Abstract
Multi-drug resistance (MDR) of pathogens is a current public health crisis exacerbated by the horizontal transfer of antibiotic resistance genes via conjugative plasmids. Factors that stabilize these plasmids in bacterial communities contribute to an even higher incidence of MDR, given the increased likelihood that a host will already contain a plasmid when it acquires another through conjugation. Here we show one such stabilizing factor is host-plasmid coevolution under antibiotic selection, which facilitated the emergence of MDR via two distinct plasmids in communities consisting of Escherichia coli and Klebsiella pneumoniae once antibiotics were removed. In our system, evolution promoted greater stability of a plasmid in its coevolved host. Further, pleiotropic effects resulted in greater plasmid persistence in both novel host-plasmid combinations and, in some cases, multi-plasmid hosts. This evolved stability favored the generation of MDR cells and thwarted their loss within communities with multiple plasmids. By selecting for plasmid persistence, the application of antibiotics may promote MDR well after their original period of use.
Introduction
Plasmids are found in bacteria as extrachromosomal pieces of DNA that replicate separately from the host chromosome. They are a common repository of genes encoding antibiotic resistance, and conjugative plasmids can facilitate the spread of such genes via horizontal transfer1. The acquisition of a new plasmid can be costly for its host due to the burden imposed by plasmid-related processes such as replication, conjugation and gene expression, or the “interference” associated with interactions between plasmid-encoded proteins and cell housekeeping functions2–4. In the presence of an antibiotic, these costs can be outweighed by the benefit of plasmid-encoded resistance. In its absence, however, the costs are predicted to give a competitive advantage to plasmid-free cells. If plasmid loss occurs due to improper segregation of the plasmid during cell division (i.e. segregational loss), such costs could lead to a selective decrease in the fraction of plasmid-bearing cells in the population. In this light it is puzzling that plasmids encoding antibiotic resistance often show high persistence5, here defined as the capacity of a population, wherein all cells initially contain the plasmid, to maintain a high fraction of plasmid-bearing cells over time in the absence of drugs.
Solutions to this “plasmid paradox”6 involve processes that counterbalance, disrupt, or diminish selection against the plasmid. For instance, high rates of plasmid conjugation can transform plasmid-free cells into plasmid-containing cells, thereby counterbalancing selection for segregants and contributing to plasmid persistence7,8. Low rates of segregational loss or the incorporation of a post-segregational killing mechanism that inhibits growth of plasmid-free cells can disrupt selection against the plasmid; these mechanisms thus contribute to plasmid persistence by ensuring a dearth of (fitter) plasmid-free competitors9,10. Finally, compensatory mutations occurring in the host chromosome or the plasmid during coevolution can alleviate plasmid costs, which diminishes the strength of selection against the plasmid11–21.
Mutations that enhance conjugation, diminish segregational loss, or relieve the fitness cost of plasmids encoding antibiotic resistance allow the plasmids to better persist in the absence of drugs. These mutations could improve persistence strictly within the original coevolutionary context or additionally in novel host-plasmid combinations. Either way, these mutants serve as a stable source that can spread resistance to new strains and species within the microbial community. If recipient hosts are already resistant to a different antibiotic, novel MDR results. Therefore, in bacterial communities where prior evolution led to greater persistence of conjugative plasmids encoding resistance to different antibiotics, we expect the likelihood of hosts acquiring multiple distinct plasmids to be higher, thereby priming the emergence of MDR.
Antibiotic usage is on the rise, resulting in higher incidences of resistance in species of high clinical importance within the family Enterobacteriaceae22–24. This resistance is often caused by multiple self-transmissible plasmids, even leading to extended-resistant E. coli and pan-resistant K. pneumoniae25,26. Indeed, evolution of MDR in K. pneumoniae is mainly driven by the acquisition of multiple resistance plasmids27, which was reported to be particularly the case in clones found in clinical outbreaks28,29. It is thus critical to explore the effects of evolution under antibiotic selection on the subsequent likelihood of emergence of novel MDR via horizontally transmitted resistance genes in Enterobacteriaceae communities.
Predicted effects of coevolution
To explore how plasmid dynamics affect the emergence of MDR in bacterial communities, we consider a system involving two bacterial species and two conjugative plasmids, each encoding resistance to a different antibiotic. For simplicity, in this example we focus on the cost of plasmid carriage as the determinant of plasmid persistence. However, we note that the rate of plasmid conjugation or segregational loss could also be contributing factors. For any host-plasmid pair in this example, we predict that the plasmid-associated cost will affect the rate of plasmid loss from a population of hosts propagated in the absence of antibiotics. If the magnitude of the cost is high, de novo segregants will displace plasmid-bearing cells quickly (Figure 1b), producing a steep plasmid decay curve (Figure 1c). Yet evolution in the presence of the relevant antibiotic (Figure 1a) may lead to a reduction in the cost, which would then result in greater plasmid persistence (Figures 1d and 1e). Such predictions are consistent with the results from previous studies10–13. By incorporating a second host-plasmid pair, we expand upon these prior studies by exploring the emergence of MDR under drug-free conditions when the two evolved bacterial species, each harboring a distinct plasmid, are now mixed. We predict that if plasmid persistence is low, the incidence of MDR will be low (Figures 2b and 2c). However, if each bacterial species first coevolved with a distinct plasmid separately before coming together as a community (Figure 2a), the incidence of MDR would be higher (Figures 2d and 2e). Once a cell harbors both plasmids, MDR could be maintained either due to maintenance of both plasmids, or by the incorporation of the resistance genes into the chromosome or a single maintained plasmid. Regardless, we assume that by creating more stable sources of plasmids, the opportunities for conjugation between host types increase once they are in a mixed community, contributing to an increase in novel MDR. A more rigorous treatment of these topics via a mathematical model supports these predictions (see Supplementary Information I; Extended Data 1, Extended Data 2).
Figure 1. Predictions of the effects of host-plasmid coevolution on plasmid persistence.

(a) A hypothetical population of bacteria evolving for many generations (represented as a culture propagated via serial batch transfer) in the presence of an antibiotic (red-shaded medium) selecting for maintenance of a plasmid (red circle) encoding resistance. Evolutionary changes are represented by progressively darker shading of the bacteria over the sequence. (b) An isolate from the ancestral population is grown and propagated without the antibiotic (yellow-shaded medium) over a small number of transfers. (c) When the plasmid is costly, we predict the proportion of plasmid-containing cells decreases rapidly as they are outcompeted by plasmid-free cells generated via segregational loss (dashed line). (d) If evolutionary changes include mutations that compensate for the cost of plasmid carriage, we predict the proportional loss of plasmid-containing cells is slower (solid line), as would occur when (e) an isolate from the evolved population with compensatory mutations is grown and propagated without the antibiotic.
Figure 2. Predictions on the effects of host-plasmid coevolution on MDR emergence.

(a) Two different species of bacteria are considered. Cells of the first species are represented as rectangles, while cells of the second are given as rods. Cells from each species possess a distinct plasmid represented by the different colored circles. Both species evolve independently for many generations in the presence of an antibiotic that selects for maintenance of the plasmid (blue-shaded medium selects for blue plasmid and red-shaded medium selects for red plasmid). (b) Isolates from each ancestral population are mixed in medium without antibiotics and tracked over a small number of transfers, in which case (c) we predict low numbers of MDR cells (containing both plasmids) arising (dashed line). (d) However, we predict the incidence of MDR cells is higher (solid line), when (e) isolates from the evolved populations are mixed in antibiotic-free medium.
Results
Evolution increases plasmid persistence
To empirically test our predictions, we introduced conjugative plasmid pALTS28 (hereafter “p1”) encoding tetracycline resistance into an Escherichia coli host (hereafter “E”) and conjugative plasmid pALTS29 (hereafter “p2”) encoding chloramphenicol resistance into a Klebsiella pneumoniae host (hereafter “K”). We denote these strains as E(p1) and K(p2), where the host species is listed first and the plasmid inside the host is given in parentheses. Plasmid-free cells are indicated by E(Ø) and K(Ø) and cells that contain both plasmids are denoted E(p1,p2) and K(p1,p2). As shown in Figure 2a, each plasmid-bearing host was propagated in the presence of the relevant antibiotic for approximately 400 generations. To signify that evolution has taken place, and to convey information about the context of evolution, we add subscripts to both the host and the plasmid. Thus, E1(p1E) is an E. coli cell from a bacterial lineage that evolved with plasmid p1 currently possessing plasmid p1 from a plasmid lineage that evolved in E. coli. The reason for the complex notation is that mutations may occur in either the host chromosome or the plasmid17,30, and that we need to specify the evolutionary histories of both hosts and plasmids and indicate new plasmid-host combinations generated by conjugation. For instance, K2(p1E,p2K) is a K. pneumoniae cell with a coevolutionary history with plasmid p2, which currently contains both this coevolved plasmid p2 and plasmid p1 that evolved in the E. coli host.
Both plasmids were unstable in the absence of antibiotics prior to coevolving with their hosts: p1 was rapidly lost from E. coli (Figure 3a) and p2 from K. pneumoniae (Figure 3c). That is, separate populations of E(p1) and K(p2) were rapidly overtaken by E(Ø) and K(Ø) cells, respectively. However, after evolution, plasmids p1 and p2 each persisted to a greater degree in their respective co-evolved hosts across all replicates (Figures 3b and 3d). Statistical support for these differences was obtained using a plasmid population dynamic model previously described31–33 to fit and compare plasmid persistence curves (see Methods; Supplementary Information IV). All aspects of this protocol were also completed for the other plasmid-host combinations, E(p2) and K(p1), and nearly all evolved lines exhibited a similar increase in persistence compared to their ancestors (Extended Data 3).
Figure 3. Plasmid persistence in the absence of antibiotics increases after coevolution of plasmids with their hosts.

Dashed lines (left graphs) indicate ancestral strains. Solid lines (right graphs) indicate evolved strains. (a) The proportion of cells containing plasmid p1 decreased rapidly in an ancestral E(p1) population in the absence of the relevant antibiotic (tetracycline). However, host-plasmid coevolution in two replicate populations with tetracycline led to (b) greater plasmid persistence in the absence of antibiotic for both E1(p1E) populations. (c) The proportion of cells containing p2 similarly decreased swiftly in an ancestral K(p2) population in the absence of the relevant antibiotic (chloramphenicol). Host-plasmid coevolution in six replicate populations with chloramphenicol resulted in (d) greater plasmid persistence in the absence of antibiotic for all K2(p2K) populations. Note that for E1(p1E), isolates from only two of six evolved populations were included in this assay due to inadvertent appearance of K. pneumoniae cells in the remaining four E1(p1E) populations. In these graphs, every point is the mean of the three replicate persistence assays conducted for each isolate, one from each evolved population, with upward-pointing triangles used to represent points for p1-containing populations, and downward-pointing triangles used to represent points for p2-containing populations. Bars indicate the standard error. E. coli and K. pneumoniae icons are represented as rectangles and rods, respectively. The two “★” symbols denotes the persistence profiles for the evolved lineages selected to be used for all further assays in this paper.
Evolution increases emergence of MDR
We then asked how plasmid-host coevolution would affect the emergence of novel MDR in a mixed-species culture. As diagrammed in Figure 2b and 2e, we co-cultured three replicates of the ancestral species E(p1) with K(p2), and three replicates of the evolved species E1(p1E) with K2(p2K), in serial batch culture in the absence of antibiotics. Note that the evolved isolates used were those that correspond to the persistence profiles indicated by the “★” symbols in Figure 3. Using selective plating to track the eight potential cell types that could arise in such a co-culture, we found a higher cumulative incidence of K. pneumoniae cells containing both plasmids in the evolved assemblage (two-tailed Welch’s t test, t = −7.904, 95% CI = [−11199698, −3304356], df = 2, P = 0.0156, α=0.025 after a Bonferroni correction for two comparisons (i.e., E. coli and K. pneumoniae); Supplementary Information VI). This is a difference of 7.25 × 106 cells and approximately three orders of magnitude (Figure 4). This entire protocol was carried out three more times: (i) comparing the ancestral E(p2)-K(p1) co-culture to the evolved E2(p2E)-K1(p1K) co-culture (Extended Data 4), (ii) comparing the E. coli specific E(p1)-E(p2) and E1(p1E)-E2(p2E) co-cultures (Extended Data 5), and (iii) comparing the K. pneumoniae specific K(p1)-K(p2) and K1(p1E)-K2(p2E) co-cultures (Extended Data 6). While MDR E. coli arose in some of the co-cultures, the majority of all MDR cells were K. pneumoniae. Regardless, in each comparison, greater MDR emerged in all the co-cultures that consisted of evolved pairs, supporting the predictions laid out in Figure 2.
Figure 4. The emergence of MDR in mixed species co-cultures increases after coevolution of plasmids with their hosts.
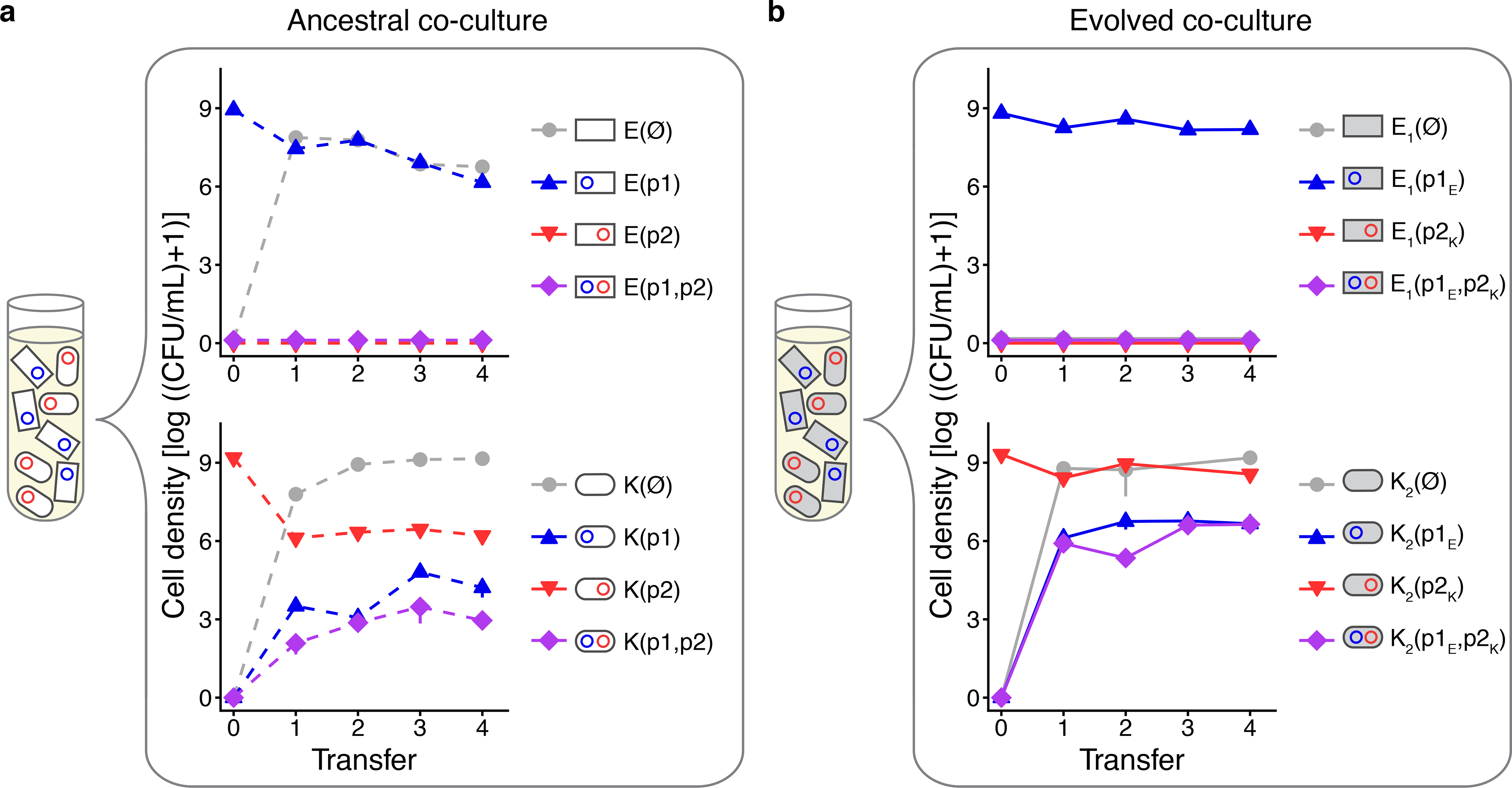
(a) Three replicate co-cultures of ancestral strains E(p1) and K(p2) were propagated over 4 transfers in the absence of antibiotics, with the initial starting co-culture composition shown in the tube. Within each co-culture, E. coli (upper graph) and K. pneumoniae (lower graph) were tracked via daily selective plating for each host-plasmid combination. Note that all plasmid-free, single- and double-plasmid-containing cell types become possible in either host species within the co-culture due to segregational loss and conjugation. (b) Three replicate co-cultures of evolved strains E1(p1E) and K2(p2K) were propagated and tracked in an identical manner. In all plots, blue lines indicate a host containing only a p1-type plasmid (p1 or p1E); red lines indicate a host containing only a p2-type plasmid (p2 or p2K); purple lines indicate an MDR host containing both plasmid types ((p1, p2) or (p1E, p2K)); and grey lines indicate plasmid free cells. Bars indicate the standard error of the mean of three replicate co-cultures. Dashed and solid lines indicate ancestral and evolved cells, respectively. The key comparison here is between the solid purple and dashed purple trajectories.
Evolution generates pleiotropic effects
In our original predictions (Figure 2 & Extended Data 2), we assumed that mutations that compensated for the cost of the plasmid, whether plasmid-born or chromosomal, only altered fitness of the host-plasmid combination that had coevolved. However, mutant plasmids may persist longer in novel hosts34 and mutant hosts may better retain novel plasmids. Indeed, Loftie-Eaton et al. (2017) showed that compensatory chromosomal mutations that increased persistence of a co-evolved plasmid also enabled the same mutant host to better retain alternate plasmids, an example of synergistic pleiotropy and a phenomenon termed “plasmid permissiveness”14. Expanding upon our mathematical model used to support our prior predictions, we explored how this might affect the emergence of MDR in bacterial communities with multiple plasmids. Our model predicted that when compensatory mutations that generate greater plasmid persistence in one host simultaneously improve plasmid persistence in a different host, the incidence of multi-drug resistance is predicted to increase (Supplementary Information I and Extended Data 7).
Did synergistic pleiotropy contribute to greater persistence of evolved host-plasmid pairs in our mixed species community? Because the vast majority of cells containing both plasmids in our co-cultures were K. pneumoniae, we focused on this species. In addition to evolution increasing the persistence of the p2K plasmid in its coevolved host K2 as seen in Fig 3, (ΔBIC = −714.3, Supplementary Information IV), persistence of p1 was higher in that same host (K2(p1)) than in the ancestral host K(p1) (ΔBIC = −151.7; Extended Data 8, panel a; Supplementary Information VII). Thus, we see evidence of increased plasmid permissiveness: mutations in the host chromosome stabilized the coevolved plasmid p2K as well as a novel plasmid p1. Similarly, we observed increased permissiveness of K. pneumoniae evolved with plasmid p1 (K1) towards plasmid p2 (ΔBIC = −765.7; Extended Data 8, panel b; Supplementary Information VII). These data suggest that evolutionary changes that stabilize one particular host-plasmid relationship can have pleiotropic effects, whereby the same benefits are also experienced in novel host-plasmid combinations. These interactions can then further maintain the source pool of plasmids in the community that are available to generate more MDR cells.
Evolution can increase MDR persistence
In our study, the emergence of MDR is certainly facilitated by a higher likelihood of formation of cells with multiple plasmids due to the greater plasmid persistence in populations of donors and recipients containing unique plasmids. However, it may also be the case that once MDR cells are formed, they have a higher likelihood of being maintained in the population. Thus, we consider how previous host-plasmid coevolution affects the decay of plasmid-mediated antibiotic resistance in our MDR cells when selection for the plasmids is removed. In our system, this process includes transitions first from double-plasmid-containing cells to single-plasmid-containing cells and then from single-plasmid-containing cells to plasmid-free cells (Figure 5a). To understand this first transition we measured plasmid persistence in a population of double-plasmid-containing cells under single antibiotic exposure, which allowed the population to lose only one of the two plasmids. Specifically, in the presence of tetracycline selecting for the p1-type plasmid (p1 or p1E), we measured the persistence of the p2-type plasmid (p2 or p2K) in the ancestral host with both ancestral plasmids, K(p1, p2), as well as the evolved host with both evolved plasmids, K2(p1E, p2K) (Figure 5b). Additionally, in the presence of chloramphenicol selecting for the p2-type plasmid, we measured the persistence of p1-type plasmid in each of the same two double-plasmid-containing hosts (Figure 5c). Coevolution resulted in slower rates of plasmid loss in both double-plasmid-containing contexts (Figure 5b and 5c; ΔBIC = −19.1 and −23.9, respectively). Turning to the transition from single-plasmid-containing cells to plasmid-free cells, we find the rate was lowered after host-plasmid coevolution (Figures 5d and 5e; ΔBIC = −866.0 and −413.4, respectively; similar to that seen in Figure 3 and Extended Data 3). However, in the flipped case where the persistence of p1K and p2E were measured in the K1 host, the patterns were much muted for the transition from double-plasmid- to single-plasmid-containing cells, suggesting that host-plasmid coevolution does not always significantly reduce the loss rate of MDR cells (see Extended Data 9 and Supplementary Information VIII). Likewise, the persistence of MDR in an environment with no antibiotics had a similar trend, whereby MDR persistence was greater in the evolved context, K2(p1E, p2K), than in the ancestral context, K(p1, p2) (Extended Data 10, panel b) but the difference was not meaningful (ΔBIC = 4.5, Supplementary Information VIII). Although in the flipped case comparing K1(p1K, p2E) to the ancestor, there was a meaningful increase in persistence (ΔBIC = −2.1, Supplementary Information VIII).
Figure 5. Maintenance of antibiotic resistance and MDR increases after host-plasmid coevolution, in both coevolved pairs and novel combinations of host and plasmids.

(a) A lineage can lose MDR by first losing either one of the two plasmid types and then losing the remaining plasmid. Here we compare the rates of loss of a focal plasmid between ancestral and evolved strains for all single- and double-plasmid-containing cells. (b) and (c) represent the transition from double-plasmid-containing cells to single-plasmid-containing cells, whereas (d) and (e) show the transition from single-plasmid-containing cells to plasmid-free cells, with conclusions from (d) having already been drawn from a previous assay (Figure 3). All persistence assays were done with K. pneumoniae as the host in an ancestral context (K(p1), K(p2) or K(p1,p2)) or an evolved context (K2(p1E), K2(p2K) or K2(p1E,p2K)). In all the evolved contexts, p2K is the coevolved plasmid and p1E is recently introduced. Furthermore, for all trajectories in panels (b)-(e), the left cell in the displayed “two-cell transition icon” possesses the focal plasmid; thus, the proportion being tracked refers to this left cell (whereas the right cell refers to a host without the focal plasmid). (b, c) Persistence of the focal plasmid in a double-plasmid-containing population under selection for the alternate plasmid is higher in the evolved context than the ancestral context. (d, e) Likewise, plasmid persistence in single-plasmid-containing populations is higher in the evolved than ancestral context. We also note an interesting result regarding the effect of plasmid co-residency on plasmid persistence in the ancestral strains: the dashed lines in (b) and (d) are tracking the loss of the same plasmid but in the context of the plasmid either on its own or with a co-residing plasmid (see Supplementary Information XI). Graph background shading indicates the presence of tetracycline (blue; selecting for p1-type plasmid), chloramphenicol (red; selecting for p2-type plasmid), or no antibiotics (white) during the assay. Blue, red, and purple lines indicate hosts containing only the p1-type plasmid, only the p2-type plasmid, and both plasmid types, respectively. Dashed and solid lines indicate ancestral and evolved contexts, respectively. Bars indicate standard error.
Taken together, we observed that prior host-plasmid coevolution not only contributes to increased emergence of MDR in the absence of antibiotics (Figure 4), but in some cases can improve the maintenance of MDR (Figure 5). This result may be particularly relevant to the practice of drug combination therapy, which is often prescribed in clinical settings to hinder the emergence of strains resistant to single antibiotics35. If MDR cells exist in a population where a drug cocktail containing the relevant antibiotics is applied, this could result in populations consisting entirely of MDR cells. Our results suggest that in some cases, these MDR cells may be maintained for a longer duration if host-plasmid coevolution has occurred.
Discussion
The focal plasmids p1 and p2 used in this study are broad-host-range, antibiotic resistance plasmids that were obtained from biosolid waste that is applied on agricultural soils. Global antibiotic exposure is largely driven by agricultural practices in which antibiotics are spread on crops and given to livestock to boost food production36,37. These practices are only expected to rise38, and thus concerns are also rising about the increased incidence of plasmid-mediated antibiotic resistance genes spreading from environmental isolates to the clinic. This transition is thought to be common39 and is likely the cause of the recent transcontinental spread of resistance to colistin, an “antibiotic of last resort”40 used in livestock production41 and to combat clinical MDR infections. Therefore plasmids p1 and p2 are suitable for helping us better understand how novel MDR might transition from the environment to clinical settings, especially after sustained antibiotic exposure. Also relevant to our choice of plasmids is that they belong to different incompatability (Inc) groups. This avoids any possible instability due to sharing a common replication or partitioning mechanism when the two plasmids co-reside in the same cell42. We would expect the emergence of MDR cells to be muted in cases where two plasmids are from the same Inc group.
In the mixed-species cultures there is asymmetry in the emergence of MDR for K. pneumoniae and E. coli, with a much higher likelihood of MDR formation in K. pneumoniae. This may be largely due to poor efficiency of plasmid transfer from K. pneumoniae to E. coli, as evidenced by conjugative transfer efficiencies calculated from cell counts obtained during the first 24 hours of our emergence assays (Supplementary Information VI). Indeed, in three of the four mixed-species co-cultures, conjugation from K. pneumoniae to E. coli was never observed (Supplementary Table 5). It is also possible that K. pneumoniae is, on average, better at maintaining multiple plasmids than E. coli. This would be consistent with research suggesting that the number of plasmids per genome is higher in K. pneumoniae than in other Gram-negative pathogens28. Finally, it is possible that our clinically isolated K. pneumoniae strain is pre-disposed to better maintain multiple plasmids due to the fact that it houses three stable native plasmids of its own (Supplementary Information II). However, reasoning against the last two possibilities for this particular case, plasmids p1 and p2 were originally less persistent in our K. pneumoniae strain than in E. coli, a pattern that was largely maintained across replicates after host-plasmid coevolution (compare Figure 3 and Extended Data 3).
A list of all mutations that occurred in each evolved isolate is provided in Supplementary Information X. While we are unable to attribute increased persistence to any one of these mutations, interesting patterns emerged. For example, mutations in the DNA-binding transcriptional regulator of the TetR/AcrR family were found in a majority of the K1(p1K) and K2(p2K) isolates, whereas no such mutations were observed in K. pneumoniae hosts evolved without a plasmid (i.e., KØ(Ø); Supplementary Data 2). We note that mutations in acrR have been previously associated with a reduction in plasmid cost in the E. coli strain MG165543, the same strain used in our study. In addition, it is interesting to consider mutations that would have multiple effects on community-level plasmid dynamics. For example, mutations in the conjugation-associated tra genes may alleviate plasmid cost but also inhibit conjugation. Porse, et al. (2016) found that deletions in tra genes on the plasmid ameliorated costs, and in three of our six K1(p1K) isolates we observed large deletions of tra genes (Supplementary Data 2). This was not the case for the K1(p1K) isolate used to explore the emergence of MDR in our study, but in specific cases where compensatory mutations during host-plasmid coevolution occur in tra genes, we might expect a decrease in MDR emergence due to abolished conjugation.
Our finding that the improved persistence of two environmental drug resistance plasmids after a short period of coevolution with their new hosts resulted in emergence of novel MDR warrants future research into the consequences of enhanced co-residency of multiple plasmids inside single host cells. One side-effect may be a greater opportunity for genetic rearrangement between co-residing plasmids. Host-plasmid coevolution could thus contribute to increased generation of novel MDR plasmids that could then conjugate into new cells as a single transferrable unit. In Lam et al. 2019, the authors specifically voice concerns that MDR and virulence genes could easily become associated on a single plasmid in K. pneumoniae, due to the common co-occurrence of multiple plasmid types within that species44. Although we did not examine the MDR K. pneumoniae cells generated in this study for the incidence of co-integration of plasmids, this is a fruitful direction for further research as to how (and how quickly) novel MDR plasmids can originate.
Given the prevalence of antibiotic usage across the globe, we expect a wide range of bacteria to commonly experience selection for antibiotic resistance in their natural environments. Such exposure may be transient due to a defined period of drug usage or due to the migration of bacteria from environments with antibiotics to environments without drugs45. Our and previous studies suggest this type of exposure could lead to stabilized mobile plasmids encoding antibiotic resistance. Here we show that such coevolution can then contribute to a higher incidence of novel MDR through at least two mechanisms: by combining the resistance genes from more persistent plasmid sources in single cells at higher frequencies, and, in some cases, by limiting the rate of loss of these resistance genes from MDR cells. In this way, antibiotic usage does not only immediately select for drug resistance, but simultaneously generates conditions favoring the emergence of multi-drug resistance even after antibiotics are removed.
Methods
More detailed strain descriptions and methods (including a description of our mathematical models, statistical approaches, and genomic analysis) can be found in Supplementary Information.
Hosts and plasmids.
Bacterial hosts included strains of two Enterobacteriaceae species Escherichia coli and Klebsiella pneumoniae: strains MG1655_SR and Kp08_R. We use the first letter of the genus name (E and K) to refer to these strains throughout. The two plasmids are conjugative, broad-host-range plasmids that were isolated from biosolids: the 61 kilobase (kb) plasmid pALTS28 (MN366357) from the plasmid group PromA, carrying genes conferring resistance to tetracycline (tetX), sulfonamide (sul2), MLS (mph(E) and msr(E)), and aminoglycosides (aph(3’’)-Ib and aph(6)-Id); and the 54 kb plasmid pALTS29 (MN366358) from the plasmid group IncP-1β, carrying genes conferring resistance to chloramphenicol (cmlA1), MLS (mph(E) and msr(E)), sulfonamide (sul1), and a gene encoding a class D beta-lactamase (blaOXA). We refer to these plasmids as p1 and p2 throughout. We note that both of our plasmids encode resistance to multiple drugs and thus MDR is conferred when a host contains either one. However, in this paper we are focused on the origin of an expanded set of resistances facilitated by plasmids with distinct resistance profiles coming together in the same cell. Thus, when we discuss the emergence of MDR we are referring to the co-residency of two plasmids (each encoding resistance to distinct antibiotics) within the same cell.
Evolution of host-plasmid pairs.
Replicate populations initiated with either K(p1), K(p2), E(p1), E(p2), K(Ø), or E(Ø) were evolved in 300 μl Lysogeny broth (LB) in microtiter plate wells for 68 culture transfers involving a 1:60 dilution (approximately 400 generations). Plasmid-containing strains were propagated in the presence of either 10 μg/ml tetracycline or 25 μg/ml chloramphenicol to select for p1 or p2, respectively. At the last transfer, each evolving population was diluted, plated and a haphazardly chosen colony served as the “evolved isolate” for the replicate lineage for subsequent assays.
Single-plasmid persistence and permissiveness assays.
All ancestral and evolved plasmid-containing strains were inoculated under antibiotic selection for the plasmid into 3 replicate microtiter plate wells. Each culture was then passaged daily for 9 transfers in the absence of antibiotic selection. Every three transfers, each culture was diluted and plated on LB agar to obtain single colonies. The proportion of plasmid-containing colonies out of 50 was then phenotypically determined via streaking on selective and non-selective agar. Differences between the ancestral and evolved persistence profiles were determined using a Bayesian Information Criterion (BIC) model selection approach (see Supplementary Information for details). This method is in accordance with that used in previous studies measuring plasmid persistence, which is also sometimes termed “plasmid stability”3,14,17,30,34.
MDR emergence assay.
Plasmid-containing strains contributing to each mixture were grown in liquid cultures overnight under antibiotic selection for the appropriate plasmid. One ml of each saturated culture was spun down for 5 minutes at 6000 rpm, the supernatant was replaced with 0.086% saline, and the cells were mixed thoroughly. This was repeated three times. K. pneumoniae cultures were then diluted 10-fold to ensure the mixed-species cultures were started with a similar number of cells per species. Both of the relevant strains were mixed in equal volumes, 5μl of which was used to inoculate 3 replicate microtiter plate wells containing 295 μl fresh LB. All replicates were thereafter grown in the absence of antibiotics and propagated via a 1:60 dilution every 24 hours for 4 days. All possible host- and plasmid-specific cell types were tracked via selective plating daily.
Double-plasmid persistence assays.
Double-plasmid-containing K. pneumoniae strains were obtained via plate matings between the recipient K. pneumoniae host and an E.coli donor. They were then tracked for the loss of each plasmid via streaking colonies onto separate antibiotic-containing plates. When the double-plasmid-containing populations were tracked for the loss of a single plasmid in the background of the alternate plasmid (i.e., the enforced presence of the non-focal plasmid), the LB growth medium was supplemented with the antibiotic concentration that inhibited the growth of any cells that did not contain the non-focal plasmid. Differences between the ancestral and evolved persistence profiles were determined using BIC model selection approaches (see Supplementary Information for details).
Data availability.
All sequencing data pertaining to this project have been made available at the National Center for Biotechnology Information (SRA accession number PRJNA552385). All other data that support the findings of this study are available at https://github.com/livkosterlitz/Figures-Jordt-et-al-2020.
Code availability.
The StabilityToolkit package used to analyze persistence data via our plasmid population dynamic model and corresponding instructions are available at https://github.com/jmponciano/StabilityToolkit/blob/master/RunningStabToolsPack.zip. The code used for the non-linear Beta-binomial regression model (Supplementary Information IX) can be found at https://github.com/jmponciano/JordtEtAl2020. The code used for simulations of our mathematical model is available at https://github.com/evokerr/Jordt_et_al_Gillespe_Code. Code used to generate all figures can be found at https://github.com/livkosterlitz/Figures-Jordt-et-al-2020.
Extended Data
Extended Data Fig. 1. Model simulation of plasmid persistence.

(a) The Gillespie algorithm was used to simulate population dynamics with μ* = 0.7, c = 0.2, KR = 0.004, λ = 0.05, τ = 0.00001, and 1/ψ = 0.000002, and the dynamic variables were initialized with R0 = 0.02, A0 = 0, and Aa,0 = 200. Many of the parameter values used here are similar to those from a previous parameterized model2. The average plasmid-bearing proportion of the simulated population over 10 replicates is shown as points, with each point located at the time closest to the end of the relevant 8-hour transfer period, after which a 60-fold dilution and resource replenishment occurred. The dashed line indicates that ancestral populations are being tracked. (b) Evolution is integrated by a reduction in the cost of plasmid carriage. Here we set p = 0.99, which makes the effective cost of the plasmid two orders of magnitude lower. Consequently, the loss of the plasmid from the evolved population is slower compared to its ancestor in part a. The solid line indicates that evolved populations are being tracked.
Extended Data Fig. 2. Model simulation of MDR emergence.

(a) The Gillespie algorithm was used to simulate community dynamics with parameters as in Supplementary Figure 1, and initial non-zero variables set to Aa,0 = 200, Bb,0 = 200, and R0 = 0.02. The average cell densities for the simulated population over 10 replicates are shown as points, with each point located at the time closest to the end of the relevant transfer period. The dashed lines indicate that ancestral populations are being tracked. (b) Evolution is incorporated by a reduction in the plasmid cost (p = 0.99) for both plasmids in their native hosts. Consequently, the density of MDR cells is higher compared to the ancestral community in part a. The solid lines indicate that evolved populations are being tracked.
Extended Data Fig. 3. Plasmid persistence in the absence of antibiotics increases after coevolution of plasmids with their hosts.

Dashed lines (left graphs) indicate ancestral strains. Solid lines (right graphs) indicate evolved strains. (a) The proportion of cells containing plasmid p2 decreased in an ancestral E(p2) population in the absence of the relevant antibiotic (chloramphenicol). However, coevolution of this host and plasmid in six replicate populations with chloramphenicol led to (b) greater plasmid persistence in the absence of antibiotic for nearly all of the E2(p2E) populations. (c) The proportion of cells containing p1 similarly decreased in an ancestral K(p1) population in the absence of the relevant antibiotic (tetracycline). Host-plasmid coevolution in six replicate populations with tetracycline resulted in (d) greater plasmid persistence in the absence of antibiotic for all K1(p1K) populations. In these graphs, every point is the mean of the three replicate persistence assays conducted for each isolate, with upward-pointing triangles used to represent points for p1-containing populations, and downward-pointing triangles used to represent points for p2-containing populations. Bars indicate the standard error. E. coli and K. pneumoniae icons are represented as rectangles and rods, respectively. The two “★” symbols denotes the persistence profiles for the evolved strains selected to be used for all further assays.
Extended Data Fig. 4. The emergence of MDR in mixed species co-cultures increases after coevolution of plasmids with their hosts.

(a) Three replicate co-cultures of ancestral strains E(p2) and K(p1) were propagated over 4 transfers in the absence of antibiotics. Within each co-culture, E. coli (upper graph) and K. pneumoniae (lower graph) were tracked via daily selective plating for each host-plasmid combination. (b) Three replicate co-cultures of evolved strains E2(p2E) and K1(p1K) were propagated and tracked in an identical manner. In all plots, blue lines indicate a host containing only a p1-type plasmid (p1 or p1K); red lines indicate a host containing only a p2-type plasmid (p2 or p2E); purple lines indicate an MDR host containing both plasmid types ((p1, p2) or (p1K, p2E)); and grey lines indicate plasmid free cells. Bars indicate the standard error of the mean of three replicate co-cultures. Dashed and solid lines indicate ancestral and evolved cells, respectively. Note that the point outlined in black in the plasmid-free cell trajectory indicates that the point was interpolated using the previous and following points on the trajectory, due to missing data (see Supplementary Information VI, section a). The key comparison here is between the solid purple and dashed purple trajectories. Cumulatively, there are significantly more MDR K. pneumoniae in evolved than ancestral co-cultures (t = −19.565, 95% CI = [−44541787, −28484560], df = 2, P = 0.0026), by a difference of 3.65 × 107 cells. MDR E. coli exhibits a similar trend, although the mean difference of 5.83 × 102 cells is not significant (t = −2.6576, 95% CI = [−1527.7, 361.1], df = 2, P = 0.1172).
Extended Data Fig. 5. The emergence of MDR in E. coli co-cultures increases after coevolution of plasmids with their hosts.

(a) Three replicate co-cultures of ancestral strains E(p1) and E(p2) were propagated over 4 transfers in the absence of antibiotics. Within each co-culture, E. coli cells were tracked via daily selective plating for each host-plasmid combination. (b) Three replicate co-cultures of evolved strains E1(p1E) and E2(p2E) were propagated and tracked in an identical manner. The notation E* indicates either host E1 or E2. In all plots, blue lines indicate a host containing only a p1-type plasmid (p1 or p1E); red lines indicate a host containing only a p2-type plasmid (p2 or p2E); purple lines indicate an MDR host containing both plasmid types ((p1, p2) or (p1E, p2E)); and grey lines indicate plasmid free cells. Bars indicate the standard error of the mean of three replicate co-cultures. Dashed and solid lines indicate ancestral and evolved cells, respectively. Note that the point outlined in black in the double-plasmid containing cell trajectory indicates that the point was interpolated using the previous and following points on the trajectory, due to missing data (see Supplementary Information VI, section a). The key comparison here is between the solid purple and dashed purple trajectories. Cumulatively there are significantly more MDR E. coli in evolved than ancestral co-cultures by a difference of 1.53 × 106 cells (t = −27.465, 95% CI = [−1767743, −1288990], df = 2.0005, P = 0.001321).
Extended Data Fig. 6. The emergence of MDR in K. pneumoniae co-cultures increases after coevolution of plasmids with their hosts.

(a) Three replicate co-cultures of ancestral strains K(p1) and K(p2) were propagated over 4 transfers in the absence of antibiotics. Within each co-culture, K. pneumoniae cells were tracked via daily selective plating for each host-plasmid combination. (b) Three replicate co-cultures of evolved strains K1(p1K) and K2(p2K) were propagated and tracked in an identical manner. The notation K* indicates either host K1 or K2. In all plots, blue lines indicate a host containing only a p1-type plasmid (p1 or p1K); red lines indicate a host containing only a p2-type plasmid (p2 or p2K); purple lines indicate an MDR host containing both plasmid types ((p1, p2) or (p1K, p2K)); and grey lines indicate plasmid free cells. Bars indicate the standard error of the mean of three replicate co-cultures. Dashed and solid lines indicate ancestral and evolved cells, respectively. Note that the open diamonds in the ancestral double-plasmid-containing cell trajectories indicate that the colony counts fell below our false positive threshold (see Supplementary Information VI, section a). The key comparison here is between the solid purple and dashed purple trajectories. Cumulatively there are significantly more MDR K. pneumoniae in evolved than ancestral co-cultures by a difference of 1.08 × 105 cells (t = −7.174, 95% CI = [−172891.2, −43255.5], df = 2, P = 0.01888).
Extended Data Fig. 7. Model simulation of MDR emergence with pleiotropic effects.

(a) The Gillespie algorithm was used to simulate community dynamics with parameters and initial variable settings as in Supplementary Figures 1 and 2. The average cell densities for the simulated population over 10 replicates are shown as points, with each point located at the time closest to the end of the relevant transfer period. Here we assume that evolutionary changes have led to a reduction in the plasmid cost (p = 0.5) for both plasmids in their native hosts, but note for any non-native configuration there is no cost reduction (q = 0). (b) Here we allow for pleiotropic effects—namely a reduction of plasmid cost in non-native configurations (q = 0.5). Although the difference is not substantial, the density of MDR cells is greater at every transfer. (c) More generally, we show a (literal) density plot for different p - q combinations. The gray level for each square is the log of the cumulative density of MDR cells at the end of 8 transfers for each combination of direct (p) and pleiotropic (q) effects of compensatory mutations. Greater reductions of plasmid costs in either native or non-native contexts correspond to higher incidence of MDR.
Extended Data Fig. 8. Hosts evolved with one plasmid became more permissive towards a novel plasmid.

Longer dashed lines indicate a host that was coevolved with the alternate plasmid. Shorter dashed lines represent ancestral host-plasmid pairs and are identical to those seen in Figure 3 and Supplementary Figure 4. (a) Persistence of the ancestral plasmid p1 was greater in K2(p1) than it was in K(p1) (ΔBIC = −151.7), indicating that changes in the host due to evolution with alternate plasmid type p2 have allowed it to better retain novel plasmid p1 (i.e., permissiveness is observed). (b) Persistence of the ancestral plasmid p2 was greater in K1(p2) than it was in K(p2) (ΔBIC = −765.7), again indicating permissiveness. Each point is the mean of three replicate persistence assays. Bars indicate the standard error of the mean.
Extended Data Fig. 9. The dynamics of antibiotic resistance and MDR persistence after host-plasmid coevolution, in both coevolved pairs and novel combinations of host and plasmids.

(a) A lineage can lose MDR by first losing either one of the two plasmid types and then losing the remaining plasmid. Here we compare the rates of loss of a focal plasmid between ancestral and evolved strains for all single- and double-plasmid-containing cells. (b) and (c) represent the transition from double-plasmid-containing cells to single-plasmid-containing cells, whereas (d) and (e) show the transition from single-plasmid-containing cells to plasmid-free cells, with conclusions from (d) having already been drawn from a previous assay (Supplementary Figures 4c and 4d). All persistence assays were done with K. pneumoniae as the host in an ancestral context (K(p1), K(p2) or K(p1,p2)) or an evolved context (K1(p1K), K1(p2E) or K1(p1K,p2E)). In all the evolved contexts, p1K is the coevolved plasmid and p2E is recently introduced. Furthermore, for all trajectories in panels (b)-(e), the left cell in the displayed “two-cell transition icon” possesses the focal plasmid; thus, the proportion being tracked refers to this left cell (whereas the right cell refers to a host without the focal plasmid). (b) Persistence of the focal plasmid in a double-plasmid-containing population under selection for the alternate plasmid is initially higher and ultimately different in the evolved context compared to the ancestral context for the p1-type plasmid (ΔBIC = −27.7, Supplementary Table 7) and (c) higher but not meaningfully different compared to the ancestral context for the p1-type plasmid (ΔBIC = 11.7, Supplementary Table 7) . (d) Plasmid persistence in a single-plasmid-containing population is higher in the evolved context than the ancestral context for the p1-type plasmid (ΔBIC = −552.3, Supplementary Table 7) and (e) p2-type plasmid (ΔBIC = −893.7, Supplementary Table 7). We also note an interesting result regarding the effect of plasmid co-residency on plasmid persistence in the ancestral strains: the dashed lines in (b) and (d) are tracking the loss of the same plasmid but in the context of the plasmid either on its own or with a co-residing plasmid (see Supplementary Information XI). Graph background shading and line colors are identical to Figure 5. Dashed and solid lines indicate ancestral and evolved contexts, respectively. Bars indicate standard error.
Extended Data Fig. 10. The dynamics of MDR persistence in the absence of antibiotics.

(a) A K. pneumoniae cell lineage loses MDR by first losing either one of the two plasmid types and then losing whatever plasmid type remains. The diagrams in this figure are repeated from Figures 5a and Supplementary Figure 10a. Here we compare the rates of plasmid loss between ancestral and evolved strains for all double-plasmid-containing cells as their lineages transition to further plasmid loss. White cell backgrounds indicate ancestral hosts and plasmids. Grey cell backgrounds indicate evolved hosts and plasmids. Plasmid persistence is measured as the transition from double-plasmid-containing cells to either single-plasmid-containing cells or plasmid-free cells. Plasmid persistence assays were done with K. pneumoniae as the host in an ancestral context (K(p1,p2); dashed line) or in an evolved context (K1(p1K,p2E); solid line). While there appears to be greater MDR persistence in the evolved context, there is no meaningful difference between the two persistence curves (ΔBIC = 4.5) according to a non-linear Beta-binomial regression model (see Supplementary Information IX). (b) We compare the same ancestral persistence curve (dashed line) to the alternate evolved context (K2(p1E,p2K); solid line), in which case there is a meaningful difference between the curves (ΔBIC = −2.1; Supplementary Information IX) . No antibiotics were present during the assay. Purple lines indicate MDR hosts containing both plasmid types. Bars indicate standard error.
Supplementary Material
Aknowledgements
This work is supported by the National Institute of Allergy and Infectious Diseases Extramural Activities grant R01 AI084918 of the National Institutes of Health (NIH), and through the National Science Foundation (NSF) under Cooperative Agreement No. DBI-0939454. H.J. was supported in part by Public Health Service, National Research Service Award, T32GM007270, from the National Institute of General Medical Sciences. O.K. was supported in part by the NSF Graduate Research Fellowship grant no. DGE-1762114. B.K. was supported in part by the NSF Career award DEB-0952825. We thank Wesley Loftie-Eaton for aiding in initial training of experimental techniques, and the entire Kerr and Top labs for useful suggestions on the manuscript.
Footnotes
Competing Interests Statement
The authors declare no competing interests.
References
- 1.Norman A, Hansen LH & Sørensen SJ Conjugative plasmids: vessels of the communal gene pool. Philos Trans R Soc Lond B Biol Sci 364, 2275–2289 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.San Millan A & MacLean RC Fitness Costs of Plasmids: a Limit to Plasmid Transmission. Microbiol Spectr 5, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yano H et al. Evolved plasmid-host interactions reduce plasmid interference cost. Mol. Microbiol. 101, 743–756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogwill T & MacLean RC The genetic basis of the fitness costs of antimicrobial resistance: a meta-analysis approach. Evol Appl 8, 284–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergstrom CT, Lipsitch M & Levin BR Natural selection, infectious transfer and the existence conditions for bacterial plasmids. Genetics 155, 1505–1519 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison E & Brockhurst MA Plasmid-mediated horizontal gene transfer is a coevolutionary process. Trends in Microbiology 20, 262–267 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lopatkin AJ et al. Persistence and reversal of plasmid-mediated antibiotic resistance. Nature Communications 8, 1689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner PE, Cooper VS & Lenski RE Tradeoff Between Horizontal and Vertical Modes of Transmission in Bacterial Plasmids. Evolution 52, 315 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Li Y et al. A Post-segregational Killing Mechanism for Maintaining Plasmid PMF1 in Its Myxococcus fulvus Host. Front. Cell. Infect. Microbiol. 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahlberg C & Chao L Amelioration of the cost of conjugative plasmid carriage in Eschericha coli K12. Genetics 165, 1641–1649 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouma JE & Lenski RE Evolution of a bacteria/plasmid association. Nature 335, 351–352 (1988). [DOI] [PubMed] [Google Scholar]
- 12.Starikova I et al. Fitness costs of various mobile genetic elements in Enterococcus faecium and Enterococcus faecalis. J. Antimicrob. Chemother. 68, 2755–2765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dionisio F, Conceição IC, Marques ACR, Fernandes L & Gordo I The evolution of a conjugative plasmid and its ability to increase bacterial fitness. Biol. Lett. 1, 250–252 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftie-Eaton W et al. Compensatory mutations improve general permissiveness to antibiotic resistance plasmids. Nature Ecology & Evolution 1, 1354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridenhour BJ et al. Persistence of antibiotic resistance plasmids in bacterial biofilms. Evol Appl 10, 640–647 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison E, Guymer D, Spiers AJ, Paterson S & Brockhurst MA Parallel compensatory evolution stabilizes plasmids across the parasitism-mutualism continuum. Curr. Biol. 25, 2034–2039 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Stalder T et al. Emerging patterns of plasmid-host coevolution that stabilize antibiotic resistance. Scientific Reports 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.San Millan A et al. Positive selection and compensatory adaptation interact to stabilize non-transmissible plasmids. Nat Commun 5, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porse A, Schønning K, Munck C & Sommer MOA Survival and Evolution of a Large Multidrug Resistance Plasmid in New Clinical Bacterial Hosts. Mol Biol Evol 33, 2860–2873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos-Lopez A et al. Compensatory evolution facilitates the acquisition of multiple plasmids in bacteria. bioRxiv 187070 (2017) doi: 10.1101/187070. [DOI] [Google Scholar]
- 21.Wein T, Hülter NF, Mizrahi I & Dagan T Emergence of plasmid stability under non-selective conditions maintains antibiotic resistance. Nat Commun 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Exner M et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg Infect Control 12, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliphant CM & Eroschenko K Antibiotic Resistance, Part 2: Gram-negative Pathogens. The Journal for Nurse Practitioners 11, 79–86 (2015). [Google Scholar]
- 24.WHO | Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug resistant bacterial infections, including tuberculosis. WHO; http://www.who.int/medicines/areas/rational_use/prioritization-of-pathogens/en/. [Google Scholar]
- 25.McGann P et al. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrob. Agents Chemother. 60, 4420–4421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man T. J. B. de et al. Genomic Analysis of a Pan-Resistant Isolate of Klebsiella pneumoniae, United States 2016. mBio 9, e00440–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navon-Venezia S, Kondratyeva K & Carattoli A Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 41, 252–275 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Wyres KL & Holt KE Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Curr. Opin. Microbiol. 45, 131–139 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Wyres KL & Holt KE Klebsiella pneumoniae Population Genomics and Antimicrobial-Resistant Clones. Trends Microbiol. 24, 944–956 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Sota M et al. Shifts in Host Range of a Promiscuous Plasmid through Parallel Evolution of its Replication Initiation Protein. ISME J 4, 1568–1580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelder LD et al. Combining Mathematical Models and Statistical Methods to Understand and Predict the Dynamics of Antibiotic-Sensitive Mutants in a Population of Resistant Bacteria During Experimental Evolution. Genetics 168, 1131–1144 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponciano JM, Gelder LD, Top EM & Joyce P The Population Biology of Bacterial Plasmids: A Hidden Markov Model Approach. Genetics 176, 957–968 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loftie-Eaton W et al. Evolutionary Paths That Expand Plasmid Host-Range: Implications for Spread of Antibiotic Resistance. Mol Biol Evol 33, 885–897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Gelder L, Williams JJ, Ponciano JM, Sota M & Top EM Adaptive Plasmid Evolution Results in Host-Range Expansion of a Broad-Host-Range Plasmid. Genetics 178, 2179–2190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tängdén T Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci 119, 149–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manyi-Loh C, Mamphweli S, Meyer E & Okoh A Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 23, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaz‐Moreira I, Ferreira C, Nunes OC & Manaia CM Sources of Antibiotic Resistance. in Antibiotic Drug Resistance 211–238 (John Wiley & Sons, Ltd, 2019). doi: 10.1002/9781119282549.ch10. [DOI] [Google Scholar]
- 38.Van Boeckel TP et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112, 5649–5654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crofts TS, Gasparrini AJ & Dantas G Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 15, 422–434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaye KS, Pogue JM, Tran TB, Nation RL & Li J Agents of Last Resort: Polymyxin Resistance. Infect. Dis. Clin. North Am. 30, 391–414 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Schwarz S & Johnson AP Transferable resistance to colistin: a new but old threat. J. Antimicrob. Chemother. 71, 2066–2070 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Novick RP Plasmid incompatibility. Microbiology and Molecular Biology Reviews 51, 381–395 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bottery MJ, Wood AJ & Brockhurst MA Adaptive modulation of antibiotic resistance through intragenomic coevolution. Nat Ecol Evol 1, 1364–1369 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam MMC et al. Convergence of virulence and MDR in a single plasmid vector in MDR Klebsiella pneumoniae ST15. J Antimicrob Chemother 74, 1218–1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellie Harrison, Hall James P. J. & Brockhurst Michael A. Migration promotes plasmid stability under spatially heterogeneous positive selection. Proceedings of the Royal Society B: Biological Sciences 285, 20180324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data pertaining to this project have been made available at the National Center for Biotechnology Information (SRA accession number PRJNA552385). All other data that support the findings of this study are available at https://github.com/livkosterlitz/Figures-Jordt-et-al-2020.


