Abstract
Recent work demonstrated that sympathetic neurons innervate the skeletal muscle near the neuromuscular junction (NMJ), and muscle sympathectomy and sympathomimetic agents strongly influence motoneuron synaptic vesicle release ex vivo. Moreover, reports attest that the pontine nucleus locus coeruleus (LC) projects to preganglionic sympathetic neurons and regulates human mobility and skeletal muscle physiology. Thus, we hypothesized that peripheral and central sympathetic neurons projecting directly or indirectly to the skeletal muscle regulate NMJ transmission. The aim of this study was to define the specific neuronal groups in the peripheral and central nervous systems that account for such regulation in adult mice in vivo by using optogenetics and NMJ transmission recordings in 3–5-month-old, male and female ChR2(H134R/EYFP)/TH-Cre mice. After detecting ChR2(H134R)/EYFP fluorescence in the paravertebral ganglia and LC neurons, we tested whether optostimulating the plantar nerve near the lumbricalis muscle or LC neurons effectively modulates motor nerve terminal synaptic vesicle release in living mice. Nerve optostimulation increased motor synaptic vesicle release in vitro and in vivo, while the presynaptic adrenoceptor blockers propranolol (β1/β2) and atenolol (β1) prevented this outcome. The effect is primarily presynaptic since miniature end-plate potential (MEPP) kinetics remained statistically unmodified after stimulation. In contrast, optostimulation of LC neurons did not regulate NMJ transmission. In summary, we conclude that postganglionic sympathetic neurons, but not LC neurons, increased NMJ transmission by acting on presynaptic β1-adrenergic receptors in vivo.
Keywords: Skeletal muscle, Sympathetic nervous system, Neuromuscular junction, Neuromuscular transmission
1. Introduction
Sarcopenia, or loss of muscle mass, strength, and power, is inherent to aging, contributing to disability and impairing quality of life (Alley et al., 2010; Bean et al., 2003; Clark and Manini, 2012; Doherty, 2003; Guralnik and Kritchevsky, 2010; Janssen, 2006; Lynch and Ryall, 2008; Manini and Clark, 2012; Onder et al., 2005; Onder et al., 2002; Rantanen et al., 1999). Interventions to increase muscle mass can improve strength and power in older adults (Fiatarone and Evans, 1993; Hunter et al., 2004; Latham et al., 2004; Liu and Latham, 2009). The discovery of adrenoceptors, which mediate the effects of the sympathetic nervous system (SNS) neurotransmitter noradrenaline (NA) on specific tissues, sparked the development of sympathomimetics that have profound influence on skeletal muscle mass (Lynch and Ryall, 2008). However, chronic administration has serious side effects that preclude their use for muscle-wasting conditions (Baker et al., 2006). Interventions that can adjust NA release to changing physiological demands will depend on understanding how the SNS, including peripheral and central relays, regulates two interrelated processes: skeletal muscle innervation and neuromuscular junction (NMJ) transmission.
SNS failure is common in old age (Lipsitz and Novak, 2008) and neurodegenerative diseases (Kaufmann and Biaggioni, 2003) that impair adaptation to common physiological stressors. We and others found that sympathetic axons innervate skeletal muscle fibers (Barker and Saito, 1981; Chan-Palay et al., 1982; Khan et al., 2016; Rodrigues et al., 2018) and maintain the integrity of skeletal muscle composition and function at the presynaptic and postsynaptic NMJ (Khan et al., 2016; Rodrigues et al., 2018; Wang et al., 2020). We also demonstrated that (a) SNS impairment leads to skeletal muscle motor denervation (Rodrigues et al., 2018); (b) both the SNS and sympathomimetics regulate motoneuron synaptic vesicle release and postsynaptic molecular composition (Rodrigues et al., 2019); and (c) aging blunts SNS influence on NMJ transmission (Wang et al., 2020). These data support the strong influence of the SNS on motoneuron, NMJ transmission, and the dynamic relationship between motoneuron and skeletal muscle fiber across the lifespan (Wang et al., 2020).
Probing deeper, we found that the SNS and sympathomimetics regulate motoneuron synaptic vesicle release via extracellular Ca2+ and such molecular targets as TRPV1 and P/Q- and N-type voltage-activated Ca2+ channels (Rodrigues et al., 2019). Recently, we demonstrated that the β1-adrenoceptor is expressed in motoneuron axonal terminals and its levels decline significantly with aging (Wang et al., 2020). These studies unveil the molecular substrate that accounts for the influence of peripheral sympathetic neurons on NMJ transmission in young mice and its decline with aging.
However, we must still define the specific neuronal groups in the peripheral and central nervous system that account for SNS influence on NMJ transmission. For example, we do not know whether or how postganglionic sympathetic neurons regulate synaptic vesicle release in vivo or the central autonomic relays regulate NMJ transmission. Previous studies have related LC with human mobility, motility and skeletal muscle physiology (Buchman et al., 2012; Del Tredici and Braak, 2013; Dobbins and Feldman, 1994; Jacobs et al., 1991; Robinson, 1978; Xiang et al., 2014). Here, we focus on lumbar postganglionic sympathetic neurons, and the pontine locus coeruleus (LC) (a.k.a. A6 nucleus), a subdivision of which projects to preganglionic neurons in the spinal cord intermediolateral (IML) column.
We reported that the plantar-lumbricalis nerve preparation is amenable to testing the influence of sympathetic neuron activity on motor axon terminal synaptic vesicle release in vitro (Wang et al., 2020); however, whether it is a viable approach for in vivo experiments is unknown. Here, for the first time, we tested this approach. While the in vitro studies entailed direct stimulation of ganglia neurons to define their role in modulating NMJ transmission (Wang et al., 2020) their direct stimulation in vivo is traumatic because they are located near the aorta and retroperitoneal organs. Instead, we optostimulated sympathetic neuron axons near where they enter the lumbricalis muscle. Combining optogenetics with NMJ transmission recordings provides a unique opportunity to determine the precise role of postganglionic sympathetic neurons and the LC nucleus in NMJ transmission in adult living mice.
This study is the first to show in living mice that postganglionic sympathetic neurons regulate neuromuscular transmission by activating β1-AR. It elucidates the cross-talk between the motoneuron and SNS and underscores their relevance to aging and neurodegeneration.
2. Materials and methods
2.1. Animals and ethics statement
We generated a mouse model expressing ChR2/EYFP fusion protein in sympathetic neurons (Figs. 1, 2) by crossing B6.Cg-Gt(ROSA) 26Sortm32(CAG-COP4*H134R/EYFP)Hze/J, (a.k.a.Ai32(RCL-ChR2(H134R/EYFP)) (Madisen et al., 2012) with B6.Cg-7630403G23RikTg(Th-cre) 1Tmd/J (a.k.a. TH-Cre) (Savitt et al., 2005) mice, both from Jackson Laboratories (Bar Harbor, Maine, USA). Supplementary Fig. 1 shows the ChR2(H134R/EYFP), TH-Cre, and ChR2(H134R/EYFP)/TH-Cre mouse genotyping. Only mice heterozygous for both alleles were used for experiments. The primers used for genotyping mouse B6.Cg-Gt(ROSA) 26Sortm32(CAG-COP4*H134R/EYFP)Hze/J were: AAG GGA GCT GCA GTG GAG TA (wild type forward), CCG AAA ATC TGT GGG AAG TC (wild type reverse), ACA TGG TCC TGC TGG AGT TC (forward), and GGC ATT AAA GCA GCG TAT CC (reverse). Primers for mouse B6.Cg-7630403G23RikTg(Th-cre)1Tmd/J were: AGT GGC CTC TTC CAG AAA TG (internal positive control forward), TGC GAC TGT GTC TGA TTT CC (internal positive control reverse), GAG ACA GAA CTC GGG ACC AC (transgenic forward), and AGG CAA ATT TTG GTG TAC GG (transgene reverse).
Fig. 1.

Relationship between the pontine locus coeruleus, paravertebral sympathetic ganglia and motor- and sympathetic-skeletal muscle innervation. The diagram shows the dual sympathetic and motor innervation of the skeletal muscle fiber. The locus coeruleus (LC) neurons project to the spinal cord intermediolateral (IML) column and dorsal horn. The sympathetic preganglionic neurons at IML synapse with paravertebral sympathetic postganglionic neurons, which in turn innervate the myofiber. Sympathetic neuron axons display periodic bulbous enlargements also known as varicosities. Lumbar spinal cord motoneurons innervate the skeletal muscle at the neuromuscular junction (NMJ). Framed paravertebral sympathetic ganglia ①, terminal sympathetic and motoneuron axons ②, and LC ③ are imaged in Figs. 2A and B and 4A, respectively.
Fig. 2.
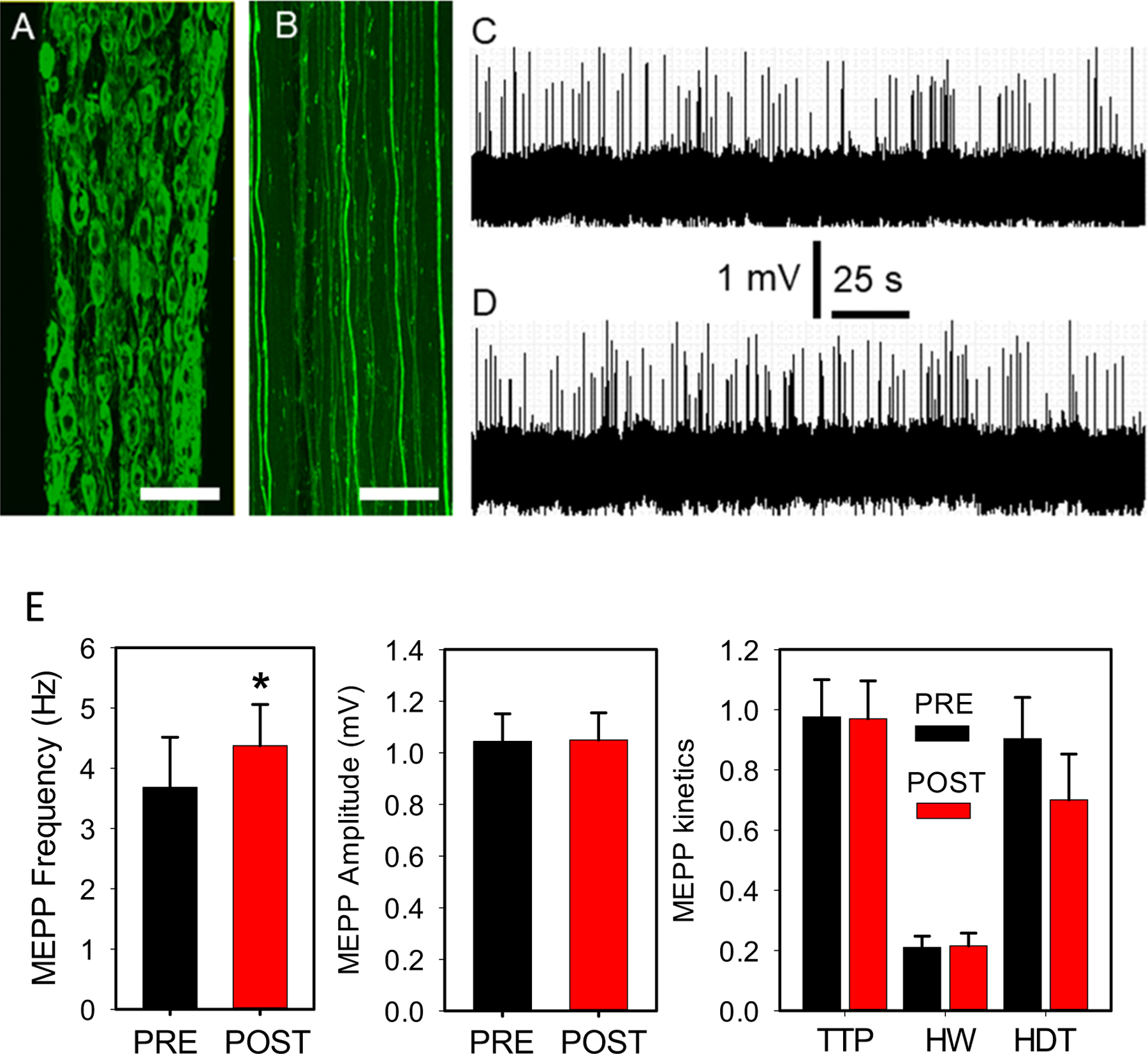
Postganglionic sympathetic neuron optostimulation enhances NMJ transmission in vitro.
Confocal z-stack maximum intensity projections showing that ChR2(H134)/EYFP fusion protein expression in postganglionic sympathetic neurons (A) reaches distal segments of their axons, at the plantar nerve (B). Sympathetic neuron preparation in vitro. A 100-s fragment from a continuous 5-min recording of spontaneous ACh release at baseline (C), and after sympathetic neuron terminal optostimulation at 60-Hz for 10-s in 8 fibers from 7 mice (D). Calibration bar = 500 μm (A) and 300 μm (B). (E) MEPP frequency, amplitude and kinetics recorded before (PRE, black column) and after (POST, red column) optostimulation. The asterisk denotes a statistical significant difference (P = 0.032). P-values for MEPP amplitude, time to peak (TTP), half-width (HW), and half-decay time (HDT) are 0.805, 0.456, 0.466, and 0.174, respectively.
We illuminated young (3–5-month-old), male and female mice with a blue light laser for rapid in vivo and in vitro activation of excitable cells (see Optostimulation, below). The mice were housed in the pathogen-free facility of the Wake Forest School of Medicine (WFSM) Animal Research Program. All mice were fed chow ad libitum and had continuous access to drinking water. Experimental procedures were conducted in compliance with National Institutes of Health laboratory animal care guidelines. We made every effort to minimize suffering. The WFSM Institutional Animal Care and Use Committee approved the study protocol.
2.2. Transgenic ChR2-GFP expression in paravertebral ganglia sympathetic neuron soma, terminals, and LC
To verify ChR2-GFP expression, we dissected paravertebral sympathetic ganglia, and neuronal terminals at the plantar nerve next to the lumbricalis muscle and sliced the pontine LC nucleus. The anatomical organization of the lumbricalis into few myofiber layers allowed us to fully image the intact muscle and distal plantar nerve as a whole-mount, avoiding cryosection distortions (Rodrigues et al., 2018). Whole lumbricalis muscles and the nerve were dissected and freed from surrounding tissues. The tendons were pinned in a dish coated with Sylgard 184 silicone (Dow Corning, Midland, MI), fixed in 4% paraformaldehyde (PFA) at 4 °C overnight. The next day the preparation was washed 3 times in phosphate buffer solution (PBS). Tendons were cut, and the plantar nerve-lumbricalis muscle preparation was mounted between a glass slide and a coverslip using fluorescence mounting medium (S3023, Dako, Carpinteria, CA).
Similarly, the paravertebral sympathetic ganglia chain was dissected, freed from surrounding tissues, and fixed overnight in 4% PFA. The next day, ganglia were mounted as described above.
To detect LC fluorescence, mice were transcardially perfused with, first cold PBS, then 4% PFA. The dissected brain stem was kept in 4% PFA for 2 days, rinsed in PBS three times, and sectioned (40 μm) using a vibratome. The three preparations were initially visualized with an inverted, fluorescent microscope (Olympus IX81, Tokyo, Japan) with an Orca-R2 Hamamatsu CCD camera (Hamamatsu, Japan). MetaMorph Imaging System software (Olympus) controlled the camera driver and image acquisition. Tissue samples were then imaged using a confocal microscope.
2.3. Localization of immunofluorescence expression in motor, sympathetic, or sensory neurons
Nerves were dissected and freed from surrounding fat and blood vessels. After incubation in 4% PFA, they were cryopreserved in 10, 20, and 30% sucrose, covered with optimal cutting-temperature compound (Sakura O.C.T., Ted Pella, Redding, CA), quick frozen in cold isopentane in dry ice, and stored at −80 °C until cut into 10-μm sections at −20 °C with a Leica CM3050 S cryostat (Heidelberg, Germany). For immunofluorescence staining, cryosections mounted on slides were rinsed in phosphate-buffered saline (PBS) and blocked with 10% goat serum in PBS for 1 h. Axons were identified using the following specific antibodies: tyrosine hydroxylase (cat. AB152, MilliporeSigma, Burlington, MA, dilution 1:200); choline acetyl transferase (ChAT) (cat. AB144P MilliporeSigma, dilution 1:200); and calcitonin-gene-related protein (CGRP) (cat. ab81887, Abcam, dilution 1:200). Nerve sections were incubated with primary mouse antibodies and 10% goat serum in PBS for 2 h at room temperature. After washing with PBS twice for 5 min, the sections were incubated with secondary goat antibodies diluted in PBS for 1 h at room temperature followed by two 5-min washes with PBS. All secondary antibodies were purchased from Invitrogen (Thermo Fisher, Carlsbad, CA). Tissue sections were mounted using fluorescence mounting medium (S3023, Dako, Carpinteria, CA) and visualized with an IX81 Olympus fluorescence microscope. Control experiments included (i) immunofluorescent nerve staining in the absence of the primary antibody, which abolished the specific immunoreactive signal; and (ii) fluorescence-signal detection at the wavelength expected for the secondary antibody’s emission spectrum and in no other fluorescence channel.
2.4. Imaging of whole-mount paravertebral sympathetic ganglia and plantar nerve
Lumbar paravertebral sympathetic chain in the mid-belly region of a ganglion and plantar nerves were imaged using an inverted, motorized Olympus FV1200/IX83 spectral laser scanning confocal microscope. We acquired image z-stacks with a UPLSAP020X objective, NA: 0.75. All ganglia were inspected in the z-dimension at 1.16 μm/slice resolution, and the step-size analysis avoided counting cells more than once. Images were acquired at 0.397 μm/pixel. Images of the pontine LC nucleus were visualized by epifluorescence microscopy as described (Schmidt et al., 2019).
2.5. NMJ transmission recording
We recorded NMJ transmission at baseline and after optostimulating (a) the plantar nerve-lumbricalis preparation (Rodrigues et al., 2019) where the nerve enters the lumbricalis muscle in vivo and in vitro, and (b) the LC in vivo. For the in vitro recordings, the lumbricalis muscle was dissected with its plantar nerve attached as described (Rodrigues et al., 2018; Rodrigues et al., 2019). For the in vivo recordings, the mouse connected to the isoflurane system was positioned on the side of the microscope stage, and the paw with the dissected plantar nerve and lumbricalis muscle, was pinned to the bottom of a Sylgard chamber, continually perfused, and transilluminated for NMJ and sharp electrode visualization. The intracellular electrode was placed near the NMJ. The NMJ preparation was incubated in μ-conotoxin GIIIB (Alomone Labs, Jerusalem, Israel) to a final concentration of 1-μM for 30 min before recording started, to prevent muscle contraction. NMJ transmission was then recorded intracellularly in oxygenated normal mammalian Ringer’s solution (in mM, 135 NaCl, 5 KCl, 1 MgSO4, 15 NaHCO3, 1 Na2HPO4, 11 D-glucose, 2.5 calcium gluconate, pH 7.4) using a TEV-200A amplifier (Dagan Co., Minneapolis, MN), DigiData 1322A, and pClamp10.5 software (MDS Analytical Technologies, Sunnyvale, CA). The intracellular electrodes (~40mΩ) were filled with 2 M K-citrate and 10 mM K-chloride and mounted on the stage of a MP-285 micromanipulator (Sutter Instruments, Novato, CA). We examined the preparation using an upright, fixed-stage Zeiss Axioscope FS microscope (Thornwood, NY) with a 10 or 20×, water immersion FLUAR objective (Zeiss), mounted on a microscope translator (Sutter Instruments). The recorded miniature endplate potentials (MEPPs) were baseline corrected, and their frequency, amplitude, time-to-peak, half-duration, and half-decay time were analyzed using Clampfit 10.7 (MDS Analytical Technologies). Data were transferred to Microsoft Excel for basic analysis. We used the Transfer Trace application in Clampfit to illustrate electrophysiological recordings.
2.6. Viral vector construct and delivery
To drive GFP expression to the LC in the absence of ChR2, we used a specific NA neuron promoter and an adeno-associated virus (AAV) to build the AAV9-PRSx8-EGFP (pBK228) vector. We selected an AAV-serotype 9 (AAV9) because it displayed the strongest expression of all AAVs in a comparative study of various serotypes carrying the same reporter gene (Arimura et al., 2014; Berns and Linden, 1995; Zincarelli et al., 2008). The capsid gene of AAV9 was pseudotyped by cloning into helper plasmids containing the AAV2 rep gene.
The packaging vector contained AAV2-ITRs (inverted terminal repeat sequences) and the PRSx8-driven EGFP transgene. The PRSx8 promoter consisted of tandem repeats of transcription factor Phox 2a/2b binding sites. PRSx8 promoter was followed by a transcription start site from the hDBH gene (Hwang et al., 2001). This hybrid transcomplementing construct, encoding replication genes from AAV2 and capsid genes from the AAV9 serotype displays AAV9 cell tropism. The virus was produced by the triple-transfection method using HEK293 cells as described (Haberman et al., 1998; Zincarelli et al., 2008). Viral vector (5 × 1011 vg of AAV9 in PBS supplemented with 5% sorbitol) was delivered by LC injection to isofluorane-anesthetized mice using Paxinos’s coordinates (Paxinos and Franklin, 2019).
2.7. Stereotaxic virus injection
Mice were anesthetized using ketamine hydrochloride (80 mg/kg, i.p.) and xylazine hydrochloride (10 mg/kg, i.p.). Once they were placed in a stereotaxic frame, their scalps were shaved, wiped with iodine, and incised down the center to reveal the skull. A small hole was drilled for a screw to stabilize the cap, and into another on the right side, an optic-fluid cannula (OFC) (Doric Lenses, Quebec, Canada) was implanted (DV, 4.0 mm) for virus injection. Dental cement was used to cover the exposed skull. We returned mice to their cages for recovery after the cement dried.
Since LC is most prominent where the cerebellum and inferior colliculus meet, the virus was injected −5.52 posterior of the bregma, lateral 1 mm (Schmidt et al., 2019). This viral vector restricts ChR2 expression to noradrenaline neurons in LC. We sacrificed all mice injected with the virus vector in LC to visualize its expression and to locate the optic fiber as described next.
2.8. Optostimulation
For peripheral sympathetic neuron optostimulation, we micropositioned an optic fiber in close contact with the distal plantar nerve near the lumbricalis muscle. The laser, set at wavelength 473 nm (Optotronics, LLC, Mead, CO), had a 150-mW maximum power output. A programmable function generator (Agilent 33120A, 1-Mz function/arbitrary waveform generator) provided control signals to modulate the laser via the TTL input control port on the power supply. The function generator produced single pulses at 1-, 10-, or 60-Hz for 10-ms. Individual pulse duration within each series of pulses was 4-ms. We used a power meter (Thorlabs Model S121C, Newton, NJ) to measure the laser power output.
For central sympathetic neuron optostimulation, we micropositioned an optic fiber in the LC using Paxinos’s stereotaxic coordinates (Paxinos and Franklin, 2019). The laser settings, including spectrum wavelength, maximum power output, and pulse stimulation protocols, are the same as for nerve stimulation.
2.9. Continuous breath-rate monitoring
We digitally recorded mouse thoracic expansion with a Sony CCD-IRIS (SSC-C374) video camera to monitor breath rate before and after LC optostimulation, Respiratory pattern and NMJ transmission were recorded simultaneously and analyzed off-line.
2.10. Statistical analysis
All experiments and analyses were conducted blind to treatment group. No statistical methods were used to predetermine sample sizes, but they are similar to those reported in recently published studies (Rodrigues et al., 2018; Rodrigues et al., 2019). Sigma Plot version 12.5 (Systat Software, Inc., San Jose, CA) and Microsoft Excel were used to perform the analyses. Results were tested for normal distribution before selecting the appropriate statistical test. Student’s t-test was used to compare two groups, and analysis of variance (ANOVA) followed by Bonferroni’s post-hoc analysis were used to compare three or more. An ANOVA repeated measures test was used to compare means across one or more variables that were based on repeated observations. Data were expressed as mean ± S.E.M. A p-value < 0.05 was considered significant.
3. Results
To examine whether exclusively stimulating sympathetic neurons would increase NMJ transmission, we used a mouse model that crossed ChR2/EGFP mice with Th-Cre mice (Supplementary Fig. 1). TH-Cre mice have the rat tyrosine hydroxylase (TH) promoter, which directs Cre recombinase expression to catecholaminergic cells (Savitt et al., 2005), while ChR2(H134) mice carry a single point mutation at position H134, which generates larger photocurrents than wild-type ChR2 (Madisen et al., 2012). This mouse model was amenable to test the influence of exclusive sympathetic neuron activation on NMJ transmission using optogenetics. Supplementary Fig. 2 shows that ChR-EYFP is expressed in tyrosine hydroxylase (TH) immunoreactive sympathetic axons, but not in choline acetyl transferase (ChAT) motoneuron or calcitonin-gene-related protein (CGRP) sensory axons. ChAT (A) and CGRP (B) immunoreactivity is unrelated to EYFP, while TH and EYFP (C) overlap extensively. These results have been confirmed in nerves from another mouse.
Since we detected the fusion protein ChR2(H134R)/EYFP in the paravertebral ganglia neurons and their axons (Fig. 2A, B), we next tested whether optostimulation of the plantar nerve near the lumbricalis muscle effectively modulates motor nerve terminal synaptic vesicle release.
The diagram in Fig. 1 shows the relationship between the sympathetic structures examined in this study and indicates where the tissues illustrated in Figs. 2A and B and 4A reside.
3.1. Direct, high-frequency optostimulation of the plantar nerve terminal increases motor synaptic vesicle release in vitro
Fig. 2 shows that ChR2(H134)/EYFP expression in paravertebral sympathetic neurons (A) reaches distal segments of their axons at the plantar nerve near the lumbricalis muscle (B). Fig. 1 (frames 1 and 2) indicates where the paravertebral sympathetic ganglia and neuron terminals were imaged. Thus, our in vitro study focused on this region. Fig. 2 also shows a 100-s fragment selected from a continuous 5-min recording of spontaneous ACh release at baseline (C) and after sympathetic neuron terminal optostimulation at 60 Hz for 10 s (D). MEPP frequency seems to increase after the catecholaminergic neuron terminals were optostimulated. MEPP frequency, but not amplitude, time-to-peak, half-width, or half-decay time increased significantly. The increase in MEPP frequency was ~18% (E). The laser activation rate (60 Hz) was chosen based on the maximal response obtained in a similar in vitro preparation where sympathetic neurons were electrically stimulated (Wang et al., 2020). Due to potential exhaustion of the neurotransmitters (see Discussion), we recorded each preparation once, with the exception of one mouse, from which we obtained two recordings. All experiments lasted at most 2 h after the nerve/muscle preparation was dissected. To avoid any time limitation, we performed the remaining experiments in living mice.
3.2. Optostimulation of paravertebral sympathetic ganglia neuron terminals modulates presynaptic motor vesicle release in vivo
These experiments tested whether plantar nerve optostimulation enhances synapse transmission at the plantar nerve-lumbricalis muscle in an anesthetized mouse. This approach assures that all the nervous and vascular connections and the influence of the humoral milieu are preserved. We also varied the range of optic pulses (1-, 10-, or 60-Hz) applied to the nerve.
Fig. 2(A, B) shows that ChR2(H134)/EYFP expression in paravertebral ganglia is uniform along the sympathetic neuron. Fig. 3 shows that optostimulation of the plantar nerve at 1 Hz significantly enhanced MEPP frequency, but not amplitude, time-to-peak, half-width, or half-decay time. MEPP frequency increased ~33% at 1 Hz, almost twice that achieved in vitro at 60 Hz. Propranolol, a β1-β2 adrenergic receptor (AR) blocker, completely prevented this effect, probably due to presynaptic motoneuron β1-AR blockade. To confirm that norepinephrine released from sympathetic neuron terminals acts on preterminal β1-ARs, we tested a specific β1-AR blocker, atenolol, which also strongly suppressed AR-dependent enhanced synaptic vesicle release (Supplementary Table 1).
Fig. 3.

Postganglionic sympathetic neuron optostimulation enhances NMJ transmission in living mice.
MEPP frequency, amplitude and kinetics recorded before (PRE, black column) and after (POST, red column) optostimulation at 1 Hz (A), 10 Hz (B), and 60 Hz (C). (A) The asterisk denotes a statistical significant difference in MEPP frequency (P = 0.035). P-values for MEPP amplitude, time to peak (TTP), half-width (HW), and half-decay time (HDT) are 0.081, 0.764, 0.58, and 0.517, respectively. (B) MEPP frequency is statistically different after optostimulation at 10 Hz (P = 0.014). P-values for MEPP amplitude, time to peak (TTP), half-width (HW), and half-decay time (HDT) are 0.356, 0.318, 0.637, and 0.336, respectively. (C) MEPP frequency is significantly different (P = 0.007). P-values for MEPP amplitude, time to peak (TTP), half-width (HW), and half-decay time (HDT) are 0.931, 0.707, 0.84, and 0.216, respectively.
Optostimulation frequency at 10 Hz (Fig. 3B) and 60 Hz (Fig. 3C) further increased MEPP frequency to ~46% and ~ 80%, respectively. However, MEPP amplitude, time-to-peak, half-width, and half-decay time did not change significantly. Both propranolol and atenolol prevented any effects on synaptic vesicle release at 10 Hz and 60 Hz (Supplementary Tables 2 and 3).
Recordings in the peroneal nerve of ChR2-negative mice showed no significant modifications in MEPP frequency at any stimulation frequency (post/pre stimulation at 1 Hz: 0.992 ± 0.038; n = 8 fibers/7 mice, p = 0.468; 20 Hz: 1.02 ± 0.095; n = 6 fibers/7 mice, p = 0.462; and 60 Hz: 0.081 ± 0.098; n = 5 fibers/7 mice, p = 0.274). These results indicate that nerve optostimulation in the absence of ChR2 expression does not increase MEPP frequency.
3.3. LC optostimulation does not modify the frequency of synaptic vesicle release at the NMJ in vivo
These experiments examined whether stimulating the central sympathetic nucleus, LC, regulates NMJ transmission. Fig. 4 shows ChR2/EYFP fusion protein expression in the pontine LC nucleus (A) (see Fig. 1, frame 3). Optostimulation of these neurons in vivo at 60 Hz did not modify baseline MEPP frequency or other properties (Fig. 4B–D). MEPP frequency, amplitude, time-to-peak, half-width, and half-decay-time recorded at rest and after stimulation at 1, 10, and 60 Hz for 10 s did not differ significantly (Supplementary Table 4). After recordings concluded, the mice were sacrificed, cardiac-perfused, and their brain stems were sectioned with a vibratome (see Methods) to determine the location of the optic fiber at LC. If the optic fiber was misplaced, data from the mouse were removed from further analysis and statistical considerations. The mice in which optic fibers were incorrectly positioned in the LC did exhibit a high breathing rate, which indicates that other regions of the brainstem sympathetic network regulate the breathing pattern (Feldman et al., 2003; Stornetta et al., 2006). However, identifying them was beyond the goal of our study.
Fig. 4.
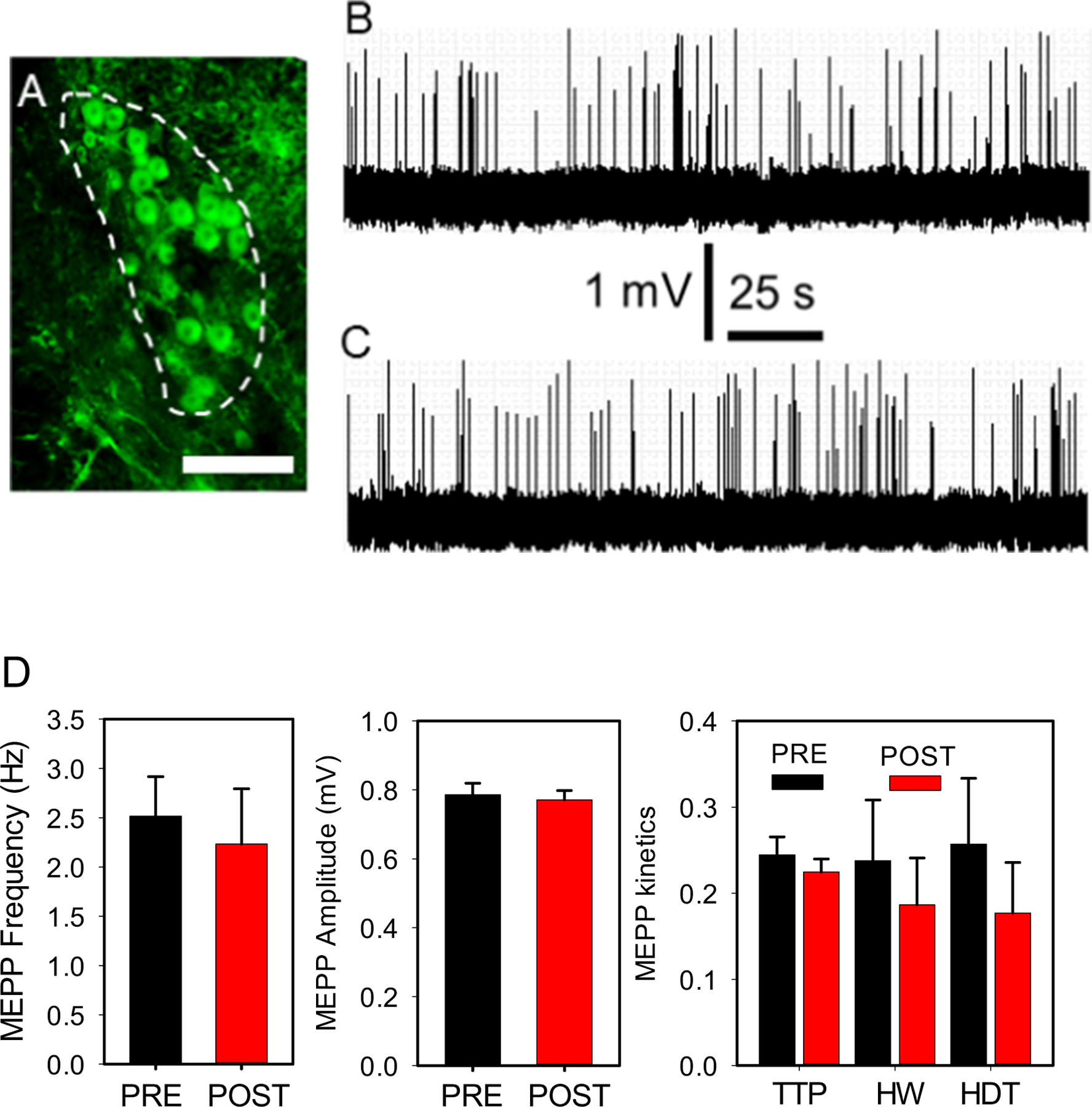
Locus coeruleus optostimulation does not significantly modify NMJ transmission in vivo.
ChR2(H134)/EYFP fusion protein expression at the LC nucleus outlined by a white-dashed line (A). Calibration bar = 500 μm. Optostimulation of these neurons in vivo did not modify baseline MEPP properties. A 100-s fragment from a continuous 5-min recording of spontaneous ACh release at baseline (B), and after sympathetic neuron terminal optostimulation at 60 Hz for 10-s (C). (D) MEPP frequency, amplitude and kinetics recorded before (PRE, black column) and after (POST, red column) optostimulation. P-values for MEPP frequency, amplitude, time to peak (TTP), half-width (HW), and half-decay time (HDT) are 0.289, 0.365, 0.227, 0.307, 0.345, respectively.
Since LC regulates respiration, we continuously monitored mouse breath rate to determine the neurons’ responsiveness to ChR2(H134)/EYFP optostimulation. Under deep isoflurane anesthesia, breath rate was 38 ± 3/min at baseline (n = 74 mice) and increased to 59 ± 8/min (n = 22), 68 ± 9/min (n = 19), and 81 ± 11/min (n = 14) after LC optostimulation at 1, 10, and 60 Hz, respectively. To determine the specificity of the respiratory response, we transfected LC with an AAV9-PRSx8-EGFP viral vector injected using stereotaxic coordinates (see Methods). Neither NMJ transmission (Supplementary Table 5) nor respiratory pattern significantly changed after LC optostimulation. The breath rate was 42 ± 5/min at baseline (n = 19 mice) and 49 ± 9/min (n = 8), 52 ± 7/min (n = 7), and 45 ± 8/min (n = 6) after LC optostimulation at 1, 10, and 60 Hz, respectively. These results indicate that the increased respiratory response stems from optostimulation of LC neurons since the AAV9-PRSx8-EGFP viral vector is devoid of the channelrhodopsin encoding gene.
4. Discussion
4.1. A novel approach to investigate the role of peripheral and central sympathetic relays in NMJ transmission in living adult mice
Previous studies from our laboratory tested the influence of the SNS on NMJ transmission. We used in vitro preparations including either the plantar and lumbricalis muscle (Rodrigues et al., 2018; Rodrigues et al., 2019) or a more complex dissection maintaining the anatomical relationship of the NMJ with the paravertebral sympathetic ganglia chain, which we called SNLP preparation (Wang et al., 2020). The first is similar to the one used for the experiments included in Fig. 2. We performed all of the remaining experiments using a novel plantar nerve-lumbricalis NMJ preparation in vivo. It enabled us to record, for the first time, spontaneous synaptic vesicle release under conditions that maintain nervous system and humoral systemic influences.
The influence of the peripheral and central SNS on NMJ transmission has not been investigated in vivo. Although the SNLP preparation significantly advanced our investigation of the influence of postganglionic sympathetic neurons on NMJ transmission (Wang et al., 2020), we questioned whether their electrical stimulation might regulate lumbar organs other than hindlimb muscles. Of the few studies that have reported on NMJ transmission in vivo, many recordings were performed blind. Measurements in the rat hemidiaphragm (Muchnik et al., 1975) are irrelevant to the study of limb sarcopenia, while the cat soleus muscle preparation (Haberman et al., 1998) impedes a combined electrophysiological and optogenetics approach. In contrast, our novel in vivo plantar nerve-lumbricalis muscle preparation allows us to combine optogenetic studies and electrophysiological recordings of NMJ transmission and permits inspection of fluorescence-tagged molecules at the nerve and muscle in addition to the NMJ.
4.2. Optogenetics allows us to define the role of specific peripheral and central sympathetic neuron pools in NMJ transmission in living mice
Since Ai32(RCL-ChR2(H134R/EYFP))-TH-Cre transgenic mice exhibit ChR2(H134R/EYFP) protein expression in peripheral and central sympathetic neurons they are eminently suitable to test the role of both peripheral and central sympathetic relays in NMJ transmission. The Ai32 ChR2(H134R-EYFP) mouse has been successfully used for optogenetics analysis of cortical pyramidal neurons (Madisen et al., 2012) and direct optical activation of skeletal muscle fibers (Magown et al., 2015), among other applications. In turn, the TH-Cre mouse (Savitt et al., 2005) has been amply used to target the expression of a variety of genes to sympathetic neurons (You et al., 2019), and the overwhelming majority of GFP-expressing neurons are TH-immunopositive. To our knowledge, this mouse has not been used in similar studies.
It has one limitation. In contrast to focused viral vector delivery, the mouse model used in this study expresses ChR2-EYFP transgene in sympathetic neurons in brainstem regions other than the LC (i.e., the paraventricular nucleus, suprachiasmatic nucleus, among others; data not shown). However, despite the transgene’s widespread expression, its presence in the LC was found consistent across mice.
We propose that this mouse is a valuable tool to interrogate the role of the SNS in various targets’ functions, especially NMJ transmission. We have not examined other groups of brain stem neurons that project to the IML (Bruinstroop et al., 2012), but investigating their role in NMJ transmission will require this transgenic mouse together with viral vectors to pinpoint the autonomic neurons that control peripheral and central targets.
4.3. Sympathetic axon optostimulation enhances NMJ transmission in vitro and in living mice
We previously reported that sympathomimetic agents modulate plantar nerve terminal synaptic vesicles release in vitro (Rodrigues et al., 2019). To prove that activating only the sympathetic nerve terminals modulates NMJ transmission, we optostimulated the plantar nerve near its entrance into the lumbricalis muscle in vitro. We chose 60 Hz as the stimulation frequency because it has been shown to elicit the maximal response (Wang et al., 2020). The fact that MEPP frequency, but not amplitude, time-to-peak, half-width, or half-decay time, increased significantly indicates that activating the catecholaminergic terminals influences NMJ transmission at the preterminal synapse, with negligible impact on the postterminal.
In a previous study we showed that MEPP kinetics are affected in sympathectomized mice (Rodrigues et al., 2018); however, in the current study there are no significant differences on MEPP kinetics after activation of sympathetic nerves or application of β-AR blockers. Skeletal muscle sympathectomy affects both pre- and postsynaptic structure and molecular composition (Rodrigues et al., 2018) and consequently MEPP kinetics, but sympathetic neuron optostimulation or β-AR blockers blockers act at the presynapse, modulating NA levels, and thus restricting the influence of the neurotransmitter mainly to acetylcholine release.
Although this approach was useful as a proof of concept, it has limitations. First, repetitive stimulation may exhaust the catecholaminergic and/or acetylcholine neuron pools. Second, we cannot draw physiological conclusions because the systemic influence of the humoral milieu on the subcellular structures and molecular components involved in NMJ transmission is suppressed (Wang et al., 2020). The difference between NMJ-transmission enhancement in this study (18%) and the previous one (80%) (Wang et al., 2020) recorded at 60 Hz could be explained by the site of sympathetic neuron stimulation. In the earlier study (Wang et al., 2020), we directly stimulated the sympathetic ganglia electrically (see Fig. 1, ref. (Wang et al., 2020)), while in the present study we optostimulated a peroneal nerve stump near the lumbricalis muscle in vitro. We speculate that sympathetic neuron integrity is required for its proper function. We were unable to directly optostimulate the sympathetic neuron soma for anatomical reasons. The paravertebral ganglia chain is located near the aorta and critical blood vessels on the dorsal abdomen, which seriously limits its study in live mice. However, note that optostimulation of sympathetic axons in the peroneal nerve at 60 Hz enhanced MEPP frequency ~80% in vivo (Fig. 3C).
We did not test the effect of sympathetic optostimulation on end-plate potential (EPP) and quantal content because prior work recorded no changes after electrical stimulation of ganglia sympathetic neurons (Wang et al., 2020). The most plausible explanation for this lack of response is that the electrical pulses required to elicit EPP stimulate all axons running along the nerve, including the sympathetic ones. Thus, EPP represents motoneuron/muscle transmission evoked by an electrical pulse under the strong activation of the sympathetic nervous system, so the sympathetic neuron influences EPP both before and after sympathetic neuron optostimulation.
Although, together with MEPP, EPP recording has proven useful to measure acetylcholine quantal content before and after perfusion with a pharmacological agent, we found it useless under the present experimental conditions. EPPs recorded before sympathetic neuron optostimulation are influenced by sympathetic axon activation because the electrical stimulation delivered to generate an EPP activates motor, sensory, and sympathetic axons simultaneously. Thus, baseline EPP recordings are already influenced by noradrenaline (NA) modulation of motoneuron ACh release (Wang et al., 2020). Thus, differences in EPP recordings at baseline and after neuron optostimulation are markedly attenuated.
4.4. β1-ARs mediate catecholamines’ influence on motoneuron synaptic vesicle release in living mice
Using specific receptor toxins and nerve immunohistochemistry, we found that both sympathetic and motor neuron terminals express β1-AR, which is susceptible to propranolol blockade (Rodrigues et al., 2019; Wang et al., 2020). This receptor mediates Ca2+ influx through N-type Ca2+ channels at the sympathetic neuron and through P/Q-type Ca2+ channels in motoneurons (Rodrigues et al., 2019; Wang et al., 2020). However, whether β1-AR mediate sympathetic neuron-elicited increased motoneuron synaptic vesicle release in vivo has not been tested before. The concentrations chosen for these experiments were previously tested and indicate that 1 μM of propranolol effectively blocks the influence of NA on MEPP frequency (Rodrigues et al., 2019), while 10 μM of atenolol blocks synchronized quanta secretion (Bukharaeva et al., 2000). Here, we found that propranolol and atenolol can prevent catecholamine enhancement of synaptic vesicle release and reduce ACh release by acting on the motoneuron terminal β1-AR in living mice.
4.5. The master sympathetic pontine LC nucleus does not regulate NMJ transmission
The conclusion that LC did not modulate MEPPs is based on (1) the abundant expression of ChR2 in this pontine nucleus (Fig. 4A) and nerve sympathetic axons (Fig. 2A, B, and Supplementary Fig. 2); (2) the lack of significant changes in MEPP frequency in response to LC optostimulation evoked by an optic fiber located at the nucleus and verified postmortem for each mouse (see Results under LC optostimulation does not modify the frequency of synaptic vesicles release at the NMJ in vivo); and (3) the increase in mouse breath rate in response to LC optostimulated at 1, 10, and 60 Hz. Also note that neurons in the LC fire tonically at 1–3 Hz, while high-frequency stimulation at 5 Hz or higher causes loss of muscle tone. We have stimulated LC at frequencies below and above 5 Hz, with no obvious response, which indicates that by innervating the muscle spindle (Radovanovic et al., 2015), LC modulates posture (Carter et al., 2010) but not NMJ transmission (this study).
The fact that LC optostimulation did not increase MEPP frequency raises questions about the functional role of its anatomical projections to the IML. Previous work using a retrograde pseudorabies virus (PRV) or adenoviral constructs concluded that LC neurons predominantly project to the dorsal horn. After injecting PRV into sympathetically innervated organs, a subpopulation of neurons located in the ventral part of the LC were consistently infected, suggesting a direct projection to preganglionic sympathetic neurons in the IML (Cano et al., 2004; Sly et al., 1999). The adenoviral approach also identified LC projections in the IML (Bruinstroop et al., 2012).
Consequently, the lack of LC-evoked modulation of NMJ transmission implies that LC projections to the IML do not play a functional role, while its influence on the mouse respiratory pattern indicates an effective technical activation. Since confocal z-stack projections of sections through the spinal cord showed that the injected AAV2/rh8-PRSx8-EGFP-W vector appears at both the dorsal horn and the IML (Bruinstroop et al., 2012), an alternative explanation is that LC projections to the IML do not establish en passant synapse with preganglionic sympathetic neurons.
Despite documented interaction between the brainstem nucleus and spinal cord preganglionic sympathetic neurons, their functional relevance is unknown. Our study indicates that they do not play a physiological role in controlling NMJ transmission, but we cannot rule out the possibility that other brainstem autonomic relays control spinal cord sympathetic efflux toward the mouse hindlimb NMJ.
Detailed analysis of central neuroanatomical circuitries show that several brainstem noradrenergic regions project to the spinal cord intermediolateral column (IML), including noradrenergic bulbospinal A1 and A2 areas (Rodovalho et al., 2020) and pontine A5, A6, and A7 (Bruinstroop et al., 2012). The functional relevance of these CNS projections for NMJ transmission and muscle motor innervation remains unknown.
5. Conclusions
The main accomplishments of the present study are: (1) developing an approach method to investigate the role of peripheral and central sympathetic relays on NMJ transmission in living adult mice; (2) using optogenetics to define the role of specific peripheral and central sympathetic neuron pools in NMJ transmission in living mice; and discovering that (3) selective optoactivation of postganglionic sympathetic neurons increases NMJ transmission in vitro and in vivo; (4) β1-ARs mediate the influence of catecholamines on motoneuron synaptic vesicle release under physiological conditions; and (5) LC neurons projecting to preganglionic sympathetic neurons (IML), do not regulate NMJ transmission.
Supplementary Material
Acknowledgments
The National Institutes of Health grants R01AG057013 and R01AG057013-02S1 to Osvaldo Delbono, the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332) supported this research, and R01AA022449 to Evgeny Budygin.
Footnotes
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcn.2020.103563.
References
- Alley DE, Koster A, Mackey D, Cawthon P, Ferrucci L, Simonsick EM, Yu B, Hardy S, Goodpaster B, Sarkisian C, Houston DK, Kritchevsky SB, Cummings S, Lee JS, Tylavsky FA, Newman A, Harris T, 2010. Hospitalization and change in body composition and strength in a population-based cohort of older persons. J. Am. Geriatr. Soc 58, 2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura S, Okada T, Tezuka T, Chiyo T, Kasahara Y, Yoshimura T, Motomura M, Yoshida N, Beeson D, Takeda S, Yamanashi Y, 2014. Neuromuscular disease. DOK7 gene therapy benefits mouse models of diseases characterized by defects in the neuromuscular junction. Science 345, 1505–1508. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Constantin-Teodosiu D, Jones SW, Timmons JA, Greenhaff PL, 2006. Chronic treatment with the beta(2)-adrenoceptor agonist prodrug BRL-47672 impairs rat skeletal muscle function by inducing a comprehensive shift to a faster muscle phenotype. J. Pharmacol. Exp. Ther 319, 439–446. [DOI] [PubMed] [Google Scholar]
- Barker D, Saito M, 1981. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc. R. Soc. Lond. B Biol. Sci 212, 317–332. [DOI] [PubMed] [Google Scholar]
- Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L, 2003. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J. Gerontol. A Biol. Sci. Med. Sci 58, 728–733. [DOI] [PubMed] [Google Scholar]
- Berns KI, Linden RM, 1995. The cryptic life style of adeno-associated virus. Bioessays 17, 237–245. [DOI] [PubMed] [Google Scholar]
- Bruinstroop E, Cano G, Vanderhorst VGJM, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB, 2012. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J. Comp. Neurol 520, 1985–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, Schneider JA, Bennett DA, 2012. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov. Disord 27, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukharaeva EA, Kim KK, Nikol’skii EE, Vyskochil F, 2000. Synchronization of evoked secretion of quanta of mediator as a mechanism facilitating the action of sympathomimetics. Neurosci. Behav. Physiol 30, 139–146. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF, 2004. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol 471, 462–481. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L, 2010. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci 13, 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V, Engel AG, Palay SL, Wu JY, 1982. Synthesizing enzymes for four neuroactive substances in motor neurons and neuromuscular junctions: light and electron microscopic immunocytochemistry. Proc. Natl. Acad. Sci. U. S. A 79, 6717–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Manini TM, 2012. What is dynapenia? Nutrition 28, 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, Braak H, 2013. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J. Neurol. Neurosurg. Psychiatry 84, 774–783. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL, 1994. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol 347, 64–86. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, 2003. Invited review: aging and sarcopenia. J. Appl. Physiol 95, 1717–1727. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE, 2003. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci 26, 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA, Evans WJ, 1993. The etiology and reversibility of muscle dysfunction in the aged. J Gerontol 48, 77–83 Spec No. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Kritchevsky SB, 2010. Translating research to promote healthy aging: the complementary role of longitudinal studies and clinical trials. J. Am. Geriatr. Soc 58 (Suppl. 2), S337–S342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman RP, McCown TJ, Samulski RJ, 1998. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 5, 1604–1611. [DOI] [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, Bamman MM, 2004. Effects of resistance training on older adults. Sports Med 34, 329–348. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA Jr., Isacson O, Kim KS, 2001. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum. Gene Ther 12, 1731–1740. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Abercrombie ED, Fornal CA, Levine ES, Morilak DA, Stafford IL, 1991. Single-unit and physiological analyses of brain norepinephrine function in behaving animals. Prog. Brain Res 88, 159–165. [DOI] [PubMed] [Google Scholar]
- Janssen I, 2006. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J. Am. Geriatr. Soc 54, 56–62. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Biaggioni I, 2003. Autonomic failure in neurodegenerative disorders. Semin. Neurol 23, 351–363. [DOI] [PubMed] [Google Scholar]
- Khan MM, Lustrino D, Silveira WA, Wild F, Straka T, Issop Y, O’Connor E, Cox D, Reischl M, Marquardt T, Labeit D, Labeit S, Benoit E, Molgó J, Lochmüller H, Witzemann V, Kettelhut IC, Navegantes LCC, Pozzan T, Rudolf R, 2016. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci 113, 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham NK, Bennett DA, Stretton CM, Anderson CS, 2004. Systematic review of progressive resistance strength training in older adults. J. Gerontol. A Biol. Sci. Med. Sci 59, 48–61. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Novak V, 2008. Aging and autonomic function, Third ed. Mayo Foundation, Rochester. Liu, C.J., Latham, N.K., 2009. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 8, 1–273. [Google Scholar]
- Liu C-J, Latham NK, 2009. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst. Rev. 2009 (3), CD002759. 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Ryall JG, 2008. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev 88, 729–767. [DOI] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo J. m., Berenyi A, Fujisawa S, Hsu Y-WA, Garcia Iii AJ, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H, 2012. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci 15, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magown P, Shettar B, Zhang Y, Rafuse VF, 2015. Direct optical activation of skeletal muscle fibres efficiently controls muscle contraction and attenuates denervation atrophy. Nat. Commun 6, 8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC, 2012. Dynapenia and aging: an update. J. Gerontol. A Biol. Sci. Med. Sci 67, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchnik S, Kotsias BA, Arrizurieta de Muchnik EE, 1975. In vivo and in vitro miniature end-plate potentials at various external K concentrations. Am. J. Phys 228, 1733–1737. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor M, 2002. Change in physical performance over time in older women: the Women’s Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci 57, M289–M293. [DOI] [PubMed] [Google Scholar]
- Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M, 2005. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci 60, 74–79. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ, 2019. The Mouse Brain in Stereotaxic Coordinates. Academic Press, London, UK. [Google Scholar]
- Radovanovic D, Peikert K, Lindström M, Domellöf FP, 2015. Sympathetic innervation of human muscle spindles. J. Anat 226, 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP, 1999. Disability, physical activity, and muscle strength in older women: the Women’s Health and Aging Study. Arch. Phys. Med. Rehabil 80, 130–135. [DOI] [PubMed] [Google Scholar]
- Robinson TE, 1978. Electrical stimulation of the brain stem in freely moving rats: I. effects on behavior. Physiol. Behav 21, 223–231. [DOI] [PubMed] [Google Scholar]
- Rodovalho GV, Drummond LR, Coimbra CC, 2020. Involvement of brainstem noradrenergic system in cutaneous heat loss during exercise. Brain Res. Bull 164, 372–379. 10.1016/j.brainresbull.2020.08.029. Epub 2020 Sep 3. [DOI] [PubMed] [Google Scholar]
- Rodrigues ACZ, Messi ML, Wang Z-M, Abba MC, Pereyra A, Birbrair A, Zhang T, O’Meara M, Kwan P, Lopez EIS, Willis MS, Mintz A, Files DC, Furdui C, Oppenheim RW, Delbono O, 2018. The Sympathetic Nervous System Regulates Skeletal Muscle Motor Innervation and Acetylcholine Receptor Stability Acta Physiologica. 225. pp. e13195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AZC, Wang Z-M, Messi ML, Delbono O, 2019. Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca2+ channels. Mol. Cell. Neurosci 95, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt JM, Jang SS, Mu W, Dawson VL, Dawson TM, 2005. Bcl-x is required for proper development of the mouse Substantia Nigra. J. Neurosci 25, 6721–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Bari B, Ralle M, Washington-Hughes C, Muchenditsi A, Maxey E, Lutsenko S, 2019. Localization of the Locus Coeruleus in the Mouse Brain. (J Vis Exp). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Colvill L, McKinley MJ, Oldfield BJ, 1999. Identification of neural projections from the forebrain to the kidney, using the virus pseudorabies. J. Auton. Nerv. Syst 77, 73–82. [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG, 2006. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci 26, 10305–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-M, Rodrigues ACZ, Messi ML, Delbono O, 2020. Aging blunts sympathetic neuron regulation of motoneurons synaptic vesicle release mediated by β1- and α2B-adrenergic receptors in geriatric mice. The Journals of Gerontology: Series A 75, 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H-B, Liu C, Liu T-T, Xiong J, 2014. Central circuits regulating the sympathetic outflow to lumbar muscles in spinally transected mice by retrograde transsynaptic transport. Int. J. Clin. Exp. Pathol 7, 2987–2997. [PMC free article] [PubMed] [Google Scholar]
- You Y, Botros MB, Enoo AAV, Bockmiller A, Herron S, Delpech JC, Ikezu T, 2019. Cre-inducible Adeno associated virus-mediated expression of P301L mutant tau causes motor deficits and neuronal degeneration in the Substantia Nigra. Neuroscience 422, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE, 2008. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther 16, 1073–1080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


