Abstract
Aim: Both oxidative stress and inflammation are involved in the pathogenesis of cardiovascular disease (CVD). The serum level of derivatives of reactive oxygen metabolites (d-ROMs) is a measure of the total amount of hydroperoxides serving as a marker of oxidative stress. We investigated whether d-ROMs could predict the clinical outcomes in hemodialysis patients and whether the associations of d-ROMs with the outcomes are independent of a marker of inflammation, C-reactive protein (CRP).
Methods: This was a prospective cohort study in hemodialysis patients. The key exposures were the serum levels of d-ROMs and CRP. The outcome measures were all-cause mortality and new CVD events.
Results: A total of 517 patients were analyzed. d-ROMs correlated positively with CRP. During follow-up for 5 years, 107 patients died, and 190 patients experienced new CVD events. In the Kaplan–Meier analyses, both higher d-ROMs and higher CRP levels predicted higher risks for mortality and CVD events. By Cox proportional-hazard regression analysis adjusted for potential confounders excluding CRP, d-ROMs exhibited a significant association with all-cause mortality, but this association was no longer significant after further adjustment for CRP. Using the same model, CRP exhibited a significant association with all-cause mortality, but this association was no longer significant after further adjustment for d-ROMs. When we analyzed new CVD events as the outcome, CRP was a significant predictor, whereas the level of d-ROMs was not.
Conclusions: Although d-ROMs predicted mortality and CVD events in unadjusted models, the associations of d-ROMs with these outcomes were not independent of CRP. Oxidative stress and inflammation appear to share common causal pathways.
Keywords: Oxidative stress, Hemodialysis, Cardiovascular disease, Clinical epidemiology, d-ROMs
See editorial vol. 28: 227–229
Introduction
Patients with chronic kidney disease (CKD) show advanced morphological and functional vascular changes1, 2). The risk of cardiovascular disease (CVD) and all-cause mortality is increased in CKD3, 4), particularly in the CKD stage G5D5). In patients under-going dialysis, the risk of death from CVD is 10–30-fold higher compared with the general population5). The increased risk of CVD in CKD is not fully explained by the traditional risk factors, and the excess risk may be attributable to nontraditional risk factors3, 6), such as renal anemia, CKD–mineral bone disorder (CKD-MBD), protein-energy wasting (PEW), inflammation, and increased oxidative stress. There is a correlation between the markers of oxidative stress and the markers of inflammation in CKD7, 8) and also in the general population9).
CKD, particularly the advanced stages of CKD, is regarded as pro-oxidant state10), and previous studies revealed elevated levels of oxidative stress markers in various stages of CKD7, 11–14). However, as yet, there is only quite limited amount of evidence indicating that oxidative stress is involved in the increased risk of mortality15, 16). Furthermore, there is lack of previous studies which investigated whether oxidative stress could predict the risk of CVD events in patients with CKD, including hemodialysis patients.
Oxidative stress is defined as an impaired balance between the production and elimination of reactive oxygen species (ROSs)17). ROSs, such as superoxide radical and hydroxyl radical, are difficult to measure in clinical specimens due to their very short half-lives. Therefore, more stable biomarkers have been utilized in clinical studies. For example, thiobarbituric acid reactive substances (TBARS)18), advanced oxidation protein products (AOPP)12), and 8-hydroxydeoxy-guanodine (8-OHdG)19) are measured as markers of oxidative modification of lipids, proteins, and nucleic acids, respectively.
The assay of serum derivatives of reactive oxygen metabolites (d-ROMs) is a measure of total hydroperoxides of lipids, proteins, and nucleic acids20), which serves as an integrated marker of oxidative modification of biomolecules in the serum. A higher level of d-ROMs was shown to predict worse clinical outcomes in previous cohort studies in the general population9, 21), in patients with diabetes mellitus22), and in patients with cardiovascular disease23), but not yet in patients with CKD. In addition, the level of d-ROMs was reported to be increased in patients undergoing hemodialysis compared with the healthy control group24).
In the present study, we hypothesized that the level of d-ROMs predicts these clinical outcomes in a cohort of hemodialysis patients. We also examined whether the associations of d-ROMs with the outcomes are independent of a marker of inflammation, C-reactive protein (CRP).
Methods
Study Design
This is an observational study which includes cross-sectional and longitudinal analyses in hemodialysis patients. In the cross-sectional analyses, we examined the associations between serum d-ROMs levels and other clinical variables. In the longitudinal analysis, we conducted a prospective cohort study to examine whether d-ROMs can predict all-cause mortality and new CVD events.
Hemodialysis Patients
The hemodialysis patients in this study were selected from a total of 518 patients in our prospective observational cohort named DREAM (Dialysis-Related Endocrine And Metabolic changes affecting cardiovascular disease) at Inoue Hospital, Suita, Osaka, Japan. We included hemodialysis patients who were treated at Inoue Hospital but excluded (1) those who were treated with peritoneal dialysis, (2) those who were hospitalized at the start of the observation, and (3) those with dementia and/or severe disability due to difficulty in obtaining informed consent; (4) those who did not give informed consent were also excluded. This study was conducted in accordance to the Declaration of Helsinki, and the protocol of this observational study was reviewed and approved by the ethics committee of Inoue Hospital (Approval No. 121). The participants were enrolled in December 2004 and provided written informed consent. The DREAM cohort was registered at UMIN-CTR (ID UMIN000006168, http://www.umin.ac.jp/ctr/index.htm), and some results of this cohort have been published25–28). For this analysis, we excluded patient(s) if the d-ROMs measurement was missing.
Blood Sampling, Storage, and Assay of d-ROMs
Blood samples were collected from the arteriovenous fistula just before the start of the first hemodialysis session of the week. Routine laboratory tests were conducted within the day of blood sampling using an automated analyzer, whereas additional measurements, including that of d-ROMs, were performed afterward using freshly frozen samples kept at −80°C.
We performed d-ROMs assays within 3 months after blood sampling via the method described by Italian researchers20) using the assay kit (d-ROMs test) and the device (FREE system) by Diacron Co. Ltd., Milan, Italy, which were purchased from Wismerll Co. Ltd., Tokyo, Japan. The results were expressed in Carratelli Units (Carr U), which was named after the inventor of the assay, Mauro Carratelli. One Carr U corresponds to 0.08 mg of hydrogen peroxide per 100 mL of the sample29).
Definition of CVD Events
CVD events in the DREAM cohort comprised of ischemic heart disease, ischemic stroke, hemorrhagic stroke, peripheral artery disease, pulmonary edema, and cardiac valve disease. The CVD events included incident and recurrent CVD and interventions for CVD, as described elsewhere25–28) and in the supplemental material (Supplemental Table 1). The same definitions were used for preexisting CVD as of December 2004 and the new-onset CVD during the follow-up. We considered sudden deaths as new and fatal CVD events.
Supplemental Table 1. Definitions of CVD events in the DREAM cohort.
| CVD event | Definition |
|---|---|
| Ischemic heart disease (IHD) |
|
| Stroke |
|
| Peripher alartery disease (PAD) |
|
| Congestive heart failure (CHF) |
|
| Valve disease |
|
Sudden deaths are considered as fatal CVD events.
Abbreviations: IHD, ischemic heart disease; MRI, magnetic resonance imaging; CT, computed tomography; PAD, peripher alartery disease; CHF, congestive heart failure.
Other Variables
We recorded age, sex, dialysis duration, clinical diagnosis of underlying renal disease (diabetic nephropathy or not, not necessarily biopsy-proven), and preexisting CVD as case-mix variables. As traditional risk factors, we included the current smoking status, hypertension, high-density lipoprotein cholesterol (HDL-C), and non-high-density lipoprotein cholesterol (non-HDL-C). Hypertension was defined as blood pressure of 140/90 mmHg or higher, and/or the use of any anti-hypertensive medication. Antihypertensive medications were divided into renin–angiotensin system inhibitors (RASi) and others (non-RASi). Non-HDL-C was calculated by subtracting HDL-C from the total cholesterol. The use of a statin was included in the analysis. As indicators of inflammation and wasting, the body mass index (BMI) after dialysis, CRP, and serum albumin were documented. Serum calcium, phosphate, intact parathyroid hormone (PTH), and use of oral and/or intravenous vitamin D receptor activator (VDRA) were also recorded as CKD-MBD parameters. In 2004, calcium-sensing receptor agonists were not yet available for clinical use in Japan. With regard to renal anemia, we included hematocrit, dose of erythropoiesis-stimulating agent (ESA), and use of intravenous iron injections at baseline. In 2004, we used only recombinant human erythropoietin preparations as ESA.
Follow-Up
The cohort was followed up for up to 5 years until the end of 2009. At the end of each year, the attending physicians filled an annual follow-up sheet to report new onsets, if any, of CVD using the same definitions as indicated above. The annual follow-up sheet also included the dates of death (cause of death), renal transplantation, switching to peritoneal dialysis, and moving away to other dialysis unit.
Statistics
The patients for analysis were divided into quartiles of d-ROMs, and the baseline data were summarized as numbers and percentages for categorical variables or medians and interquartile ranges for continuous variables. CRP values below the detection limit (0.01 mg/dL) were handled as 0.01 mg/dL.
Unadjusted correlations of d-ROMs with other clinical variables were examined by Spearman's rank correlation. Moreover, multivariable non-linear regression analysis was conducted to assess the adjusted association between d-ROMs and CRP, which were adjusted for the following 22 potential confounders: age, sex, diabetic nephropathy or not, dialysis duration, prior CVD, systolic blood pressure, diastolic blood pressure, use of RASi, use of non-RASi, smoking status, HDL-C, non-HDL-C, use of statin, calcium, phosphate, intact PTH, use of vitamin D, hematocrit, dose of ESA, use of intravenous iron, BMI, and serum albumin. CRP variable was used with log base-10 transformation.
The cumulative probabilities of all-cause mortality and new CVD events were calculated by the Kaplan–Meier method for the subject groups divided by the quartiles of d-ROMs, which were compared using the log-rank test. And the adjusted associations of d-ROMs with the all-cause mortality and new CVD events were evaluated by multivariable Cox proportional-hazard regression analysis with adjustment for the potential confounders, in which the non-linear effect of d-ROMs on outcome was taken into consideration, separately. Furthermore, we conducted similar analyses including CRP as well as the potential confounders as covariates to estimate the independent association of d-ROMs with outcomes in models considering CRP value as an additional covariate. To estimate the association of CRP with all-cause mortality and new CVD events, similar regression analysis was performed using models described above with and without adjustment for d-ROMs separately.
In the statistical hypothesis testing, we used two-sided 5% significance level. And all statistical estimations were performed using Windows personal computers, utilizing the statistical software JMP 12 (SAS Institute Japan, Tokyo, Japan) and R software version 3.6.0 (https://www.r-project.org/fuundation/).
Results
Baseline Characteristics of the Hemodialysis Cohort
The 517 hemodialysis patients with d-ROMs measurement at baseline were used for analysis (Fig. 1), and Table 1 presents their baseline characteristics by the quartiles of d-ROMs.
Fig. 1.
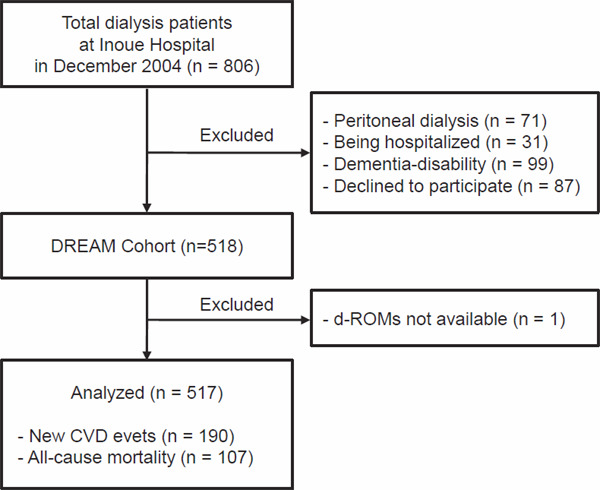
Flow of patients analyzed for this study
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; CVD, cardiovascular disease.
Table 1. Baseline characteristics of the hemodialysis cohort by quartile of d-ROMs.
| No. of participants with data | d-ROMs quartile |
||||||
|---|---|---|---|---|---|---|---|
| Overall, N = 517 |
Q1 N = 130 |
Q2 N = 129 |
Q3 N = 129 |
Q4 N = 129 |
P values across quartiles |
||
| d-ROMs | 517 | 294 (250–350) | 220 (186–236) | 275 (263–284) | 318 (308–331) | 385 (366–419) | < 0.001 |
| Age (years) | 517 | 61 (54–68) | 56 (49–65) | 60 (54–65) | 62 (56–70) | 64 (59–69) | < 0.001 |
| Sex (%male) | 517 | 62.9 | 73.1 | 67.4 | 57.4 | 53.5 | 0.004 |
| Dialysis duration (month) | 517 | 114 (47–194) | 92 (35–171) | 116 (47–194) | 123 (53–202) | 131 (68–201) | 0.03 |
| Diabetic nephropathy (%) | 517 | 21.3 | 26.2 | 19.4 | 17.8 | 21.7 | 0.4 |
| Pre-existing CVD (%) | 517 | 33.5 | 30.8 | 29.5 | 29.5 | 44.2 | 0.03 |
| Systolic BP (mmHg) | 517 | 154 (141–164) | 153 (141–164) | 155 (142–166) | 153 (143–163) | 151 (134–163) | 0.2 |
| Diastolic BP (mmHg) | 517 | 79 (74–82) | 79 (74–84) | 79 (74–83) | 78 (73–82) | 77 (72–81) | 0.01 |
| RASi use (%) | 517 | 36.0 | 48.5 | 34.1 | 34.1 | 27.1 | 0.004 |
| Non-RASi use(%) | 517 | 64.4 | 66.9 | 73.6 | 61.2 | 55.8 | 0.002 |
| Hypertension (%) | 517 | 86.3 | 90.0 | 89.1 | 88.4 | 77.5 | 0.01 |
| Smoker (%) | 517 | 41.0 | 36.2 | 42.6 | 43.4 | 41.9 | 0.6 |
| HDL-C (mg/dL) | 517 | 44.2 (36–54.2) | 43 (36–54) | 45 (37–58) | 45 (37.4–54.2) | 44 (36–52) | 0.6 |
| Non-HDL-C (mg/dL) | 517 | 115 (91–138) | 103 (88–135) | 110 (87–137) | 119 (95–146) | 120 (98–137) | 0.07 |
| Statin use (%) | 517 | 4.8 | 7.7 | 3.9 | 5.4 | 2.3 | 0.2 |
| BMI (kg/m2) | 517 | 21.5 (19.6–23.4) | 21.9 (19.9–23.2) | 21.8 (19.6–24.1) | 21.5 (19.5–23.4) | 21.5 (19.3–23.3) | 0.7 |
| Serum albumin (g/dL) | 517 | 3.7 (3.5–3.9) | 3.8 (3.6–4) | 3.7 (3.6–4) | 3.8 (3.6–3.9) | 3.6 (3.4–3.8) | < 0.001 |
| CRP (mg/dL) | 517 | 0.14 (0.05–0.41) | 0.05 (0.03–0.12) | 0.09 (0.05–0.2) | 0.15 (0.06–0.33) | 0.45 (0.19–1.25) | < 0.001 |
| Calcium (mg/dL) | 517 | 9.1 (8.6–9.8) | 9.1 (8.7–9.7) | 9.2 (8.8–9.9) | 9.1 (8.3–9.9) | 9.1 (8.4–9.7) | 0.2 |
| Phosphate (mg/dL) | 517 | 5.8 (5.0–6.6) | 5.6 (5–6.4) | 6 (5.4–6.7) | 5.8 (4.9–6.6) | 5.6 (4.7–6.6) | 0.02 |
| Intact PTH (pg/mL) | 517 | 118 (41–215) | 109 (43–214) | 130 (52–235) | 127 (44–201) | 97 (30–211) | 0.5 |
| VDRA use (%) | 517 | 44.3 | 46.2 | 39.5 | 48.1 | 43.4 | 0.5 |
| Hematocrit (%) | 517 | 30.7 (28.6–32.4) | 30.7 (29.2–32.2) | 31.4 (29.8–32.9) | 30.8 (28.5–32.4) | 29.1 (27–31.6) | < 0.001 |
| ESA dose (x1000 units/week) | 517 | 9.0 (7.5–9.0) | 9.0 (7.5–9.0) | 9.0 (7.5–9.0) | 9.0 (7.5–9.0) | 9.0 (7.5–9.0) | 0.8 |
| IV iron use (%) | 517 | 58.2 | 51.5 | 47.3 | 66.7 | 67.4 | 0.001 |
The table gives median (interquartile range) for continuous variables and percentages for categorical variables.
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; Q, quartile; CVD, cardiovascular disease; BP, blood pressure; RASi, reninangiotensin system inhibitors; Non-RASi, antihypertensive medications other than RAS inhibitors; HDL, high-density lipoprotein; BMI, body mass index; CRP, C-reactive protein; PTH, parathyroid hormone; VDRA, vitamin D receptor activator; ESA, erythropoiesis stimulating agent; IV-iron, intravenous iron preparation.
Conversion factors for units: HDL-C and Non-HDL-C in mg/dL to mmol/L, × 0.02586; phosphorus in mg/dL to mmol/L, × 0.3229.
Relationship of d-ROMs with Other Variables
Table 2 summarizes the cross-sectional analyses of d-ROMs at baseline. The serum level of d-ROMs was higher in women than in men, in those with prior CVD than in those without, and in those without hypertension than in those with hypertension. The serum level of d-ROMs was lower in RASi and in non-RASi users, whereas it was higher in the users of intravenous iron preparations compared with the non-user counterparts. The serum level of d-ROMs correlated positively with age, dialysis duration, non-HDL-C, total protein, and CRP, whereas it inversely correlated with diastolic blood pressure, serum albumin, and hematocrit. Fig. 2 shows the distribution of d-ROMs and its nonlinear relationship with CRP levels adjusted for the 22 potential confounders.
Table 2. Unadjusted association of d-ROMs with other variables in hemodialysis patients.
| (1) Comparison between subgroups | |||
|---|---|---|---|
| Subgroups | Median d-ROMs | ||
| N | (inter-quartile range) | P value | |
| Men | 325 | 283 (243 to 339) | |
| vs.Women | 192 | 312 (275 to 364) | < 0.001 |
| Prior CVD (+) | 173 | 306 (260 to 366) | |
| vs. Prior CVD (−) | 344 | 291 (246 to 337) | 0.03 |
| DN | 110 | 290 (241 to 354) | |
| vs. Other than DN | 407 | 297 (254 to 350) | 0.6 |
| Smoking (+) | 212 | 299 (255 to 352) | |
| vs. Smoking (−) | 305 | 291 (245 to 348) | 0.3 |
| Hypertension (+) | 446 | 291 (248 to 343) | |
| vs. Hypertension (−) | 71 | 324 (268 to 383) | 0.008 |
| RASi use (+) | 186 | 278 (235 to 330) | |
| vs. RASi use (−) | 331 | 304 (260 to 362) | < 0.001 |
| Non-RASi use (+) | 333 | 289 (249 to 339) | |
| vs. Non-RASi use (−) | 184 | 309 (257 to 365) | 0.02 |
| Statin use (+) | 25 | 282 (220 to 321) | |
| vs. Statin use (−) | 492 | 296 (252 to 352) | 0.06 |
| VDRA use (+) | 186 | 299 (245 to 351) | |
| vs. VDRA use (−) | 331 | 292 (252 to 350) | 0.9 |
| IV iron use (+) | 301 | 309 (259 to 363) | |
| vs. IV iron use (−) | 216 | 281 (242 to 332) | < 0.001 |
| (2) Correlation with other variables | ||
|---|---|---|
| Variables | Spearman's correlation coefficient | P value |
| Age | 0.298 | < 0.001 |
| Dialysis duration | 0.119 | 0.007 |
| Systolic BP | −0.052 | 0.2 |
| Diastolic BP | −0.140 | 0.001 |
| HDL-C | −0.01 | 0.8 |
| Non-HDL-C | 0.120 | 0.006 |
| BMI | −0.027 | 0.6 |
| Serum albumin | −0.230 | < 0.001 |
| C-reactive protein | 0.526 | < 0.001 |
| Calcium | −0.053 | 0.2 |
| Phosphate | −0.078 | 0.08 |
| Intact PTH | −0.038 | 0.4 |
| Hematocrit | −0.178 | < 0.001 |
| Total protein | 0.094 | 0.03 |
| ESA dose | 0.02 | 0.6 |
The table gives correlations in 517 total hemodialysis patients.
Abbreviations are the same as indicated in the footnote of Table 1.
Fig. 2.
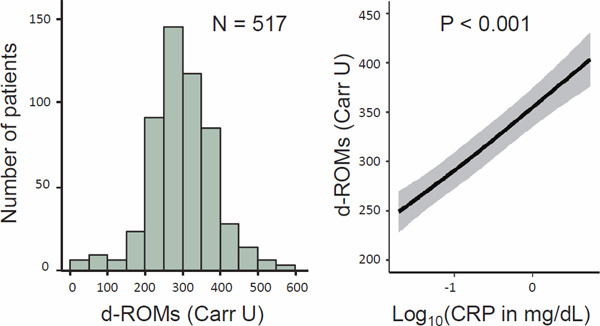
Distribution of d-ROMs and its adjusted association with CRP at baseline
The left panel of the figure presents the histogram of d-ROMs. The right panel shows the association between d-ROMs and CRP using restricted cubic spline curve adjusted for 22 potential confounders, including age, sex, diabetic nephropathy or not, dialysis duration, prior CVD, systolic blood pressure, diastolic blood pressure, use of RASi, use of non-RASi, smoking status, HDL-C, non-HDL-C, use of statin, calcium, phosphate, intact PTH, use of vitamin D receptor activator, hematocrit, dose of ESA, use of intravenous iron, BMI, and serum albumin.
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; CRP, C-reactive protein; CVD, cardiovascular disease; RASi, renin-angiotensin system inhibitor; non-RASi, anti-hypertensive medications other than RASi; HDL, high-density lipoprotein; PTH, parathyroid hormone; ESA, erythropoiesis-stimulating agent; BMI, body mass index.
Associations of d-ROMs with All-Cause Mortality and New CVD Events
There were 107 all-cause deaths and 190 new CVD events during the 5-year follow-up. Kaplan–Meier curves show that the risks of death and new CVD events were significantly different across the quartiles of d-ROMs (Fig. 3).
Fig. 3.
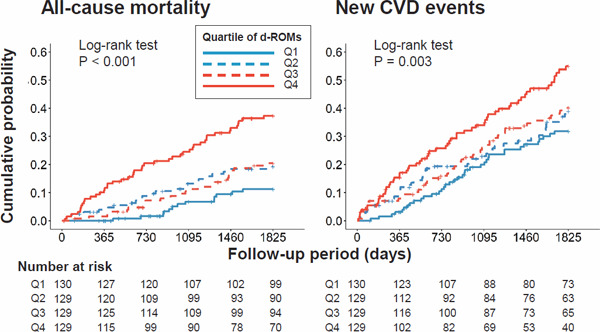
Kaplan–Meier curves showing associations of d-ROMs with all-cause mortality and new CVD events
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; CVD, cardiovascular disease; Q, quartile; P, level of significance
Independent Associations of d-ROMs with All-Cause Mortality
The association of d-ROMs with all-cause mortality was significant in the Cox analysis using restricted cubic spline adjusted for potential confounders, excluding CRP. However, this association was no longer significant after further adjustment for CRP. Using the same model without adjustment for d-ROMs, the association of CRP with all-cause mortality was significant. However, this association was not significant when adjusted for d-ROMs (Fig. 4).
Fig. 4.
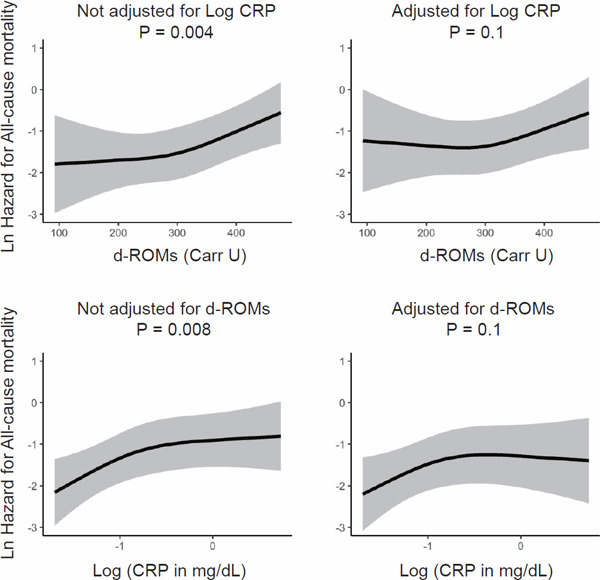
Independent associations of d-ROMs and CRP with all-cause mortality
The upper panels show the multivariable-adjusted association between d-ROMs and hazard for all-cause mortality with and without further adjustment for CRP. The lower panels show the multivariable-adjusted association between CRP and hazard for all-cause mortality with and without further adjustment for d-ROMs. Adjustment was done for the 22 potential confounders indicated in the legend of Fig. 2.
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; CRP, C-reactive protein; Ln, natural logarithm (log e); Log, common logarithm (log 10).
Independent Associations of d-ROMs with New CVD Events
The association of d-ROMs with new CVD events was not significant in the Cox analysis adjusted for the potential confounders either excluding or including CRP (Fig. 5). The association of CRP with new CVD events was significant in the Cox analysis adjusted for the potential confounders excluding d-ROMs, and the association remained almost significant (P = 0.06) after further adjustment for d-ROMs.
Fig. 5.
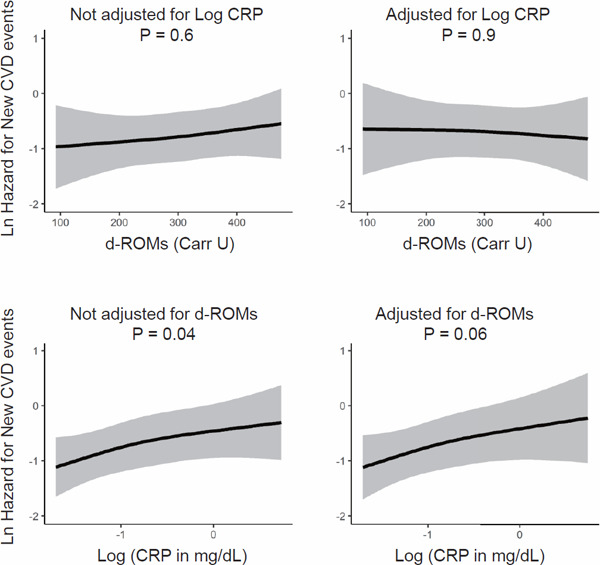
Independent associations of d-ROMs and CRP with new CVD events
The upper panels show the multivariable-adjusted association between d-ROMs and hazard for new CVD events with and without further adjustment for CRP. The lower panels show the multivariable-adjusted association between CRP and hazard for new CVD events with and without further adjustment for d-ROMs. Adjustment was done for the 22 potential confounders indicated in the legend of Fig. 2.
Abbreviations: d-ROMs, derivatives of reactive oxygen metabolites; CRP, C-reactive protein; Ln, natural logarithm (log e); Log, common logarithm (log 10).
Factors Independently Associated with d-ROMs
To further explore the unadjusted associations between d-ROMs and some variables found in Table 2, we conducted an additional analysis to examine whether or not these associations remained significant after multivariable adjustment using a multivariable-adjusted regression model, in which the non-linear association was considered between d-ROMs and Log CRP. This model included Log CRP and the 22 potential confounders used in the Cox analysis above. The results indicated that (1) d-ROMs level was positively associated with age, being female, Log CRP, and use of IV-iron injection; that (2) d-ROMs level was inversely associated with hematocrit, use of RASi, and use of statin; and that (3) the other factors were not significantly associated with d-ROMs.
Discussion
This study examined the possible associations of serum level of d-ROMs with all-cause mortality and new CVD events in a prospective cohort of hemodialysis patients. At baseline, serum levels of d-ROMs and CRP exhibited a positive correlation. Although d-ROMs predicted mortality and CVD events in unadjusted model, the associations of d-ROMs with these outcomes were not independent of CRP.
Previous cohort studies reported that oxidative stress markers predicted all-cause mortality in hemodialysis patients. Kalantar-Zadeh et al.8) revealed that high serum levels of myeloperoxidase predicted allcause mortality in 356 hemodialysis patients. Lin et al.30) demonstrated that gene polymorphism of glutathione S-transferase M1 was a risk factor of death in 488 hemodialysis patients. Xu et al.15) found that serum concentration of 8-OHdG was associated with death in 220 patients treated with hemodialysis. In contrast, we failed to show an independent association of another marker of oxidative stress, serum d-ROMs, with all-cause mortality in this study. The discrepancy between this study and the previous ones may arise from the differences in study populations, characteristics of the markers, or statistical methods. It is crucial to note that none of the above previous studies adjusted for CRP or other markers of inflammation.
We found attenuation of the association between d-ROMs and worse clinical outcomes when adjustment was done for CRP in this hemodialysis cohort; this was also reported in the studies in the general population. A meta-analysis of individual participant data of 10,622 men and women (age range, 45–85 years) from population-based cohorts from Germany, Poland, Czech Republic, and Lithuania9) revealed that adjustment for CRP attenuated the association of d-ROMs with all-cause mortality, although the association remained significant. Another report of the pooled analysis of large European cohort studies revealed that d-ROMs predicted CVD events, including myocardial infarction and stroke21), although this association was no longer significant after adjustment for CRP.
In this study, d-ROMs and CRP showed a close positive association. The association between d-ROMs and all-cause mortality was significant in a multivariable-adjusted model without CRP, but the association was no longer significant when the model was further adjusted for CRP. Conversely, CRP exhibited a significant association with all-cause mortality in a multivariable-adjusted model without d-ROMs, whereas this association disappeared when the model was further adjusted for d-ROMs level. These results indicate similar roles of oxidative stress and inflammation in the pathways to mortality. These two pathophysiological mechanisms could enhance each other.
We noticed that the association of d-ROMs with new CVD events was somewhat different from its association with all-cause mortality. Serum d-ROMs level was not significantly associated with new CVD events in a multivariable-adjusted model, even without including CRP. In contrast, the association of CRP with new CVD events was significant in a model adjusted for 22 variables excluding d-ROMs, and it remained almost significant (P = 0.06) after further adjustment for d-ROMs. These results suggest that inflammation is more important than oxidative stress as the mechanism for CVD. Atherosclerosis is regarded as inflammatory disease characterized by infiltration of inflammatory cells, such as macrophages, lymphocytes, and other leukocytes, in atherosclerotic plaques31). Inflammation is also involved in the pathogenesis of non-atherosclerotic CVD, such as left ventricular hypertrophy and myocardial fibrosis32).
In the additional analysis, being female was an independent factor associated with a higher level of d-ROMs. Although the pathophysiological mechanisms for this observation are unknown, similar findings were reported by other studies33, 34). We also found that the use of some medications, such as RAS inhibitors and statins, were factors independently associated with lower d-ROMs levels, whereas the use of intravenous iron preparations was an independent factor associated with a higher d-ROMs level. The use of VDRA or dose of ESA was not associated with serum level of d-ROMs. We should be careful in interpreting these associations, because randomized controlled trials failed to reduce CVD outcomes by RAS inhibitors35) or statins36, 37) in hemodialysis patients. A recent randomized trial in hemodialysis patients38) revealed that a high dose (versus low dose) of intravenous iron for renal anemia reduced the primary CVD composite outcome. A randomized trial with alfacalcidol as a VDRA showed no reduction in CVD risk in hemodialysis patients39).
This study has some limitations. First, the results were based on d-ROMs measurements at a single time point. This may underestimate or overestimate the true associations with the outcomes. Second, the d-ROMs assays were performed, not with fresh serum but with serum samples freshly frozen and stored for 3 months at −80°C. The storage of serum may have affected the results of d-ROMs. However, previous studies 9, 40) reported the long-term stability of d-ROMs measurements for 3–10 years when stored at −80°C. Third, we did not include serum ferritin concentrations41) or transferrin saturation levels, which may be related to oxidative stress. Fourth, we are not sure whether the results of this study can be also true in CKD patients other than those treated with hemodialysis. And finally, due to the observational design of this study, which is similar to other cohort studies, we should be careful in interpreting the causality of the findings.
In conclusion, we did not observe an association of the serum d-ROMs level with all-cause mortality or new CVD events in a cohort of hemodialysis patients independent of CRP. However, CRP and d-ROMs exhibited similar associations with all-cause mortality, suggesting that inflammation and oxidative stress share common causal pathways. Further studies are needed to confirm our results in other populations with CKD.
Acknowledgements
This study was supported by a grant for TS from the Japan Dialysis Outcome Research Foundation, Tokyo, Japan (No. 002). A part of this study was presented as a poster (FP720, June 14, 2019) at the 56th ERA-EDTA Congress in Budapest, Hungary, and the abstract was published.
Conflicts of Interest
All authors declared no competing interests relevant to this study.
References
- 1). Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, Ishimura E, Tabata T and Nishizawa Y: Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol, 2001; 12: 2117-2124 [DOI] [PubMed] [Google Scholar]
- 2). Shoji T, Emoto M, Tabata T, Kimoto E, Shinohara K, Maekawa K, Kawagishi T, Tahara H, Ishimura E and Nishizawa Y: Advanced atherosclerosis in predialysis patients with chronic renal failure. Kidney Int, 2002; 61: 2187-2192 [DOI] [PubMed] [Google Scholar]
- 3). Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ and Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation, 2003; 108: 2154-2169 [DOI] [PubMed] [Google Scholar]
- 4). Go AS, Chertow GM, Fan D, McCulloch CE and Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med, 2004; 351: 1296-1305 [DOI] [PubMed] [Google Scholar]
- 5). Foley RN, Parfrey PS and Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis, 1998; 32: S112-S119 [DOI] [PubMed] [Google Scholar]
- 6). Wanner C, Amann K and Shoji T: The heart and vascular system in dialysis. Lancet, 2016; 388: 276-284 [DOI] [PubMed] [Google Scholar]
- 7). Tsuchikura S, Shoji T, Shimomura N, Kakiya R, Emoto M, Koyama H, Ishimura E, Inaba M and Nishizawa Y: Serum C-reactive protein and thioredoxin levels in subjects with mildly reduced glomerular filtration rate. BMC Nephrol, 2010; 11: 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Kalantar-Zadeh K, Brennan ML and Hazen SL: Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis, 2006; 48: 59-68 [DOI] [PubMed] [Google Scholar]
- 9). Schottker B, Brenner H, Jansen EH, Gardiner J, Peasey A, Kubinova R, Pajak A, Topor-Madry R, Tamosiunas A, Saum KU, Holleczek B, Pikhart H and Bobak M: Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med, 2015; 13: 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C and Zoccali C: Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant, 2003; 18: 1272-1280 [DOI] [PubMed] [Google Scholar]
- 11). Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW and Blumberg JB: Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int, 2001; 59: 1960-1966 [DOI] [PubMed] [Google Scholar]
- 12). Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillere-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drueke T and Descamps-Latscha B: Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol, 1998; 161: 2524-2532 [PubMed] [Google Scholar]
- 13). Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA and Himmelfarb J: Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int, 2004; 65: 1009-1016 [DOI] [PubMed] [Google Scholar]
- 14). Pupim LB, Himmelfarb J, McMonagle E, Shyr Y and Ikizler TA: Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int, 2004; 65: 2371-2379 [DOI] [PubMed] [Google Scholar]
- 15). Xu H, Watanabe M, Qureshi AR, Heimburger O, Barany P, Anderstam B, Eriksson M, Stenvinkel P and Lindholm B: Oxidative DNA damage and mortality in hemodialysis and peritoneal dialysis patients. Perit Dial Int, 2015; 35: 206-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Goncalves LL, Ramkissoon A and Wells PG: Prostaglandin H synthase-1-catalyzed bioactivation of neurotransmitters, their precursors, and metabolites: oxidative DNA damage and electron spin resonance spectroscopy studies. Chem Res Toxicol, 2009; 22: 842-852 [DOI] [PubMed] [Google Scholar]
- 17). Sies H: Oxidative stress: oxidants and antioxidants. Exp Physiol, 1997; 82: 291-295 [DOI] [PubMed] [Google Scholar]
- 18). Murillo-Ortiz B, Ramirez Emiliano J, Hernandez Vazquez WI, Martinez-Garza S, Solorio-Meza S, Albarran-Tamayo F, Ramos-Rodriguez E and Benitez-Bribiesca L: Impact of Oxidative Stress in Premature Aging and Iron Overload in Hemodialysis Patients. Oxid Med Cell Longev, 2016; 2016: 1578235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Kuo KL, Hung SC, Wei YH and Tarng DC: Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol, 2008; 19: 1817-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R and Nicolaides A: A simple test to monitor oxidative stress. Int Angiol, 1999; 18: 127-130 [PubMed] [Google Scholar]
- 21). Xuan Y, Bobak M, Anusruti A, Jansen E, Pajak A, Tamosiunas A, Saum KU, Holleczek B, Gao X, Brenner H and Schottker B: Association of serum markers of oxidative stress with myocardial infarction and stroke: pooled results from four large European cohort studies. Eur J Epidemiol, 2019; 34: 471-481 [DOI] [PubMed] [Google Scholar]
- 22). Xuan Y, Gao X, Anusruti A, Holleczek B, Jansen E, Muhlack DC, Brenner H and Schottker B: Association of Serum Markers of Oxidative Stress With Incident Major Cardiovascular Events, Cancer Incidence, and All-Cause Mortality in Type 2 Diabetes Patients: Pooled Results From Two Cohort Studies. Diabetes Care, 2019; 42: 1436-1445 [DOI] [PubMed] [Google Scholar]
- 23). Vassalle C, Boni C, Di Cecco P and Landi P: Elevated hydroperoxide levels as a prognostic predictor of mortality in a cohort of patients with cardiovascular disease. Int J Cardiol, 2006; 110: 415-416 [DOI] [PubMed] [Google Scholar]
- 24). Gerardi G, Usberti M, Martini G, Albertini A, Sugherini L, Pompella A and Di LD: Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin Chem Lab Med, 2002; 40: 104-110 [DOI] [PubMed] [Google Scholar]
- 25). Kakiya R, Shoji T, Hayashi T, Tatsumi-Shimomura N, Tsujimoto Y, Tabata T, Shima H, Mori K, Fukumoto S, Tahara H, Koyama H, Emoto M, Ishimura E, Nishizawa Y and Inaba M: Decreased serum adrenal androgen dehydroepiandrosterone sulfate and mortality in hemodialysis patients. Nephrol Dial Transplant, 2012; 27: 3915-3922 [DOI] [PubMed] [Google Scholar]
- 26). Shoji T, Kakiya R, Hayashi T, Tsujimoto Y, Sonoda M, Shima H, Mori K, Fukumoto S, Tahara H, Shioi A, Tabata T, Emoto M, Nishizawa Y and Inaba M: Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am J Kidney Dis, 2013; 62: 568-576 [DOI] [PubMed] [Google Scholar]
- 27). Okute Y, Shoji T, Hayashi T, Kuwamura Y, Sonoda M, Mori K, Shioi A, Tsujimoto Y, Tabata T, Emoto M and Inaba M: Cardiothoracic Ratio as a Predictor of Cardiovascular Events in a Cohort of Hemodialysis Patients. J Atheroscler Thromb, 2017; 24: 412-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Kuwamura Y, Shoji T, Okute Y, Yamazaki Y, Motoyama K, Morioka T, Mori K, Fukumoto S, Tsujimoto Y, Shioi A, Emoto M and Inaba M: Altered Serum n-6 Polyunsaturated Fatty Acid Profile and Risks of Mortality and Cardiovascular Events in a Cohort of Hemodialysis Patients. J Ren Nutr, 2018; 28: 54-63 [DOI] [PubMed] [Google Scholar]
- 29). Verde V, Fogliano V, Ritieni A, Maiani G, Morisco F and Caporaso N: Use of N,N-dimethyl-p-phenylenediamine to evaluate the oxidative status of human plasma. Free Radic Res, 2002; 36: 869-873 [DOI] [PubMed] [Google Scholar]
- 30). Lin YS, Hung SC, Wei YH and Tarng DC: GST M1 polymorphism associates with DNA oxidative damage and mortality among hemodialysis patients. J Am Soc Nephrol, 2009; 20: 405-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med, 1999; 340: 115-126 [DOI] [PubMed] [Google Scholar]
- 32). Liu Y, Liu X, Chen J, Zhang K, Huang F, Wang JF, Tang W and Huang H: Apocynin Attenuates Cardiac Injury in Type 4 Cardiorenal Syndrome via Suppressing Cardiac Fibroblast Growth Factor-2 With Oxidative Stress Inhibition. J Am Heart Assoc, 2015; 4: e001598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Kohata Y, Ohara M, Nagaike H, Fujikawa T, Osaka N, Goto S, Fukase A, Kushima H, Hiromura M, Terasaki M, Mori Y, Fukui T, Ouchi M, Suzuki T, Hirano T and Yamagishi SI: Association of Hemoglobin A1c, 1,5-Anhydro-D-Glucitol and Glycated Albumin with Oxidative Stress in Type 2 Diabetes Mellitus Patients: A Cross-Sectional Study. Diabetes Ther, 2020; 11: 655-665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Fukui T, Yamauchi K, Maruyama M, Yasuda T, Kohno M and Abe Y: Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res, 2011; 34: 1041-1045 [DOI] [PubMed] [Google Scholar]
- 35). Iseki K, Arima H, Kohagura K, Komiya I, Ueda S, Tokuyama K, Shiohira Y, Uehara H, Toma S and Olmesartan Clinical Trial in Okinawan Patients Under OG: Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with longterm haemodialysis: a randomized controlled trial. Nephrol Dial Transplant, 2013; 28: 1579-1589 [DOI] [PubMed] [Google Scholar]
- 36). Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E, German D and Dialysis Study I: Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med, 2005; 353: 238-248 [DOI] [PubMed] [Google Scholar]
- 37). Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F and Group AS: Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med, 2009; 360: 1395-407 [DOI] [PubMed] [Google Scholar]
- 38). Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CRV, Wheeler DC, Winearls CG, Ford I, Investigators P and Committees: Intravenous Iron in Patients Undergoing Maintenance Hemodialysis. N Engl J Med, 2019; 380: 447-458 [DOI] [PubMed] [Google Scholar]
- 39). Shoji T, Inaba M, Fukagawa M, Ando R, Emoto M, Fujii H, Fujimori A, Fukui M, Hase H, Hashimoto T, Hirakata H, Honda H, Hosoya T, Ikari Y, Inaguma D, Inoue T, Isaka Y, Iseki K, Ishimura E, Itami N, Ito C, Kakuta T, Kawai T, Kawanishi H, Kobayashi S, Kumagai J, Maekawa K, Masakane I, Minakuchi J, Mitsuiki K, Mizuguchi T, Morimoto S, Murohara T, Nakatani T, Negi S, Nishi S, Nishikawa M, Ogawa T, Ohta K, Ohtake T, Okamura M, Okuno S, Shigematsu T, Sugimoto T, Suzuki M, Tahara H, Takemoto Y, Tanaka K, Tominaga Y, Tsubakihara Y, Tsujimoto Y, Tsuruya K, Ueda S, Watanabe Y, Yamagata K, Yamakawa T, Yano S, Yokoyama K, Yorioka N, Yoshiyama M and Nishizawa Y: Effect of Oral Alfacalcidol on Clinical Outcomes in Patients Without Secondary Hyperparathyroidism Receiving Maintenance Hemodialysis: The J-DAVID Randomized Clinical Trial. JAMA, 2018; 320: 2325-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Cavalleri A, Colombo C, Venturelli E, Miceli R, Mariani L, Cornelli U, Pala V, Berrino F and Secreto G: Evaluation of reactive oxygen metabolites in frozen serum samples. Effect of storage and repeated thawing. Int J Biol Markers, 2004; 19: 250-253 [DOI] [PubMed] [Google Scholar]
- 41). Shoji T, Niihata K, Fukuma S, Fukuhara S, Akizawa T and Inaba M: Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin Exp Nephrol, 2017; 21: 685-693 [DOI] [PMC free article] [PubMed] [Google Scholar]


