Abstract
Background:
Although most patients with cutaneous melanoma are non-Hispanic whites (NHWs), minorities consistently suffer worse melanoma-specific survival (MSS). Much of the literature comes from analyses of registries from the 1990s and 2000s.
Objective:
We sought to evaluate whether and to what degree racial disparity in MSS persists since 2010.
Methods:
We analyzed 381,035 patients from the Surveillance, Epidemiology, and End Results registry. Race categories included Hispanic, NHW, non-Hispanic black (NHB), non-Hispanic Asian or Pacific Islander (NHAPI), and non-Hispanic American Indian/Alaska Native (NHAIAN). We evaluated the association between MSS and race in 3 time periods: before the year 2000, 2000 to 2009, and 2010 or later. NHW was the reference group for all analyses.
Results:
Racial disparity worsened from before the year 2000 to 2010 or later for Hispanic (P<.001), NHB (P = .024), and NHAPI (P <.001) patients. Across all minority groups, patients with localized disease suffered increasing disparity (P = .010 for Hispanic, P<.001 for NHB, P = .023 for NHAPI, and P = .042 for NHAIAN patients). Among those with regional and distant disease, Hispanic patients were the only minority to experience worsening disparity (P = .001 and P = .019, respectively).
Limitations:
Lack of immunotherapy and targeted treatment information.
Conclusions:
Racial disparity in MSS is worsening. Improving postdiagnosis management for minorities with localized disease is imperative to mitigate disparity and improve survival.
Keywords: disparities, melanoma, race/ethnicity, SEER, survival
There are long-standing and well-documented racial disparities for patients with melanoma in the United States. Compared with whites, racial and ethnic minorities consistently receive diagnoses at more advanced disease stages and consequently suffer worse morbidity and mortality.1–9 These discrepancies exist despite the higher annual incidence of melanoma in whites versus nonwhites. Multiple studies have reported on the challenges to early detection of melanoma in minorities, including a constellation of biologic, socioeconomic, and cultural factors.10,11 However, the determinants of timely access to health care provide only a partial explanation for disparate outcomes. Racial and ethnic minorities have consistently lower rates of health insurance coverage compared with whites and continue to be underrepresented in melanoma clinical trials, both of which beget further survival disadvantages.12,13
Given ongoing demographic changes in the United States, it is imperative that clinicians recognize the barriers to primary, secondary, and tertiary prevention of melanoma for minority patients.14 The ability to do so requires a thorough understanding of how presenting features and outcomes vary according to race and ethnicity. Yet much of the extant literature comes from population-based analyses of national and state registries from the 1990s and 2000s. There is a paucity of research describing trends in melanoma presentation and survival by race since the introduction of immunotherapy around 2010. We hypothesized that this phenomenon suggests that racial and ethnic minority groups continue to suffer worse outcomes. To test this hypothesis, we assessed the association between racial group and melanoma-specific survival (MSS) by using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry and adjusting for up-to-date information on demographics and clinical characteristics.
METHODS
Patient population
We queried SEER 18 for cutaneous, mucosal, and uveal melanoma cases from 1975 to 2016.15 Melanomas were classified according to the International Classification of Disease for Oncology, 3rd edition. In total, we identified 381,035 cutaneous, 4592 mucosal, and 12,407 uveal cases. We extracted race, ethnicity, age, gender, year of diagnosis, primary tumor site, histologic subtype, and disease stage. We categorized race/ethnicity as Hispanic, non-Hispanic white (NHW), non-Hispanic black (NHB), non-Hispanic Asian or Pacific Islander (NHAPI), and non-Hispanic American Indian/Alaska Native (NHAIAN). Using the SEER summary staging system, disease stage was classified as localized, regional, or distant. The primary outcome was MSS. We evaluated MSS in 3 time periods: before 2000 (<2000; 1975–1999), 2000 to 2009, and 2010 or later (≥2010; 2010–2016).
Statistical analyses
We used analysis of variance to analyze continuous variables and chi-square tests for categorical variables. Kaplan–Meier survival curves were compared with log-rank tests. We evaluated the association of race/ethnicity category with MSS by Cox proportional hazards regression models including racial groups, categories of year of diagnosis (<2000, 2000–2009, and ≥2010), and their interactions. The interaction terms specifically test whether racial disparities increased in melanoma diagnosed from <2000 to ≥2010. We adjusted for age, gender, stage at diagnosis, histologic subtype, and primary site location. To assess the robustness of the persistent racial disparity secular trend, and to confirm that our results were not the effect of selecting to analyze the above 3 time periods, we performed sensitivity analysis using the year of diagnosis as a continuous variable. In all analyses, NHW was used as the reference category. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported with P values obtained by 2-sided Wald tests. P<.05 was considered statistically significant. All statistical analyses were performed using R software (v 4.0.1; available at: http://www.R-project.org/). Assessment of study quality was performed using the Strengthening the Reporting of Observational Studies in Epidemiology checklist.
RESULTS
Different demographic and clinical characteristics between NHW and minority patients with melanoma
In total, there were 398,034 patients (Table I). Of these, 381,035 had cutaneous, 12,407 had uveal, and 4592 had mucosal melanoma. A majority of patients was NHW (95.4%). There were several significant differences between racial groups. A majority of NHW patients was male (57.6%) whereas a majority of NHB and Hispanic patients was female (54.7% and 57.5%, respectively). NHW patients had superficial spreading melanoma more frequently than any minority population; NHB patients more frequently had acral lentiginous melanoma (ALM) than any other group. The most common primary site was the lower limb or hip for Hispanic, NHAPI, and NHB patients (25.9%, 31.4%, and 42.0%, respectively). NHWs more frequently developed melanoma on the trunk or upper limb and shoulder (31.6% and 23.7%, respectively). A higher percentage of minorities had mucosal melanoma compared with NHWs. Across all races, a majority of patients presented with localized disease. However, compared with NHWs, significantly more minorities presented at later disease stages. In total, 12.6% of NHWs presented with regional or distant disease whereas 21.0% of Hispanic, 34.1% of NHBs, 28.6% of NHAPIs, and of 18.6% NHAIANs were diagnosed at more advanced stages. There was a significant difference in tumor thickness between races (P < .001) as well as in ulceration status (P <.001). NHWs had the lowest mean thickness (1.22 mm; 0.93 mm for localized stage) and percent with ulcerated tumor (12.8%; 9.5% for localized stage).
Table I.
Demographics and clinical characteristics of melanoma patients from the Surveillance, Epidemiology, and End Results registry
| Characteristics | NHW | Hispanic | NHB | NHAPI | NHAIAN | P value* |
|---|---|---|---|---|---|---|
| N (%) | 379,736 (95.40) | 12,290 (3.09) | 2286 (0.57) | 2913 (0.73) | 809 (0.20) | |
| Age at diagnosis, y, mean (SD) | 60.25 (16.97) | 55.08 (18.08) | 61.87 (17.70) | 59.02 (18.48) | 55.55 (17.22) | <.001 |
| Male gender, n (%) | 218,849 (57.6) | 5223 (42.5) | 1035 (45.3) | 1385 (47.5) | 414 (51.2) | <.001 |
| Year of diagnosis, n (%) | <.001 | |||||
| <2000 | 82,126 (21.6) | 2113 (17.2) | 584 (25.5) | 647 (22.2) | 139 (17.2) | |
| 2000–2009 | 156,649 (41.3) | 5113 (41.6) | 915 (40.0) | 1192 (40.9) | 323 (39.9) | |
| ≥2010 | 140,961 (37.1) | 5064 (41.2) | 787 (34.4) | 1074 (36.9) | 347 (42.9) | |
| Primary site, n (%) | <.001 | |||||
| Skin of trunk | 119,928 (31.6) | 2932 (23.9) | 298 (13.0) | 565 (19.4) | 218 (26.9) | |
| External ear | 10,874 (2.9) | 294 (2.4) | 14 (0.6) | 40 (1.4) | 25 (3.1) | |
| Unspecified parts of the face | 34,906 (9.2) | 1074 (8.7) | 89 (3.9) | 133 (4.6) | 71 (8.8) | |
| Skin of the scalp and neck | 28,466 (7.5) | 645 (5.2) | 95 (4.2) | 109 (3.7) | 55 (6.8) | |
| Skin of the upper limb and shoulder | 90,130 (23.7) | 2333 (19.0) | 262 (11.5) | 444 (15.2) | 161 (19.9) | |
| Skin of the lower limb and hip | 63,176 (16.6) | 3181 (25.9) | 961 (42.0) | 914 (31.4) | 162 (20.0) | |
| Other skin | 17,142 (4.5) | 806 (6.6) | 231 (10.1) | 240 (8.2) | 61 (7.5) | |
| Mucosal | 3569 (0.9) | 441 (3.6) | 232 (10.1) | 327 (11.2) | 23 (2.8) | |
| Uveal | 11,545 (3.0) | 584 (4.8) | 104 (4.5) | 141 (4.8) | 33 (4.1) | |
| Histologic subtype, n (%) | <.001 | |||||
| Superficial spreading melanoma | 118,737 (31.3) | 3150 (25.6) | 295 (12.9) | 549 (18.8) | 228 (28.2) | |
| Malignant melanoma, NOS | 187,137 (49.3) | 6510 (53.0) | 1308 (57.2) | 1595 (54.8) | 420 (51.9) | |
| Nodular melanoma | 26,858 (7.1) | 1061 (8.6) | 188 (8.2) | 241 (8.3) | 60 (7.4) | |
| Lentigo maligna melanoma | 25,497 (6.7) | 421 (3.4) | 54 (2.4) | 95 (3.3) | 29 (3.6) | |
| Acral lentiginous melanoma | 2551 (0.7) | 495 (4.0) | 303 (13.3) | 250 (8.6) | 23 (2.8) | |
| Desmoplastic melanoma | 4008 (1.1) | 95 (0.8) | 26 (1.1) | 32 (1.1) | 9 (1.1) | |
| Spindle cell melanoma, NOS | 4999 (1.3) | 167 (1.4) | 38 (1.7) | 44 (1.5) | 11 (1.4) | |
| Other | 9949 (2.6) | 391 (3.2) | 74 (3.2) | 107 (3.7) | 29 (3.6) | |
| Stage, n (%) | <.001 | |||||
| Localized | 292,655 (77.1) | 8174 (66.5) | 1169 (51.1) | 1707 (58.6) | 560 (69.2) | |
| Regional | 33,522 (8.8) | 1744 (14.2) | 468 (20.5) | 531 (18.2) | 104 (12.9) | |
| Distant | 14,277 (3.8) | 840 (6.8) | 310 (13.6) | 304 (10.4) | 46 (5.7) | |
| Unstaged | 39,282 (10.3) | 1532 (12.5) | 339 (14.8) | 371 (12.7) | 99 (12.2) | |
| Skin thickness, mm, mean (SD) | 1.22 (1.70) | 1.69 (2.20) | 2.80 (2.84) | 2.13 (2.52) | 1.49 (1.96) | <.001 |
| Skin thickness and localized stage, mm, mean (SD) | 0.93 (1.21) | 1.11 (1.41) | 1.74 (1.95) | 1.34 (1.60) | 1.06 (1.29) | <.001 |
| Skin ulceration, n (%) | 27,288 (12.8) | 1330 (19.6) | 354 (37.3) | 307 (23.0) | 73 (16.2) | <.001 |
| Skin ulceration and localized stage, n (%) | 16,184 (9.5) | 591 (12.2) | 151 (26.6) | 137 (14.8) | 36 (10.8) | <.001 |
| Follow-up, mo, median (IQR) | 86 (34–155) | 71 (24–141) | 77 (26–152) | 76 (26–153) | 74 (27–150) | <.001 |
IQR, Interquartile range; NHAIAN, non-Hispanic American Indian/Alaska Native; NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic black; NHW, non-Hispanic white; NOS, not otherwise specified; SD, standard deviation.
Estimated by analysis of variance for continuous variables and chi-squared tests for categorical variables to compare clinical and demographic characteristics among the racial groups.
Increasing racial disparity in prognosis for patients with cutaneous melanoma despite improving survival
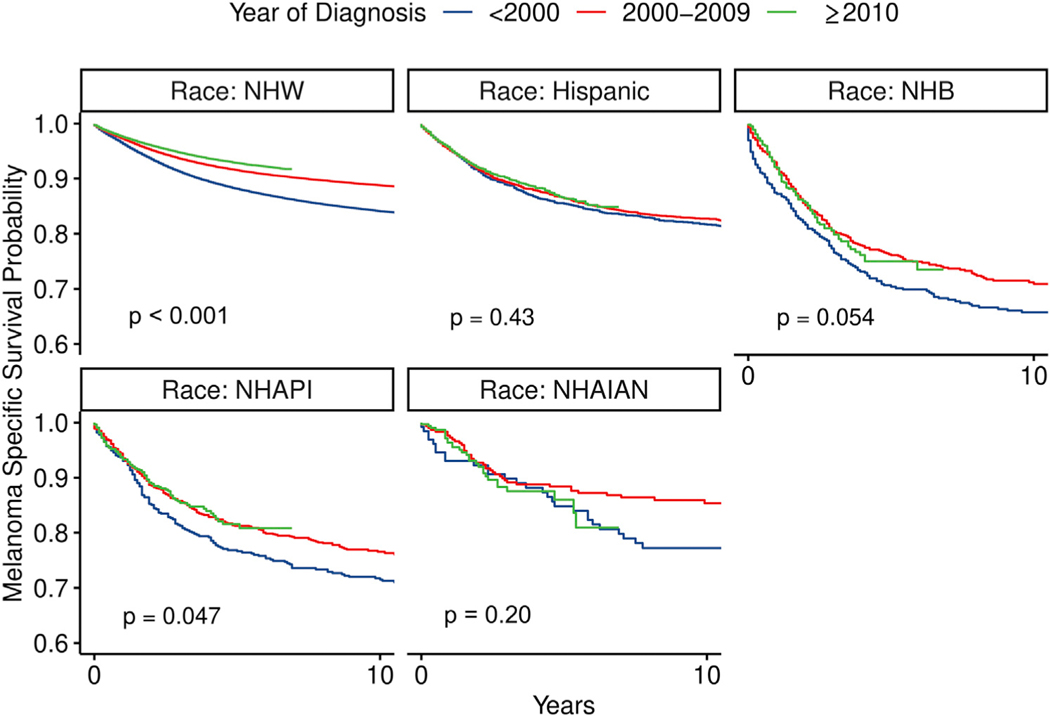
MSS improved for most racial groups from the <2000 period to the ≥2010 period, although it was most significant for NHWs (P <.001, P = .054, and P = .047 for NHWs, NHBs, and NHAPIs, respectively; Fig 1). The improvement in MSS was not significant for Hispanics and NHAIANs. The 5-year MSS rate for NHWs increased from 88.1% (95% CI 87.9–88.4) for melanomas diagnosed <2000 to 92.9% (95% CI 92.7–93.1) for those diagnosed ≥2010. Less improvement was observed for minorities over the same 2 time periods. For Hispanic patients, it improved from 85.4% (95% CI 83.8–87.1) to 86.5% (95% CI 85.0–87.9). In NHBs, the improvement was from 70.4% (95% CI 66.2–74.8) to 75.0% (95% CI 70.5–79.8). In NHAPIs, it was from 76.6% (95% CI 73.0–80.4) to 81.6% (95% CI 77.9–85.4). In NHAIANs, it was from 84.9% (95% CI 78.8–91.4) to 86.0% (95% CI 80.8–91.7). Despite these improvements, minority patients had significantly worse MSS than NHWs (P<.001).
Fig 1.
Estimated survival curves for melanoma-specific survival in different racial groups from the Surveillance, Epidemiology, and End Results cohort and stratified by year of diagnosis. P values were estimated by log-rank test comparing survival outcomes for each racial group in the diagnosis period. NHAIAN, non-Hispanic American Indian/Alaska Native; NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic black; NHW, non-Hispanic white.
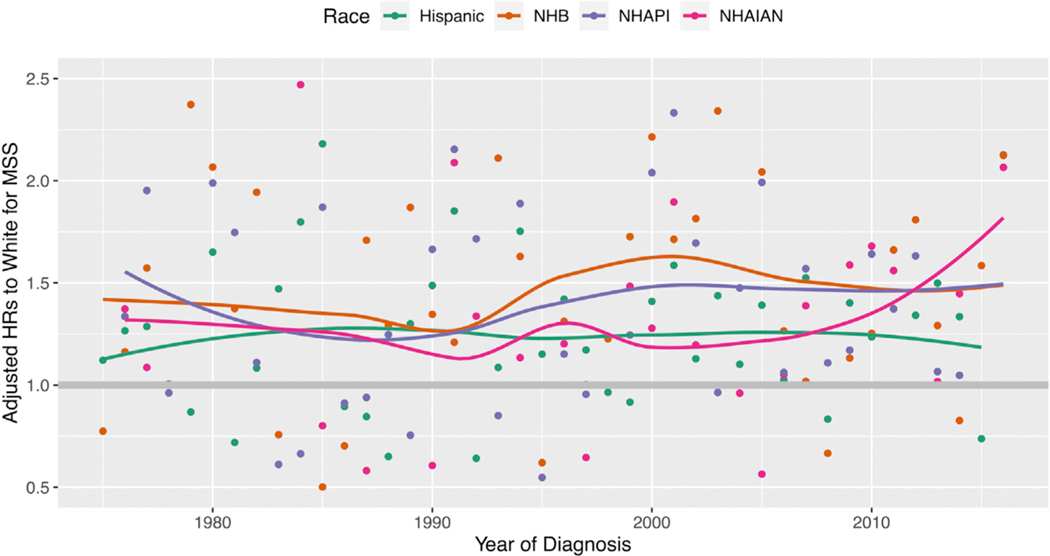
Racial disparity in MSS significantly worsened for Hispanics, NHBs, and NHAPIs from the <2000 period to the ≥2010 period, but not for NHAIANs. The unadjusted HR are shown in Table II. The adjusted HRs (aHRs) are shown in Table III. The aHR for Hispanics compared with NHWs increased from 0.95 (95% CI 0.86–1.06) to 1.56 (95% CI 1.41–1.73; interaction term P <.001). The aHR for NHBs increased from 1.40 (95% CI 1.20–1.64) to 1.87 (95% CI 1.54–2.28; interaction term P = .024). The aHR for NHAPIs increased from 1.20 (95% CI 1.02–1.41) to 1.97 (95% CI 1.62–2.40; interaction term P <.001). Persistent disparity for MSS was observed when we estimated the aHRs of minorities stratified by year of diagnosis as a continuous variable (Fig 2). In a subset analysis of ALM, disparity worsened for Hispanic (P = .001) and NHBs (P = .003) but not NHAPIs (P = .92) or NHAIANs (P = .37; Supplemental Table I available via Mendeley at https://doi.org/10.17632/9hrjwmspc3.1). In mucosal melanoma, there was no significant worsening in disparity (P = .69 for Hispanic; P = .27 for NHB; P = .61 for NHAPI; and P = .49 for NHAIAN patients; Supplemental Table II). This was also true in uveal melanoma (P = .94 for Hispanic; P = .71 for NHB; and P = .77 for NHAPI patients (NHAIANs were not evaluated because of sample size); Supplemental Table III.
Table II.
Unadjusted hazard ratio and 95% confidence intervals for melanoma-specific survival of different racial/ethnicity groups compared with non-Hispanic whites in the Surveillance, Epidemiology, and End Results cohort and across 3 diagnostic time periods for different stages for cutaneous melanoma
| <2000 |
2000–2009 |
≥2010 |
||||
|---|---|---|---|---|---|---|
| Race | HR (95% CI) for MSS | HR (95% CI) for MSS | P for interaction | HR (95% CI) for MSS | P for interaction | |
| Localized stage* | ||||||
| Hispanic | 0.93 (0.79–1.09) | 1.24 (1.08–1.43) | .007 | 1.35 (1.06–1.72) | .012 | |
| NHB | 1.62 (1.22–2.15) | 2.26 (1.70–3.02) | .10 | 4.29 (2.84–6.47) | <.001 | |
| NHAPI | 1.18 (0.89–1.57) | 1.47 (1.10–1.96) | .29 | 2.21 (1.42–3.43) | .020 | |
| NHAIAN | 0.82 (0.44–1.53) | 1.02 (0.58–1.80) | .61 | 1.98 (0.94–4.15) | .076 | |
| Regional stage* | ||||||
| Hispanic | 0.98 (0.81–1.18) | 1.24 (1.10–1.40) | .042 | 1.46 (1.22–1.75) | .003 | |
| NHB | 1.72 (1.30–2.27) | 1.48 (1.18–1.84) | .41 | 1.80 (1.28–2.52) | .84 | |
| NHAPI | 1.20 (0.90–1.59) | 1.31 (1.06–1.62) | .61 | 1.24 (0.82–1.88) | .88 | |
| NHAIAN | 2.25 (1.24–4.07) | 1.15 (0.72–1.83) | .080 | 2.72 (1.57–4.69) | .65 | |
| Distant stage* | ||||||
| Hispanic | 0.80 (0.64–1.00) | 1.01 (0.87–1.17) | .088 | 1.14 (0.96–1.35) | .014 | |
| NHB | 1.18 (0.89–1.56) | 0.78 (0.60–1.00) | .032 | 0.96 (0.70–1.32) | .34 | |
| NHAPI | 1.19 (0.87–1.63) | 1.02 (0.80–1.30) | .45 | 1.69 (1.28–2.25) | .10 | |
| NHAIAN | 1.51 (0.72–3.16) | 0.73 (0.35–1.54) | .18 | 0.82 (0.39–1.71) | .25 | |
| All stages* | ||||||
| Hispanic | 1.13 (1.02–1.25) | 1.61 (1.49–1.73) | <.001 | 1.96 (1.76–2.17) | <.001 | |
| NHB | 2.32 (1.99–2.72) | 2.88 (2.51–3.31) | .044 | 3.85 (3.16–4.68) | <.001 | |
| NHAPI | 1.77 (1.51–2.07) | 2.23 (1.95–2.55) | .029 | 2.76 (2.27–3.36) | .001 | |
| NHAIAN | 1.30 (0.91–1.86) | 1.34 (0.99–1.81) | .89 | 2.09 (1.45–3.01) | .067 | |
CI, Confidence interval; HR, hazard ratio; MSS, melanoma-specific survival; NHAIAN, non-Hispanic American Indian/Alaska Native; NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic black; NHW, non-Hispanic white.
For each of the 4 models (localized stage, regional stage, distant stage, and all stages), the unadjusted HRs for MSS and P values were calculated from Cox proportional hazard models with race, year of diagnosis, and the interaction terms. P values in 2000–2009 showed significance comparing the HRs to NHWs for melanomas diagnosed in 2000–2009 and those for melanomas diagnosed <2000. P values in ≥2010 showed significance comparing the HRs to NHWs for melanomas diagnosed in ≥2010 and those for melanomas diagnosed before 2000.
Table III.
Adjusted hazard ratio and 95% confidence intervals for melanoma-specific survival of different racial/ethnicity groups compared with non-Hispanic whites in the Surveillance, Epidemiology, and End Results cohort and across 3 diagnostic time periods for different stages for cutaneous melanoma
| <2000 |
2000–2009 |
≥2010 |
||||
|---|---|---|---|---|---|---|
| Race | aHR (95% CI) for MSS | aHR (95% CI) for MSS | P for interaction | aHR (95% CI) for MSS | P for interaction | |
| Localized stage* | ||||||
| Hispanic | 1.05 (0.90–1.24) | 1.44 (1.26–1.66) | .004 | 1.54 (1.21–1.96) | .010 | |
| NHB | 1.49 (1.12–1.98) | 1.92 (1.44–2.57) | .21 | 4.08 (2.70–6.16) | <.001 | |
| NHAPI | 1.19 (0.90–1.58) | 1.46 (1.09–1.94) | .33 | 2.19 (1.41–3.40) | .023 | |
| NHAIAN | 0.79 (0.42–1.46) | 1.20 (0.68–2.12) | .32 | 2.14 (1.02–4.50) | .042 | |
| Regional stage* | ||||||
| Hispanic | 1.01 (0.83–1.22) | 1.32 (1.17–1.49) | .021 | 1.56 (1.30–1.87) | .001 | |
| NHB | 1.62 (1.22–2.15) | 1.49 (1.19–1.86) | .64 | 1.74 (1.24–2.45) | .76 | |
| NHAPI | 1.17 (0.88–1.56) | 1.26 (1.02–1.56) | .69 | 1.28 (0.85–1.93) | .73 | |
| NHAIAN | 2.30 (1.27–4.16) | 1.19 (0.75–1.90) | .087 | 2.86 (1.66–4.94) | .60 | |
| Distant stage* | ||||||
| Hispanic | 0.83 (0.66–1.05) | 1.06 (0.92–1.23) | .081 | 1.17 (0.99–1.38) | .019 | |
| NHB | 1.33 (1.00–1.76) | 0.78 (0.60–1.01) | .007 | 1.01 (0.74–1.39) | .21 | |
| NHAPI | 1.18 (0.86–1.62) | 1.03 (0.81–1.32) | .51 | 1.68 (1.27–2.23) | .10 | |
| NHAIAN | 1.25 (0.59–2.62) | 0.64 (0.31–1.35) | .21 | 0.77 (0.37–1.61) | .36 | |
| All stages* | ||||||
| Hispanic | 0.95 (0.86–1.06) | 1.35 (1.25–1.46) | <.001 | 1.56 (1.41–1.73) | <.001 | |
| NHB | 1.40 (1.20–1.64) | 1.30 (1.13–1.50) | .48 | 1.87 (1.54–2.28) | .024 | |
| NHAPI | 1.20 (1.02–1.41) | 1.32 (1.15–1.51) | .39 | 1.97 (1.62–2.40) | <.001 | |
| NHAIAN | 1.12 (0.78–1.60) | 1.15 (0.85–1.55) | .92 | 1.61 (1.12–2.32) | .16 | |
aHR, Adjusted hazard ratio; CI, confidence interval; MSS, melanoma-specific survival; NHAIAN, non-Hispanic American Indian/Alaska Native; NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic black; NHW, non-Hispanic white.
For each of the 4 models (localized stage, regional stage, distant stage, and all stages), the adjusted HRs for MSS and P values were calculated from Cox proportional hazard models with race, year of diagnosis, and the interaction terms, adjusting for age, gender, primary site, histologic subtype, and stage. P values in 2000–2009 showed significance comparing the adjusted HRs to NHWs for melanomas diagnosed in 2000–2009 and those for melanomas diagnosed <2000. P values in ≥2010 showed significance comparing the adjusted HRs to NHWs for melanomas diagnosed in ≥2010 and those for melanomas diagnosed before 2000.
Fig 2.
Trend of adjusted hazard ratios (HRs) for melanoma-specific survival (MSS) of minorities to non-Hispanic whites along years of melanoma diagnosis in the Surveillance, Epidemiology, and End Results cohort. Adjusted HRs were calculated from multivariable Cox proportional hazard models, adjusting for racial groups, age, gender, primary site, histologic subtype, and stage. The same model was performed for samples of melanoma patients diagnosed in each year. The adjusted HRs of each minority group to non-Hispanic whites are presented on the Y axis and the diagnosis year is presented on the X axis (dots). Loess curves were estimated for the adjusted HRs along years of diagnosis for each racial group. NHAIAN, Non-Hispanic American Indian/Alaska Native; NHAPI, non-Hispanic Asian or Pacific Islander; NHB, non-Hispanic black.
Stage-specific worsening of racial disparity in MSS
Across all disease stages, the disparity in MSS worsened for Hispanics, NHBs, and NHAPIs compared with NHWs from <2000 to ≥2010 (Table III). In addition, for patients specifically with localized disease, there has been worsening disparity in MSS for all minority races compared with NHWs from <2000 to ≥2010 (interaction terms P = .010, P<.001, P = .023, and P = .042 for Hispanics, NHBs, NHAPIs, and NHAIANs, respectively). However, in patients with regional and distant disease, only Hispanic individuals suffered worsening disparity in MSS from <2000 to ≥2010. For patients with regional disease, the aHR for Hispanics compared with NHWs increased from 1.01 (95% CI 0.83–1.22) to 1.56 (95% CI 1.30–1.87; interaction term P = .001). For patients with distant disease, the aHR for Hispanics compared with NHWs increased from 0.83 (95% CI 0.66–1.05) to 1.17 (95% CI 0.99–1.38; interaction term P = .019). In a subset analysis of patients ≥65 years of age, Hispanics and NHBs with localized disease had relative worsening in MSS from <2000 to ≥2010 (P = .046 and P<.001, respectively), but NHAPIs and NHAIANs did not (P = .96 and P = .45, respectively); Supplemental Table IV.
DISCUSSION
In this study, we tested the hypothesis that racial disparities in MSS persist in the United States in the contemporary treatment era. Our analysis confirmed that Hispanic, NHB, and NHAPI patients continue to suffer worse outcomes than their NHW counterparts. In fact, that discrepancy significantly increased from the <2000 period to the ≥2010 period despite the introduction of immunotherapy and targeted therapies. Interestingly, this recent trend toward worsening disparity occurred despite almost universal improvement in melanoma outcomes. We found that the 5-year disease-specific survival rate improved for most races in the ≥2010 period. This reflects progress on multiple fronts, such as changes in screening guidelines and advances in disease management. In line with our own findings, 2 recent studies also show that mortality for patients with advanced melanoma has significantly improved since 2011, when immunotherapies and targeted agents began to receive approval by the U.S. Food and Drug Administration.16,17
Importantly, our data suggest that the degree of improvement in survival for minorities has been significantly worse compared with NHWs. There are several possible explanations. Foremost, minorities may not have equal access to contemporary treatments. Since most patients with melanoma are white, minorities are repeatedly underrepresented in clinical trials. For example, CheckMate-067 and CheckMate-037 included <0.7% African Americans and <1.1% patients of Asian descent.18,19 This disparity permeates translational cancer research. In the Cancer Genome Atlas cohort, only 7 African American patients with melanoma were included out of the approximately 1100 cases. Such stark underrepresentation naturally limits the generalizability of study findings to the broader American population, which translates into less frequent prescription of immunotherapy for minorities in the community setting. This conclusion is supported by Haque et al,20 who showed that black patients are less likely to receive immunotherapy compared with white patients. There is an urgent need for clinical and translational studies to include larger minority cohorts.
Several previously established etiologies for racial disparity in melanoma are redemonstrated in our article. First, we found that lower extremity melanoma occurs most often in NHBs and least often in NHWs. The lower extremities are less frequently exposed to the sun, which can provide false reassurance to patients and providers that these anatomic loci are immune to developing melanoma. As a result, they may be overlooked during physical examinations, which can lead to presentation at later disease stages. The early diagnosis of melanoma may be more challenging in darkly pigmented skin, which can also beget later presentation for minorities. Consistent with previous reports, we observed that NHW patients were more likely than minorities to present with early stage melanoma.3,10,21–24 Early-stage disease is one of the most important factors contributing to significantly better MSS for NHWs compared with minorities.25 Increased tumor thickness and ulceration occur with delayed diagnosis and portend worse survival. Here, we found significant racial disparity in both. Finally, we found that ALM, which is often detected late, was more common in minorities than in NHWs. Disparity in MSS significantly worsened for Hispanics and NHBs with ALM but not NHAPIs or NHAIANs. In previous work, we showed that ALM is an independent negative predictor of survival.26 Collectively, these data suggest that the predilection for minorities to more frequently develop ALM contributes to their worse outcomes. However, because not all minorities with ALM suffered worsening disparity, and because minorities do not appear to be more susceptible to ALM now versus <2000, this biologic predisposition is unlikely to be the sole factor driving worsening outcomes. Mucosal melanoma is also more common in minorities, which we redemonstrated here.27 However, we found no worsening disparity in MSS, likely because mucosal melanoma develops and progresses in occult locations irrespective of race.
Notably, we found that disparity in MSS worsened for NHB, NHAPI, and NHAIAN patients with local disease but not for those with regional or distant disease. Minorities with early-stage disease suffer worse outcomes than their white counterparts because of multiple factors. A longer time from diagnosis to definitive surgery is associated with worse survival, and several studies show that minorities more often have a longer time from diagnosis to definitive surgery.28–30 Insurance status may play a role because it too is associated with time from diagnosis to definitive surgery. One study of patients in North Carolina found that privately insured patients were least likely to experience delays in surgery, followed by Medicare and then Medicaid patients.31 In a similar vein, lower socioeconomic status is associated with worse overall survival and MSS.32 While these findings highlight opportunities to improve the management of melanoma, they do not explain the worsening disparity, which should be elucidated in future research. Since early-stage melanoma carries a favorable prognosis, efforts to improve postdiagnosis care for minorities with local disease may substantially improve survival and concomitantly mitigate health care costs.33
In contrast to NHBs and NHAPIs, Hispanics suffered increasing disparity in MSS across all disease stages from <2000 to ≥2010. The universal worsening is particularly concerning considering how rapidly this ethnic group is growing in the United States. Among Hispanics, a lack of awareness about melanoma contributes to both development of disease and delayed presentation. Public health initiatives may not have effectively reached the Hispanic population. Educational programs represent an opportunity for primary and secondary disease prevention. In 2 recent studies that proposed educational curricula, the postintervention surveys reflected significant improvement in knowledge about melanoma,34,35 which speaks to the potential impact of disseminating melanoma information. As is the case for other minorities, Hispanics with advanced disease may have limited access to immunotherapy and targeted therapy. It is imperative that forthcoming randomized trials include larger cohorts of Hispanic patients.
There are several limitations to this study. SEER does not have information on immunotherapy or targeted therapy, which were introduced around 2010 and have dramatically improved survival in melanoma. Thus, we could not analyze racial disparity in MSS in patients who received either treatment. While our study calls attention to worsening racial disparity in melanoma, we did not have the granular socioeconomic information needed to uncover clear etiologies for this trend. This includes data such as income and insurance coverage. For instance, we found that Hispanics and NHBs ≥65 years of age with localized disease had relative worsening in MSS but NHAPIs and NHAIANs did not. This age group can access Medicare, but that information was not available through SEER and we could not determine whether Medicare enrollment accounts for these discrepancies between minorities.
In conclusion, the almost universal improvement in MSS across racial and ethnic groups is encouraging, but there is persistent and worsening racial disparity in outcomes. Based on our findings, identifying and mitigating barriers to postdiagnosis care is essential to further improve outcomes for minorities with early-stage disease.
Supplementary Material
CAPSULE SUMMARY.
Racial disparity in melanoma survival persists since 2010. In all minority groups, patients with localized disease suffered worsening disparity. In patients with regional or distant disease, Hispanic patients were the only minority to suffer increasing disparity.
Improving postdiagnosis management for minorities with localized disease is imperative to improve survival outcomes.
Acknowledgments
Supported by the New York University Melanoma Spore (P50CA016087) and the Laura and Isaac Perlmutter Cancer Center Support Grant (P30CA016087).
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Derm. 2009; 145(12):1369–1374. [DOI] [PubMed] [Google Scholar]
- 2.Dawes SM, Tsai S, Gittleman H, Barnholtz-Sloan JS, Bordeaux JS. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75(5):983–991. [DOI] [PubMed] [Google Scholar]
- 3.Hu S, Soza-Vento RM, Parker DF, Kirsner RS. Comparison of stage at diagnosis of melanoma among hispanic, black, and white patients in Miami-Dade County, Florida. Arch Derm. 2006;142(6):704–708. [DOI] [PubMed] [Google Scholar]
- 4.Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 5.Battie C, Gohara M, Verschoore M, Roberts W. Skin cancer in skin of color: an update on current facts, trends, and misconceptions. J Drugs Dermatol. 2013;12(2):194–198. [PubMed] [Google Scholar]
- 6.Gupta AK, Bharadwaj M, Mehrotra R. Skin cancer concerns in people of color: risk factors and prevention. Asian Pac J Canc Prev. 2016;17(12):5257–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kailas A, Solomon JA, Mostow EN, Rigel DS, Kittles R, Taylor SC. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74(5):1020–1021. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman BP, Alexis AF. Skin cancer mortality in patients with skin of color. Cutis. 2017;99(5):307–308. [PubMed] [Google Scholar]
- 9.Ozdemir BC, Dotto GP. Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer. 2017;3(3):181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korta DZ, Saggar V, Wu TP, Sanchez M. Racial differences in skin cancer awareness and surveillance practices at a public hospital dermatology clinic. J Am Acad Dermatol. 2014;70(2): 312–317. [DOI] [PubMed] [Google Scholar]
- 11.Myles ZM, Buchanan N, King JB, et al. Anatomic distribution of malignant melanoma on the non-hispanic black patient, 19982007. Arch Dermatol. 2012;148(7):797–801. [DOI] [PubMed] [Google Scholar]
- 12.Harvey VM, Patel H, Sandhu S, Wallington SF, Hinds G. Social determinants of racial and ethnic disparities in cutaneous melanoma outcomes. Cancer Control. 2014;21(4):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2017 National Population Projections Datasets. United States Census Bureau. Available at: https://www.census.gov/data/datasets/2017/demo/popproj/2017-popproj.html. Accessed March 23, 2020.
- 15.Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1975–2016), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. www.seer.cancer.gov.
- 16.Berk-Krauss J, Stein JA, Weber J, Polsky D, Geller AC. New systemic therapies and trends in cutaneous melanoma deaths among US whites, 1986–2016. Am J Public Health. 2020;110(5): 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobry AS, Zogg CK, Hodi FS, Smith TR, Ott PA, Iorgulescu JB. Management of metastatic melanoma: improved survival in a national cohort following the approvals of checkpoint blockade immunotherapies and targeted therapies. Cancer Immunol Immunother. 2018;67:1833–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. A phase 3 study of nivolumab or nivolumab plus ipilimumab versus ipilimumab alone in previously untreated advanced melanoma (CheckMate 067). Available at: https://clinicaltrials.gov/ct2/show/study/NCT01844505. Accessed March 29, 2020.
- 19.ClinicalTrials.gov. A study to compare BMS-936558 to the physician’s choice of either dacarbazine or carboplatin and paclitaxel in advanced melanoma patients that have progressed following anti-CTLA-4 therapy (CheckMate 037). Available at: https://clinicaltrials.gov/ct2/show/results/NCT01721746. Accessed March 29, 2020.
- 20.Haque W, Verma V, Butler EB, Teh BS. Racial and socioeconomic disparities in the delivery of immunotherapy for metastatic melanoma in the United States. J Immunother. 2019;42(6):228–235. [DOI] [PubMed] [Google Scholar]
- 21.Perez MI. Skin cancer in hispanics in the United States. J Drugs Dermatol. 2019;18(3):117–120. [PubMed] [Google Scholar]
- 22.Rouhani P, Hu S, Kirsner RS. Melanoma in hispanic and black Americans. Cancer Control. 2008;15(3):248–253. [DOI] [PubMed] [Google Scholar]
- 23.Shoo BA, Kashani-Sabet M. Melanoma arising in African-, Asian-, Latino- and Native-American populations. Semin Cutan Med Surg. 2009;28(2):96–102. [DOI] [PubMed] [Google Scholar]
- 24.Wich L, Ma MW, Price LS, et al. Impact of socioeconomic status and sociodemographic factors on melanoma presentation among ethnic minorities. J Community Health. 2011;36(3):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45(6):791–801. [DOI] [PubMed] [Google Scholar]
- 26.Gumaste PV, Fleming NH, Silva I, et al. Analysis of recurrence patterns in acral versus nonacral melanoma: should histologic subtype influence treatment guidelines? J Natl Compr Canc Netw. 2014;12(12):1706–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altieri L, Wong MK, Peng DH, Cockburn M. Mucosal melanomas in the racially diverse population of California. J Am Acad Dermatol. 2017;76(2):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020; 83(3):854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson AS, Zhou L, Baggett CD, et al. Association of delays in surgery for melanoma with insurance type. JAMA Dermatol. 2017;153(11):1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel-Rahman O Prognostic impact of socioeconomic status among patients with malignant melanoma of the skin: a population-based study. J Dermatolog Treat. 2020;31(6): 571–575. [DOI] [PubMed] [Google Scholar]
- 33.Guy GP Jr, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections – United States, 1982–2030. Morb Mortal Wkly Rep. 2015;64(21): 591–596. [PMC free article] [PubMed] [Google Scholar]
- 34.Roman CJ, Guan X, Barnholtz-Sloan JS, et al. A trial online educational melanoma program aimed at the Hispanic population improves knowledge and behaviors. Dermatol Surg. 2016;42(5):672–676. [DOI] [PubMed] [Google Scholar]
- 35.Chung GY, Brown G, Gibson D. Increasing melanoma screening among Hispanic/Latino Americans: a community-based educational intervention. Health Educ Behav. 2015;42(5): 627–632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.