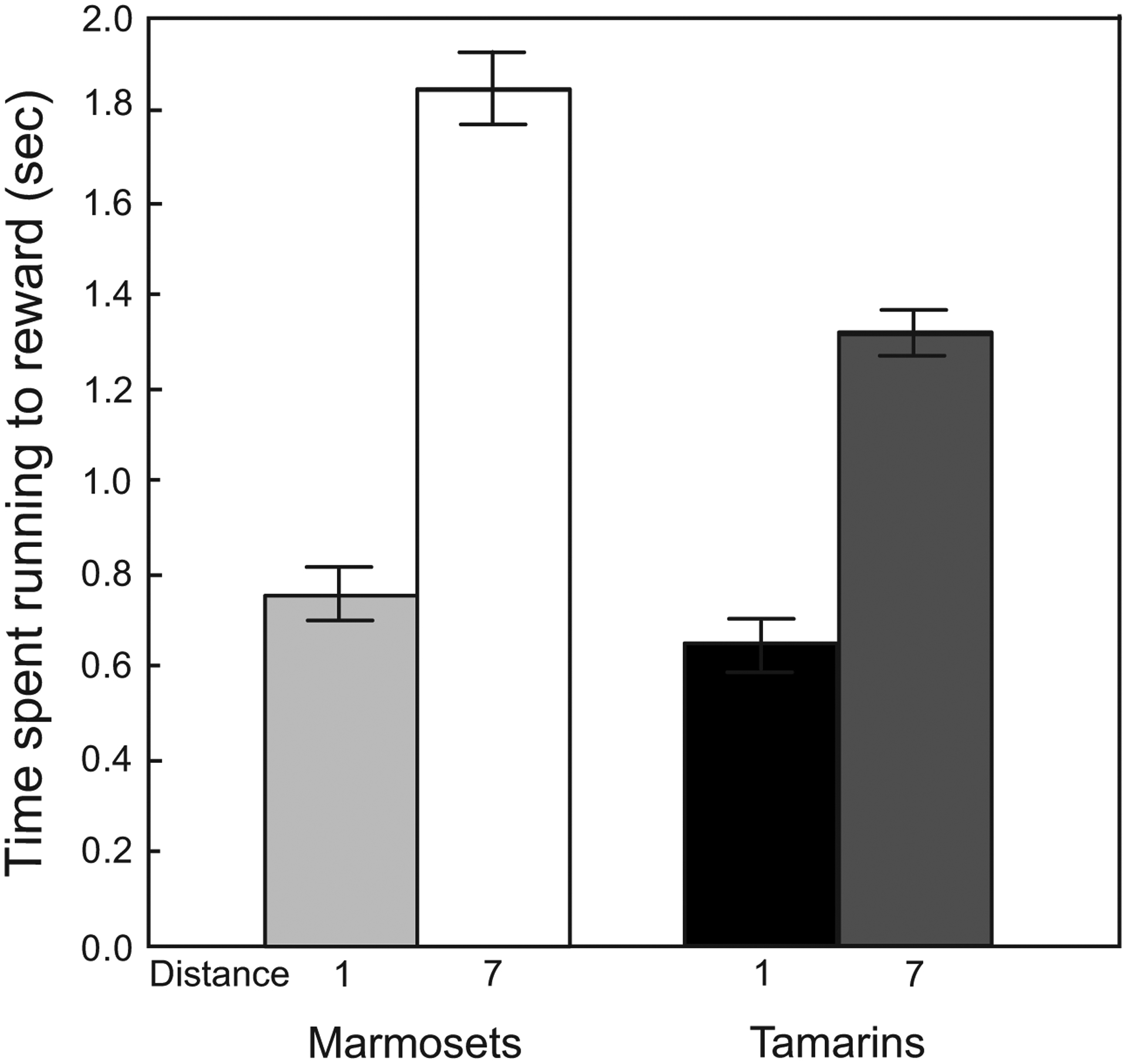
Tamarins and marmosets took the same amount of time to travel to the short distance (increment 1; 35 cm). Tamarins, however, traveled to the farthest distance (increment 7; 245 cm) significantly faster than marmosets. Error bars represent standard error of the mean.
To determine whether reward magnitude influences tamarin and marmoset discounting, we compared sessions in which subjects chose between one and three pellets to those in which they chose between two and six pellets. Subjects showed no significant difference in preference for the larger reward across the two magnitude conditions (F1,6 = 1.55, p = 0.26). There was also no significant interaction between magnitude and distance (F5,29 = 1.50, p = 0.22) or between magnitude and species (Figure 4—F1,6 = 0.03, p = 0.88). Therefore, changes in absolute magnitude did not influence discounting in these monkeys when the ratio between rewards remained constant.
Figure 4: Effect of species and reward magnitude on choice.
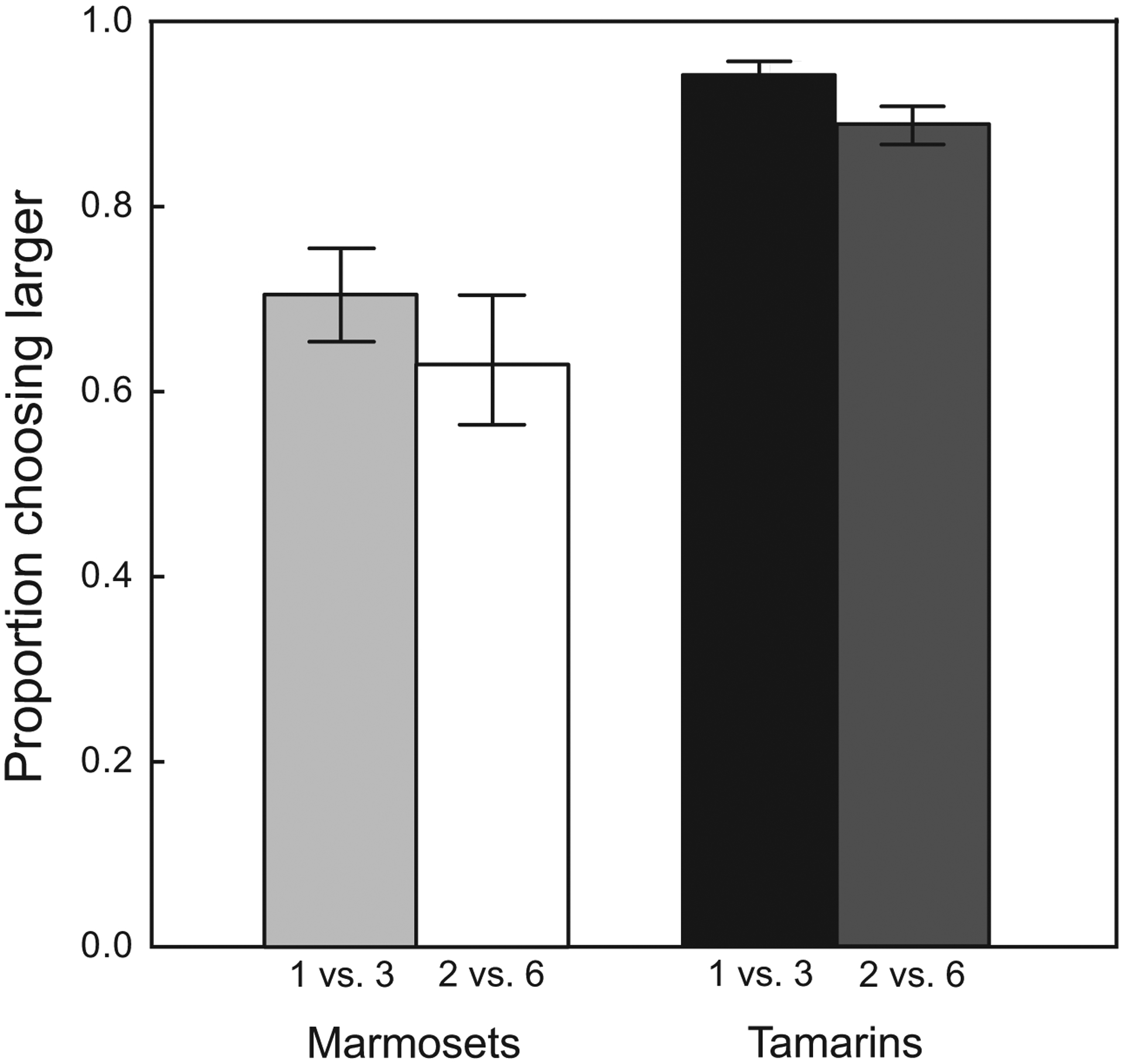
Neither tamarins nor marmosets altered their preferences for the larger reward when choosing between one and three pellets or between two and six pellets. Therefore, they maintained their preferences for the same ratio of rewards but ignored absolute magnitude. Error bars represent standard error of the mean.
Space and time
Faced with the same sets of decisions between smaller, closer rewards and larger, more distant rewards, tamarins traveled farther for rewards than marmosets. While marmosets reduced their preference for the large reward at the farthest distances (210–245 cm), tamarins did not discount at these distances. This demonstrates a reversal from the previous findings in which marmosets waited longer in a temporal discounting task [5]. Because the tamarins did not discount over these distances, we cannot determine whether spatial context in particular and context more generally, affects their discounting. However, both the disparity between the marmosets’ preferences in these two tasks and a quantitative analysis of their temporal discounting levels implies that context influenced their decision making—spatial discounting was not equivalent to temporal discounting. As a result, something in addition to time must have played a role in their spatial discounting decisions.
Two other factors may account for the observed differences: energetic costs of traveling and predation risk. Models of temporal discounting behavior that take only reward quantity and time delays into account may make good approximations of animal choice because the metabolic cost of waiting for a food reward to appear may be negligible. However, when animals must actively work to obtain food, the associated energetic costs are no longer trivial. For example, European starlings (Sturnus vulgaris) adjusted their preference to account for both the gain associated with rewards and the travel cost of obtaining those rewards by means of walking as opposed to flying [16]. Additionally, brown capuchin monkeys (Cebus apella) demonstrated a rapid decrease in preferences for distant rewards, perhaps due to energetic costs of movement as well as temporal aspects of intake rate [20, 21]. It is likely that tamarins and marmosets also include the energetic costs of traveling in their spatial discounting decisions.
Concerning predation risk, Waite [17] found that grey jays (Perisoreus canadensis) were more reluctant to retrieve a large food reward deep inside a tube when they previously had to travel only half-way into the tube for the same reward amount. Waite interpreted this result as a reflection of the increased predation risk associated with traveling farther into the tube. Although the tamarins and marmosets in our study were all born in captivity, they have observed free-flying raptors outside of their colony room and have experienced direct exposure to approaches by a trained Northern goshawk (Accipiter gentiles) (A. Palleroni, C. Sproul, M.D. Hauser, unpublished data). Consequently, they might have perceived a potential predation risk when entering our apparatus.
Our results suggest that, at least in marmosets, discounting behavior is context-specific: they will wait for food longer than tamarins but will not travel as far. A major selective force that underlies foraging decisions is ecological context. Previously, we ascribed the differences in tamarin and marmoset temporal discounting to ecological pressures, and in particular, aspects of their feeding behavior [5] (see [34] for a similar argument for memory differences in other tamarin and marmoset species). A key difference between the two species is the primary food items in their diet: tamarins specialize on insects, whereas marmosets specialize on gum and sap exuding from trees [6–9]. This difference in foraging ecology aligns with the temporal discounting results: tamarins primarily consume an ephemeral food source that requires impulsive action, whereas marmosets prefer to feed on a food source that requires scratching tree bark and then patiently waiting for sap to exude. These foraging differences may also account for ranging differences between species [9]. Because tamarins feed on an ephemeral, dispersed food source, they travel through large territories to find insects. Marmosets, however, feed on a localized, immobile food source and, consequently, face little pressure to travel long distances for food [9]. As a result, the territory sizes of these species are non-overlapping, with tamarins averaging 7.8 to 10 ha and marmosets 0.5 to 5 ha [7, 8]; moreover, tamarins travel farther on a daily basis (1700 m) than marmosets (700 m) [35, 36]. As such, these two discounting tasks may actually trigger different discounting strategies in the two species and reflect the innate preferences each species has for one foraging mode over the other.
Although our data are consistent with the foraging ecology hypothesis, we cannot completely exclude other hypotheses. It is possible that foraging ecology has shaped the cost/benefit functions of the species, such that these species differ in their travel costs or predation risk and, therefore, have different optimal strategies. Alternatively, other differences between these species may account for our results, such as general activity level, muscle mass, limb length, or basal metabolic rate. However, gummivory is a very powerful selective force that has led to adaptive specializations in tooth morphology and digestive physiology in marmosets [6, 37]. The far-reaching effects of gummivory on other aspects of physiology and behavior are difficult to disentangle from other selective forces.
Reward magnitudes
Reward magnitude does not appear to influence tamarin or marmoset preferences: both species discounted at the same rate regardless of whether they chose between one and three pieces of food or between two and six pieces of food. This corroborates previous studies of animal discounting levels in which there is no effect of magnitude on choice behavior [3, 27, 28]. As of yet, only humans reliably demonstrate a magnitude effect in discounting tasks, discounting smaller rewards more highly than large rewards [14, 31, 32].
This divergence between the human and animal data may result from different methodologies used to study discounting. Studies of human discounting use monetary rewards which are often hypothetical. As such, these rewards can be much larger than any feasible food reward. For example, in two studies that found a magnitude effect, one [14] titrated large money amounts ranging from $100 to $100,000, and the other [31] used reward amounts ranging up to one million dollars. Thus, animals may not demonstrate a magnitude effect simply because the phenomenon in humans is an artifact of presenting extremely large reward quantities—quantities that are both impossible to offer animals in the laboratory, and unlikely to occur in the wild, including our own species’ early history.
Experimental Procedures
Experimental design
Four cotton-top tamarins (three females and one male) and four common marmosets (two males and two females) of mixed experimental history participated in this experiment. Each subject experienced seven distance comparisons. For all distance comparisons we placed the small reward one distance increment (35 cm) from the front of the enclosure, while we placed the larger reward progressively farther away from the subject on subsequent sessions. Initially, the distance to the larger reward was the same as the distance to the smaller reward (35 cm); in the next session, the larger reward was moved two distance increments (70 cm) away while the smaller reward remained at one distance increment. This process continued until the larger reward was placed seven distance increments (245 cm) away, the total length of the enclosure (Figure 1c).
Subjects completed these seven distances for two reward magnitude comparisons: one versus three banana-flavored food pellets (Research Associates 45 mg purified primate diet pellets) and two versus six food pellets. We counter-balanced the order of presenting the two magnitude conditions across subjects.
Apparatus and setup
We placed subjects in a small transport cage (30×30×30 cm) abutting the front of the large, Plexiglas test enclosure (240×120×45 cm). A transparent Plexiglas door allowed the subjects to see into the enclosure. The enclosure consisted of opaque white walls and a transparent Plexiglas ceiling (Figure 1a). In addition, the enclosure had a movable back wall that we adjusted such that it was placed 70 cm behind the far reward for distance increments 1–6 and 35 cm behind the far reward for increment 7.
Trial and session procedures
The experimenter placed two black boxes (20×11×11 cm) that contained the food rewards in the enclosure (Figure 1b). We lined up the food rewards in an array on a ledge inside the box, each piece approximately 1 cm apart. After placing food in the boxes at the appropriate distances, the experimenter waited 10 seconds for the subject to view the choices and then removed the door. After removing the door, the subject had one minute to leave the transport box and enter the apparatus, and then had 30 seconds to make a decision. As soon as the subject made a choice (by touching a pellet) in a free session, the experimenter used a remote control to close the non-chosen reward box, eliminating the possibility of obtaining these food pellets. We trained subjects to return to the starting transport box after consuming their chosen reward.
For each distance increment subjects first completed a forced-choice session of eight trials. In these sessions, subjects received only one option per trial and thus gained experience with both distances and reward contingencies. We presented four smaller, closer reward trials and four larger, farther reward trials in randomized order. The following day, subjects completed a free-choice session of eight trials at the same distance increment, in which we allowed them to choose between the two options. In both session types, we randomly assigned the side of the enclosure for larger and smaller rewards for each trial. Please see Supplementary Materials for further details on experimental methodology.
Supplementary Material
Acknowledgements
We are grateful for funding from the National Research Service Award (NIH) to JRS, the Harvard College Research Program to AGR, and an NSF-ROLE grant to MDH. We thank Jen Gong, Sarah Heilbronner, Teddy Jones, and Jenny Pegg for assistance in conducting the experiments and Matt Kamen for building the apparatus. We appreciate comments on the manuscript from Fiery Cushman, Sarah Heilbronner, Tim O’Donnell, and three anonymous referees.
References
- 1.Mazur JE (1987). An adjusting procedure for studying delayed reinforcement. In Quantitative Analyses of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value, Volume 5,Commons ML,Mazur JE,Nevin JA andRachlin H, eds. (Hillsdale, NJ: Lawrence Erlbaum Associates; ), pp. 55–73. [Google Scholar]
- 2.Bateson M, and Kacelnik A (1996). Rate currencies and the foraging starling: the fallacy of the averages revisited. Behav. Ecol 7, 341–352. [Google Scholar]
- 3.Richards JB, Mitchell SH, de Wit H, and Seiden LS (1997). Determination of discount functions in rats with an adjusting-amount procedure. J. Exp. Anal. Behav 67, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens DW, and Anderson D (2001). The adaptive value of preference for immediacy: when shortsighted rules have farsighted consequences. Behav. Ecol 12, 330–339. [Google Scholar]
- 5.Stevens JR, Hallinan EV, and Hauser MD (2005). The ecology and evolution of patience in two New World primates. Biol. Lett 1, 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coimbra-Filho AF, and Mittermeier RA (1976). Exudate-eating and tree-gouging in marmosets. Nature 262, 630–630. [Google Scholar]
- 7.Stevenson MF, and Rylands AB (1988). The marmosets, genus Callithrix. In Ecology and Behavior of Neotropical Primates, Volume 2,Mittermeier RA,Rylands AB,Coimbra-Filho AF andFonseca GAB, eds. (Washington, DC: World Wildlife Fund; ), pp. 131–222. [Google Scholar]
- 8.Snowdon CT, and Soini P (1988). The tamarins, genus Saguinus. In Ecology and Behavior of Neotropical Primates, Volume 2,Mittermeier RA,Rylands AB,Coimbra-Filho AF andFonseca GAB, eds. (Washington, DC: World Wildlife Fund; ), pp. 223–298. [Google Scholar]
- 9.Harrison ML, and Tardif SD (1994). Social implications of gummivory in marmosets. Amer. J. Phys. Anthropol 95, 399–408. [DOI] [PubMed] [Google Scholar]
- 10.Frederick S, Loewenstein G, and O’Donoghue T (2002). Time discounting and time preference: a critical review. J. Econ. Lit XL, 351–401. [Google Scholar]
- 11.Kacelnik A (2003). The evolution of patience. In Time and Decision: Economic and Psychological Perspectives on Intertemporal Choice, Loewenstein G, Read D and Baumeister RF, eds. (New York: Russell Sage Foundation; ), pp. 115–138. [Google Scholar]
- 12.Tobin H, Logue AW, Chelonis JJ, and Ackerman KT (1996). Self-control in the monkey Macaca fascicularis. Anim. Learn. Behav 24, 168–174. [Google Scholar]
- 13.Szalda-Petree AD, Craft BB, Martin LM, and Deditius-Island HK (2004). Self-control in rhesus macaques (Macaca mulatta): controlling for differential stimulus exposure. Percept. Motor Skill 98, 141–146. [DOI] [PubMed] [Google Scholar]
- 14.Green L, Myerson J, and McFadden E (1997). Rate of temporal discounting decreases with amount of reward. Mem. Cognition 25, 715–723. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds B, and Schiffbauer R (2004). Measuring state changes in human delay discounting: an experiential discounting task. Behav. Proc 67, 343–356. [DOI] [PubMed] [Google Scholar]
- 16.Bautista LM, Tinbergen J, and Kacelnik A (2001). To walk or to fly? How birds choose among foraging modes. Proc. Nat. Acad. Sci. USA 98, 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waite TA (2001). Background context and decision making in hoarding gray jays. Behav. Ecol 12, 318–324. [Google Scholar]
- 18.Evenden JL, and Ryan CN (1996). The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128, 161–170. [DOI] [PubMed] [Google Scholar]
- 19.Evenden JL (1999). Varieties of impulsivity. Psychopharmacology 146, 348–361. [DOI] [PubMed] [Google Scholar]
- 20.Janson CH (1998). Experimental evidence for spatial memory in foraging wild capuchin monkeys, Cebus apella. Anim. Behav 55, 1229–1243. [DOI] [PubMed] [Google Scholar]
- 21.Janson CH (2000). Spatial movement strategies: theory, evidence, and challenges. In On the Move: How and Why Animals Travel in Groups,Boinski S andGarber PA, eds. (Chicago: University of Chicago Press; ), pp. 165–203. [Google Scholar]
- 22.Mischel W, Shoda Y, and Rodriguez ML (1989). Delay of gratification in children. Science 244, 933–938. [DOI] [PubMed] [Google Scholar]
- 23.Navarick DJ (2004). Discounting of delayed reinforcers: measurement by questionnaires versus operant choice procedures. Psychol. Rec 54, 85–94. [Google Scholar]
- 24.Wilson M, and Daly M (2004). Do pretty women inspire men to discount the future? Proc. R. Soc. Lond B (Suppl.) 271, 177–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samuelson PA (1937). A note on measurement of utility. Rev. Econ. Stud 4, 155–161. [Google Scholar]
- 26.Stephens DW, and Krebs JR (1986). Foraging Theory, (Princeton: Princeton University Press; ). [Google Scholar]
- 27.Grace RC (1999). The matching law and amount-dependent exponential discounting as accounts of self-control choice. J. Exp. Anal. Behav 71, 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green L, Myerson J, Holt DD, Slevin JR, and Estle SJ (2004). Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? J. Exp. Anal. Behav 81, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrar AM, Kieres AK, Hausknecht KA, de Wit H, and Richards JB (2003). Effects of reinforcer magnitude on an animal model of impulsive behavior. Behav. Proc 64, 261–271. [DOI] [PubMed] [Google Scholar]
- 30.Ong EL, and White KG (2004). Amount-dependent temporal discounting? Behav. Proc 66, 201–212. [DOI] [PubMed] [Google Scholar]
- 31.Raineri A, and Rachlin H (1993). The effect of temporal constraints on the value of money and other commodities. J. Behav. Decis. Making 6, 77–94. [Google Scholar]
- 32.Kirby KN, and Marakovic NN (1996). Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychon. B. Rev 3, 100–104. [DOI] [PubMed] [Google Scholar]
- 33.Mazur JE (1984). Tests of an equivalence rule for fixed and variable reinforcer delays. J. Exp. Psychol. Anim. B 10, 426–436. [PubMed] [Google Scholar]
- 34.Platt ML, Brannon EM, Briese TL, and French JA (1996). Differences in feeding ecology predict differences in performance between golden lion tamarins (Leontopithecus rosalia) and Wied’s marmosets (Callithrix kuhli) on spatial and visual memory tasks. Anim. Learn. Behav 24, 384–393. [Google Scholar]
- 35.Hubrecht RC (1985). Home-range size and use and territorial behavior in the common marmoset, Callithrix jacchus jacchus, at the Tapacura Field Station, Recife, Brazil. Inter. J. Primatol 6, 533–550. [Google Scholar]
- 36.Neyman PF (1977). Aspects of the ecology and social organization of free-ranging cotton-top tamarins (Saguinus oedipus) and the conservation status of the species. In The Biology and Conservation of the Callitrichidae,Kleiman DG, ed. (Washington, DC: Smithsonian Institution Press; ), pp. 39–71. [Google Scholar]
- 37.Power ML, and Oftedal OT (1996). Differences among captive callitrichids in the digestive responses to dietary gum. Amer. J. Primatol 40, 131–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


