Abstract
Maternal mortality rates have been steadily increasing in the United States; and cardiovascular mortality is the leading cause of death among pregnant and post-partum women. Maternal stroke accounts for a significant burden of cardiovascular mortality. Data suggest that rates of maternal stroke have been increasing in recent years. Advancing maternal age at the time of birth and increasing prevalence of traditional cardiovascular risk factors as well as other risk factors such as hypertensive disorders of pregnancy, migraine, and infections may contribute to increased rates of maternal stroke. In this document, we provide an overview of the epidemiology of maternal stroke, explore mechanisms that may explain increasing rates of stroke among pregnant women, and identify key knowledge gaps for future investigation in this area.
Keywords: hemorrhagic stroke, intracerebral hemorrhage, ischemic stroke, pregnancy, preeclampsia, post-partum, subarachnoid hemorrhage
Introduction:
Maternal mortality remains a global health care concern (1). While low- and middle-income countries account for more than 90% of maternal mortality globally, rates in the United States (US) are on the rise and are considered the highest among developed countries (1,2). Cardiovascular disease is the most prevalent preventable cause of maternal mortality in developed countries, and also contributes substantially to the high maternal mortality rates in low-and middle-income countries (3). In the US, one-third of pregnancy-related deaths are attributed to cardiovascular conditions, and approximately 60% of these deaths are deemed preventable (4).
Stroke is the second leading cause of death worldwide, the fourth leading cause in US women (5), and is recognized as the leading etiology of long-term physical and cognitive disability in adults (6). Maternal stroke is an infrequent but debilitating complication of pregnancy and is the most frequent cause of serious long-term disability after pregnancy (5). It accounts for at least 7.7% of pregnancy-related deaths in the US (7), and results in residual neurological deficits in approximately half of maternal stroke survivors (8), potentially affecting their ability to care for a newborn.
Specific pregnancy-related conditions often trigger maternal stroke, many of which are potentially preventable (9). In this review, we provide an overview of the epidemiology, risk factors, mechanisms, and therapies for maternal stroke, as well as focus on potential preventive strategies. However, many important knowledge gaps remain regarding maternal stroke, so another focus of this review is to highlight these areas and provide some directions for future research.
Epidemiology of maternal stroke:
The risk of stroke among pregnant and post-partum women is ~3 times increased compared with non-pregnant women of similar age (10). In a meta-analysis of 11 studies including >85 million pregnant and post-partum women, from high-income countries, the incidence of maternal stroke was ~30 per 100,000 pregnancies; most occurred during the post-partum period (up to 6 weeks) (10). Although there is a paucity of robust large-scale studies, small studies from some countries indicate an alarmingly higher incidence of maternal stroke in low- and middle-income countries. In one study > 5,500 deliveries in Tanzania from 2009 to 2010, the incidence of maternal stroke was 89 per 100,000 deliveries (11). Another study of > 39,000 deliveries in India from 2006 to 2008 revealed that the incidence of cerebrovascular complications was 66 per 100,000 deliveries (12).
Maternal stroke is a frequent cause of strokes among younger women (i.e., maternal stroke accounts for 18% of strokes in women aged 12–35 years compared with 1.4% of strokes in women aged 35–55 years) (13). This difference is partially explained by distinct stroke mechanisms across the age spectrum. Strokes in older age groups are typically related to cardio-embolic events, large artery atherosclerosis, or cerebral small vessel disease, while strokes in younger women may result from rarer mechanisms such as cervical artery dissection (14), venous infarction or hemorrhage due to cerebral venous thrombosis, primary hypercoagulable states, and reversible cerebral vasoconstriction syndrome (13). In addition, unlike strokes in the general population which are 87% ischemic (6), half of maternal strokes are hemorrhagic (due to intracerebral hemorrhage [ICH] or subarachnoid hemorrhage [SAH]) (10).
Overall, the incidence of stroke in the US population has declined in recent years, which could partly be attributed to the improved control of some traditional cardiovascular risk factors (15). However, an opposite trend has been observed with maternal stroke. In an analysis of the National Inpatient Sample (NIS) of >37 million hospitalizations for pregnancy and post-partum conditions from 2007 to 2015, the rates of acute stroke or transient ischemic attack (TIA) did not change over time (42.8 per 100,000 hospitalizations in 2007 versus with 42.2 per 100,000 hospitalizations in 2015). In a secondary analysis excluding TIA and specific codes for stroke during pregnancy, there was a small but significant increase in the rates of maternal stroke (29.8 per 100,000 hospitalizations in 2007 versus 33.0 per 100,000 hospitalizations in 2015), suggesting that the rates of maternal stroke might be on the rise (16). This suggestion is supported by a Canadian cohort of >3.9 million pregnant and post-partum women from 2003–2016, where there was also a temporal increase in the age-adjusted incidence of maternal stroke (10.8 per 100,000 deliveries in 2003, to 16.6 per 100,000 deliveries in 2016) (17).
The rates of maternal stroke appear higher in the US compared with other developed nations (Table 1) (8,12,16–21). In addition to increasing maternal age and rising trends in the prevalence of cardiovascular risk factors among young women, significant health disparities by race and ethnicity may contribute to the increased rate of maternal stroke in the US. Black women have been shown to have higher risk of maternal stroke and higher maternal stroke -associated in-hospital mortality (16). In another analysis of the NIS of > 65 million pregnant and post-partum women, among those with hypertensive disorders of pregnancy (HDP), Blacks and Hispanics had double the risk of maternal stroke during delivery admissions compared with non-Hispanic White women (Blacks: adjusted risk ratio [aRR], 2.07; 95% confidence interval [CI], 1.86–2.30; Hispanics: aRR, 2.19; 95% CI, 1.98–2.43). Among those with chronic hypertension, all minority women had a higher stroke risk (Blacks: aRR, 1.71; 95% CI, 1.30–2.26; Hispanics: aRR, 1.75; 95% CI, 2.32–5.63; Asian/Pacific Islanders: aRR, 3.62; 95% CI, 2.32–5.63). Among normotensive women, Black women, but not women in other minority groups, had a 17% excess risk of maternal stroke versus White women (18). Studies consistently indicate that Black women suffer disproportionately high risk of adverse maternal outcomes compared to other groups. While genetic and environmental factors may also be contributing factors, systemic racism must be confronted to address these troublesome trends (22).
Table 1:
Rates of maternal stroke across different nations
| Study | Country | Enrollment period | Pregnant/post-partum women, n | Incidence of maternal stroke (per 100,000) | Mean age of women with stroke, years |
|---|---|---|---|---|---|
| Elgendy et al.16 | USA | 2007–2015 | 37,360,772 | 45 | 30 |
| Liu et al.17 | Canada | 2003–2016 | 3,907,262 | 13.4 | NR |
| Yoshida et al.19 | Japan | 2012–2013 | 2,115,949 | 10.2 | 32.2 |
| Sharshar et al.8 | France | 1989–1992 | 669,680 | 4.6 | 30.6 |
| Bashiri et al.20 | Israel | 1988–2004 | 173,803 | 9.2 | 35.5 |
| Liang et al.21 | Taiwan | 1992–2004 | 66,781 | 47.9 | 30.1 |
| Prabhu et al.12 | India | 2006–2008 | 39,211 | 66 | 22 |
NR: not reported
Timing and risk factors for maternal stroke
Timing of maternal stroke:
The majority of maternal strokes occur in the post-partum period (defined variously as up to 12 weeks after delivery), often after women have left the hospital (10). In a study using administrative data from the US Healthcare Cost and Utilization Project’s Nationwide Readmissions Database from 2013–2014, the median time to readmission for stroke after delivery was 8 days (23). The risk of thromboembolic events in the 6 weeks postpartum has been estimated to be 15–35 times higher in the first week postpartum, compared with non-pregnant women, and the risk remains elevated up to 12 weeks postpartum (24). Hemorrhagic strokes, too, occur most frequently post-partum; a case-crossover study using administrative data from New York, California, and Florida found a 9-fold increased rate of ICH in the 12 weeks post-partum (rate ratio, 9.15; 95% CI, 5.16–16.23), compared with the non-pregnant state (25).
Maternal stroke risk factors:
Risk factors for maternal stroke may be broadly classified as traditional cardiovascular or other risk factors. Traditional risk factors include older age, obesity, smoking, chronic hypertension, hyperlipidemia, and heart disease. Other risk factors include HDP, migraine, infections, and hypercoagulable states (Figure 1).
Figure 1:
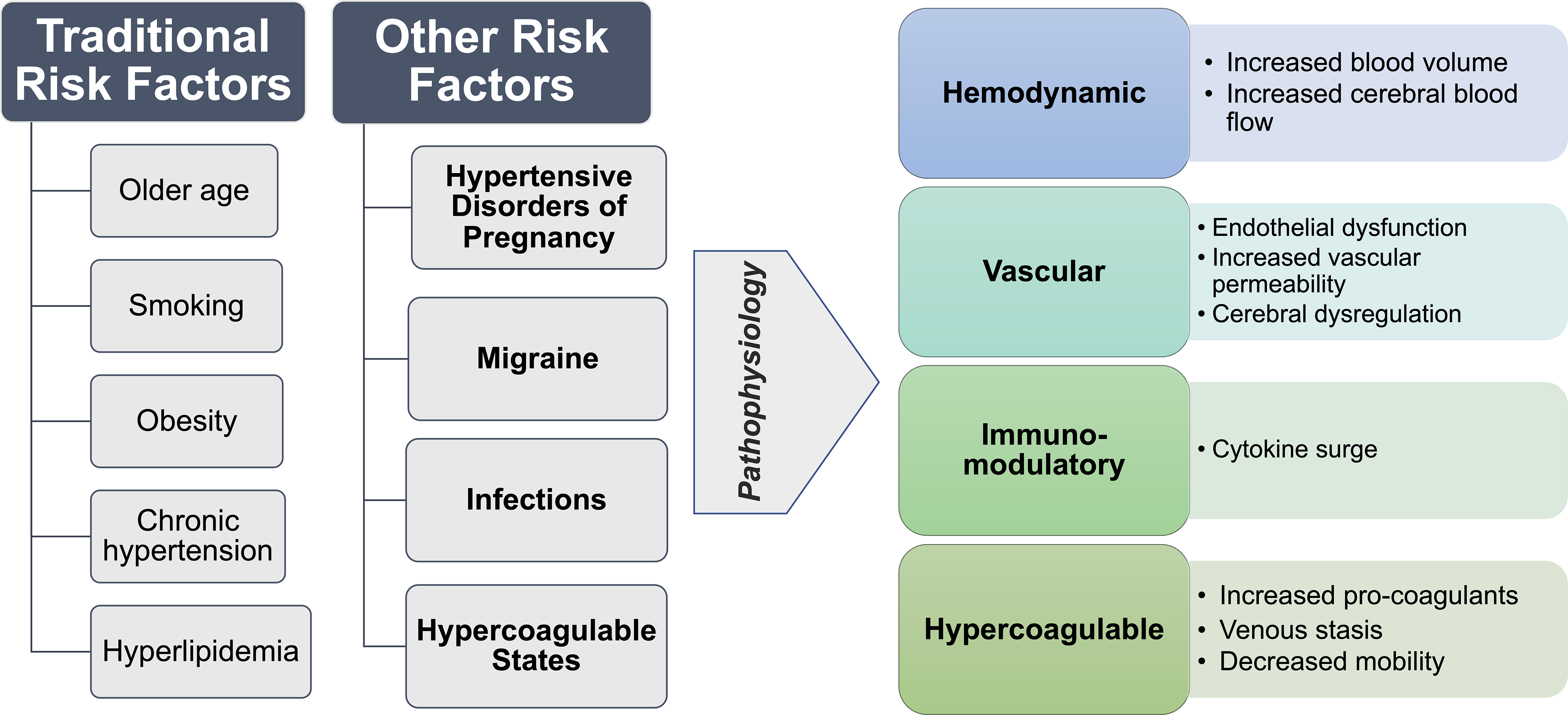
Risk factors and pathophysiology of maternal stroke.
Risk factors for maternal stroke are traditional and other risk factors including hypertensive diseases of pregnancy, migraine, infections, and hypercoagulable states. The pathophysiological mechanisms implicated in maternal stroke involve hemodynamic, vascular, immune-modulatory, and hypercoagulable changes.
1. Traditional modifiable cardiovascular risk factors
While the rising trend in the incidence of maternal stroke might be partly attributed to advancing maternal age (26), recently there has also been a notable increase in the prevalence of traditional modifiable cardiovascular risk factors in younger patients (27). Of these, hypertension remains the most prevalent modifiable risk factor for stroke among the general population as well as pregnant and post-partum women (6). The prevalence of most other traditional cardiovascular risk factors including obesity, smoking, and hyperlipidemia is also increasing among women with maternal stroke (16). Nevertheless, these risk factors remain less prevalent among pregnant women with stroke compared with non-pregnant women with stroke, suggesting that underlying pathophysiological mechanisms for maternal stroke involve unique pregnancy-related mechanisms (28).
2. Hypertensive disorders of pregnancy (HDP)
HDP, defined as gestational hypertension, preeclampsia/eclampsia, and chronic hypertension with or without superimposed preeclampsia, are associated with a higher risk for all stroke types during pregnancy and the post-partum period. Preeclampsia and stroke are known to share common risk factors such as obesity, metabolic syndrome, heightened inflammatory responses, hypercoagulable states, and endothelial dysfunction (29). In addition, mechanisms specific to HDP may contribute to maternal stroke risk (see Mechanisms, below). HDP confer a higher attributable risk to maternal stroke compared with traditional cardiovascular risk factors. In an analysis of ~82 million pregnancy-related hospitalizations from the NIS, women with HDP were ~5 times more likely to have an ischemic or hemorrhagic stroke (30). In a meta-analysis of 22 studies, comprising of >6.4 million women including ~250,000 women with pre-eclampsia, pre-eclampsia was independently associated with a 2-fold increased risk of maternal stroke after adjusting for other potential confounders including traditional cardiovascular risk factors (31).
3. Migraine
The association between migraine and both ischemic and hemorrhagic stroke in the general population has been demonstrated in multiple studies (32–33). This association appears to hold true among pregnant and post-partum women with migraine. In an analysis of the NIS including ~18 million pregnancy hospitalizations, migraine was strongly associated with maternal stroke after adjusting for other risk factors (odds ratio [OR] 15.05, 95% CI 8.26–27.4) (34). Notably, migraine might not be easily distinguished from pre-eclampsia in administrative datasets, which could potentially introduce misclassification bias (35). Like HDP, migraine predisposes to endothelial dysfunction and increased platelet activation and aggregation (36). In fact, HDP has been suggested to mediate the association between migraine and stroke. In an analysis of an administrative database from California including >3 million pregnant and postpartum women including ~26,000 with migraine, migraine was independently associated with HDP (aRR 1.6; 95% CI 1.6–1.7), as well as stroke during pregnancy or delivery (aRR 6.8; 95% CI 4.7–9.8) and stroke during the post-partum period (aRR 2.1, 95% CI 1.2–3.7) (37). Importantly, HDP, especially pre-eclampsia, mediated one-fourth of the excess risk of maternal stroke associated with migraine, which indicates the role of pregnancy-related hemodynamic and circulatory changes in the pathogenesis of stroke among women with migraine (37).
4. Infection
Infections are now recognized as a trigger for strokes in people of all ages (38,39), but this association may be even stronger among younger patients, including children (40,41) and young adults (42). This association has also been observed among pregnant and post-partum women. Infections have been recognized as a risk factor for maternal stroke even among women with pre-eclampsia (43). The risk appears to be higher with genitourinary infections and sepsis (44). An analysis of the National Readmission Database including 17.2 million pregnant and post-partum women showed that infections were associated with higher rates of ischemic stroke but not hemorrhagic stroke at 30-days. This effect was mainly observed among women without HDP (45).
Proposed pathophysiological pathways for the association between infections and maternal stroke include activation of the inflammatory cascade, causing a surge in inflammatory cytokines leading to platelet activation and aggregation; increased oxidative stress; and impaired endothelial function, all of which are linked with maternal stroke (45).
5. Hypercoagulable states:
Hypercoagulable states predispose pregnant and postpartum women to arterial and venous thrombosis as well as hemorrhagic stroke (43). An analysis of the New York State Department of Health inpatient database, comprising of >88,000 women with preeclampsia, showed that prothrombotic states including systemic lupus erythematosus and sickle cell disease were associated with higher risk of maternal stroke (OR 3.5; 95% CI 1.3–9.2) (43). Pregnancy with antiphospholipid syndrome (APS) increases the risk of recurrent ischemic stroke, preeclampsia and preterm delivery (46). A prospective analysis of 33 pregnant women with APS showed that strongest predictive factors for thrombotic event in pregnancy were the presence of lupus anticoagulant and a past thrombotic event (47).
Mechanisms of maternal stroke
Pregnancy is associated with a broad range of stroke subtypes (Figure 2). Arterial ischemic strokes may be caused by arterial occlusions due to cardio-embolism (48), paradoxical emboli in the setting of patent foramen ovale (49,50), cervical artery dissections (51), or severe vasospasm (28). Large artery atherosclerosis is rarely seen in this population due to their young age, but other causes of intracranial arterial stenosis such as moyamoya disease have been described in association with maternal stroke (52). Hemorrhagic strokes account for ~60% of maternal stroke (53), a dramatic difference compared with the general population where 87% of strokes are ischemic (6). Hemorrhagic stroke mechanisms include ICH and SAH, which have distinct etiologies. ICH can occur either as a result of ruptured small blood vessels, often in the setting of uncontrolled hypertension, or in the presence of underlying vascular abnormalities such as arteriovenous malformations, cerebral cavernous malformations, or fragile moyamoya collaterals. Similarly, SAH may occur due either to rupture of vascular lesions such as intracranial aneurysms or dural arteriovenous fistulae (54,55), as a result of the reversible cerebral vasoconstriction syndrome, or from venous hemorrhages. Interestingly, the majority of pregnancy-associated ICH and SAH do not appear to be associated with underlying vascular lesions (25,56). Cerebral venous sinus thrombosis (thrombosis of the cerebral veins and/or dural sinuses) may also lead to maternal stroke due to cerebral venous congestion which can result in venous infarction, with or without hemorrhagic conversion (57).
Figure 2:

Examples for cases of maternal stroke.
(A) Right frontal intracerebral hemorrhage with surrounding edema and brain herniation in a post-partum woman with the HELLP syndrome. No underlying vascular lesion was identified, and the stroke was felt like due to hypertension and HELLP-related coagulopathy; (B) Arterial ischemic stroke due to paradoxical embolus related to patent foramen ovale, although no deep venous thrombosis was identified to left posterior cerebral artery in a post-partum woman; (C) Multifocal vasogenic edema due to the posterior reversible encephalopathy syndrome (PRES) in a woman with eclampsia. Note that not all lesions are posterior. A small area of infarction on diffusion-restricted imaging is noted, occasionally seen in association with PRES. (D) Cerebral angiogram demonstrating multifocal vasospasm due to reversible cerebral vasoconstriction syndrome in a postpartum woman with pre-eclampsia. She developed ischemic strokes distal to the areas of vasospasm, as well as intracerebral hemorrhage.
Immunomodulatory and vascular changes in pregnancy may contribute to the mechanisms of both ischemic and hemorrhagic maternal strokes. Pregnancy is associated with changes in both innate immunity and T-cell-mediated immunity. Increased expression of Th17, a highly inflammatory subtype of regulatory T cells, is also seen, especially in the presence of infection (58,59). Similar inflammatory mechanisms have been shown to contribute to the pathogenesis of ischemic stroke outside of pregnancy (60). Furthermore, the cerebral vasculature may be more susceptible to ischemic or hemorrhagic stroke during pregnancy and postpartum. Animal studies have shown pregnancy-related physiological changes, including arteriolar dilatation and remodeling, decreased cerebrovascular resistance, and increased blood-brain barrier permeability (61). Even more extreme disruptions have been observed in animal models of preeclampsia, including dramatic increases in blood-brain barrier permeability, disruption cerebral autoregulatory function, and impaired arteriolar response to neurovascular signaling (62,63). Small clinical studies have corroborated these findings, finding changes in cerebral autoregulatory function both in healthy pregnant women and women with HDP (64,65). Independent of immunomodulatory mechanisms, the altered cerebral autoregulation associated with HDP may elevate cerebral wall tension in the fragile vessel walls and increase vulnerability to maternal stroke (66,67) (Figure 3). A severe form of preeclampsia is characterized by hemolysis, elevated liver enzymes, and a low platelet count, known as HELLP syndrome. The combination of blood-brain barrier damage in the setting of hypertension enhances the risk of hemorrhagic stroke in women with HELLP (68). The pathogenesis of preeclampsia is thought to be linked, at least in part, to altered expression of placental anti-angiogenic factors which induces endothelial dysfunction, contributing to increased risk for proteinuria and hypertension (69). Endothelial dysfunction, both systemic and cerebral, is a pivotal mechanism that results in the disruption of vascular tone, increased vessel reactivity, and, in some cases, vasogenic edema (70). In addition, preeclampsia has been linked with enhanced platelet aggregation (71).
Figure 3:
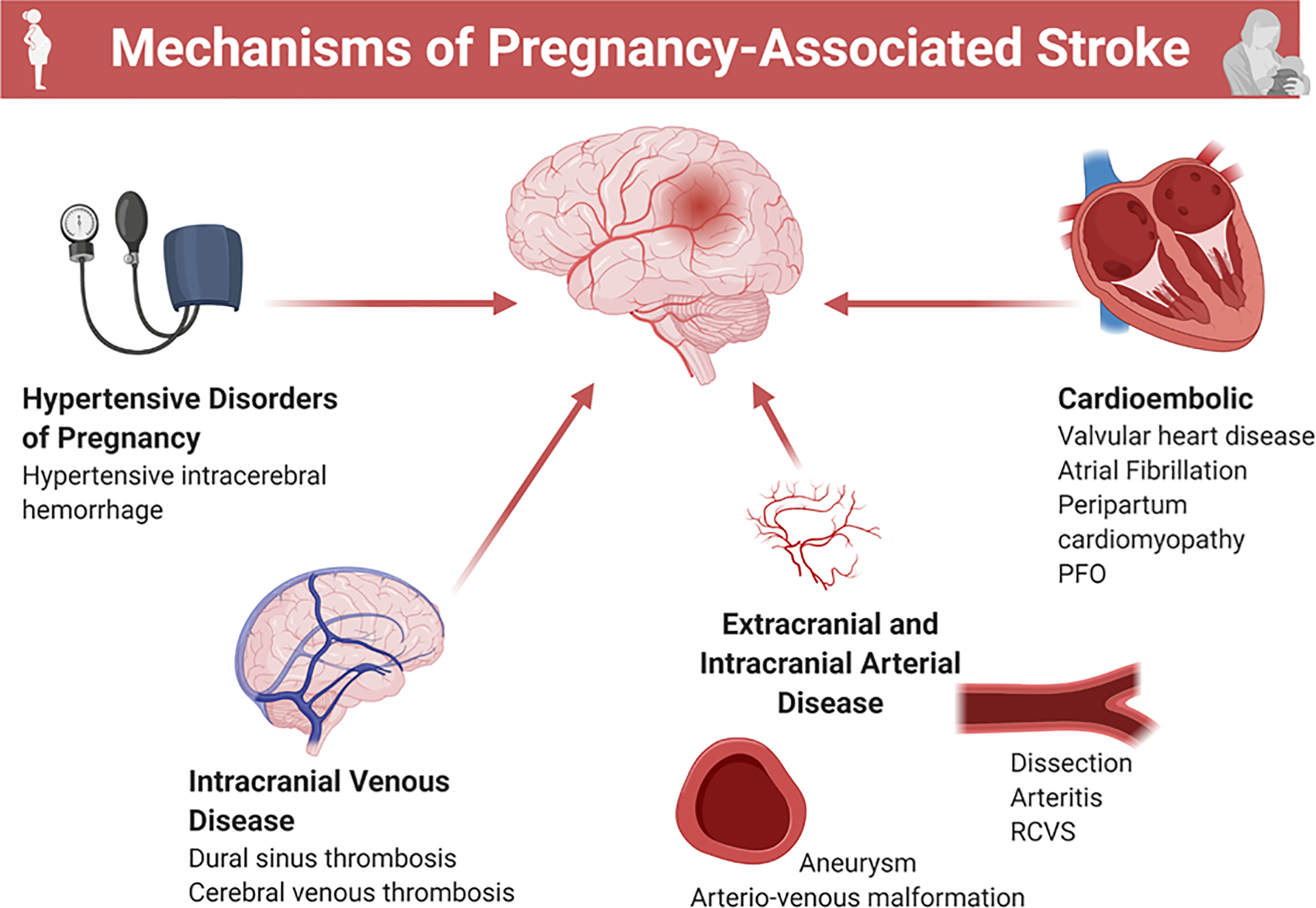
Potential mechanisms of maternal stroke.
Summary of possible mechanisms of maternal stroke.
PFO= patent foramen ovale; RCVS= reversible cerebral vasoconstriction syndrome
Clinically, preeclampsia and eclampsia are highly associated with the reversible cerebral vasoconstriction syndrome (RCVS) and the posterior reversible encephalopathy syndrome (PRES) (72), which may lead to ischemic stroke due to vasospasm-related hypoperfusion, as well as subarachnoid and intracerebral hemorrhage due to increased cerebral perfusion pressure, inflammation, and impaired compensatory autoregulatory mechanisms (73).
Cerebral venous thrombosis is another cause of maternal stroke, usually occurring in the postpartum period (28,74). During pregnancy and the post-partum period, hormonally mediated changes in the coagulation system shift the balance to a hypercoagulable state (75). Hypercoagulability, together with other mechanisms such as hypervolemic circulatory dynamics, venous stasis, and vascular endothelial injury in the setting of delivery increase the risk of thrombogenicity and may potentiate ischemic stroke risk (76), particularly in the postpartum period. In addition, increased venous capacitance and venous pooling with resultant stasis, as well as reduced mobility, may play a role in the increased risk of thrombotic events during pregnancy and the post-partum period (77).
Management of maternal stroke
All randomized trials of therapies for acute ischemic stroke have excluded pregnant and postpartum women. In general, pregnant women with acute stroke are best managed in tertiary stroke centers whenever feasible (78). A multidisciplinary team approach including obstetricians, obstetric anesthesiologists, and neurologists is recommended (78). Emergent neuro-imaging should be obtained either with computed tomography (CT) or magnetic resonance imaging (MRI), in accordance with best practices for all acute stroke patients (79). Iodinated contrast for CT may be given when clinically indicated (80). Gadolinium contrast for MRI can cross the placenta and has been linked with still birth and neonatal death, and thus should be avoided; in any case it is rarely needed for management of acute stroke (81). Both pregnant women and their care providers often express concerns about the fetal risk associated with neuroimaging and ionizing radiation. Given the magnitude of maternal and fetal risk caused by potential delay in diagnosis of stroke, particularly given the higher proportion of hemorrhagic strokes among pregnant women, and the low risk to the fetus of CT, neuroimaging with CT scanning is acceptable and often the fastest or only available option (78).
Among pregnant women with acute ischemic stroke and no other contraindications to systemic thrombolysis, systemic intravenous thrombolysis with alteplase is recommended if they present within 4.5 hours of symptom onset when the benefit outweighs the risk (78). Since alteplase is a large molecule, it does not cross the placental barrier (78). Few case reports and series suggest that intravenous thrombolysis is associated with favorable outcomes during all trimesters including symptomatic improvement, uneventful deliveries and low incidence of symptomatic intracerebral bleed that is comparable with non-pregnant women (82). Thrombolytic therapy in the early postpartum period (≤ 48 hours) remains controversial because of increased risk of bleeding (79). A multi-disciplinary approach is needed to weigh the risks and benefits of thrombolytic strategy in such circumstances, and management needs to be individualized. Endovascular mechanical thrombectomy with stent retrievers has revolutionized the management of acute ischemic stroke among select patients with proximal large vessel intracranial arterial occlusions (83). In an analysis of 338 pregnant and post-partum women from the Get With the Guidelines Stroke-Registry treated with reperfusion therapy (i.e., systemic thrombolysis, catheter-based thrombolysis or thrombectomy), there was no difference in the short-term outcomes between pregnant and postpartum women compared with non-pregnant women (84), despite the maternal stroke group having more severe strokes.
In women with hemorrhagic stroke, for both ICH or SAH, treatment involves initial stabilization measures as blood pressure control (i.e., systolic blood pressure ≤160 mm Hg), reversal of anticoagulation, and identification of the etiology and source control if feasible (78). Emergent procedures like aneurysm clipping/coiling, arteriovenous malformation embolization, and surgical resection can be performed, regardless of pregnancy status (78). Among patients with maternal stroke due to cerebral venous thrombosis, therapeutic anticoagulation with low molecular weight or unfractionated heparin remains the mainstay therapy (85), regardless of the presence of intracerebral hemorrhage. Intra-sinus thrombolysis or endovascular thrombectomy have been reported in some severe cases (86).
Mode of delivery among pregnant women with stroke:
Maternal stroke does not preclude vaginal delivery, provided that there is no other obstetric contraindication. The etiology of stroke and risks posed to the mother and the fetus by the Valsalva efforts are important considerations for decision making regarding the mode of delivery (78). In women for whom a mild increase in the intracranial pressure could be tolerated, vaginal births without assisted delivery can be a safe option (78). Women in whom increased intracranial pressure is a concern may be candidates for assisted vaginal delivery (78). Cesarean delivery is usually necessitated by standard obstetric indications, irrespective of severity of the stroke, which include maternal (e.g., pelvic deformity and abruptio placenta) and fetal (e.g., malpresentation and fetal asphyxia) indications. Additionally, cesarean delivery may also be considered in women with intracranial bleeding, or those who are at high risk of intracranial bleeding including those with unsecured aneurysm, untreated or partially treated symptomatic arteriovenous malformation (AVM) and acute ischemic stroke with hemorrhagic transformation (78). Data regarding the mode of delivery for women with intracranial aneurysms are limited. In an analysis of NIS years 1988–2009 including ~20 million pregnancies, the estimated risk of aneurysmal rupture was 1.4% during pregnancy and only 0.05% during delivery which were comparable with the risk in the general population (87). Though data is insufficient, vaginal delivery is generally recommended for most cardiac conditions including patent foramen ovale.
The process of decision-making regarding mode of delivery should be determined on an individualized basis. Maternal safety and outcomes should be prioritized, recognizing that what is best for maternal health is usually though not always best for fetal health as well (78). Important factors to consider include the viability of the fetus, the health of the mother and the health of the fetus, recognizing that death or disability of the mother is not in either her or her child’s interest (88).
Primary prevention of maternal stroke
Although maternal stroke is a relatively rare condition, it may lead to potential devastating consequences; thus, prevention remains of paramount importance. Since a number of maternal stroke risk factors are potentially manageable, a key aspect of prevention involves multidisciplinary careful recognition of these risk factors and addressing them appropriately in a timely manner. There are currently no available risk prediction tools to identify women with increased risk for maternal stroke.
With the rise in prevalence of traditional risk factors among pregnant women in the recent years, optimal management of these factors is a pivotal step in the prevention of maternal stroke. Early identification and optimal management of risk factors during pregnancy and postpartum period are important. Women with a history of chronic hypertension should be identified, monitored closely for superimposed preeclampsia and treated to a targeted blood pressure goal depending on their other underlying cardiovascular conditions. With regards to obesity, excessive weight gain during pregnancy is commonly seen in women with a higher pre-pregnancy BMI and is associated with adverse pregnancy outcomes including HDP (89). These women will probably benefit most from lifestyle intervention strategies and counselling, including regular exercise, a healthy diet, achieving a desirable body weight and discontinuing smoking prior to conceiving. An increase in physical activity during and before pregnancy not only reduces blood pressure but might also reduce the incidence of preeclampsia (90).
Prevention, early recognition and treatment of HDP, especially preeclampsia, may be key for primary prevention of maternal stroke. Prophylactic use of aspirin, before 16 weeks of gestation, in patients with one high or two or more moderate risk factors for preeclampsia has been found to reduce the risk of preeclampsia (Table 2) (91,92). A meta-analysis of five trials with a total of 556 women concluded that aspirin initiated at or before 16 weeks of gestation was associated with a major reduction in the risk of preterm preeclampsia (RR 0.11, 95% CI 0.04–0.33), although no significant effect was seen on term preeclampsia (RR 0.98, 95% CI 0.42–2.33) (91). Another meta-analysis including the results of 13 studies, reported that low-dose aspirin (60–100 mg) use was associated with a 24% reduction in preeclampsia (RR 0.76, 95% CI 0.62–0.95), when initiated from 12 to 16 weeks of gestation (92). Results from the ASPRE trial, which enrolled 1776 women with singleton pregnancies who were at high risk for preterm preeclampsia, showed that low-dose aspirin (150 mg per day), initiated from 11–14 weeks of gestation until 36 weeks of gestation, reduced the risk of delivery with preeclampsia before 37 weeks of gestation, compared with placebo (OR 0.38, 95% CI 0.20–0.74), and without an increased risk of adverse events (93).
Table 2:
Risk factors for preeclampsia
| High risk factors: |
| History of preeclampsia |
| Multifetal gestation |
| Renal disease |
| Autoimmune disease |
| Type 1 or type 2 diabetes |
| Chronic hypertension |
| Moderate risk factors: |
| First pregnancy |
| Maternal age of 35 years or older |
| Body mass index greater than 30 |
| Family history of preeclampsia |
| Sociodemographic risk factors (African American race, low socioeconomic status) |
| Personal history factors (low birth weight or small for gestational age, previous adverse pregnancy outcome, more than 10-year pregnancy interval) |
Prophylactic low dose aspirin is recommended before 16 weeks of gestation until the day of delivery among women with one high risk factors or two or more moderate risk factors
Systolic blood pressure rises between days 3–6 postpartum, typically peaking around day 6–7 (94). Up to 30% of HDP may occur postpartum, often associated with headaches and/or visual changes (95). The postpartum period is also the most common time for hemorrhagic stroke, most often associated with HDP (31,95). A study from Calgary, Canada identified that 6-week postpartum HDP are often not accurately identified and treated in the emergency department (95). Timely identification and treatment of blood pressure is recommended to reduce the risk of cardiovascular complications including stroke among women with HDP. Studies have revealed that a large proportion of deaths attributable to maternal stroke could possibly be prevented with earlier transfer to higher level of care and aggressive blood pressure treatment (78). However, unlike in hypertensive patients without HDP, there is no consensus on ideal target blood pressure goal in HDP. There is a need for robust studies to determine the impact of aggressive blood pressure control in HDP on the prevention of maternal stroke (96).
Epidemiological data suggest racial and ethnic disparities in the risk of maternal stroke, as Black and Hispanic populations have a higher incidence of maternal stroke than Whites (16,18,25). There are multiple possible reasons to account for these differences including socioeconomic differences, disproportionate access to health facilities, systemic racism, differences in prevalence of co-morbidities including obesity and high blood pressure (13,30). Additionally, non-hypertensive Black women remained at increased risk of stroke, suggesting the possibility of additional mechanisms contributing to increased maternal stroke in this group, including the impact of systemic racism and clinician bias (18,22). This calls for coordinated efforts to explore the underlying reasons for these disparities, reduce overall maternal stroke burden by addressing modifiable risk factors in these populations, and address clinician biases which contribute to differences in the quality of care provided. Further efforts should be directed at developing an individualized prenatal care plan for high-risk populations, focusing on a more aggressive risk factor control strategies, careful counselling, and community-based preventive measures to modify the risk of maternal stroke.
The dynamics of preventive efforts may differ in low-and middle-income countries from those witnessed in developed countries, and besides the personalized primary preventive strategies, a community-based approach for the prevention of maternal stroke in low-and middle-income countries might be more effective. This includes improved healthcare infrastructure and facilities for the pregnant women, strong physician-patient dynamics in terms of extensive counseling and regular follow-up visits, and early screening and diagnosis of HDP. There is a need for community-based initiatives and public education regarding the importance of maternal health, and awareness campaigns with regards to symptoms of stroke and highlighting steps needed for the primary prevention.
Secondary prevention of maternal stroke
Women with a history of stroke in general are at risk for recurrent strokes. One study, of 441 young women with prior stroke, found that the overall risk of recurrent ischemic stroke was low in subsequent pregnancies (absolute risk 1.8%; 95%CI 0.5–7.5) (97). In a systematic review of 13 studies including 217 pregnant and post-partum women with cerebral venous thrombosis recurrence showed that the pooled estimate for recurrent cerebral venous thrombosis was 9 per 1000 pregnancies (98). Similar data on the subsequent risk of hemorrhagic stroke are lacking.
Long-term postpartum monitoring is crucial among women who develop maternal stroke for risk factor assessment and secondary prevention. While proper counseling is imperative, a history of maternal stroke should not be considered as a contraindication for subsequent pregnancy. These women should be encouraged to discuss their future pregnancy plans with their healthcare provider for adequate pre-pregnancy counselling. Lifestyle modification is a pivotal component of the secondary prevention strategy for all stroke subtypes, with emphasis on healthy diet, regular exercise, weight loss, smoking cessation, and blood pressure control. Secondary preventive strategies should be tailored to the stroke subtype and etiology. For women with a history of ischemic stroke, contraceptive counseling is important. Systemic estrogen-containing contraceptives or hormone replacement therapy can increase the risk of thromboembolic events, and therefore alternative methods of contraception should be considered (99). Women who develop maternal stroke secondary to cerebral venous thrombosis might benefit from low–molecular weight heparin thromboprophylaxis during subsequent pregnancies (99).
Conclusions and Future Directions:
Maternal stroke is an important cause of maternal morbidity and mortality and is potentially preventable. The prevalence of maternal stroke is higher in the US compared with other developed nations, and this prevalence appears to be rising. To address gaps in knowledge, further research is needed to broaden our understanding of the mechanisms, risk factors, and management options along with devising robust preventive strategies (Table 3). Thrombolytic therapy for ischemic stroke appears to be safe among pregnant women, but there is insufficient evidence to support thrombolysis for post-partum women within the first 48 hours. Mechanical thrombectomy can be safely performed in both pregnant and post-partum women. Future studies addressing therapies for ischemic stroke might consider enrolling pregnant and post-partum women. There is no current risk prediction tool to identify women at risk for maternal stroke, and studies are needed to risk stratify women for early identification and timely risk factor management of higher risk women. Prevention should focus on controlling traditional risk factors prior to conception and early recognition and treatment of HDP. Given our recognition of racial disparities in the risk of maternal stroke, the employment of aggressive strategies in terms of risk factor control, proper counseling, and community preventative measures are critically important in high-risk populations. The risk of recurrent stroke in future pregnancies depends on the initial stroke mechanism, and secondary prevention strategies must be individualized, ideally with the help of a multidisciplinary team including vascular neurologists, obstetricians, and other specialists depending on the stroke mechanism. There is also a need to implement aggressive healthcare policies that ensure accessibility of standardized healthcare for all pregnant women (Table 4). Filling these knowledge gaps could help provide physicians with better understanding of maternal stroke, make them better equipped to manage maternal stroke and improve outcomes for pregnant and post-partum women.
Table 3:
Knowledge gaps related to maternal stroke
| 1. | Mechanisms of maternal stroke |
| A. Understanding mechanisms related to known risk factors including HDP, migraine and infections | |
| B. Identification of other risk factors. | |
| 2. | Development and validation of risk prediction tools to identify women who are at increased risk for maternal stroke. |
| 3. | Consideration of enrollment of pregnant and post-partum in future randomized controlled trials of therapies for acute ischemic stroke. |
| 4. | Risk factor control strategies, including individualized blood pressure goals, prenatal care plan and preventive approaches for high-risk groups and racial groups. |
| 5. | Investigating the impact of aggressive blood pressure control in HDP on the prevention of maternal stroke. |
| 6. | Need for large-scale studies to evaluate the risk of stroke recurrence in women with maternal stroke, especially hemorrhagic stroke. |
HDP= hypertensive disorders of pregnancy
Table 4:
Call for action- Clinical practice and healthcare settings
| 1. | Improved prenatal patient education of cardiovascular risk factors, symptoms of cardiovascular complications of pregnancy, and the importance of long-term preventative care among reproductive age women. |
| 2. | Implementation of multidisciplinary healthcare team education and maternal stroke toolkits to improve recognition of cardiovascular complications and standardization of maternal healthcare delivery. |
| 3. | Reduction of socioeconomic disparities in maternal cardiovascular outcomes, through increased access to healthcare coverage for pregnant and postpartum women, increased access to maternal healthcare in rural areas, and efforts to address systemic racism. |
| 4. | Targeted efforts to reduce knowledge gaps in maternal cardiovascular health, through increased funding for maternal cardiovascular research and increased inclusion of pregnant and postpartum women in clinical trials. |
Acknowledgments
Disclosures: Dr. Miller receives funding from the National Institutes of Health, National Institute of Neurological Disorders and Stroke (K23NS107645) and the Louis V. Gerstner Jr Foundation (Gerstner Scholars Program). The other authors have no disclosures.
Non-standard Abbreviations and Acronyms:
- APS
antiphospholipid syndrome
- AVM
arteriovenous malformation
- aRR
adjusted risk ratio
- CI
confidence interval
- CT
computed tomography
- HDP
hypertensive disorders of pregnancy
- ICH
intracerebral hemorrhage
- MRI
magnetic resonance imaging
- NIS
National Inpatient Sample
- OR
odds ratio
- PRES
the posterior reversible encephalopathy syndrome
- RCVS
reversible cerebral vasoconstriction syndrome
- SAH
subarachnoid hemorrhage
- TIA
transient ischemic attack
- US
United States
References:
- 1.Trends in maternal mortality: 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Geneva: World Health Organization; 2019. Available at: https://apps.who.int/iris/handle/10665/327596. Accessed on November 17, 2020. [Google Scholar]
- 2.MacDorman MF, Declercq E, Cabral H, Morton C. Is the United States maternal mortality rate increasing? Disentangling trends from measurement issues. Obstet Gynecol. 2016;128:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, Syverson C, Seed K, Shapiro-Mendoza CK, Callaghan WM, et al. Vital signs: pregnancy-related deaths, United States, 2011–2015, and strategies for prevention, 13 states, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Vital Statistics System: Leading causes of death. Available at: https://www.cdc.gov/nchs/nvss/leading-causes-of-death.htm Accessed on September 1, 2020.
- 6.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–596. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention: Pregnancy Mortality Surveillance System. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed on September 1, 2020.
- 8.Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium: a study in public hospitals of Ile de France. Stroke. 1995;26:930–936. [DOI] [PubMed] [Google Scholar]
- 9.Katsuragi S, Tanaka H, Hasegawa J, Nakamura M, Kanayama N, Nakata M, Murakoshi T, Yoshimatsu J, Osato K, Tanaka K, et al. Analysis of preventability of stroke-related maternal death from the nationwide registration system of maternal deaths in Japan. J Matern Fetal Neonatal Med. 2018;31:2097–2104. [DOI] [PubMed] [Google Scholar]
- 10.Swartz RH, Cayley ML, Foley N, Ladhani NN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke. 2017;12:687–697. [DOI] [PubMed] [Google Scholar]
- 11.Ndaboine EM, Kihunrwa A, Rumanyika R, Im HB, Massinde AN. Maternal and perinatal outcomes among eclamptic patients admitted to Bugando Medical Centre, Mwanza, Tanzania. Afr J Reprod Health. 2012;16:35–41. [PubMed] [Google Scholar]
- 12.Prabhu TR. Cerebrovascular complications in pregnancy and puerperium. J Obstet Gynaecol India. 2013;63:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Elkind MS, Willey JZ. Risk of pregnancy-associated stroke across age groups in New York state. JAMA Neurol. 2016;73:1461–1467. [DOI] [PubMed] [Google Scholar]
- 14.Omran SS, Parikh NS, Poisson S, Armstrong J, Merkler AE, Prabhu M, Navi BB, Riley LE, Fink ME, Kamel H. Association between Pregnancy and Cervical Artery Dissection. Ann Neurol. 2020;88:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, Coresh J. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 16.Elgendy IY, Gad MM, Mahmoud AN, Keeley EC, Pepine CJ. Acute stroke during pregnancy and puerperium. J Am Coll Cardiol. 2020;75:180–190. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Chan WS, Ray JG, Kramer MS, Joseph KS, Canadian Perinatal Surveillance System (Public Health Agency of Canada). Stroke and cerebrovascular disease in pregnancy: incidence, temporal trends, and risk factors. Stroke. 2019;50:13–20. [Google Scholar]
- 18.Miller EC, Zambrano Espinoza MD, Huang Y, Friedman AM, Boehme AK, Bello NA, Cleary KL, Wright JD, D’Alton ME. Maternal Race/Ethnicity, Hypertension, and Risk for Stroke During Delivery Admission. J Am Heart Assoc. 2020;9:e014775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida K, Takahashi JC, Takenobu Y, Suzuki N, Ogawa A, Miyamoto S. Strokes associated with pregnancy and puerperium: a nationwide study by the Japan Stroke Society. Stroke. 2017;48:276–282. [DOI] [PubMed] [Google Scholar]
- 20.Bashiri A, Lazer T, Burstein E, Smolin A, Lazer S, Perry ZH, Mazor M. Maternal and neonatal outcome following cerebrovascular accidents during pregnancy. J Matern Fetal Neonatal Med. 2007;20:241–247. [DOI] [PubMed] [Google Scholar]
- 21.Liang CC, Chang SD, Lai SL, Hsieh CC, Chueh HY, Lee TH. Stroke complicating pregnancy and the puerperium Eur J Neurol. 2006;13:1256–1260. [DOI] [PubMed] [Google Scholar]
- 22.Infographic: Racial/Ethnic Disparities in Pregnancy-Related Deaths — United States, 2007–2016. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/disparities-pregnancy-related-deaths/infographic.html. Accessed on November 17, 2020.
- 23.Too G, Wen T, Boehme AK, Miller EC, Leffert LR, Attenello FJ, Mack WJ, D’Alton ME, Friedman AM. Timing and risk factors of postpartum stroke. Obstet Gynecol. 2018;131:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS. Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med. 2014;370:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeks JR, Bambhroliya AB, Alex KM, Sheth SA, Savitz SI, Miller EC, McCullough LD, Vahidy FS. Association of Primary Intracerebral Hemorrhage With Pregnancy and the Postpartum Period. JAMA Network Open. 2020;3:e202769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheen JJ, Wright JD, Goffman D, Kern-Goldberger AR, Booker W, Siddiq Z, D’Alton ME, Friedman AM. Maternal age and risk for adverse outcomes. Am J Obstet Gynecol. 2018;219:390-e1–15. [DOI] [PubMed] [Google Scholar]
- 27.Bardenheier BH, Imperatore G, Devlin HM, Kim SY, Cho P, Geiss LS. Trends in pre-pregnancy diabetes among deliveries in 19 US states, 2000− 2010. Am J Prev Med. 2015;48:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EC, Yaghi S, Boehme AK, Willey JZ, Elkind MS, Marshall RS. Mechanisms and outcomes of stroke during pregnancy and the postpartum period: a cross-sectional study. Neurol Clin Pract. 2016;6:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, Giles WH, Kittner SJ. Preeclampsia and the risk of ischemic stroke among young women: results from the Stroke Prevention in Young Women Study. Stroke. 2006;37:1055–1059. [DOI] [PubMed] [Google Scholar]
- 30.Leffert LR, Clancy CR, Bateman BT, Bryant AS, Kuklina EV. Hypertensive disorders and pregnancy-related stroke: frequency, trends, risk factors, and outcomes. Obstet Gynecol. 2015;125:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew-Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. [DOI] [PubMed] [Google Scholar]
- 32.Elgendy IY, Nadeau SE, Bairey Merz CN, Pepine CJ. Migraine Headache: An Under-Appreciated Risk Factor for Cardiovascular Disease in Women. J Am Heart Assoc. 2019;8:e014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoud AN, Mentias A, Elgendy AY, Qazi A, Barakat AF, Saad M, Mohsen A, Abuzaid A, Mansoor H, Mojadidi MK, et al. Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bushnell CD, Jamison M, James AH. Migraines during pregnancy linked to stroke and vascular diseases: US population based case-control study. BMJ. 2009;338:b664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wabnitz A, Bushnell C. Migraine, cardiovascular disease, and stroke during pregnancy: systematic review of the literature. Cephalalgia. 2015;35:132–139. [DOI] [PubMed] [Google Scholar]
- 36.Lee ST, Chu K, Jung KH, Kim DH, Kim EH, Choe VN, Kim JH, Im WS, Kang L, Park JE, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70:1510–1517. [DOI] [PubMed] [Google Scholar]
- 37.Bandoli G, Baer RJ, Gano D, Pawlowski LJ, Chambers C. Migraines During Pregnancy and the Risk of Maternal Stroke. JAMA Neurol. 2020;77:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller EC, Elkind MS. Infection and stroke: an update on recent progress. Curr Neurol Neurosci Rep. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boehme AK, Luna J, Kulick ER, Kamel H, Elkind MS. Influenza-like illness as a trigger for ischemic stroke. Ann Clin Transl Neurol. 2018;5:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, deVeber GA, et al. Herpesvirus infections and childhood arterial ischemic stroke: results of the VIPS study. Circulation. 2016;133:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fullerton HJ, Hills NK, Elkind MS, Dowling MM, Wintermark M, Glaser CA, Tan M, Rivkin MJ, Titomanlio L, Barkovich AJ, et al. Infection, vaccination, and childhood arterial ischemic stroke: results of the VIPS study. Neurology. 2015;85:1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. [DOI] [PubMed] [Google Scholar]
- 43.Miller EC, Gatollari HJ, Too G, Boehme AK, Leffert L, Marshall RS, Elkind MS, Willey JZ. Risk factors for pregnancy-associated stroke in women with preeclampsia. Stroke. 2017;48:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller EC, Gallo M, Kulick ER, Friedman AM, Elkind MS, Boehme AK. Infections and risk of peripartum stroke during delivery admissions. Stroke. 2018;49:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller EC, Wen T, Elkind MS, Friedman AM, Boehme AK. Infection during delivery hospitalization and risk of readmission for postpartum stroke. Stroke. 2019;50:2685–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischer-Betz R, Specker C, Brinks R, Schneider M. Pregnancy outcome in patients with antiphospholipid syndrome after cerebral ischaemic events: an observational study. Lupus. 2012;21:1183–1189. [DOI] [PubMed] [Google Scholar]
- 47.Stone S, Hunt BJ, Khamashta MA, Bewley SJ, Nelson-Piercy C. Primary antiphospholipid syndrome in pregnancy: an analysis of outcome in a cohort of 33 women treated with a rigorous protocol. J Thromb Haemost. 2005;3:243–245. [DOI] [PubMed] [Google Scholar]
- 48.Skidmore FM, Williams LS, Fradkin KD, Alonso RJ, Biller J. Presentation, etiology, and outcome of stroke in pregnancy and puerperium. J Stroke Cerebrovasc Dis. 2001;10:1–10. [DOI] [PubMed] [Google Scholar]
- 49.Miller BR, Strbian D, Sundararajan S. Stroke in the young: patent foramen ovale and pregnancy. Stroke. 2015;46:e181–183. [DOI] [PubMed] [Google Scholar]
- 50.Mojadidi MK, Zaman MO, Elgendy IY, Mahmoud AN, Patel NK, Agarwal N, Tobis JM, Meier B. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol. 2018;71:1035–1043. [DOI] [PubMed] [Google Scholar]
- 51.Salehi Omran S, Parikh NS, Poisson S, Armstrong J, Merkler AE, Prabhu M, Navi BB, Riley LE, Fink ME, Kamel H. Association Between Pregnancy and Cervical Artery Dissection. Ann Neurol. 2020;88:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyakoshi K, Matsuoka M, Yasutomi D, Tanaka M, Yakubo K, Fukuiya T, Yoshimura Y. Moyamoya-disease-related ischemic stroke in the postpartum period. J Obstet Gynaecol Res. 2009;35:974–977. [DOI] [PubMed] [Google Scholar]
- 53.Aoyama K, Ray JG. Pregnancy and Risk of Intracerebral Hemorrhage. JAMA Netw Open 2020;3:e202844. [DOI] [PubMed] [Google Scholar]
- 54.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, Fonarow GC, Schwamm LH, Kuklina EV, George MG. Patient characteristics and outcomes after hemorrhagic stroke in pregnancy. Circ Cardiovasc Qual Outcomes. 2015;8:S170–178. [DOI] [PubMed] [Google Scholar]
- 55.Miller EC, Sundheim KM, Willey JZ, Boehme AK, Agalliu D, Marshall RS. The impact of pregnancy on hemorrhagic stroke in young women. Cerebrovasc Dis. 2018;46:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bateman BT, Olbrecht VA, Berman MF, Minehart RD, Schwamm LH, Leffert LR. Peripartum subarachnoid hemorrhage: nationwide data and institutional experience. Anesthesiology. 2012;116:324–333. [DOI] [PubMed] [Google Scholar]
- 57.Cantu C, Barinagarrementeria F. Cerebral venous thrombosis associated with pregnancy and puerperium. Review of 67 cases. Stroke. 1993;24:1880–1884. [DOI] [PubMed] [Google Scholar]
- 58.Ito M, Nakashima A, Hidaka T, Okabe M, Bac ND, Ina S, Yoneda S, Shiozaki A, Sumi S, Tsuneyama K, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 60.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12:594. [DOI] [PubMed] [Google Scholar]
- 61.Cipolla MJ, Sweet JG, Chan SL. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J Appl Physiol. 2011;110:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson AC, Cipolla MJ. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvasc Res. 2018;119:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci. 2019;9:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Veen TR, Panerai RB, Haeri S, van den Berg PP, Zeeman GG, Belfort MA. Changes in cerebral autoregulation in the second half of pregnancy and compared to non-pregnant controls. Pregnancy Hypertens. 2016;6:380–383. [DOI] [PubMed] [Google Scholar]
- 65.van Veen TR, Panerai RB, Haeri S, Singh J, Adusumalli JA, Zeeman GG, Belfort MA. Cerebral autoregulation in different hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2015;212:513–e1. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–376. [DOI] [PubMed] [Google Scholar]
- 67.Williams KP, Galerneau F, Wilson S. Changes in cerebral perfusion pressure in puerperal women with preeclampsia. Obstet Gynecol. 1998;92:1016–1019. [DOI] [PubMed] [Google Scholar]
- 68.Martin JN, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254. [DOI] [PubMed] [Google Scholar]
- 69.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 70.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller EC. Preeclampsia and cerebrovascular disease: the maternal brain at risk. Hypertension. 2019;74:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018;49:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Warrington JP, Drummond HA, Granger JP, Ryan MJ. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: role of TNF-α. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kashkoush AI, Ma H, Agarwal N, Panczykowski D, Tonetti D, Weiner GM, Ares W, Kenmuir C, Jadhav A, Jovin T, et al. Cerebral venous sinus thrombosis in pregnancy and puerperium: a pooled, systematic review. J Clin Neurosci. 2017;39:9–15. [DOI] [PubMed] [Google Scholar]
- 75.Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73:31–36. [DOI] [PubMed] [Google Scholar]
- 76.Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension. 2016;67:754–762. [DOI] [PubMed] [Google Scholar]
- 77.Edouard DA, Pannier BM, London GM, Cuche JL, Safar ME. Venous and arterial behavior during normal pregnancy. Am J Physiol. 1998;274:H1605–1612. [DOI] [PubMed] [Google Scholar]
- 78.Ladhani NNN, Swartz RH, Foley N, Nerenberg K, Smith EE, Gubitz G, Dowlatshahi D, Potts J, Ray JG, Barrett J, et al. Canadian stroke best practice consensus statement: acute stroke management during pregnancy. Int J Stroke. 2018;13:743–758. [DOI] [PubMed] [Google Scholar]
- 79.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–418. [DOI] [PubMed] [Google Scholar]
- 80.Committee on Obstetric Practice. Committee opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2017;130:e210. [DOI] [PubMed] [Google Scholar]
- 81.Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316:952–961. [DOI] [PubMed] [Google Scholar]
- 82.Tassi R, Acampa M, Marotta G, Cioni S, Guideri F, Rossi S, Cerase A, Martini G. Systemic thrombolysis for stroke in pregnancy. Am J Emerg Med. 2013;31:448.e1–3. [DOI] [PubMed] [Google Scholar]
- 83.Elgendy IY, Kumbhani DJ, Mahmoud A, Bhatt DL, Bavry AA. Mechanical thrombectomy for acute ischemic stroke: a meta-analysis of randomized trials. J Am Coll Cardiol. 2015;66:2498–2505. [DOI] [PubMed] [Google Scholar]
- 84.Leffert LR, Clancy CR, Bateman BT, Cox M, Schulte PJ, Smith EE, Fonarow GC, Kuklina EV, George MG, Schwamm LH. Treatment patterns and short-term outcomes in ischemic stroke in pregnancy or postpartum period. Am J Obstet Gynecol. 2016;214:723–e1. [DOI] [PubMed] [Google Scholar]
- 85.Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis–endorsed by the European Academy of Neurology. Eur Stroke J. 2017;2:195–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Siddiqui FM, Banerjee C, Zuurbier SM, Hao Q, Ahn C, Pride GL, Wasay M, Majoie CB, Liebeskind D, Johnson M, et al. Mechanical thrombectomy versus intrasinus thrombolysis for cerebral venous sinus thrombosis: a non-randomized comparison. Interv Neuroradiol. 2014;20:336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim YW, Neal D, Hoh BL. Cerebral aneurysms in pregnancy and delivery: pregnancy and delivery do not increase the risk of aneurysm rupture. Neurosurgery. 2013;72:143–149. [DOI] [PubMed] [Google Scholar]
- 88.Smok D, Prager KM. The ethics of neurologically complicated pregnancies. In Handbook of Clinical Neurology 2020. January 1 (Vol. 171, pp. 227–242). Elsevier. [DOI] [PubMed] [Google Scholar]
- 89.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18:234–239. [DOI] [PubMed] [Google Scholar]
- 90.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25:331–343. [DOI] [PubMed] [Google Scholar]
- 91.Roberge S, Villa P, Nicolaides K, Giguère Y, Vainio M, Bakthi A, Ebrashy A, Bujold E. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31:141–146. [DOI] [PubMed] [Google Scholar]
- 92.Henderson JT, O’Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann Intern Med. 2014;161:613–614. [DOI] [PubMed] [Google Scholar]
- 93.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. [DOI] [PubMed] [Google Scholar]
- 94.Lopes Perdigao J, Hirshberg A, Koelper N, Srinivas SK, Sammel MD and Levine LD. Postpartum blood pressure trends are impacted by race and BMI. Pregnancy Hypertens. 2020;20:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahajan A, Kemp A, Hawkins TL, Metcalfe A, Dowling S and Nerenberg K. Postpartum hypertensive disorders in the Emergency Department - A retrospective review of local practice in Calgary, Alberta. Pregnancy Hypertens. 2020;19:212–217. [DOI] [PubMed] [Google Scholar]
- 96.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol. 2020;135:e237–e260. [DOI] [PubMed] [Google Scholar]
- 97.Lamy C, Hamon JB, Coste J, Mas JL. Ischemic stroke in young women: risk of recurrence during subsequent pregnancies. Neurology. 2000;55:269–274. [DOI] [PubMed] [Google Scholar]
- 98.Aguiar de Sousa D, Canhão P, Ferro JM. Safety of pregnancy after cerebral venous thrombosis: a systematic review. Stroke. 2016;47:713–718. [DOI] [PubMed] [Google Scholar]
- 99.Swartz RH, Ladhani NNN, Foley N, Nerenberg K, Bal S, Barrett J, Bushnell C, Chan WS, Chari R, Dowlatshahi D, et al. Canadian stroke best practice consensus statement: secondary stroke prevention during pregnancy. Int J Stroke. 2018;13:406–419. [DOI] [PubMed] [Google Scholar]


