Abstract
INTRODUCTION:
To evaluate the diagnostic performance of ultrasound attenuation parameter (UAP) and liver stiffness measurement (LSM) by FibroTouch for diagnosis of hepatic steatosis and fibrosis in patients with nonalcoholic fatty liver disease (NAFLD).
METHODS:
We recruited 237 patients undergoing FibroTouch and liver biopsy within 2 weeks. The pathological findings of liver biopsy were scored by Nonalcoholic Steatohepatitis Clinical Research Network, and the diagnostic accuracy of UAP for steatosis and LSM for fibrosis was evaluated by area under the receiver operating characteristic curve (AUROC). The impacts of histological parameters on UAP and LSM were analyzed, and diagnostic performance of FibroTouch UAP and LSM was compared with other noninvasive biomarkers.
RESULTS:
The success rate of FibroTouch examination was 96.51%. The AUROC of UAP for diagnosis of steatosis ≥S1, ≥S2, and S3 was 0.88, 0.93, and 0.88, and the cutoff values were 244, 269, and 296 dB/m, respectively. The AUROC of LSM for the diagnosis of fibrosis stages ≥F2, ≥F3, and F4 was 0.71, 0.71, and 0.77, and the cutoff values were 9.4, 9.4, and 11 kPa, respectively. Multiple regression analysis showed that LSM was positively correlated with degree of fibrosis and NAFLD activity score. UAP was positively correlated with liver steatosis. The diagnostic performance of UAP for steatosis was significantly superior to that of the hepatic steatosis index.
DISCUSSION:
FibroTouch has a low failure rate with moderate to high diagnostic performance for discriminating the steatosis degree and fibrosis stage and is suitable for clinical evaluation and monitoring of patients with NAFLD.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of chronic liver diseases (CLDs) and should be the leading cause of end-stage liver disease in the coming decades (1). The global prevalence of NAFLD is 25.24%, with the highest prevalence in the Middle East (31.79%) and South America (30.45%), followed by Asia (27.37%), North America (24.13%), and Europe (23.71%), whereas NAFLD is less common in Africa (13.48%) (2). With change of lifestyle, the increase of NAFLD in China has become a major public health concern, and the prevalence of NAFLD in Shanghai has been as high as 38.17% (3). It is predicted that by 2030, the number of NAFLD cases will be around 314.58 million, suggesting the tremendous impacts of NAFLD in the later decades (4).
NAFLD might progress to liver cirrhosis and even hepatocellular carcinoma. It was found that in China, 58.9% of patients with NAFLD had nonalcoholic steatohepatitis (NASH) morphology; significant fibrosis and advanced fibrosis account for 40% and 13.7%, respectively (5). NAFLD is also closely associated with increased risk of extrahepatic diseases, such as diabetes mellitus, cardiovascular disease, and metabolic syndrome, and is also positively correlated with incidence of extrahepatic tumors (6). Therefore, detection of liver steatosis and fibrosis in NAFLD is clinically significant. Transient elastography FibroTouch (FT; FibroTouch-FT5000, iLivTouch series, Wuxi Hisky Medical Technologies, China) measures the degree of hepatic fibrosis by detecting the liver stiffness measurement (LSM) based on the vibration-controlled instantaneous elastography. Liver steatosis is quantitatively assessed by measuring the extent of attenuation of ultrasound signal occurs in liver, referred as the ultrasound attenuation parameter (UAP). Transient elastography could be a promising technique for the clinical diagnosis and evaluation of hepatic steatosis and fibrosis (7). However, there is still a lack of large-scale clinical research for NAFLD with FT, comparing with liver biopsy as gold standard. Therefore, a large collaborative prospective study including 9 medical centers was performed for assessing the accuracy of UAP and LSM by FT in patients with NAFLD. It might provide a basis for the noninvasive clinical diagnosis of hepatic steatosis and fibrosis by FT in patients with NAFLD.
MATERIALS AND METHODS
Study design and participants
This was a multicenter study to assess the diagnostic performance of UAP for hepatic steatosis and LSM for liver fibrosis by using FT. By comparing the results of FT with that of liver biopsy, the specificities, sensitivities, and accuracies for diagnosis of liver steatosis and fibrosis would be defined. The methods used and the ranges of results followed the Standards for Reporting Diagnostic Accuracy Studies guidelines (8). Consecutive patients in 9 medical centers in China from April 1, 2014, to September 30, 2018, were recruited.
The study (Clinical Trials No: NCT02456766) was approved by Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, and was conducted in accordance with the declaration of Helsinki, and all the participating subjects had provided their written informed consent.
Study population
Patients with clinically suspicion to have NAFLD and who required liver biopsy meeting the following inclusion and exclusion criteria were enrolled.
Inclusion criteria included (i) subject age 18–65 years, sex not limited, (ii) accepting liver biopsy within 2 weeks of FT examination, (iii) willing and able to abide all the principles and complete all the study procedures, and (iv) willing to provide written informed consent either by patient or patient's legal guardian.
Exclusion criteria included (i) subject with active or suspected cancer or history of malignant tumor within 5 years, (ii) with a history of significant alcohol consumption or drug abuse, (iii) hepatitis C infection, (iv) pregnant or nursing woman, (v) with history of organ transplantation or functional grafts, (vi) with nonhealing wound on the right upper abdomen at this moment, (vii) unsuitable to participate in the study as having other serious disease or a history of other serious illness, and (viii) participating in other clinical trial at the same time. Note that the definition of significant alcohol consumption was 40 g/d for male and 20 g/d for female. Alcohol intake (g) = the volume of drinking (mL) × alcohol percentage (%, vol/vol) × 0.8 (g/mL).
Patient characteristics
The following demographic data were recorded: age, sex, height, weight, and body mass index (BMI). An overnight fasting blood sample was collected on the day of the FT procedure for the assessment of following laboratory parameters: alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin (TBIL), direct bilirubin, total protein, albumin, gamma-glutamyl transferase, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, glycated hemoglobin A1c, fasting plasma glucose, alpha-fetoprotein, hemoglobin, white blood cells, and platelets.
Histopathologic evaluation
In this study, liver histology served as the gold standard for evaluating the diagnostic accuracy of FT. All the patients received liver biopsy within 2 weeks after FT examination. A quick-cut (purchased from Japan) or Menghini needle (16G) was used in this procedure, and tissue sample was obtained. The biopsy specimen was fixed with 10% formalin, routinely embedded in paraffin, and tissue sections were processed with hematoxylin and eosin, and Masson trichrome staining. A minimum length of 1.0 cm of the liver tissue with at least 6 portal tracts was required for the diagnosis. All the sections were blindly and independently assessed by 3 pathologists. When the 3 pathologists did not agree with each other, the specimen was re-examined to analyze the discrepancies for reaching a consensus. Pathological examination was performed by Xiqi Hu, Hongguang Zhu, and Xiuping Liu from Department of pathology, Shanghai Medical College, Fudan University, Shanghai, China.
Steatosis, hepatocyte ballooning, lobular inflammation, and fibrosis were scored using the NASH Clinical Research Network scoring system (9). Fibrosis of other liver diseases was staged according to the Scheuer system (10).
FibroTouch UAP and LSM
FT measurements were performed by experienced and certificated physicians (who had performed more than 500 examinations) without knowledge of the results of hepatic histology. FT integrates a 2-dimensional image-guided system for precise positioning and measures the speed of the shear wave propagation. Velocity indicates the stiffness depending on the quantity of fibrotic tissues. Therefore, liver stiffness can be measured with specific scientific algorithms, and LSM could reflect the stage of liver fibrosis. Meanwhile, FT tracks the energy attenuation of ultrasound signal during the propagation process and obtain the quantitative result of UAP quickly, which indirectly assesses the degree of liver steatosis. According to manufacturer instructions, the patient was at supine position with the right hand behind head to expand the intercostal space. An image-guided probe was selected to detect the region through the seventh–ninth intercostal space avoiding the cysts and blood vessels in the liver. The probe was kept in a vertical position to the skin surface with pressure maintained the appropriate range. The detection started when M waveform intensity distributed uniform and A waveform was linear. The median value of the 10 acceptable LSMs (kPa) and UAPs (dB/m) with interquartile range was expressed as the representative measurement of FT. In this study, LSM and UAP were considered as reliable only if 10 successful measurements were obtained, and with an interquartile range/median of LSM and UAP of ≤30% and a success rate of ≥60% (11).
Sample size estimation
It was hypothesized that the estimated area under the receiver operating characteristic curve (AUROC) of diagnostic performance for FT to detect steatosis (S > S0) is not less than 0.80 (12), and a 95% confidence interval (CI) for P value to be no wider than 0.07, so the required sample size was 223. We anticipated 240 patients would enroll this study with an expected 7% drop-out rate.
Statistical analysis of the data was performed with SAS9.4 software (SAS Institute, Cary, NC) and stata12.0 (StataCorp LLC, College Station, TX). The data measured were described as mean ± SD for normal distribution and as median (lower quartile–upper quartile) if not normally distributed. Categorical variables were described using frequency (percentage). Differences between steatosis grades were compared using 1-way analysis of variance (ANOVA), and the Student–Newman–Keuls (SNK) test was used for the multiple comparisons between different grades. Differences between fibrosis stages were compared using the Kruskal–Wallis test; the aligned rank transform technique was used to perform multiple comparisons of SNK. P < 0.05 was considered as statistically significant. The diagnostic efficacy of UAP and LSM was evaluated using AUROC. The cutoff values for UAP and LSM were selected to maximize Youden index, sensitivity (Se) 90%, and specificity (Sp) 90% (13). For each cutoff value, Se, Sp, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, negative likelihood ratio and corresponding 95% CI were calculated. PPV and NPV were also detected for different prevalence rates. Independent variable candidates and steatosis, balloon degeneration, intralobular inflammation and portal inflammation, and hepatic fibrosis were regressed against the dependent variable UAP and LSM, respectively, using stepwise regression with an entry and exit significance level of 0.05 to find the best histological parameters and assess their effects on UAP and LSM.
RESULTS
General information and histopathologic evaluation
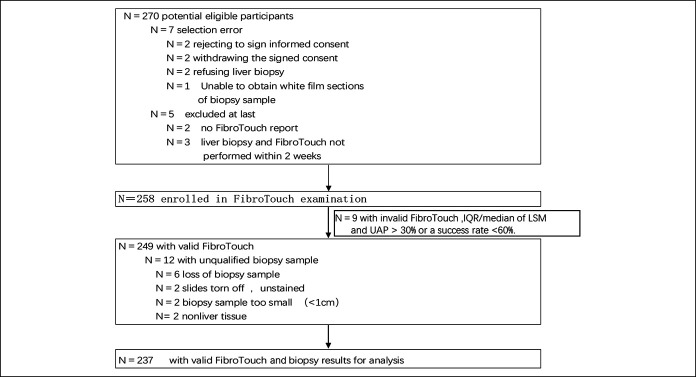
Among 270 potential eligible participants, 258 were enrolled in FT examination. The success rate of FT was 96.51% (249/258); 9 patients had ineligible FT results. Twelve patients were excluded for unqualified biopsy sample, therefore, a total of 237 patients were finally evaluated for analyzing the accuracy of UAP and LSM in detecting liver steatosis and fibrosis, respectively (Figure 1). Among 237 patients, 140 had steatosis from S1 to S3. The mean age was 42 years. The mean BMI was 25.65 kg/m2, ranging from 15.6 to 44.73 kg/m2 (Table 1). Besides, 14 patients have the history of type 2 diabetes.
Figure 1.
Flow chart of study design and patient enrollment. Of 270 patients enrolled, 12 were eligible, 258 had the FibroTouch examination performed, and 249 had a valid FibroTouch examination. Twelve cases had unqualified biopsy samples. Eventually, 237 patients had a valid UAP and LSM with liver biopsy. IQR, interquartile range; LSM, liver stiffness measurement; UAP, ultrasound attenuation parameter.
Table 1.
Patients' characteristics: demographic data and laboratory findings (n = 237)
| Characteristics | Distribution |
| Age (yr) | 41.71 ± 12.49 |
| Female | 90 (37.97) |
| Height (cm) | 166.37 ± 7.91 |
| Weight (kg) | 71.30 ± 14.27 |
| BMI (kg/m2) | 25.65 ± 4.27 |
| AST (U/L) | 40 [26.40–62.45] |
| ALT (U/L) | 55 [29.5–97.0] |
| TBIL (μmol/L) | 15 [11.0–21.4] |
| DBIL (μmol/L) | 4.3 [3.1–6.7] |
| ALP (U/L) | 89 [72–119] |
| GGT (U/L) | 53 [31–102] |
| TPr (g/L) | 71.05 ± 6.70 |
| ALB (g/L) | 42.66 ± 5.16 |
| TC (mmol/L) | 4.94 ± 2.25 |
| TG (mmol/L) | 1.85 ± 1.22 |
| HbA1c (%) | 5.75 ± 1.08 |
| HDL (mmol/L) | 1.21 ± 0.40 |
| LDL (mmol/L) | 2.85 ± 0.83 |
| FPG (mmol/L) | 5.19 ± 1.15 |
| AFP (μg/L) | 3.22 [2.01–6.67] |
| HB (g/L) | 141.93 ± 20.23 |
| WBC (×109/L) | 6.11 ± 1.74 |
| PLT (×109/L) | 195.78 ± 61.44 |
| UAP (dB/m) | 252.59 ± 49.75 |
| LSM (kPa) | 8.4 [6.1–13.6] |
| Fibrosis stage | |
| F0 | 19 (13.57) |
| F1 | 36 (25.71) |
| F2 | 35 (25.00) |
| F3 | 45 (32.14) |
| F4 | 5 (3.57) |
| Steatosis grade | |
| S0 | 97 (40.93) |
| S1 | 56 (23.63) |
| S2 | 56 (23.63) |
| S3 | 28 (11.81) |
| Ballooning grade | |
| B0 | 32 (13.5) |
| B1 | 75 (31.65) |
| B2 | 130 (54.85) |
| Lobular inflammation grade | |
| I0 | 27 (11.39) |
| I1 | 84 (35.44) |
| I2 | 45 (18.99) |
| I3 | 81 (34.18) |
| Portal inflammation | 85 (35.86) |
| Pathological diagnosis | |
| No steatosis group | 97 (S = 0) |
| NAFLD group | 140 (S ≥ 1) |
Distribution is expressed as mean ± SD or median [lower quartile–upper quartile] or figure (percentage).
AFP, alpha-fetoprotein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase AST, aspartate aminotransferase; BMI, body mass index; DBIL, direct bilirubin; FPG, fasting plasma glucose; GGT, gamma-glutamyl transferase; HB, hemoglobin; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LSM, liver stiffness measurement; NAFLD, nonalcoholic fatty liver disease; PLT, platelets; TBIL, total bilirubin; TC, cholesterol; TG, triglyceride; TPr, total protein; UAP, ultrasound attenuation parameter; WBC, white blood cell.
All the 237 patients received liver biopsy and had valid UAP. The prevalence and grades of lobular inflammation, fibrosis, steatosis, ballooning, portal inflammation, and NAS score are shown in Table 1. For hepatic steatosis, 97 (40.93%) were S0, 56 (23.63%) were S1, 56 (23.63%) were S2, and 28 (11.81%) were S3. There were no serious complications in all the patients after liver biopsy. Statistical analysis was conducted to evaluate the accuracy of LSM for hepatic fibrosis based on liver biopsy.
Assessment of steatosis using UAP
The mean ± SD of UAP in 4 groups (S0–S3) was 214.63 ± 28.12, 244.84 ± 34.70, 295.96 ± 33.89, and 312.89 ± 32.54 dB/m, respectively. Statistically, 1-way ANOVA showed that significant differences in UAP among these 4 groups. Post hoc analysis with the SNK test showed that each pair comparison also reached significant difference.
AUROC as well as the diagnostic performance of UAP with cutoff value optimized using the Youden index with a 0.90 Se and a 0.90 Sp for ≥S1, ≥S2, and S3 was shown in Table 2. Accuracy was the highest at the ≥S2 threshold, with an AUROC of 0.93 (95% CI 0.89–0.97), and Se of 0.87, Sp of 0.90 at a threshold of 269 dB/m selected by maximizing the Youden index. Accuracy dropped at ≥S1 threshold with AUROC of 0.88 (95% CI 0.84–0.92) and the corresponding Se of 0.79 and Sp of 0.86 at the threshold of 244 dB/m selected by maximizing the Youden index. At S3 threshold, the AUROC was 0.88 (95% CI 0.81–0.94), with the corresponding Se of 0.89, and Sp of 0.83 at the threshold of 296 dB/m selected by maximizing the Youden index. The AUROC for each grade of hepatic steatosis and the UAP box plots for each grade of hepatic steatosis were shown in Figure 2.
Table 2.
Diagnostic performance of UAP for hepatic steatosis S ≥ S1, S ≥ S2, and S = S3
| S ≥ S1 (≥5%) | S ≥ S2 (≥34%) | S = S3 (≥67%) | |
| AUROC (95% CI) | 0.88 (0.84–0.92) | 0.93 (0.89–0.97) | 0.88 (0.81–0.94) |
| Prevalence (N) | 0.59 (140/237) | 0.35 (84/237) | 0.12 (28/237) |
| Youden index | |||
| Cutoff (dB/m) | 244 | 269 | 296 |
| Se (95% CI) | 0.79 (0.73–0.86) | 0.87 (0.80–0.94) | 0.89 (0.78–1.00) |
| TP/(TP + FN) | 111/(111 + 29) | 73/(73 + 11) | 25/(25 + 3) |
| Sp (95% CI) | 0.86 (0.79–0.93) | 0.90 (0.85–0.94) | 0.83 (0.78–0.88) |
| TN/(TN + FP) | 83/(83 + 14) | 137/(137 + 16) | 173/(173 + 36) |
| PPV (95% CI) | 0.89 (0.83–0.94) | 0.82 (0.74–0.90) | 0.41 (0.29–0.53) |
| NPV (95% CI) | 0.74 (0.66–0.82) | 0.93 (0.88–0.97) | 0.98 (0.96–1.00) |
| LR+ (95% CI) | 5.49 (3.36–8.98) | 8.31 (5.19–13.31) | 5.18 (3.75–7.17) |
| LR− (95% CI) | 0.24 (0.17–0.34) | 0.15 (0.08–0.25) | 0.13 (0.04–0.38) |
| Se = 0.90 | |||
| Cutoff (dB/m) | 214 | 260 | 296 |
| Se (95% CI) | 0.91 (0.86–0.96) | 0.90 (0.84–0.97) | 0.89 (0.78–1.00) |
| TP/(TP + FN) | 127/(127 + 13) | 76/(76 + 8) | 25/(25 + 3) |
| Sp (95% CI) | 0.54 (0.44–0.64) | 0.86 (0.80–0.91) | 0.83 (0.78–0.88) |
| TN/(TN + FP) | 52/(52 + 45) | 131/(131 + 22) | 173/(173 + 36) |
| PPV (95% CI) | 0.74 (0.67–0.80) | 0.78 (0.69–0.86) | 0.41 (0.29–0.53) |
| NPV (95% CI) | 0.80 (0.70–0.90) | 0.94 (0.90–0.98) | 0.98 (0.96–1.00) |
| LR+ (95% CI) | 1.96 (1.57–2.44) | 6.29 (4.25–9.32) | 5.18 (3.75–7.17) |
| LR− (95% CI) | 0.17 (0.10–0.30) | 0.11 (0.06–0.22) | 0.13 (0.04–0.38) |
| Sp = 0.90 | |||
| Cutoff (dB/m) | 254 | 275 | 311 |
| Se (95% CI) | 0.69 (0.61–0.76) | 0.82 (0.74–0.90) | 0.71 (0.55–0.88) |
| TP/(TP + FN) | 96/(96 + 44) | 69/(69 + 15) | 20/(20 + 8) |
| Sp (95% CI) | 0.90 (0.84–0.96) | 0.90 (0.85–0.95) | 0.90 (0.86–0.94) |
| TN/(TN + FP) | 87/(87 + 10) | 138/(138 + 15) | 188/(188 + 21) |
| PPV (95% CI) | 0.91 (0.85–0.96) | 0.82 (0.74–0.90) | 0.49 (0.33–0.64) |
| NPV (95% CI) | 0.66 (0.58–0.75) | 0.90 (0.85–0.95) | 0.96 (0.93–0.99) |
| LR+ (95% CI) | 6.65 (3.66–12.09) | 8.38 (5.13–13.69) | 7.11 (4.45–11.36) |
| LR− (95% CI) | 0.35 (0.27–0.45) | 0.20 (0.12–0.31) | 0.32 (0.18–0.57) |
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; FP, false positive; FN, false negative; LR+, positive likelihood ratio; LR−, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity; TP, true positive; TN, true negative; UAP, ultrasound attenuation parameter.
Figure 2.
Receiver operating characteristic (ROC) curve of ultrasound attenuation parameter (UAP) for identifying hepatic steatosis ≥S1 (a), ≥S2 (b), and S3 (c). Box plots of UAP and hepatic steatosis grade (Student–Newman–Keuls for paired comparisons, both statistically significant, P < 0.0001) (d).
For determining influence of serum ALT, TBIL, and BMI on UAP diagnostic performance, patients were stratified by ALT values (≤1xULN, >1xULN), TBIL (≤1xULN, >1xULN), and BMI (BMI <25, 25 ≤ BMI < 30 and ≥30 kg/m2). However, results showed that the stratification did not influence UAP AUROC (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A526).
In the diagnosis of liver steatosis, UAP was significantly superior to that of the hepatic steatosis index (HSI) (14) (S ≥ S1 [P = 0.0001], S ≥ S2 [P = 0.0000]). Although the difference was not significant in S3 (P = 0.0532), the AUROC was numerically higher than that of HSI (see Supplementary Table 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A527). The AUROC plots for comparison between the 2 groups are presented in Figure 3.
Figure 3.

Comparison of AUROC of UAP and HSI for diagnosis of hepatic steatosis groups ≥S1 (a), ≥S2 (b), and S3 (c). AUROC, area under the receiver operating characteristic curve; HSI, hepatic steatosis index; UAP, ultrasound attenuation parameter.
Among 140 patients with NAFLD, 83 cases were diagnosed as liver steatosis by ultrasound with an accuracy rate of only 59.3%. Using UAP 214 dB/m as cutoff, 127 cases can be diagnosed by FT and sensitivity could reach 90.7%. Using 244 dB/m as cutoff, 111 cases could be diagnosed, and the sensitivity was 79.3%. Choosing 254 dB/m as cutoff to exclude NAFLD, 87 cases could be excluded by FT, with a specificity of 89.7%.
Assessment of fibrosis using LSM
Among 237 cases, 52 cases of chronic hepatitis B virus (HBV) infection and 45 cases of other causes (etiology non-NAFLD non-HBV) were excluded for assessment of fibrosis. Fibrosis stage of 140 cases with NAFLD were F0: 19 (13.57%), F1: 36 (25.71%), F2: 35 (25.00%), F3: 45 (32.14%), and F4: 5 (3.57%), respectively. SNK comparison showed that differences between F1 and F3, F1, and F4 were statistically significant.
The AUROC of LSM for the diagnosis of fibrosis stages ≥F2, ≥F3, and F4 was 0.71, 0.71, and 0.77 in the NAFLD group, 0.88, 0.89, and 0.89 in the HBV infection group, and 0.74, 0.70, and 0.60 in other groups (etiology non-NAFLD non-HBV). AUROC as well as diagnostic performance of LSM with cutoff values optimized using the Youden index, and with an Se of 0.90 and an Sp of 0.90 for ≥F2, ≥F3, and F4, was shown in Table 3.
Table 3.
Diagnostic performance of LSM for fibrosis F ≥ F2, F ≥ F3, and F = F4
| F ≥ F2 | F ≥ F3 | F = F4 | |
| AUROC (95% CI) | 0.71 (0.63–0.80) | 0.71 (0.62–0.80) | 0.77 (0.58–0.97) |
| Prevalence (N) | 0.61 (85/140) | 0.36/(50/140) | 0.04 (5/140) |
| Youden index | |||
| Cutoff (kPa) | 9.4 | 9.4 | 11 |
| Se (95% CI) | 0.58 (0.47–0.68) | 0.68 (0.55–0.81) | 0.80 (0.45–1.00) |
| TP/(TP + FN) | 49/(49 + 36) | 34/(34 + 16) | 4/(4 + 1) |
| Sp (95% CI) | 0.82 (0.72–0.92) | 0.72 (0.63–0.81) | 0.71 (0.63–0.79) |
| TN/(TN + FP) | 45/(45 + 10) | 65/(65 + 25) | 96/(96 + 39) |
| PPV (95% CI) | 0.83 (0.73–0.93) | 0.58 (0.45–0.70) | 0.09 (0.006–0.18) |
| NPV (95% CI) | 0.56 (0.45–0.66) | 0.80 (0.72–0.89) | 0.99 (0.97–1.00) |
| LR+ (95% CI) | 3.17 (1.76–5.72) | 2.45 (1.67–3.59) | 2.77 (1.66–4.62) |
| LR− (95% CI) | 0.52 (0.39–0.68) | 0.44 (0.29–0.68) | 0.28 (0.05–1.63) |
| Se = 0.90 | |||
| Cutoff (kPa) | 5.3 | 6.1 | 11 |
| Se (95% CI) | 0.92 (0.86–0.98) | 0.88 (0.79–0.97) | 0.80 (0.45–1.00) |
| TP/(TP + FN) | 78/(78 + 7) | 44/(44 + 6) | 4/(4 + 1) |
| Sp (95% CI) | 0.22 (0.11–0.33) | 0.27 (0.18–0.36) | 0.71 (0.63–0.79) |
| TN/(TN + FP) | 12/(12 + 43) | 24/(24 + 66) | 96/(96 + 39) |
| PPV (95% CI) | 0.64 (0.56–0.73) | 0.40 (0.31–0.49) | 0.09 (0.006–0.18) |
| NPV (95% CI) | 0.71 (0.49–0.92) | 0.80 (0.66–0.94) | 0.99 (0.97–1.00) |
| LR+ (95% CI) | 1.17 (1.01–1.37) | 1.20 (1.02–1.41) | 2.77 (1.66–4.62) |
| LR− (95% CI) | 0.38 (0.16–0.90) | 0.45 (0.20–1.03) | 0.28 (0.05–1.63) |
| Sp = 0.90 | |||
| Cutoff (kPa) | 13.3 | 15 | 21.4 |
| Se (95% CI) | 0.34 (0.24–0.44) | 0.40 (0.26–0.54) | 0.40 (0.03–0.83) |
| TP/(TP + FN) | 29/(29 + 56) | 20/(20 + 30) | 2/(2 + 3) |
| Sp (95% CI) | 0.91 (0.83–0.99) | 0.90 (0.84–0.96) | 0.90 (0.85–0.85) |
| TN/(TN + FP) | 50/(50 + 5) | 81/(81 + 9) | 122/(122 + 13) |
| PPV (95% CI) | 0.85 (0.73–0.97) | 0.69 (0.52–0.86) | 0.13 (0.04–0.31) |
| NPV (95% CI) | 0.47 (0.38–0.57) | 0.73 (0.65–0.81) | 0.98 (0.95–1.00) |
| LR+ (95% CI) | 3.75 (1.55–9.11) | 4.00 (1.97–8.11) | 4.15 (1.26–13.67) |
| LR− (95% CI) | 0.72 (0.61–0.86) | 0.67 (0.53–0.84) | 0.66 (0.32–1.36) |
AUROC, area under the receiver operating characteristic curve; CI, confidence interval; FP, false positive; FN, false negative; LR+, positive likelihood ratio; LR−, negative likelihood ratio; LSM, liver stiffness measurement; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity; TP, true positive; TN, true negative.
Accuracy was highest at the F4 threshold, with an AUROC of 0.77 (95% CI 0.58–0.97), and Se of 0.80, Sp of 0.71 at a threshold of 11 kPa selected by maximizing the Youden index. Accuracy dropped at ≥F3 threshold with an AUROC of 0.71 (95% CI 0.62–0.80) and corresponding Se of 0.68 and Sp of 0.72 at a threshold of 9.4 kPa selected by maximizing the Youden index. At ≥F2 threshold, the AUROC was of 0.71 (95% CI 0.63–0.80), with the corresponding Se of 0.58 and Sp of 0.82 at the threshold of 9.4 kPa selected by maximizing the Youden index. AUROC for ≥F2, ≥F3, and F4 and box plots of LSM for each stage of hepatic fibrosis are shown in Figure 4.
Figure 4.
Receiver operating characteristic (ROC) curve of liver stiffness measurement (LSM) for identifying liver fibrosis ≥F2 (a), ≥F3 (b), and F4 (c). Box plot of a LSM vs fibrosis stage (Student-Newman-Keuls comparison showed that differences between F1 and F3, F1, and F4 were statistically significant) (d).
For determining the influence of ALT and TBIL on LSM diagnostic performance, patients were stratified by ALT values (≤1x ULN, >1xULN) and TBIL (≤1x ≤ ULN, >1xULN); however, results showed that stratification did not influence AUROC of LSM (see Supplementary Table 3, Supplementary Digital Content 3, http://links.lww.com/CTG/A528).
Performance of LSM was compared with other fibrosis biomarkers, including fibrosis-4 (FIB-4) (15), Hui (16), S index (17), Forns (18), AST to platelet ratio index (19), and NAFLD fibrosis score (20). Diagnostic performances in term of AUROC for each fibrosis stage (≥F2, ≥F3, and F4) were shown in Supplementary Table 4 (see Supplementary Digital Content 4, http://links.lww.com/CTG/A529). Although AUROC in each stage of LSM was numerically higher than that of the fibrosis biomarkers, this was not statistically significant.
Impact of prevalence of steatosis and fibrosis on predictive value of LSM and UAP
The impact of prevalence of steatosis and fibrosis on PPV and NPV values was determined by using a range of different pretest probability values (prevalence). Steatosis prevalence figures used the represented values from this cohort (59%, 35%, and 12% for ≥S1, ≥S2, and S3, respectively) and values seen in patient cohorts with type 2 diabetes mellitus and the general population (21,22). For diagnosis of ≥S1, ≥S2, and S3 in general population, there was a marked reduction in PPV because the prevalence of steatosis was lower. On the contrary, there was an increase in PPV in patients with diabetes because the prevalence of steatosis was high (see Supplementary Table 5, Supplementary Digital Content 5, http://links.lww.com/CTG/A530). It showed impact of prevalence of ≥S1, ≥S2, and S3 on PPV and NPV of UAP, and for the cutoff for Se = 0.90, cutoff for the Youden index, and cutoff for Sp = 0.90. The fibrosis prevalence figures used the represent values from this cohort (61%, 36%, and 4% for ≥F2, ≥F3, and F4, respectively) and values seen in cohorts of patients with type 2 diabetes mellitus, patients at risk of liver disease and the general population (21,23–25). For diagnosis of ≥F2, ≥F3, and F4 in the general population, there was a marked reduction in PPV because the prevalence of fibrosis in general population was low (see Supplementary Table 6, Supplementary Digital Content 6, http://links.lww.com/CTG/A531).
Influence of histological parameters on UAP and LSM
The relationship between liver histological parameters and liver steatosis/fibrosis in patients with NAFLD was assessed by analyzing the influence of histological parameters on UAP and LSM, including steatosis, balloon degeneration, lobular inflammation, and portal inflammation. Univariate analysis was performed by 1-way ANOVA. Multiple stepwise regression was used for selecting influencing factors to construct the model in which interface hepatitis and liver fibrosis were the candidate independent variables, and LSM and UAP were the result variables. Results of multiple stepwise regression showed that there was a positive correlation between fibrosis stage, NAS score, and LSM. UAP was associated with hepatic steatosis, and grade (S0–S3) of hepatic steatosis was positively correlated with UAP.
DISCUSSION
NAFLD is now the leading cause of CLD and is a major hazard to people's health (26). Hepatic steatosis and its extent are closely related to inflammatory damage and fibrosis and predict the risk of metabolic disease and type 2 diabetes mellitus (26). FibroScan (FS) and FT as noninvasive diagnostic techniques are used to assess the fibrosis and steatosis. In our study, FT was found to have high diagnostic performance, and the proportion of gray zone by using the dual cutoffs (cutoff for Se ≥0.90 and cutoff for Sp <0.90 determined in the present cohort) were 28%, 6%, and 8% for the diagnosis of steatosis ≥S1, ≥S2, and S3, respectively (see Supplementary Table 7, Supplementary Digital Content 7, http://links.lww.com/CTG/A532), suggesting FT could accurately distinguish fatty liver, especially moderate and severe fatty liver. Recently, Zhu et al. (27) reported the AUROCs of UAP by FT were 0.70–0.80 for assessing hepatic steatosis severity. The different thresholds may be attributed to study design, interobserver variability, and source of patients. As a single-center study, all patients in Zhu's study are from the same hospital, and all the diagnoses of liver biopsy samples are from only 1 liver pathologist, which may lead to a higher risk of bias. Moreover, all enrolled patients were suspected to have fatty liver disease, and less than 10% cases of S0 were found in Zhu's study; therefore, sampling errors may exist because of heterogeneous fatty liver. Our previous study shown that the liver-controlled attenuation parameter (CAP) value and UAP value in 1,621 subjects by the same examiner are consistent (r = 0.62, P < 0.01), and these 2 values are closely related and can be exchanged by formula: CAP value (FT) = 134.71 + 0.456 × CAP (FS) (28). A large sample study focusing on the performance of FS for patients with CLD from multiple centers worldwide (including China) showed that the cutoff values of CAP for diagnosis of ≥S1, ≥S2, and =S3 were 248, 268, and 280 dB/m (29). Many studies used CAP for detection of moderate-severe hepatic steatosis in patients with CLD (i.e., significant steatosis in Zhu's work), and the documented optimal cutoff values range from 255 to 299 dB/m (30), which are lower than the cutoff values from Zhu's data. Our study is a prospective randomized clinical study from 9 hospitals in different regions of China, and all the eligible pathological sections were blindly and independently assessed by 3 pathologists. The indications of liver biopsy in our study included not only suspected fatty liver but also other CLD. The ratio of ≥S1 to =S0 is 140:97 in our study, which is close to the real-world population distribution in clinical practice. Therefore, the optimal cutoff values for different degrees of steatosis in our study are more accurate and reliable.
FT has an ultrasound image probe, which can assist positioning during detection, avoiding interference factors such as intrahepatic cysts, nodules, and large blood and bile vessels, and reduces errors caused by blind measurement. Skin capsular distance can affect the accuracy in patients with NAFLD by FS (31,32). FT's proprietary algorithm includes the variable of BMI and the spectral analysis of ultrasonic echo signals in determining skin capsular distance. The diagnostic accuracy of FT in quantification of liver fat is more superior because the algorithm is capable of reducing the effect of subcutaneous fat on the UAP computation. In a cohort study, it was found that the success rate of FS detection was 94.96% vs 100% for FT (28). Serra's study showed diagnostic effect of FT was not as good as FS (33), and it may be due to the fewer practices of the operators and limited technical guidance received from the manufacturers. The CAP/UAP values such as HSI can also be applicable to supplement liver biopsy and imaging diagnosis of fatty liver. The comparison of diagnostic capacity of serological markers, such as HSI and UAP, showed the significant advantages of UAP in NAFLD detection, suggesting the higher value of UAP in NAFLD diagnosis.
The progression of liver steatosis and fibrosis is dynamic and is not always parallel. Data from last few years suggested an exponential increase in the risk of liver-related mortality in NAFLD as fibrosis progressed (1,34–36). A study on the medical costs of patients with NAFLD found that the average outpatient annual cost (adjusted for inflation) increased from $2,624 ± 3,308 in 2005 to $3,608 ± 5,132 in 2010 (37). The EASL-EASO-EASD 2016 (38) guidelines recommended using biomarkers (NAFLD fibrosis score, FIB-4) and transient elastography to screen patients with NAFLD for advanced fibrosis and cirrhosis. We used FT to detect the severity of fibrosis in patients with NAFLD. The AUROC in ≥F2, ≥F3, and =F4 was 0.71, 0.71, and 0.77, respectively, and those reported by Eddowes et al. (39) were 0.77, 0.80, and 0.89, respectively, and those reported by Siddiqui et al. (40) were 0.79, 0.83, and 0.93, respectively. Considering the possibility for inclusion of less severe degree of fibrosis, our trial was based on the maximum Yonden index cutoff value of 11 KPa, whereas the latter 2 reports were 13.6 and 13.1 KPa, respectively. The proportion of gray zone by using the dual cutoffs (cutoff or Se ≥0.90 and cutoff for Sp <0.90 determined in the present cohort) were 62%, 58%, and 20% for the diagnosis of fibrosis stage ≥F2, ≥F3, and F4, respectively (see Supplementary Table 8, Supplementary Digital Content 8, http://links.lww.com/CTG/A533), similar to Eddowes et al.'s results (39), suggesting a certain overlapping area for the diagnosis of significant and advanced fibrosis. The limitations of transient elastography include overlapping LSM range and false positivity affected by inflammation, nonfasting, exercise, hepatic venous congestion, alcohol excess, cholestasis, steatosis, and portal vein thrombosis (41).
The diagnosis accuracy of fibrosis by LSM is affected by different etiologies. Although in a study of patients with CLD (mainly HBV infection), the overall diagnostic accuracy of FT for significant fibrosis, advanced fibrosis, and cirrhosis was 73.3%, 83.2%, and 84.1%, respectively, and was equivalent to FS and superior to AST to platelet ratio index and FIB-4 (42). The previous study showed that FT had high AUROC value in detecting hepatitis B–related fibrosis (43). However, the AUROC value of NAFLD is lower and may need to be validated in other NAFLD clinical trials, such as diabetic patients or multiethnic cohorts. LSM was positively correlated with NAS score, which may be the factor that causes the low value of the AUROC. A more diverse approach to study the diagnostic performance of FT and threshold setting for NASH-related fibrosis staging might be required.
PPV and NPV were significantly affected by the prevalence. The effects of the changing of prevalence were demonstrated, and the cutoff value could be selected appropriately according to the clinical situation. A negative test will be very reassuring and will play an important role in screening and secondary prevention.
This is a large, multicenter study of FT for evaluating the detection of steatosis and fibrosis stage in patients with potential NAFLD, and for recommending the borderline values for clinical use, and calculating the PPV and NPV in combination with prevalence. The liver biopsy as the reference standard is normally limited by sampling error and by interobserver variability. To reduce these limitations, we excluded all patients with biopsies that did not meet specific quality criteria and all the sections were blindly and independently assessed by 3 experienced pathologists. Another limitation of our study is that there are very few patients in steatosis S3. When comparing ROC among groups, although the difference between the areas of ROC has reached the clinical significance, because of the small sample size, the difference did not reach the statistical significance.
In conclusion, our study has demonstrated that FT is highly applicable and has a low failure rate in patients with NAFLD. We also demonstrated that the UAP value would not be affected by other histological components; the extent of fibrosis and inflammation could affect the LSM results. The cutoff values of degree of hepatic steatosis and stage of fibrosis obtained in this study provided a critical FT reference value for the diagnosis and evaluation of patients with NAFLD. FT is convenient for clinical practice.
CONFLICTS OF INTEREST
Guarantors of the article: Lun-Gen Lu, MD, and Jian-Gao Fan, MD.
Specific author contributions: Ying Qu, MD, and Yan-Yan Song, PhD, contributed equally to this article. L.-G.L. and J.-G.F.: had the original concept and contributed to the design of the study protocol. C.-W.C., Q.-C.F., J.-P.S., Y.X., Q.X., Y.-F.Y., Y.-J.Z., L.-P.L., M.-Y.X., X.-B.C., and Q.-D.Z.: assisted recruiting sites. Y.Q. and Y.-Y.S.: performed the study and generated all data for the manuscript. Y.-Y.S. and H.Y.: performed the statistical analysis. Y.Q. and L.-G.L.: drafted the manuscript. All authors approved the final version.
Financial support: This study/project is supported by the National Science and Technology Major Project of China (2017ZX10202203-007-005) and the National Science and Technology Major Special Project for Drug Development (No.2018ZX09201016); FibroTouch-FT5000 of iLivTouch series are offered by Wuxi Hisky Medical Technologies, China. The views expressed are those of the authors.
Potential competing interests: None to report.
Trial registration: ClinicalTrials.gov NCT02456766.
Study Highlights.
WHAT IS KNOWN
✓ FibroTouch vibration-controlled transient elastography is an alternative of FibroScan.
✓ Ultrasound attenuation parameter and liver stiffness measurement by FibroTouch are widely used in China.
WHAT IS NEW HERE
✓ The success rate of FibroTouch is very high in patients with chronic liver disease.
✓ FibroTouch has good diagnostic performance for hepatic steatosis and fibrosis.
TRANSLATIONAL IMPACT
✓ Ultrasound attenuation parameter and liver stiffness measurement can be used to assess steatosis and fibrosis in clinical practice.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A526, http://links.lww.com/CTG/A527, http://links.lww.com/CTG/A528, http://links.lww.com/CTG/A529, http://links.lww.com/CTG/A530, http://links.lww.com/CTG/A531, http://links.lww.com/CTG/A532, http://links.lww.com/CTG/A533.
Contributor Information
Ying Qu, Email: levine4959@163.com.
Yan-Yan Song, Email: yanyansong@sjtu.edu.cn.
Cheng-Wei Chen, Email: ccw2@163.com.
Qing-Chun Fu, Email: qcfu85@163.com.
Jun-Ping Shi, Email: 20131004@hznu.edu.cn.
Yun Xu, Email: 1309946852@qq.com.
Qing Xie, Email: xieqingrjh@163.com.
Yong-Feng Yang, Email: yyf1997@163.com.
Yong-Jian Zhou, Email: eyzhouyongjian@scut.edu.cn.
Liang-Ping Li, Email: 18981838872@163.com.
Ming-Yi Xu, Email: xumingyi@hotmail.com.
Xiao-Bo Cai, Email: caixiaobo19790719@126.com.
Qi-Di Zhang, Email: zhangqidi157626@163.com.
Hao Yu, Email: njyuhao@vip.sina.com.
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 3.Hu X, Huang Y, Bao Z, et al. Prevalence and factors associated with nonalcoholic fatty liver disease in Shanghai work-units. BMC Gastroenterol 2012;12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol 2018;69:896–904. [DOI] [PubMed] [Google Scholar]
- 5.Chan WK, Treeprasertsuk S, Imajo K, et al. Clinical features and treatment of nonalcoholic fatty liver disease across the Asia Pacific region-the GO ASIA initiative. Aliment Pharmacol Ther 2018;47:816–25. [DOI] [PubMed] [Google Scholar]
- 6.Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: A systematic review and meta-analysis. Hepatology 2019;70:1119–33. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Muir AJ, Dieterich DT, et al. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology 2017;152:1544–77. [DOI] [PubMed] [Google Scholar]
- 8.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 10.Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology 1994;19:1513–20. [PubMed] [Google Scholar]
- 11.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828–35. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Zhai F, Cheng J, et al. Evaluating the significance of viscoelasticity in diagnosing early-stage liver fibrosis with transient elastography. PLoS One 2017;12:e0170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J 2005;47:458–72. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:503–8. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 16.Hui AY, Chan HL, Wong VW, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol 2005;100:616–23. [DOI] [PubMed] [Google Scholar]
- 17.Zhou K, Gao CF, Zhao YP, et al. Simpler score of routine laboratory tests predicts liver fibrosis in patients with chronic hepatitis B. J Gastroenterol Hepatol 2010;25:1569–77. [DOI] [PubMed] [Google Scholar]
- 18.Forns X, Ampurdanes S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986–92. [DOI] [PubMed] [Google Scholar]
- 19.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518–26. [DOI] [PubMed] [Google Scholar]
- 20.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–54. [DOI] [PubMed] [Google Scholar]
- 21.Kwok R, Choi KC, Wong GL, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: A prospective cohort study. Gut 2016;65:1359–68. [DOI] [PubMed] [Google Scholar]
- 22.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 23.You SC, Kim KJ, Kim SU, et al. Factors associated with significant liver fibrosis assessed using transient elastography in general population. World J Gastroenterol 2015;21:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong VW, Chu WC, Wong GL, et al. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: A population study using proton-magnetic resonance spectroscopy and transient elastography. Gut 2012;61:409–15. [DOI] [PubMed] [Google Scholar]
- 25.Harris R, Harman DJ, Card TR, et al. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: A systematic review. Lancet Gastroenterol Hepatol 2017;2:288–97. [DOI] [PubMed] [Google Scholar]
- 26.Fan JG, Wei L, Zhuang H, et al. Guidelines of prevention and treatment of nonalcoholic fatty liver disease (2018, China). J Dig Dis 2019;20:163–73. [DOI] [PubMed] [Google Scholar]
- 27.Zhu SH, Zheng KI, Hu DS, et al. Optimal thresholds for ultrasound attenuation parameter in the evaluation of hepatic steatosis severity: Evidence from a cohort of patients with biopsy-proven fatty liver disease. Eur J Gastroenterol Hepatol 2021;33:430–5. [DOI] [PubMed] [Google Scholar]
- 28.Zeng J, Sun WL, Chen GY, et al. Efficiency of FibroScan and FibroTouch in liver stiffness measurement and fat quantification: A comparative analysis [in Chinese]. Zhonghua Gan Zang Bing Za Zhi 2016;24:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- 30.Shen F, Fan JG. Editorial: Effect of hepatic steatosis on liver stiffness in patients with chronic hepatitis B-authors' reply. Aliment Pharmacol Ther 2019;50:334–5. [DOI] [PubMed] [Google Scholar]
- 31.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology 2012;55:199–208. [DOI] [PubMed] [Google Scholar]
- 32.Shen F, Zheng RD, Shi JP, et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int 2015;35:2392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serra JT, Mueller J, Teng H, et al. Prospective comparison of transient elastography using two different devices: Performance of FibroScan and FibroTouch. Hepat Med 2020;12:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPherson S, Hardy T, Henderson E, et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- 35.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Younossi ZM, Zheng L, Stepanova M, et al. Trends in outpatient resource utilizations and outcomes for Medicare beneficiaries with nonalcoholic fatty liver disease. J Clin Gastroenterol 2015;49:222–7. [DOI] [PubMed] [Google Scholar]
- 38.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 39.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156–63.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep 2020;2:100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Liu Y, Cao Z, et al. Comparison of FibroTouch and FibroScan for staging fibrosis in chronic liver disease: Single-center prospective study. Dig Liver Dis 2019;51:1323–9. [DOI] [PubMed] [Google Scholar]
- 43.Yang XZ, Gen AW, Xian JC, et al. Diagnostic value of various noninvasive indexes in the diagnosis of chronic hepatic fibrosis. Eur Rev Med Pharmacol Sci 2018;22:479–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.