Abstract
Aim: Deep vein thrombosis (DVT) is a common complication of orthopedic surgery. Multiple lines of evidence indicate that genetic factors play an important role in the development of DVT following orthopedic surgery (DVTFOS). Recent evidence suggested that the solute carrier family 44 member 2 (SLC44A) gene may contribute to the risk of DVT. In this study, we aimed to investigate the associations of SLC44A2 and DVTFOS in Chinese Han individuals.
Methods: In the study, 2,655 subjects, including 689 DVTFOS patients and 1,966 controls, were recruited. Eighteen SNPs were genotyped in the study. Genetic association analyses were performed at both the single marker and haplotype levels. Bioinformatics analyses were conducted to predict the functional consequences of significant SNPs.
Results: SNP rs2288904 of SLC44A2 was identified as being significantly associated with DVTFOS (P = 0.0003, OR [95%CI]= 1.28[1.12–1.46]). Allelic analyses showed that the G allele of this SNP significantly elevated the risks of DVTFOS, which was replicated in the genotypic association analyses. Moreover, a two-SNP haplotype, including rs2288904, was found to be strongly correlated with the risk of DVTFOS (P = 4.15 × 10−11). Widespread effects in the expression quantitative trait loci were identified for rs2288904 in multiple tissues.
Conclusion: In summary, our results provide further supportive evidence of the association of SLC44A2 with the risk of DVTFOS, which also provide clues for understanding the important roles of the SLC44A2 gene in the pathogenesis of DVTFOS and in the development of preventive strategies.
Keywords: Single nucleotide polymorphisms, SLC44A2, Deep vein thrombosis, Case–control study, Genetic association
Introduction
Deep vein thrombosis (DVT) is a major medical disease, with an incidence of 67 per 100,000 cases every year1), and is caused by venous injury, slow blood flow, or blood hypercoagulability2). Severe DVT can result in postphlebitic syndrome, pulmonary embolism, and even death3). The results of some genetic studies have shown that genetic factors might contribute to the development of DVT4). In the past decades, family and twin studies have also confirmed that the heritability of DVT is greater than 60%5, 6). Orthopedics surgery after injury or disease is strongly associated with a risk of developing DVT. In the absence of thromboprophylaxis, venography documented DVT may occur in up to 60% of patients within 2 weeks following lower-extremity orthopedic surgery, which is far greater than the incidence observed in healthy people7). Postoperative complications of DVT were determined not only by environmental factors but also by genetic factors. Therefore, it is urgent to identify the susceptibility genes for DVT and to elucidate the exact molecular mechanisms of DVT following orthopedic surgery (DVTFOS).
With the development of high-throughput DNA sequencing techniques, genome-wide association (GWA) studies have provided supportive evidence for the polygenic nature of venous thromboembolism (VTE) susceptibility and have identified some SNPs that contribute to the risk of VTE8, 9); however, these results can explain only a small portion of the limited heritability due to a lack of biological interpretations. So far, the molecular mechanisms of VTE are still unknown. Recently, a meta-analysis of 12 GWA studies, involving 7,507 VTE cases and 52,632 controls, has identified an association between the exonic SNP rs2288904 within the solute carrier family 44 member 2 (SLC44A) gene and VTE in European populations8). Moreover, the association between SLC44A2 and thrombosis was subsequently confirmed in another independent study of a European population, which further strengthens the evidence linking SLC44A2 and VTE9).
As a common form of VTE, DVTFOS might also be associated with the SLC44A2 gene. Although there is evidence of significant associations with VTE in European-ancestry populations, the contributions of SLC44A2 to the risk of DVTFOS have not yet been fully investigated. Moreover, due to genetic heterogeneity, large-scale studies in non-European populations are necessary to confirm these results and to understand further the genetic origins of DVTFOS. In addition, current preoperative prevention strategies are not enough to prevent DVTFOS. Investigating the potential genetic markers contributing to the risk of DVTFOS would enable pre-surgery genetic screening and precision prevention for DVTFOS. Thus, we performed a hospital-based, case-controlled study to identify further the association between SLC44A2 and the risk of DVTFOS in Han Chinese individuals, which could provide clues for understanding the roles of SLC44A2 in the genetic predisposition to the development of DVTFOS and aid in the development of preventive strategies.
Materials and Methods
Study Population
In our study, 2,655 subjects who underwent orthopedic surgery on the knee or hip were recruited from Honghui Hospital of Xi’an Jiaotong University between April 2011 and May 2017. Among the subjects, 689 cases were diagnosed with DVTFOS (394 females and 295 males), and 1,966 controls experienced none of the typical symptoms or signs of DVT (1,126 females and 840 males) (Fig. 1). For each subject, anticoagulant drugs were routinely taken starting 6 hours post-operation (Rivaroxaban, 10 mg/qd). Patients with signs and symptoms suggestive of acute DVT and with a Wells score indicating a high probability of DVT underwent ultrasound examinations. DVT was assessed within 5 days postoperatively for all subjects by two independent experienced sonographers, using lower-extremity Color-Doppler ultrasound for preliminary screening by means of a GE-Logic E9 (GE Healthcare Ltd) device with a high-frequency linear transducer (10 MHz). When it was difficult for six patients to obtain an exact diagnosis, venography was applied for diagnosis confirmation. A total of 2,075 discharged patients were followed up after orthopedic surgery on the knee or hip. If DVT was not reported within 40 days after surgery, the patients were included in the controls. If the follow-up fails within 40 days after surgery, the patient was considered as a missing subject and was excluded from this study. Finally, 1,966 patients were enrolled as controls. Additionally, if the patients undergoing orthopedic surgery had a history of VTE, autoimmune disease, malignant tumors, severe respiratory and circulatory system diseases, pregnancy, oral contraceptives and hormonal therapy, acute medical illnesses, inflammatory bowel disease, and central venous catheter, we excluded them from the study. All subjects were unrelated and were restricted to the Han Chinese population. The clinical data and characteristics of all subjects were measured or recorded. Written informed consent was obtained from all subjects. This study was performed in accordance with the ethical guidelines of the Declaration of Helsinki (version 2002) and was approved by the Medical Ethics Committee of Xi'an Jiaotong University.
Fig. 1.
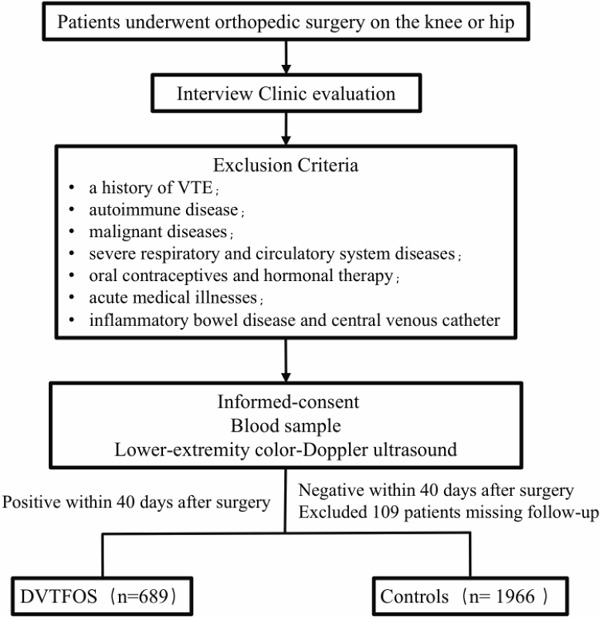
Flow diagram of cases and control selection for the study design
SNP Selection and Genotyping
We searched for all SNPs with minor allele frequencies (MAF) ≥ 0.05 within the region of the SLC44A2 gene in the 1,000 Genomes Chinese Han Beijing population. Then, MAF ≥ 0.05, with pairwise tagging, and r2 ≥ 0.7 were used as the cut-off criteria during tag SNP selection, which generated 18 tag SNPs for our study. Basic information on the 18 selected SNPs is summarized in Supplemental Table 1. Genomic DNA was isolated from peripheral blood using a Tiangen DNA extraction kit (Tiangen Biotech Co. Ltd, Beijing, China), according to the manufacturer's protocol. SNP genotyping was performed using a Sequenom MassARRAY platform with iPLEX GOLD chemistry (Sequenom, San Diego, CA, USA), based on the manufacturer's protocols. The results were processed using Sequenom Typer 4.0 software, and genotype data were generated from the samples. Genotyping was conducted by laboratory personnel blinded to the case–control status, and the genotyping results, data entry, and statistical analyses were independently reviewed by two authors10, 11). We randomly re-performed the analyses on 5% of the sample, with a concordance of 100%.
Supplemental Table 1. Basic information of the 18 selected SNPs for SLC44A2.
| CHR | SNP | POS | Alleles | GENE | FUNC |
|---|---|---|---|---|---|
| 19 | rs61127297 | 10713387 | C/G | SLC44A2 | intron |
| 19 | rs12982370 | 10715154 | A/C/T | SLC44A2 | intron |
| 19 | rs10408934 | 10716256 | A/G | SLC44A2 | intron |
| 19 | rs10413422 | 10718727 | C/T | SLC44A2 | intron |
| 19 | rs76359824 | 10721530 | A/G | SLC44A2 | intron |
| 19 | rs1865065 | 10724002 | A/G | SLC44A2 | intron |
| 19 | rs76434079 | 10726601 | G/T | SLC44A2 | intron |
| 19 | rs116957244 | 10730946 | C/T | SLC44A2 | intron |
| 19 | rs8113212 | 10732663 | A/G | SLC44A2 | intron |
| 19 | rs116979350 | 10733412 | A/G | SLC44A2 | intron |
| 19 | rs3745242 | 10736237 | C/G | SLC44A2 | intron |
| 19 | rs28860769 | 10737581 | A/G | SLC44A2 | intron |
| 19 | rs113922991 | 10738129 | A/G | SLC44A2 | intron |
| 19 | rs76638997 | 10741545 | A/G | SLC44A2 | intron |
| 19 | rs2288904 | 10742170 | C/T | SLC44A2 | missense |
| 19 | rs577950492 | 10751769 | A/G | SLC44A2 | intron |
| 19 | rs79290735 | 10752252 | A/G | SLC44A2 | intron |
| 19 | rs2288903 | 10754735 | A/C/T | SLC44A2 | untranslated-3 |
CHR: chromosome; POS: position; FUNC: function
Statistical Analyses
χ2 tests were performed to examine genetic associations at both the single marker and haplotype levels. In addition, logistic models were fitted for each SNP to adjust for the potential confounding effects of age and body mass index (BMI) by including both variables as covariates (because both variables had unbalanced distributions between DVT cases and controls). Plink was utilized for the statistical analyses mentioned above12). A Q–Q plot was made to examine the potential inflation of significant hits from single-marker-based analyses to detect the potential effects of population stratifications. Locus zoom was utilized to make a regional association plot13). Bonferroni corrections were applied to address multiple comparisons. For single-marker-based association analyses, the threshold for P values was 0.003 (0.05/18).
Bioinformatics Analyses
Functional analyses of SNPs with significant association signals were performed by two bioinformatics tools. For non-synonymous SNPs located within exons, SIFT was utilized to evaluate the functional consequences of SNPs on the protein encoded by its gene14), and we also examined the expression quantitative trait loci (eQTL) patterns of these significant association hits using the GTEx database15). In addition, we investigated the interaction network of SLC44A2 using the STRING database, which is a database of known and predicted protein–protein interactions.
Results
In the study, significant differences in age and BMI were found between DVTFOS patients and controls (Table 1). All SNPs were in Hardy–Weinberg equilibrium in the control group (P > 0.05, Supplemental Table 2). No significant clues for the inflation of −log P values can be found from the Q–Q plot (Supplemental Fig. 1). As presented in Table 2 and Fig. 1, only SNP rs2288904 of SLC44A2 was identified to be strongly associated with DVTFOS in our study subjects (P = 0.0003) after adjusting for age and BMI (Table 2 and Fig. 2). The association signal was still significant after the Bonferroni correction (Pthreshold = 0.05/18). The MAF of the G allele of this SNP was much higher in cases compared with controls, and further analyses also indicated that the G allele of rs2288904 has a positive correlation with the risk of DVTFOS (OR = 1.28, 95%CI = 1.12–1.46, Table 2). Further genotypic analyses identified the dose-dependent pattern. Compared with the CC and GC genotypes, the distribution of the GG genotype was more frequent in the patients (P = 0.00062, Table 2). Similar results were obtained in both allelic and genotypic analyses. There was no significant difference between other SNPs and the risk of DVTFOS (Supplemental Table 2).
Table 1. Characteristic and clinical information for the 2,655 study subjects.
| Cases (N = 689) | Controls (N = 1,966) | Statistics | P | |
|---|---|---|---|---|
| Age, mean ± sd | 59.6 ± 5.7 | 58.5 ± 6.5 | t = 4.3 | 1.78 × 10−5 |
| BMI, mean ± sd | 25.9 ± 1.6 | 25.4 ± 1.7 | t = 6.8 | 1.23 × 10−11 |
| Gender (%) | ||||
| Male | 295 (43) | 840 (43) | ||
| Female | 394 (57) | 1,126 (57) | χ2 = 0 | 1 |
| Site of Surgery (%) | ||||
| hip | 396 (57) | 1,146 (58) | ||
| knee | 293 (43) | 820 (42) | χ2 = 0.11 | 0.74 |
| Hypertension (%) | ||||
| Yes | 205 (30) | 555 (28) | ||
| No | 484 (70) | 1,411 (72) | χ2 = 0.51 | 0.48 |
| Hyperlipemia (%) | ||||
| Yes | 197 (29) | 490 (25) | ||
| No | 492 (71) | 1,476 (75) | χ2 = 3.39 | 0.07 |
| Diabetes (%) | ||||
| Yes | 39 (6) | 103 (5) | ||
| No | 650 (94) | 1,863 (95) | χ2 = 0.11 | 0.75 |
| Smoking (%) | ||||
| Yes | 117 (17) | 316 (16) | ||
| No | 572 (83) | 1,650 (84) | χ2 = 0.25 | 0.62 |
| Location of the thrombosis (%) | ||||
| Proximal | 586 (85) | - | ||
| Distal | 85 (12) | - | ||
| Both | 18 (3) | - | - | - |
Supplemental Table 2. Full results for single marker based association analyses.
| CHR | SNP | POS | GENE | MAF | HWE | A1 | OR | STAT | P |
|---|---|---|---|---|---|---|---|---|---|
| 19 | rs61127297 | 10713387 | SLC44A2 | 0.11 | 0.72 | G | 0.97 | −0.28 | 0.78 |
| 19 | rs12982370 | 10715154 | SLC44A2 | 0.09 | 0.56 | A | 1.04 | 0.32 | 0.75 |
| 19 | rs10408934 | 10716256 | SLC44A2 | 0.27 | 0.91 | A | 1.02 | 0.30 | 0.76 |
| 19 | rs10413422 | 10718727 | SLC44A2 | 0.28 | 0.50 | C | 0.98 | −0.28 | 0.78 |
| 19 | rs76359824 | 10721530 | SLC44A2 | 0.33 | 0.61 | A | 0.97 | −0.43 | 0.67 |
| 19 | rs1865065 | 10724002 | SLC44A2 | 0.21 | 0.89 | C | 1.03 | 0.35 | 0.73 |
| 19 | rs76434079 | 10726601 | SLC44A2 | 0.16 | 0.61 | T | 0.98 | −0.29 | 0.77 |
| 19 | rs116957244 | 10730946 | SLC44A2 | 0.10 | 0.53 | T | 1.11 | 1.00 | 0.32 |
| 19 | rs8113212 | 10732663 | SLC44A2 | 0.31 | 0.92 | A | 0.97 | −0.42 | 0.67 |
| 19 | rs116979350 | 10733412 | SLC44A2 | 0.07 | 0.58 | G | 1.09 | 0.66 | 0.51 |
| 19 | rs3745242 | 10736237 | SLC44A2 | 0.45 | 0.65 | G | 1.04 | 0.54 | 0.59 |
| 19 | rs28860769 | 10737581 | SLC44A2 | 0.24 | 0.57 | A | 0.99 | −0.17 | 0.86 |
| 19 | rs113922991 | 10738129 | SLC44A2 | 0.13 | 1.00 | G | 0.95 | −0.48 | 0.63 |
| 19 | rs76638997 | 10741545 | SLC44A2 | 0.29 | 0.96 | A | 1.03 | 0.44 | 0.66 |
| 19 | rs2288904 | 10742170 | SLC44A2 | 0.35 | 0.77 | A | 0.78 | −3.63 | 2.81 × 10−4 |
| 19 | rs577950492 | 10751769 | SLC44A2 | 0.13 | 1.00 | A | 0.97 | −0.30 | 0.76 |
| 19 | rs79290735 | 10752252 | SLC44A2 | 0.06 | 0.46 | G | 0.97 | −0.24 | 0.81 |
| 19 | rs2288903 | 10754735 | SLC44A2 | 0.15 | 0.93 | T | 1.03 | 0.35 | 0.72 |
HWE: P values of Hardy-Weinberg equilibrium tests. Significant results were highlighted in bold
Supplemental Fig. 1.
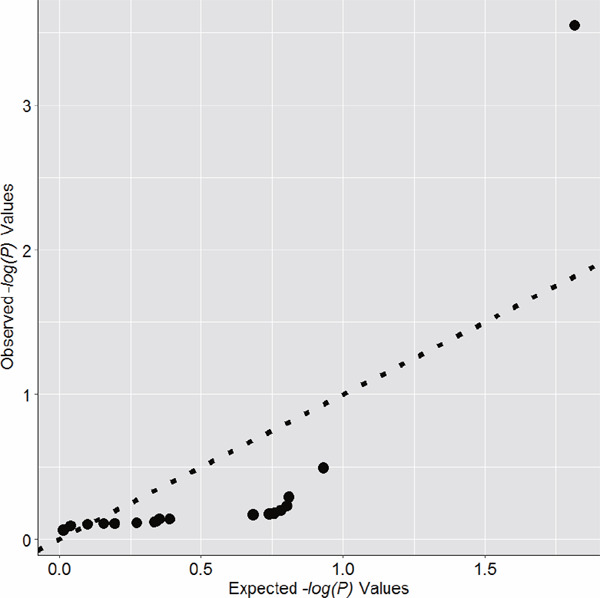
Q-Q plot for results of single marker based association analyses
Table 2. Significant SNPs identified in single marker based association analyses.
| SNPs | Status | Genotypes (%) |
Pgenotype Padj |
Alleles (%) |
χ2 |
Pallele Padj |
ORadj 95%CI |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| AA | GA | GG | A | G | ||||||
| rs2288904 | Cases (N = 689) |
58 (8.4) | 311 (45.1) | 320 (46.5) | 0.00053 | 427 (31.0) | 951 (69.0) | 0.00026 | 1.28 | |
| Controls (N = 1,966) |
264 (13.4) | 904 (46.0) | 798 (40.6) | 0.00062 | 1,432 (36.4) | 2,500 (63.6) | 13.00 | 0.00030 | (1.12–1.46) | |
Padj, ORadj: P values and OR adjusted by age and BMI.
Risk allele was highlighted in bold, and OR referred to the risk allele.
Fig. 2.
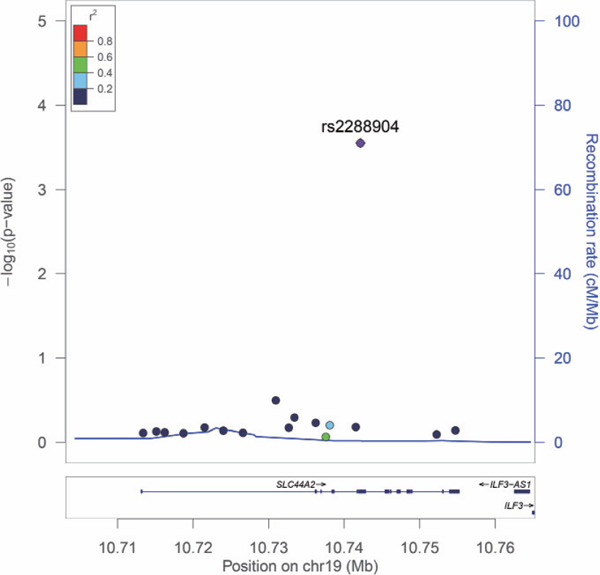
Regional association plot based on the results from the single marker-based association Analyses
Three LD blocks were constructed based on our 18 selected SNPs, and the associated SNP rs2288904 was located in block 3 (Fig. 3). Haplotypic association analyses were conducted in all LD blocks, and the results were summarized in Table 3. A two-SNP haplotype in SLC44A2 was identified to be significantly associated with DVTFOS (rs76638997-rs2288904, P = 4.15 × 10−11). The associated SNP rs2288904 was also included in this haplotype, which provided supportive evidence of the significant association of rs2288904 with the risk of DVTFOS (Table 3).
Fig. 3.
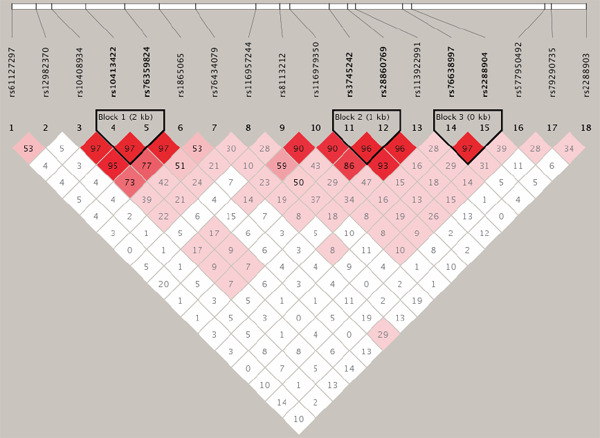
Linkage disequilibrium structure of the 18 SNPs
Values of D' are indicated in each cell.
Table 3. Results of genetic association for haplotype based analyses.
| Gene | χ2 | DF | P | SNPs |
|---|---|---|---|---|
| SLC44A2 | 0.44 | 2 | 0.80 | rs10413422-rs76359824 |
| SLC44A2 | 1.29 | 2 | 0.52 | rs3745242-rs28860769 |
| SLC44A2 | 47.81 | 2 | 4.15 × 10−11 | rs76638997-rs2288904 |
DF: degree of freedom. Significant results were highlighted in bold.
For the significant SNP, rs2288904, which results in a non-synonymous change located within the exonic region of SLC44A2, no evidence of significant functional consequences could be found using SIFT, and both alleles were classified as “tolerated”. However, widespread effects of eQTL were identified for rs2288904 in multiple tissues, including the skin, whole blood, mucosa, esophagus, and skeletal muscle. The most significant hit was from the skeletal muscle with a P-value of 10−27 (Table 4), indicating a strong effect on regulating the expression of SLC44A2. Furthermore, according to the STRING database, we found that the protein encoded by the SLC44A2 gene and the proteins encoded by the other 15 genes constructed a more complex interaction network (Fig. 4), which also increased the complexity of the effect of the SLC44A2 gene on the risk of DVTFOS to a certain extent.
Table 4. Significant eQTLs identified from data of GTEx.
| Gene | SNP | P | Effect Size | T-Statistic | Standard Error | Tissue |
|---|---|---|---|---|---|---|
| SLC44A2 | rs2288904 | 1.50 × 10−27 | −0.55 | −12.0 | 0.047 | Muscle - Skeletal |
| SLC44A2 | rs2288904 | 5.00 10−9 | 0.30 | 6.0 | 0.049 | Skin - Not Sun Exposed (Suprapubic) |
| SLC44A2 | rs2288904 | 7.70 × 10−8 | 0.19 | 5.5 | 0.034 | Skin - Sun Exposed (Lower leg) |
| SLC44A2 | rs2288904 | 3.80 × 10−7 | 0.13 | 5.2 | 0.025 | Whole Blood |
| SLC44A2 | rs2288904 | 1.90 × 10−5 | 0.15 | 4.3 | 0.034 | Esophagus - Mucosa |
Fig. 4.
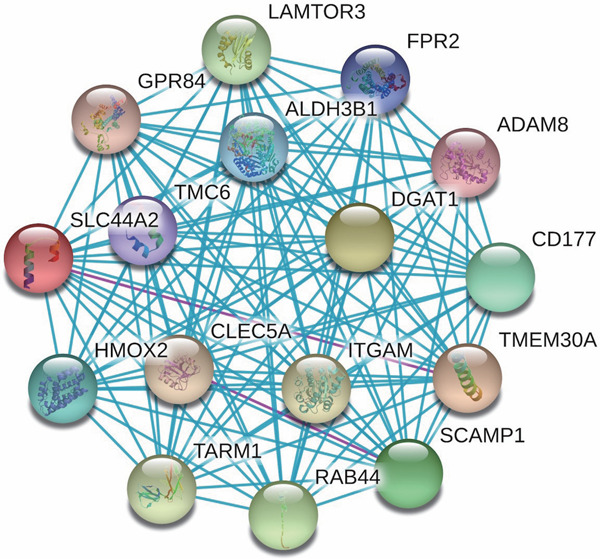
Interaction network constructed based on protein-protein interaction data
Blue and pink lines mean experimentally determined interactions.
Discussion
Previous large-scale meta-analyses and follow-up studies based on European populations have identified and confirmed the association between rs2288904 in SLC44A2 and VTE8, 9). The direction of the SNP effect of rs2288904 was the same compared with the previous meta-analysis. The effect size was also very similar between the two studies. In this sense, we have successfully replicated the finding of this previous meta-analysis for DVTFOS in the Chinese Han population. In addition, compared with the previous genetic association studies focusing on unraveling the genetic etiology of VTE, our study could provide important data to support pre-surgery genetic screening and precision prevention for DVTFOS by identifying genetic markers contributing to its risk.
The SLC44A2 gene is located at 19p13.2, and the protein encoded by this gene is the solute carrier protein 44A2, also known as transporter-like protein 2 (CTL2)16). CTL2 mutations can cause hearing loss in animals and patients and have been implicated in transfusion-related acute lung injury17, 18). Previous studies have shown that CTL2 is a binding partner for the von Willebrand factor19), which facilitates hemostasis primarily by stabilizing coagulation Factor VIII (FVIII). Increased levels of FVIII have been demonstrated to be a risk factor for first20) and recurrent21, 22) episodes of DVT. In addition, one of the primary causes of DVT is damaged blood vessel walls, resulting from oxidative stress and inflammation responses23).
Given that it is difficult to draw reliable conclusions only based on SNPs association analyses24–28), bioinformatics analyses of rs2288904 have shown that it is likely to cause very limited functional consequences of the structure of the protein encoded by SLC44A2, which indicates that the effect of rs2288904 on DVTFOS is not mediated by the disruption of its protein structure. However, eQTL analyses based on the GTEx database have identified widespread eQTL effects for rs2288904 on SLC44A2 in multiple human tissues. These findings indicate that rs2288904 might contribute to the risk of DVT by regulating the gene expression of SLC44A2. Our eQTL analysis has shown that SNP rs2288904 had significant functional consequences and was significantly associated with the gene expression of SLC44A2. Combining this evidence, we believe that this SNP may not just be a surrogate for some ungenotyped DNA variants but is a variant with the true effect on the susceptibility of DVTFOS. Nevertheless, we need to be careful in interpreting the evidence obtained from the GTEx database. Firstly, although 47 types of human tissues were examined, no targeted tissues of DVT were tested. Secondly, the gene expression pattern of SLC44A2 might be quite different in DVTFOS patients compared with controls. We cannot identify the disease status of the individuals analyzed in the GTEx database and, therefore, the eQTL signals identified using the GTEx data might not represent the real situation of DVTFOS patients. In the future, gene expression analysis for SLC44A2 conducted in DVTFOS patients is needed to clearly investigate the functional consequence of SNP rs2288904.
With the development of target sequencing, numerous susceptibility variants of complex diseases have been identified, such as schizophrenia29–31). As a candidate-gene-based association study, we only genotyped 18 SNPs in total for both loci; these SNPs are far too few to represent the structure of genetic variations for this genomic region. Large numbers of rare, low-frequency, and indel variations were not examined in this study. More interestingly, several recent studies have shown that these rare and low-frequency DNA variants might play an important role in the pathogenesis of human complex disorders32–34). Therefore, we cannot rule out the possibility that some other effective variants with independent genetic effects may be located within this region. In the future, targeted sequencing-based studies focusing on this genomic region are needed to completely unravel the genetic architectures of SLC44A2 on DVTFOS.
Population stratification is one of the most common confounding factors for genetic association studies and could be worse for research conducted in the Chinese Han population, which is an ethnic group with a large degree of heterogeneity35). In the sample recruitment process, we only included individuals with no immigration history within the last three generations. This procedure would, at least partly, restrict the genetic background and control the genetic heterogeneity of our study subjects. On the other hand, the Q–Q plot, made based on P values of single-marker-based association analyses, indicated that no signs of inflation for significance could be identified. Therefore, we believe that population stratification is not a problem for this study. In addition, although we have tried our best to exclude patients with risk factors of VTE in the sample recruitment process, it might still be not enough to control these potential risk factors. Therefore, we need to be careful in interpreting the significant hits identified in the present study, and our results should be considered to be preliminary and confirmed by functional evidence in future research.
In this study, we have obtained evidence for genetic associations between DVTFOS and gene SLC44A2. Further bioinformatics analyses have confirmed that the significant SNP had a potential functional consequence. These results suggest the important roles of SLC44A2 in the pathogenesis of DVT-FOS. Further research and wider replications should be conducted to validate in larger, preferably population-based studies to elucidate the exact molecular basis of the relationship between SLC44A2 and DVT-FOS risk, which would help to reveal the etiology of DVTFOS and provide intriguing new insight into its biology.
Sources of Funding
This work was financially supported by the China Postdoctoral Science Foundation (No. 2017M623215).
Conflict of Interest
All authors declare that they have no conflict of interest.
Author Contributions
L.Q Zhi and W.L. Feng designed the study. W.L. Feng, and J.Q. Liang carried out candidate SNPs selection and statistical analyses. J.B. Ma, Q. Zhong and L.Y. Ren conducted subject screening and contributed to the collection and preparation of control DNA samples. L.Q. Zhi drafted the manuscript, and S.Y. Yao critically revised the manuscript.
References
- 1). Huang L, Li J and Jiang Y: Association between hypertension and deep vein thrombosis after orthopedic surgery: a meta-analysis. Eur J Med Res, 2016; 21: 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Neglen P, Thrasher TL and Raju S: Venous outflow obstruction: An underestimated contributor to chronic venous disease. J Vasc Surg, 2003; 38: 879-885 [DOI] [PubMed] [Google Scholar]
- 3). Qin J, Dai J, Xu Z, Chen D, Qin J, Shi D, Teng H and Jiang Q: Genetic polymorphism of NOS3 with susceptibility to deep vein thrombosis after orthopedic surgery: a case-control study in Chinese Han population. PLoS One, 2013; 8: e70033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Souto JC, Almasy L, Borrell M, Blanco-Vaca F, Mateo J, Soria JM, Coll I, Felices R, Stone W, Fontcuberta J and Blangero J: Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Genetic Analysis of Idiopathic Thrombophilia. Am J Hum Genet, 2000; 67: 1452-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Larsen TB, Sorensen HT, Skytthe A, Johnsen SP, Vaupel JW and Christensen K: Major genetic susceptibility for venous thromboembolism in men: a study of Danish twins. Epidemiology, 2003; 14: 328-332 [PubMed] [Google Scholar]
- 6). Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR and Colwell CW: Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest, 2008; 133: 381S-453S [DOI] [PubMed] [Google Scholar]
- 7). Kakkar AK, Rushton-Smith SK: Incidence of Venous Thromboembolism in Orthopedic Surgery. In: Llau J. (eds) Thromboembolism in Orthopedic Surgery, 2013; 2: 11-17 [Google Scholar]
- 8). Marine G: Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet, 2015; 96: 532-542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Hinds DA, Buil A, Ziemek D, Martinez-Perez A, Malik R, Folkersen L, Germain M, Mälarstig A, Brown A and Soria JM: Genome-wide association analysis of selfreported events in 6,135 individuals and 252,827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet, 2016; 25: 1867-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Guan F, Zhang C, Wei S, Zhang H, Gong X, Feng J, Gao C, Su R, Yang H, Li S: Association of PDE4B polymorphisms and schizophrenia in Northwestern Han Chinese. Hum Genet, 2012; 131: 1047-1056 [DOI] [PubMed] [Google Scholar]
- 11). Guan F, Zhang B, Yan T, Li L, Liu F, Li T, Feng Z, Zhang B, Liu X, Li S: MIR137 gene and target gene CACNA1C of miR-137 contribute to schizophrenia susceptibility in Han Chinese. Schizophr Res, 2014; 152: 97-104 [DOI] [PubMed] [Google Scholar]
- 12). Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM and Lee JJ: Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience, 2015; 4: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR and Willer CJ: LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics, 2010; 26: 2336-2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Ng PC and Henikoff S: SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res, 2003; 31: 3812-3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Consortium GT: The Genotype-Tissue Expression (GTEx) project. Nat Genet, 2013; 45: 580-585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Kommareddi PK, Nair TS, Raphael Y, Telian SA, Kim AH, Arts HA, El-Kashlan HK and Carey TE: Cochlin Isoforms and Their Interaction with CTL2 (SLC44A2) in the Inner Ear. J Assoc Res Otolaryngol, 2007; 8: 435-446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Traiffort E, O'Regan S and Ruat M: The choline transporter-like family SLC44: properties and roles in human diseases. Mol Aspects Med, 2013; 34: 646-654 [DOI] [PubMed] [Google Scholar]
- 18). Le, Liu, Wang, QY, FX, Ding, XZ, Xia and LQ: Isoforms, Expression, Glycosylation, and Tissue Distribution of CTL2/SLC44A2. Protein J, 2010; 29: 417-426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Bayat B, Tjahjono Y, Berghofer H, Werth S, Deckmyn H, De Meyer SF, Sachs UJ and Santoso S: Choline Transporter-Like Protein-2: New von Willebrand Factor-Binding Partner Involved in Antibody-Mediated Neutrophil Activation and Transfusion-Related Acute Lung Injury. Arterioscler Thromb Vasc Biol, 2015; 35: 1616-1622 [DOI] [PubMed] [Google Scholar]
- 20). Koster T, Blann AD, Briet E, Vandenbroucke JP and Rosendaal FR: Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet, 1995; 345: 152-155 [DOI] [PubMed] [Google Scholar]
- 21). Roderik K, Pieternella itA, Marianne K, Pieter R, Martin P, Abraham vdE and Harry B: High Plasma Concentration of Factor VIIIc Is a Major Risk Factor for Venous Thromboembolism. Thromb Haemost, 2000; 83: 5-9 [PubMed] [Google Scholar]
- 22). Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K and Eichinger S: High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med, 2000; 343: 457-462 [DOI] [PubMed] [Google Scholar]
- 23). Akhter MS, Biswas A, Ranjan R, Sharma A, Kumar S and Saxena R: The nitric oxide synthase 3 gene polymorphisms and their association with deep vein thrombosis in Asian Indian patients. Clin Chim Acta, 2010; 411: 649-652 [DOI] [PubMed] [Google Scholar]
- 24). Guan F, Zhang T, Han W, Zhu L, Ni T, Lin H, Liu D, Chen G, Xiao J, Li T: Relationship of SNAP25 Variants With Schizophrenia and Antipsychotic-Induced Weight Change in Large-Scale Schizophrenia Patients. Schizophr Res, 2020; 215: 250-255 [DOI] [PubMed] [Google Scholar]
- 25). Li J, Zhu L, Guan F, Yan Z, Liu D, Han W, Chen T: Relationship Between Schizophrenia and Changes in the Expression of the Long Non-Coding RNAs Meg3, Miat, Neat1 and Neat2. J Psychiatr Res, 2018; 106: 22-30 [DOI] [PubMed] [Google Scholar]
- 26). Sun H, Luo C, Chen X, Tao L: Assessment of Cognitive Dysfunction in Traumatic Brain Injury Patients: A Review. Forensic Sci Res, 2017; 2: 174-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Zhang Z, Gong Q, Feng X, Zhang D, Quan L: Astrocytic Clasmatodendrosis in the Cerebral Cortex of Methamphetamine Abusers. Forensic Sci Res, 2017; 2: 139-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Zhu L, Li J, Dong N, Guan F, Liu Y, Ma D, Goh EL, Chen T: mRNA Changes in Nucleus Accumbens Related to Methamphetamine Addiction in Mice. Sci Rep, 2016; 6: 36993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Guan F, Ni T, Han W, Lin H, Zhang B, Chen G, Zhu L, Liu D, Zhang T: Evaluation of the relationships of the WBP1L gene with schizophrenia and the general psychopathology scale based on a case-control study. Am J Med Genet B Neuropsychiatr Genet, 2020; 183: 164-171 [DOI] [PubMed] [Google Scholar]
- 30). Han W, Zhang TX, Ni T, Zhu L, Liu D, Chen G, Lin H, Chen T, Guan FL: Relationship of common variants in CHRNA5 with early-onset schizophrenia and executive function. Schizophr Res, 2018; 206: 407-412 [DOI] [PubMed] [Google Scholar]
- 31). Zhang TX, Zhu L, Ni T, Liu D, Chen G, Yan Z, Lin H, Guan F, Rice JP: Voltage-gated calcium channel activity and complex related genes and schizophrenia: A systematic investigation based on Han Chinese population. J Psychiatr Res, 2018; 106: 99-105 [DOI] [PubMed] [Google Scholar]
- 32). Lotta LA, Tuana G, Yu J, Martinelli I, Wang M, Yu F, Passamonti SM, Pappalardo E, Valsecchi C, Scherer SE, Hale Wt, Muzny DM, Randi G, Rosendaal FR, Gibbs RA and Peyvandi F: Next-generation sequencing study finds an excess of rare, coding single-nucleotide variants of ADAMTS13 in patients with deep vein thrombosis. J Thromb Haemost, 2013; 11: 1228-1239 [DOI] [PubMed] [Google Scholar]
- 33). RK CY, Merico D, Bookman M, J LH, Thiruvahindrapuram B, Patel RV, Whitney J, Deflaux N, Bingham J, Wang Z, Pellecchia G, Buchanan JA, Walker S, Marshall CR, Uddin M, Zarrei M, Deneault E, D'Abate L, Chan AJ, Koyanagi S, Paton T, Pereira SL, Hoang N, Engchuan W, Higginbotham EJ, Ho K, Lamoureux S, Li W, Mac-Donald JR, Nalpathamkalam T, Sung WW, Tsoi FJ, Wei J, Xu L, Tasse AM, Kirby E, Van Etten W, Twigger S, Roberts W, Drmic I, Jilderda S, Modi BM, Kellam B, Szego M, Cytrynbaum C, Weksberg R, Zwaigenbaum L, Woodbury-Smith M, Brian J, Senman L, Iaboni A, Doyle-Thomas K, Thompson A, Chrysler C, Leef J, Savion-Lemieux T, Smith IM, Liu X, Nicolson R, Seifer V, Fedele A, Cook EH, Dager S, Estes A, Gallagher L, Malow BA, Parr JR, Spence SJ, Vorstman J, Frey BJ, Robinson JT, Strug LJ, Fernandez BA, Elsabbagh M, Carter MT, Hallmayer J, Knoppers BM, Anagnostou E, Szatmari P, Ring RH, Glazer D, Pletcher MT and Scherer SW: Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nat Neurosci, 2017; 20: 602-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Zhang T, Hou L, Chen DT, McMahon FJ, Wang JC and Rice JP: Exome sequencing of a large family identifies potential candidate genes contributing risk to bipolar disorder. Gene, 2018; 645: 119-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Chen J, Zheng H, Bei JX, Sun L, Jia WH, Li T, Zhang F, Seielstad M, Zeng YX, Zhang X and Liu J: Genetic structure of the Han Chinese population revealed by genomewide SNP variation. Am J Hum Genet, 2009; 85: 775-785 [DOI] [PMC free article] [PubMed] [Google Scholar]


