Abstract
INTRODUCTION:
In Crohn's disease (CD), the assessment of transmural inflammation and fibrosis is of utmost importance. This study aimed to quantify these parameters in CD ileal specimens and correlate them with disease progression.
METHODS:
This is a retrospective unicentric study based on the analysis of archived specimens (n = 103) of primary ileal resection. Data were retrieved from a prospective national inflammatory bowel disease registry. Two pathologists, blinded for CD phenotype and clinical indications for surgery, examined 3 sections per patient and graded inflammation and fibrosis, based on a histopathological score.
RESULTS:
Penetrating (B3, n = 74) CD exhibited significantly higher inflammation in diseased areas, compared with stricturing (B2, n = 29) disease (score 3: 96% vs 76%, P = 0.005 in inflamed areas; 78% vs 55%, P = 0.019 in most affected areas). This was also observed for the comparison of B2 CD with B3 CD with (B3s, n = 54) and without associated stricture (B3o, n = 20): B3s vs B2: 81% vs 55%, P = 0.033 in most affected areas; B3o vs B2: 100% vs 76%, P = 0.006 in inflamed areas; 70% vs 55%, P = 0.039 in most affected areas. We could not show differences in fibrosis scores between the subphenotypes. Postoperative new penetrating events occurred only in B3s (n = 6, 11%, P = 0.043) patients. The changing of biologic therapy after surgery correlated with severe inflammation at the proximal ileal margin (55% changed vs 25% not changed, P = 0.035).
DISCUSSION:
In our cohort, fibrosis scores and fibromuscular changes were comparable, irrespective of CD phenotype. Inflammation severity was the major differentiator between penetrating and stricturing disease.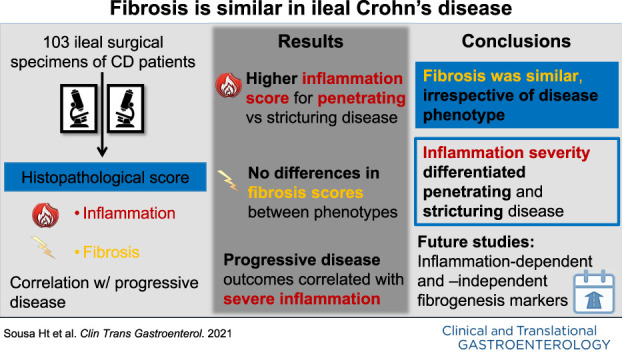
INTRODUCTION
Transmural inflammation and submucosal fibrosis are important hallmarks of Crohn's disease (CD) (1). Intestinal fibrosis concerns extracellular matrix accumulation and mesenchymal cell expansion (2,3). In this process, inflammation is the main activator of mesenchymal cells and an essential factor to initiate fibrogenesis. Still, once fibrosis is established, it may be self-propagating (3,4). In the setting of CD, patients with inflammatory lesions are considered medical therapy-responsive, while those with more fibrotic lesions will eventually need surgery (4). Hence, despite all the available therapies targeting inflammation, intestinal fibrosis remains difficult to treat and prevent (3,4).
Strictures are subdivided in fibrotic, inflammatory, and mixed forms (5). Pure fibrotic or inflammatory strictures are rare, with both components presenting overlapped histopathology (3,6–10). In CD, transmural intestinal inflammation can be assessed by cross-sectional imaging (2,11–16). On the other hand, fibrosis cannot be measured by this technique nor through biomarkers (16,17). Endoscopy or biopsy-based histology (2,11) is not feasible as tissue remodeling occurs mostly in deeper layers (18). Thus, the extent and severity of fibrosis must be evaluated by histopathological analysis of intestinal resection specimens, resorting to several histopathological scoring systems (19,20).
The main objective of our work was to characterize and quantify inflammation and fibrosis, in ileal CD resection specimens, according to a CD transmural histopathological scoring system. We also aimed to correlate inflammation and fibrosis profiles with progressive disease.
METHODS
Patients and study design
The patients included in this retrospective, single-center study were selected as depicted in Supplementary Figure (see Supplementary Digital Content 1, http://links.lww.com/CTG/A546). Patients were retrieved from the prospective database of the Portuguese Group for the Study of Inflammatory Bowel Disease (GEDII) (gediibasedados.med.up.pt), according to the following inclusion criteria: (i) definite diagnosis of CD with stricturing (B2) or penetrating (B3) phenotypes, according to Montreal criteria (21); (ii) emergent or elective ileal resection, due to CD complications, at São João University Hospital Center (CHUSJ), Porto, Portugal; and (iii) minimum postoperative follow-up of 3 years, up to January 2018.
Patients fulfilling the inclusion criteria were crossed with the digital archive of the CHUSJ Pathology Department, available since January 1998. Because of an overrepresentation of B3 phenotype with associated ileal stricture, a portion of this group was randomly (Excel's random numbers tool) excluded, to have more balanced subgroups.
Demographical, clinical, and surgical information was retrieved from the GEDII database up to September 2019. All missing data or discrepancies were obtained from clinical files. The first ileal resection was considered the index episode (e.g., index surgery). Medical therapy data were collected for the periods before and after the index surgery and after the first subsequent surgery.
Progressive disease
Progressive disease was defined as the occurrence of at least one of the postoperative outcomes described elsewhere (22). The period from index surgery to the occurrence of each outcome was recorded.
Histopathologic workout
Two pathologists (I.G. and C.C.), blinded for CD phenotype and indications for surgery, retrieved the formalin-fixed and paraffin-embedded (FFPE) blocks of the ileal resection surgical specimens. The macroscopic report, the gross picture of the specimen (when available), and the description of the location and/or lesion represented in each block were retrieved from the files of the Department of Pathology and evaluated jointly by both pathologists. On the basis of macroscopic grounds, 3 sections were selected from each specimen (Figure 1): (i) margin: proximal ileal margin; (ii) most affected: (a) narrowest caliber area of the ileal stricture, for specimens only with strictures; (b) most severely inflamed ileal area (irrespective of having an associated stricture or not), involved by fistulas, fissures, and/or deep ulcers (defined as penetrating beyond the submucosa (23)); and (iii) inflamed: (a) area of ileal stricture outside the narrowest caliber, for specimens only with strictures; (b) area of ileum with inflammatory changes outside the most inflamed area, for specimens bearing fistulas, fissures, and/or deep ulcers. If the 3 regions were not present in the macroscopic report/picture, or if the information about the exact location of each FFPE block was missing, the cases were excluded (see Supplementary Figure, Supplementary Digital Content 1, http://links.lww.com/CTG/A546). Moreover, all the layers of the ileal wall (mucosa, submucosa, muscularis propria, and serosa) were to be adequately represented and oriented on each slide.
Figure 1.
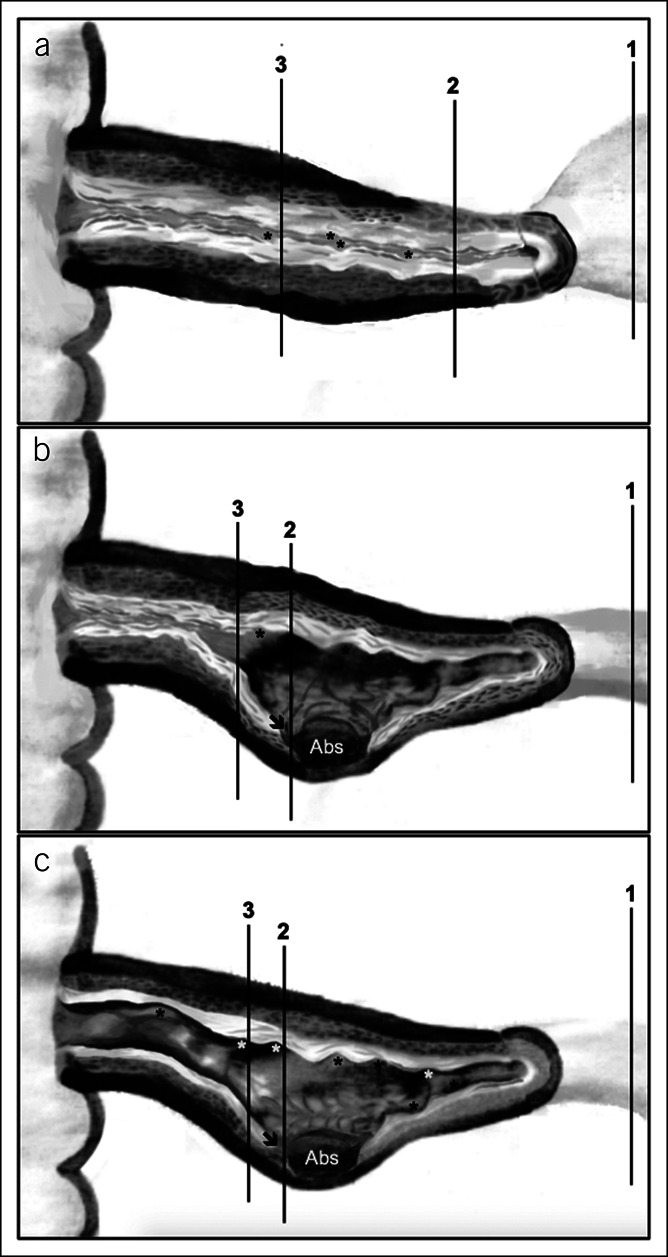
Schematic representation of anatomical locations of the 3 per-protocol sections for histopathological study, obtained from formalin-fixed and paraffin-embedded blocks of ileal resection surgical specimens (panels a to c). 1—Proximal ileal margin; 2—most affected area; and 3—inflamed area. (a) Schematic surgical specimen with strictures. (b) Schematic surgical specimen with fistulas, fissures, and/or deep ulcers and stricture. (c) Schematic surgical specimen with fistulas, fissures, and/or deep ulcers only. Abs—abscess; asterisks—superficial ulcers; arrow—deep ulcer (beyond submucosa). 1Inflammation (1–3) and fibrosis (0–2) scoring: Higher scores indicate more severe inflammation and fibrosis, respectively (23).
After a pre-evaluation of the slides to confirm the adequacy of the specimens for the study, the pathologists graded inflammation and fibrosis according to a previously described CD transmural histopathological score (23) (Table 1). Final scores were obtained by consensus. The evaluation of inflammation variables was mostly based on the histopathological analysis of hematoxylin and eosin–stained slides. Macroscopic report and pictures of the surgical specimens were considered for information on ulceration extent. Fibrosis variables were assessed on hematoxylin and eosin– and Masson trichrome–stained slides; grossing reports provided information on stricture diameter.
Table 1.
Crohn's disease transmural histopathological score (23)
| Score | Grade | Features | Score |
| Inflammation | Mild | Aphthous ulcers affected surface <50%; cryptitis <50%; inflammation limited to mucosa | 1 |
| Moderate | Large, superficial ulcers (0.5–2 cm) Ulcerated surface <50%; affected surface 50%–100% Cryptitis >50%; crypt abscesses; submucosal inflammation |
2 | |
| Severe | Deepa ulcers or ulcers >2 cm in size; circumferential ulcers; deepa fissures; transmural inflammation | 3 | |
| Fibrosis | None | None or minimal fibrosis limited to submucosa (<25% thickness) | 0 |
| Mild/moderate | Mild stricture (>15 mm) with nondilated lumen Submucosal fibrosis and muscular hyperplasia >25%b with preserved layers |
1 | |
| Severe | Massive transmural fibrosis; effacement of normal layers; severe stricture | 2 |
Deep—beyond the submucosa.
Includes muscular propria hyperplasia >25%, muscularis mucosae hyperplasia >25%, and/or splayed muscularis mucosae (without cellular hyperplasia) >25%.
As the adopted score does not specify to which layer the term “muscular hyperplasia >25%” stands for, the feature was considered to be present if found either in muscularis propria or in muscularis mucosae (MM). However, in our study, MM expansion included either true cellular hyperplasia of smooth muscle cell and/or a fibrosis-splayed layer (24). The presence of smooth muscle or adipose tissue in the submucosal layer was also evaluated. A schematic representation of the main fibromuscular changes in ileal intestinal wall in CD is presented in the Supplementary Figure (see Supplementary Digital Content 2, http://links.lww.com/CTG/A547).
As the selected histopathological score does not include a 0 (zero) score for grading inflammation, cases with absence of inflammation were signaled for descriptive purposes but excluded from correlation analyses.
Statistical considerations
Categorical variables were summarized through absolute (n) and relative (%) frequencies. Continuous variables were described as mean ± SD or median (interquartile range), minimum, and maximum. Hypotheses on the distribution of continuous variables were tested using the t test and the nonparametric Mann-Whitney and Kruskal-Wallis tests. Associations between categorical and continuous variables were tested through χ2 and Spearman correlation tests, respectively. For multiple comparison, Bonferroni correction was applied. IBM SPSS Statistics for Mac, Version 24.0 (IBM, Armonk, NY) was used to perform statistical analyses, adopting a 5% significance level.
Ethical considerations
Our study was exempt of patients' informed consent because of its retrospective nature based on archived pathological material. However, all patients gave consent for the collection of data from the GEDII database, which was endorsed by the Portuguese Data Protection Committee, authorization number 2868/2013. The study protocol conforms to the ethical guidelines of Declaration of Helsinki and was approved by the CHUSJ Ethic Committee on July 2018. Confidentiality of data was ensured.
RESULTS
Study population
From a total of 103 patients, 29 were diagnosed with B2 CD (stricturing disease) and 74 with B3 CD (penetrating disease). In the B3 subgroup, 54 patients had at least 1 associated stricture (B3s), while 20 had not (B3o). At diagnosis, B2 patients were, on average, older than B3 ones (mean age: 35 vs 28 years old, P = 0.001; age over 40 years old: 38% in B2 vs 15% in B3, P = 0.021). Regardless isolated ileal location predominating in both phenotypes, B2 CD affected more frequently the ileojejunal area when compared with B3 CD (B2 21% vs B3 3%, P = 0.002). By contrast, B3 CD involved more frequently the ileocolonic area, when compared with B3 CD (B3 39% vs B2 21%, P = 0.002) (Table 2).
Table 2.
Demographic, clinical, and surgery-related variables, per phenotype
| Demographical and clinical variables | Total | Phenotype at end of follow-up | |||
| B2 (n = 29, 28%) | B3 (n = 74, 72%) | Pa | |||
| Gender, n (%) | 0.390 | ||||
| Female | 46 (45) | 11 (38) | 35 (47) | ||
| Male | 57 (55) | 18 (62) | 39 (53) | ||
| Age at diagnosis, n (%) | 0.021 | ||||
| A1: ≤16 yr old | 11 (11) | 1 (3) | 10 (14) | ||
| A2: 17–40 yr old | 70 (68) | 17 (59) | 53 (72) | ||
| A3: >40 yr old | 22 (21) | 11 (38) | 11 (15) | ||
| Phenotype at diagnosis, n (%) | <0.001 | ||||
| B1: nonstricturing, nonpenetrating | 10 (10) | 1 (3) | 9 (12) | ||
| B2: stricturing | 37 (36) | 28 (97) | 9 (12) | ||
| B3: penetrating | 56 (54) | 0 (0) | 56 (76) | ||
| CD localization, n (%) | 0.002 | ||||
| L1 | 58 (56) | 18 (62) | 40 (54) | ||
| L1+L4 | 8 (8) | 6 (21) | 2 (3) | ||
| L3 | 32 (31) | 3 (10) | 29 (39) | ||
| L3+L4 | 5 (5) | 2 (7) | 3 (4) | ||
| Perianal disease, n (%) | 25 (24) | 5 (17) | 20 (27) | 0.324 | |
| Age at diagnosis, yr, mean (SD) | 30 (12) | 35 (13) | 28 (10) | 0.001b | |
| Total follow-up, yr, median (P25–P75) | 10 (7–12) | 12 (7–14) | 10 (7–12) | 0.024c | |
| Surgery-related variables | |||||
| Motif for first ileal surgery, n (%) | <0.001 | ||||
| Fistula/abscess | 66 (64) | 0 (0) | 66 (89) | ||
| Perforation | 5 (5) | 0 (0) | 5 (7) | ||
| Obstruction | 32 (31) | 29 (100) | 3 (4) | ||
| First ileal surgery, n (%) | >0.999 | ||||
| Segmental enterectomy | 7 (7) | 2 (7) | 5 (7) | ||
| Ileocecal resection | 86 (83) | 24 (83) | 62 (84) | ||
| Right hemicolectomy | 10 (10) | 3 (10) | 7 (9) | ||
| Motif of reoperation, n (%) | 0.050 | ||||
| Abscess | 3 (30) | 0 (0) | 3 (42) | ||
| Stricture (primary) | 4 (40) | 3 (100) | 1 (14) | ||
| Stricture (anastomotic) | 3 (30) | 0 (0) | 3 (43) | ||
| Preoperative therapy, n (%) | |||||
| 5-aminosalicylic acid | 49 (48) | 15 (52) | 34 (46) | 0.664 | |
| Steroids | 70 (68) | 23 (79) | 47 (64) | 0.122 | |
| Immunosuppressives | 60 (58) | 16 (55) | 44 (60) | 0.692 | |
| Anti-tumor necrosis factor alphad | 27 (26) | 6 (21) | 21 (28) | 0.468 | |
| Postoperative therapy, n (%) | |||||
| 5-aminosalicylic acid | 31 (30) | 11 (38) | 20 (27) | 0.278 | |
| Steroids | 32 (31) | 14 (48) | 18 (24) | 0.018 | |
| Immunosuppressives | 90 (87) | 27 (93) | 63 (85) | 0.342 | |
| Anti-tumor necrosis factor alphae | 61 (59) | 18 (62) | 43 (58) | 0.713 | |
| Other biologicsf | 11 (11) | 4 (14) | 7 (10) | 0.724 | |
| Post-re-operative therapy (n = 10), n (%) | |||||
| 5-aminosalicylic acid | 2 (20) | 1 (33) | 1 (14) | >0.999 | |
| Immunosuppressives | 7 (70) | 3 (100) | 4 (57) | 0.475 | |
| Anti-tumor necrosis factor alphag | 8 (80) | 2 (67) | 6 (86) | >0.999 | |
| Other biologicsh | 1 (10) | 1 (33) | 0 (0) | 0.300 | |
| Age at index surgery, yr, mean (SD) | 34 (13) | 40 (15) | 32 (11) | 0.008b | |
| Time from diagnosis to index surgery, yr, median (P25–P75) | 2.0 (0.5–6.0) | 3.0 (1.0–6.0) | 2.0 (0.5–6.0) | 0.273c | |
Bold entries indicate significant P values (P < 0.05).
CD, Crohn's disease; L1, terminal ileum; L1+L4, terminal ileum + upper gastrointestinal tract; L3, ileum and colon; L3+L4, ileocolonic + upper gastrointestinal tract.
χ2 test.
t test for independent samples.
Mann-Whitney test.
Infliximab (n = 22) and adalimumab (n = 2).
Infliximab (n = 51) and adalimumab (n = 10).
Vedolizumab (n = 7) and ustekinumab (n = 4).
Infliximab (n = 7) and adalimumab (n = 1).
Vedolizumab (n = 1).
Surgery-related variables and disease outcomes
The most common indication for first ileal surgery was fistula/abscess (64%); ileal resection was performed in 83% of patients. B2 patients were, on average, older at the moment of index surgery (mean age: 40 vs 32 years old, P = 0.008) (Table 2). After surgery, more B2 patients were treated with steroids than B3 ones (48% vs 24%, P = 0.018), with no differences in the number of steroid courses needed or time from surgery to the first course (Tables 2 and 3). The proportion of patients who started immunosuppressives after surgery was similar between phenotypes (41% in B2, 48% in B3). However, these were started significantly earlier in B2 patients (median: 5.8 vs 0.5 years, P = 0.028). Most patients (63%) started (39%) or changed (24%) biologic therapy (BT) (Table 3). Disease progression occurred in 75 (73%) patients, and 10 (10%) patients were reoperated at least once during follow-up, after a median period of 6.7 years (1.8–10.4). Postoperative stricturing events were reported in 23 (22%) patients. Time from index surgery to each outcome, i.e., time-to-event analysis, is displayed through Kaplan-Meier curves (see Supplementary Figure, Supplementary Digital Content 3, http://links.lww.com/CTG/A548).
Table 3.
Postoperative outcomes variables, per phenotype
| Total (n = 103) | B2 (n = 29, 28%) | B3 (n = 74, 72%) | Pa | |
| Reoperation, n (%) | 10 (10) | 3 (10) | 7 (10) | >0.999a |
| Time from index to subsequent surgery, yr, median (P25–P75)b | 6.7 (1.8–10.4) | 6.0 (1.2–7.5) | 7.8 (2.0–11.5) | 0.425c |
| Hospitalization, n (%) | 30 (29) | 10 (35) | 20 (27) | 0.454a |
| Time from index to subsequent hospitalization, yr, median (P25–P75)b | 2.0 (0.8–3.5) | 7.8 (1.2–3.0) | 2.0 (0.7–4.2) | 0.947c |
| Steroids, n (%) | 32 (31) | 14 (48) | 18 (24) | 0.018a |
| No. of postoperative steroid courses, median (min–max) | 1 (1–2) | 1 (1–4) | 1 (1–2) | 0.185c |
| Time from index surgery to first steroid course, yr, median (P25–P75)b | 2.5 (0.5–5.1) | 3.5 (0.8–7.8) | 2.3 (0.2–3.5) | 0.171c |
| Immunosuppressive (IS), n (%) | ||||
| Start IS, n (%) | 40 (39) | 12 (41) | 28 (38) | 0.740a |
| Time from index surgery to IS, yr, median (P25–P75)b | 1.0 (0.1–5.3) | 5.8 (0.6–7.7) | 0.5 (0.1–3.3) | 0.028c |
| Change IS, n (%) | 7 (7) | 2 (7) | 5 (7) | >0.999a |
| Time from index surgery to change IS, yr, median (P25–P75)b | 3.6 (1.5–4.2) | 4.3 (4.0–4.5) | 2.0 (1.5–3.6) | 0.121c |
| BT, n (%) | ||||
| Start BT, n (%) | 40 (39) | 14 (48) | 26 (35) | 0.218a |
| Time from index surgery to BT, yr, median (P25–P75)b | 5.7 (2.1–8.5) | 8.6 (1.5–12.0) | 5.3 (2.5–7.5) | 0.187c |
| Change BT, n (%) | 25 (24) | 7 (24) | 18 (24) | 0.984a |
| Time from index surgery to change BT, yr, median (P25-P75)b | 4.0 (2.4–8.7) | 8.5 (4.0–13.2) | 4 (1.5–7.6) | 0.079c |
| New event, n (%) | ||||
| Stricturing, n (%) | 23 (22) | 10 (35) | 13 (18) | 0.072a |
| Time from index surgery to new stricturing, yr, median (P25–P75)b | 3.0 (1.0–6.0) | 4.2 (2.5–7.2) | 2.2 (1.0–5.0) | >0.999c |
| Penetrating, n (%) | 6 (6) | 0 (0) | 6 (8) | 0.181a |
| Time from index surgery to new penetrating, yr, median (P25–P75)b | 2.2 (0.6–3.5) | — | 2.2 (0.6–3.5) | — |
| Perianal, n (%) | 2 (2) | 1 (3) | 1 (1) | >0.999a |
| Time from index surgery to new perianal, yr, median (P25–P50)b | 2.2 (2.0–2.5) | 2.5 (2.5–2.5) | 2.0 (2.0–2.0) | >0.999a |
| Progressive disease, n (%) | 75 (73) | 23 (79) | 52 (70) | 0.354a |
Bold entries indicate significant P values (P < 0.05).
BT, biologic therapy; IS, immunosuppressive.
χ2 test.
Time from index surgery to each outcome is considered only for patients presenting the outcome.
Mann-Whitney test.
Histopathological scoring according to section location
Overall, 10 patients (10%) showed no signs of inflammation on proximal ileal margins, and 74 (72%) showed a score of 3 in most affected regions. Histopathological scoring person can be found in Supplementary Table (see Supplementary Digital Content 4, http://links.lww.com/CTG/A549).
When comparing proximal ileal margins with inflamed areas, the inflammation scores increased in 70 (68%) patients, while fibrosis scores did not change (n = 65; 63%). Regarding inflamed and most affected areas, both inflammation and fibrosis scores remained unchanged (n = 100; 97% and n = 98; 95%, respectively). Histopathological scoring variation according to section location can be seen in Supplementary Table (see Supplementary Digital Content 5, http://links.lww.com/CTG/A550). Our study also evaluated the correlation between inflammation and fibrosis scores (see Supplementary Table, Supplementary Digital Content 6, http://links.lww.com/CTG/A551), which was weak and only in inflamed areas (r = 0.198, P = 0.045).
Histopathological scoring in B2 and B3 CD
B2 vs B3 phenotypes.
Three (15%) B2 patients and 7 (10%) B3 patients had no signs of histological inflammation in proximal ileal margins (data not shown). B3 patients had significantly higher inflammation score than B2 patients in inflamed and most affected areas (score 3: inflamed: 96% vs 76%, P = 0.005; most affected: 78% vs 55%, P = 0.019) (Figure 2a, 2b). This tendency was also observed for the total score, in both regions (score 4–5: inflamed: 93% vs 72%, P = 0.008; most affected: 79% vs 55%, P = 0.043). In terms of fibrosis, no significant differences were observed between the 2 phenotypes, in the 3 studied areas (Table 4; Figure 2c,d).
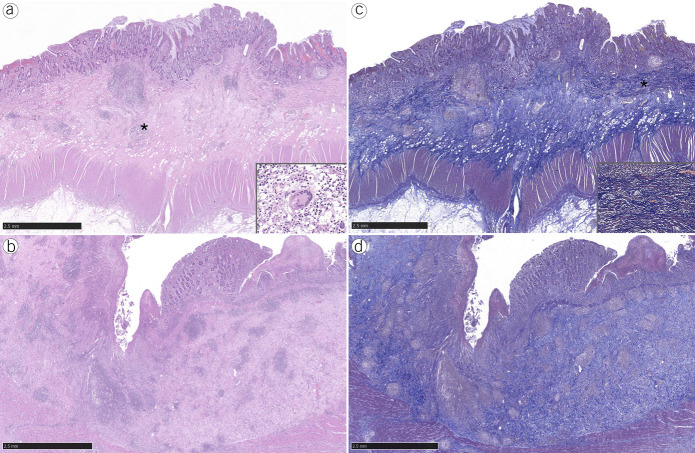
Figure 2.
Ileal resections from B2 patients (a, hematoxylin and eosin [H&E]) showed lesser inflammation when compared with B3 patients (b, H&E; large superficial ulcer). Both specimens from B2 (c, Masson trichrome [MT]) and B3 (d, MT) patients showed prominent fibrosis. Granulomatous inflammation with giant cell (a, asterisk and inset). Hyperplastic and splayed muscularis mucosae, dissected by fibrosis (c, asterisk and inset).
Table 4.
Histopathological scoring per section in stricturing (B2) and penetrating (B3) Crohn's disease
| B2, n (%) | B3, n (%) | Pa | |
| Margins | |||
| Inflammation | 0.094 | ||
| 1–2 | 21 (81) | 42 (64) | |
| 3 | 5 (19) | 24 (36) | |
| Fibrosis | 0.233 | ||
| 0 | 3 (10) | 15 (20) | |
| 1 | 26 (90) | 59 (80) | |
| 2 | 0 (0) | 0 (0) | |
| Total score | 0.061 | ||
| ≤2 | 20 (69) | 30 (43) | |
| 3 | 4 (14) | 18 (26) | |
| 4–5 | 5 (17) | 22 (31) | |
| Inflamed | |||
| Inflammation | 0.005 | ||
| 1–2 | 7 (24) | 3 (4) | |
| 3 | 22 (76) | 71 (96) | |
| Fibrosis | 0.521 | ||
| 0 | 2 (7) | 2 (3) | |
| 1 | 22 (76) | 53 (72) | |
| 2 | 5 (17) | 19 (26) | |
| Total score | 0.008 | ||
| ≤2 | 1 (4) | 0 (0) | |
| 3 | 7 (24) | 5 (7) | |
| 4–5 | 21 (72) | 69 (93) | |
| Most affected | |||
| Inflammation | 0.019 | ||
| 1–2 | 13 (45) | 16 (22) | |
| 3 | 16 (55) | 58 (78) | |
| Fibrosis | 0.774 | ||
| 0 | 0 (0) | 1 (1) | |
| 1 | 26 (90) | 68 (92) | |
| 2 | 3 (10) | 5 (7) | |
| Total score | 0.043 | ||
| ≤2 | 5 (17) | 4 (5) | |
| 3 | 8 (28) | 12 (16) | |
| 4–5 | 16 (55) | 58 (79) |
Inflammation (1–3) and fibrosis (0–2) scoring: Higher scores indicate more severe inflammation and fibrosis, respectively (23).
Bold entries indicate significant P values (P < 0.05).
χ2 test.
B2 phenotype vs B3s and B3o subphenotypes.
Three (15%) B2, 2 (10%) B3o, and 5 (9%) B3s patients did not present inflammation in proximal ileal margins (data not shown). Results for inflammation, fibrosis, and total scores for the B2 phenotype and B3o and B3s penetrating subphenotypes are listed in the Supplementary Data (Table 5 and see Supplementary Figure, Supplementary Digital Content 7, http://links.lww.com/CTG/A552).
Table 5.
Histopathological scoring per section and Crohn's disease phenotype: stricturing (B2) vs penetrating without associated stricture (B3o) vs penetrating with associated stricture (B3s)
| B2 (n = 29), n (%) | B3s (n = 54), n (%) | B3o (n = 20), n (%) | Pa | Pb | Pc | |
| Margins | ||||||
| Inflammationd | >0.999 | 0.015 | 0.044 | |||
| 1–2 | 21 (81) | 35 (71) | 7 (39) | |||
| 3 | 5 (19) | 14 (29) | 11 (61) | |||
| Fibrosis | 0.303 | >0.999 | 0.645 | |||
| 0 | 3 (10) | 13 (24) | 2 (10) | |||
| 1 | 26 (90) | 41 (76) | 18 (90) | |||
| Total scored | 0.627 | 0.081 | 0.792 | |||
| ≤2 | 17 (65) | 22 (50) | 5 (28) | |||
| 3 | 4 (15) | 15 (28) | 3 (17) | |||
| 4–5 | 5 (19) | 12 (22) | 10 (56) | |||
| Inflamed | ||||||
| Inflammation | 0.067 | 0.006 | >0.999 | |||
| 1–2 | 7 (24) | 3 (6) | 0 (0) | |||
| 3 | 22 (76) | 51 (94) | 20 (100) | |||
| Fibrosis | 0.234 | >0.999 | 0.108 | |||
| 0 | 2 (7) | 0 (0) | 2 (10) | |||
| 1 | 22 (76) | 38 (70) | 15 (75) | |||
| 2 | 5 (17) | 16 (30) | 3 (15) | |||
| Total score | 0.024 | >0.999 | >0.999 | |||
| ≤2 | 1 (4) | 0 (0) | 0 (0) | |||
| 3 | 7 (24) | 3 (6) | 2 (10) | |||
| 4–5 | 21 (72) | 51 (94) | 18 (90) | |||
| Most affected | ||||||
| Inflammation | 0.033 | 0.039 | 0.688 | |||
| 1–2 | 13 (45) | 10 (19) | 6 (30) | |||
| 3 | 16 (55) | 44 (81) | 14 (70) | |||
| Fibrosis | >0.999 | >0.999 | >0.999 | |||
| 0 | 0 (0) | 0 (0) | 1 (5) | |||
| 1 | 26 (90) | 50 (93) | 18 (90) | |||
| 2 | 3 (10) | 4 (7) | 1 (5) | |||
| Total score | 0.060 | >0.999 | >0.999 | |||
| ≤2 | 5 (17) | 2 (4) | 2 (10) | |||
| 3 | 8 (28) | 8 (15) | 4 (20) | |||
| 4–5 | 16 (55) | 44 (81) | 14 (70) |
Bold entries indicate significant P values (P < 0.05).
Inflammation (1–3) and fibrosis (0–2) scoring: Higher scores indicate more severe inflammation and fibrosis, respectively (23).
P value for B2 vs B3s with Bonferroni correction.
P value for B2 vs B3o with Bonferroni correction.
P value for B3o vs B3s with Bonferroni correction.
Does not include patients without inflammation (n = 93).
When compared with B2 patients, B3s patients presented with significantly higher inflammation score in most affected areas (inflammation score 3: 81% vs 55%, P = 0.033), whereas total score was significantly higher in inflamed areas (total score 4–5: 94% vs 72%, P = 0.024). Regarding fibrosis score, we could not find differences between the 2 groups.
The comparison between B2 and B3o patients showed that B3o patients had significantly higher inflammation scores in all 3 studied areas (score 3: proximal ileal margins: 61% vs 19%, P = 0.015; inflamed areas: 100% vs 76%, P = 0.006; most affected areas: 70% vs 55%, P = 0.039). There were no differences between the 2 groups, in terms of fibrosis and total score.
B3o patients presented significantly higher inflammation score than B3s patients at the proximal ileal margins only (score 3: 61% vs 29%, P = 0.044). Regarding fibrosis and total score, no differences were found between the 2 subphenotypes, in all areas.
The study of the contribution of individual histological features to all CD subphenotypes showed that transmural inflammation was significantly more frequent in proximal ileal margins of B3o patients (59% B3o vs 26% B3s vs 17% B2, P = 0.013), while MM splay >25% (Figure 2c) was significantly less frequent in inflamed areas (80% B3o vs 98% B3s vs 93% B2, P = 0.020). No differences between subphenotypes were found for all the other histological variables (see Supplementary Table, Supplementary Digital Content 8, http://links.lww.com/CTG/A553, which presents the association of all selected histological features per CD (subphenotype). Also, we could not evidence differences between the histopathological scores of patients with and without submucosal adipose or smooth muscle tissue. The 2 histological variables according to histopathological scoring and section location can be found in the Supplementary Data (see Supplementary Table, Supplementary Digital Content 9, http://links.lww.com/CTG/A554).
Progressive disease outcomes
Severe inflammation at proximal ileal margins was associated with postoperative change of BT (score 3: 55% changed BT vs 25% not changed BT, P = 0.035). No differences were found between histopathological scores for the other outcomes. Also, we could not establish associations between histology and postoperative outcomes (data not shown). Postoperative outcomes in the 3 CD subphenotypes are depicted in Supplementary Table (see Supplementary Digital Content 10, http://links.lww.com/CTG/A555). New penetrating events, after the index surgery, occurred exclusively in B3s patients (n = 6, 11%, P = 0.043).
DISCUSSION
In this study, we quantified and characterized inflammation and fibromuscular changes in ileal CD resection specimens according to a CD histopathological score. We confirmed pure fibrotic disease may not exist as, in most patients, both components overlapped on histopathology, irrespective of disease phenotype (25,26). Importantly, our study evidenced that the major differentiator between penetrating and stricturing disease was the degree of inflammation. Patients with penetrating disease both with (B3s) or without (B3o) associated stricture exhibited higher inflammation scores in diseased areas than pure stricturing (B2) patients, with no differences in fibrosis scores. Yet, when comparing penetrating subphenotypes, B3o patients showed a significantly higher inflammation score at the proximal ileal margin only.
Penetrating disease is believed to coexist with strictures (4). Fistula formation may be guided by both intraluminal pressure and transmural inflammation-induced changes (27). This hypothesis is supported by the higher inflammation grades observed in B3s patients when compared with B2 patients and, although uncommon, by new postoperative penetrating events occurring in B3s patients.
Regarding B2 phenotype, we found inflammation and fibrosis overlap (3,6–10) in most patients without purely fibrotic stricture in all studied areas. However, we found strictures without fibrosis in inflamed areas (n = 2 of 29 patients). These might correspond to pure inflammatory strictures because inflammation seems to be required to initiate fibrogenesis (3,4).
Overall, grade 1 fibrosis was more frequent than grade 2, demonstrating the importance of submucosal fibrosis and muscular expansion not only in stricturing (1,28), but also in penetrating disease. A deeper analysis evidenced that B3o patients presented MM splay with significantly less frequency in inflamed areas. The absence of differences in other fibromuscular variables between groups suggests that these changes are important irrespective of phenotype.
The presence of adipose tissue in the submucosa could represent a potential surrogate of adjacent nonresected creeping fat (29), which was shown to correlate with chronic inflammation (30), muscle hypertrophy, fibrosis, and strictures (31,32). In our study, adipose tissue in the submucosa was more frequent in cases of higher inflammation (score 3) and of mild to moderate fibroses (score 1). However, no differences were found between CD subphenotypes, which may be due to the reduced number of patients in this subgroup.
Our secondary aim was to correlate histopathological profiles with progressive disease. New penetrating events occurred exclusively in B3s patients. Also, postoperative need of changing BT correlated with severe inflammation at the proximal ileal margin irrespective of CD phenotype. Although the literature shows that microscopic inflammation in resection margins does not affect recurrence rates (33,34), our study suggests that severe inflammation in this area may represent a red flag for nonresponse to an ongoing biologic at the time of surgery. CD subphenotypes, histopathological cores, or variables did not correlate with the other postoperative outcomes. Although most patients (73%) presented progressive disease, the 10-year reoperation rate (10%) was lower than previously reported (33%–39%) (35–37). However, relevant methodological differences hinder direct comparisons as our study is based solely on B2 and B3 surgical specimens.
This study has some limitations. First, its retrospective and single-center study design led to many case exclusions, resulting in a small B2 patients' group and somewhat underpowered the study. To avoid potential statistical constraints, we used Bonferroni correction to preserve statistical significance regardless of subgroup size. Second, the use of archived FFPE blocks could decrease the reliability of the histopathological analyses, which may be also affected by sampling error in the choice of tissue location. To overcome this limitation, we performed a double, independent, blinded pathological assessment and resorted to a systematic inflammation and fibrosis grading based on a histopathological score (23). Moreover, we choose 3 different sections per specimen to mitigate sampling error and selection biases. Third, although this score showed high methodological quality and adequate properties (20), it was not validated and no validated scores exist for this purpose. Finally, the exclusion of cases with no inflammation in the proximal ileal resection margin could potentially introduce a bias in the analysis. However, this was not the case because the subgroup analyses including these 10 cases showed results consistent with those presented herein with no difference between phenotypes.
The strengths of this study rely on the large number of included patients and on the careful and well-defined histopathological exercise, designed to obviate the above-mentioned limitations.
In conclusion, our study innovatively demonstrated that the major differentiator between penetrating and stricturing disease was severity of inflammation because no differences were observed both in fibrosis scores and most of fibromuscular variables. We confirmed that pure fibrotic CD may not exist, with inflammation and fibromuscular changes overlapping in most patients irrespective of disease phenotype. Absence of inflammation was seldom found and only at the proximal ileal surgical margin. Thus, we herein propose that the designation “fibrostenosing disease” as a synonym for stricturing disease should be abandoned. In fact, CD should again be regarded as a mixture of inflammatory and fibromuscular changes irrespective of the phenotype, bearing in mind that higher degrees of inflammation are characteristic (but not exclusive) of a penetrating behavior. The focus of future studies should be on identification and therapeutic targeting of markers of inflammation-dependent and -independent fibrogenesis in view of preventing progression for both advanced phenotypes of CD.
CONFLICTS OF INTEREST
Guarantor of the article: Fernando Magro, MD, PhD.
Specific author contributions: H.T.S. was involved in conception and design of the study, acquisition, analysis, and interpretation of data, and was responsible for manuscript drafting. I.G. was involved in study design, acquisition and interpretation of histopathology data, histopathology image selection, manuscript drafting, and critical revision of the manuscript. C.C. was involved in study design, acquisition and interpretation of histopathology data, and critical revision of the manuscript. C.C.D. was involved in statistical analysis, interpretation of data, manuscript drafting, and critical revision of the manuscript. F.C. was involved in study design, interpretation of histopathology data, and critical revision of the manuscript for important intellectual content. F.M. was involved in conception and design of the study, interpretation of data, manuscript drafting, and critical revision of the manuscript for important intellectual content. All authors revised and approved the final manuscript for submission. All authors agreed on the accountability of all aspects of the work, thereby ensuring the accuracy and integrity of all parts.
Financial support: This study was funded an Investigation Scholarship from the Portuguese Group for the Study of IBD (GEDII—Grupo de Estudo de Doenças Inflamatórias Intestinais) received by HTS in 2016. Manuscript writing assistance was performed by PMA—Pharmaceutical Medicine Academy, which was supported by the above mentioned 2016 GEDII Investigation Scholarship.
Potential competing interests: H. Tavares de Sousa received a fee for presenting from Takeda, AbbVie, Janssen, and Pfizer. F. Magro received a fee for presenting from AbbVie, Ferring, Falk Pharma, Hospira, Pharmakern, MSD, Shering, Lab. Vitoria, Vifor, OmPharma, Janssen, Takeda, and Pfizer. F. Rieder on the advisory board or consultant for Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene/BMS, CDISC, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Origo, Pfizer, Pliant, Prometheus, Biosciences, Receptos, RedX, Roche, Samsung, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB. All other authors have no conflicts of interest to declare.
Study Highlights.
WHAT IS KNOWN
✓ The assessment of transmural intestinal fibrosis in Crohn's disease (CD) relies on surgical specimens' pathology.
✓ Inflammation and fibrosis can be quantified through a transmural histopathological score.
✓ Separate inflammation and fibrosis quantification in penetrating and stricturing CD has not been explored.
WHAT IS NEW HERE
✓ Fibrosis scores and fibromuscular changes were comparable, irrespective of CD phenotype.
✓ The major differentiator between penetrating and stricturing disease was the degree of inflammation.
TRANSLATIONAL IMPACT
✓ Therapeutic targeting of markers of inflammation-dependent and -independent fibrogenesis could prevent progression of disease both for stricturing and penetrating phenotypes of CD.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to acknowledge GEDII for their support and funding, to Maria Teresa Brito for her elegant schemes and to Paula Pinto, PharmD, PhD, from PMA—Pharmaceutical Medicine Academy for the manuscript writing assistance.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A546; http://links.lww.com/CTG/A547; http://links.lww.com/CTG/A548; http://links.lww.com/CTG/A549; http://links.lww.com/CTG/A550; http://links.lww.com/CTG/A551; http://links.lww.com/CTG/A552; http://links.lww.com/CTG/A553; http://links.lww.com/CTG/A554; and http://links.lww.com/CTG/A555.
Contributor Information
Helena Tavares de Sousa, Email: helenatsousa@gmail.com.
Irene Gullo, Email: irene.gullo12@gmail.com.
Claudia Castelli, Email: castelli911@gmail.com.
Cláudia Camila Dias, Email: ccamiladias@gmail.com.
Florian Rieder, Email: riederf@ccf.org.
Fátima Carneiro, Email: fcarneiro@ipatimup.pt.
REFERENCES
- 1.Koukoulis G, Ke Y, Henley JD, et al. Obliterative muscularization of the small bowel submucosa in Crohn disease: A possible mechanism of small bowel obstruction. Arch Pathol Lab Med 2001;125(10):1331–4. [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Latella G, Magro F, et al. European Crohn's and Colitis Organisation topical review on prediction, diagnosis and management of fibrostenosing Crohn's disease. J Crohns Colitis 2016;10(8):873–85. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Mao R, Kurada S, et al. Pathogenesis of fibrostenosing Crohn's disease. Transl Res 2019;209:39–54. [DOI] [PubMed] [Google Scholar]
- 4.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017;152(2):340–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettenworth D, Rieder F. Medical therapy of stricturing Crohn's disease: What the gut can learn from other organs—A systematic review. Fibrogenesis Tissue Repair 2014;7(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler J, Punglia DR, Dillman JR, et al. Computed tomography enterography findings correlate with tissue inflammation, not fibrosis in resected small bowel Crohn's disease. Inflamm Bowel Dis 2012;18(5):849–56. [DOI] [PubMed] [Google Scholar]
- 7.Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011;17(4):984–93. [DOI] [PubMed] [Google Scholar]
- 8.Barkmeier DT, Dillman JR, Al-Hawary M, et al. MR enterography-histology comparison in resected pediatric small bowel Crohn disease strictures: Can imaging predict fibrosis? Pediatr Radiol 2016;46(4):498–507. [DOI] [PubMed] [Google Scholar]
- 9.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn's disease complicated by strictures: A systematic review. Gut 2013;62(7):1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther 2018;48(3):347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maaser C, Sturm A, Vavricka SR, et al. ECCO Guideline/Consensus Paper ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13(2):144–64. [DOI] [PubMed] [Google Scholar]
- 12.Sousa HT de, Brito J, Magro F. New cross-sectional imaging in IBD. Curr Opin Gastroenterol 2018;34(4):194–207. [DOI] [PubMed] [Google Scholar]
- 13.Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of Crohn's disease-associated small bowel strictures and fibrosis on cross-sectional imaging: A systematic review. Gut 2019;68(6):1115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang ZN, Li XH, Lin JJ, et al. Magnetisation transfer imaging adds information to conventional MRIs to differentiate inflammatory from fibrotic components of small intestinal strictures in Crohn's disease. Eur Radiol 2020;30(4):1938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rimola J, Capozzi N. Differentiation of fibrotic and inflammatory component of Crohn's disease-associated strictures. Intest Res 2020;18(2):144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allocca M, Fiorino G, Bonifacio C, et al. Noninvasive multimodal methods to differentiate inflamed vs fibrotic strictures in patients with Crohn's disease. Clin Gastroenterol Hepatol 2019;17(12):2397–415. [DOI] [PubMed] [Google Scholar]
- 17.Higgins PDR. Measurement of fibrosis in Crohn's disease strictures with imaging and blood biomarkers to inform clinical decisions. Dig Dis 2017;35(1–2):32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn's disease strictures. Inflamm Bowel Dis 2004;10(1):55–60. [DOI] [PubMed] [Google Scholar]
- 19.Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology scoring systems of stenosis associated with small bowel Crohn's disease: A systematic review. Gastroenterology 2020;158(1):137–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavares De Sousa H, Estevinho MM, Peyrin-Biroulet L, et al. Transmural histological scoring systems in Crohn's disease: A systematic review with assessment of methodological quality and operating properties. J Crohns Colitis 2020;14(6):743–56. [DOI] [PubMed] [Google Scholar]
- 21.Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006;55(6):749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dias CC, Rodrigues PP, Coelho R, et al. Development and validation of risk matrices for Crohn's disease outcomes in patients who underwent early therapeutic interventions. J Crohns Colitis 2017;11(4):445–53. [DOI] [PubMed] [Google Scholar]
- 23.Chiorean MV, Sandrasegaran K, Saxena R, et al. Correlation of CT enteroclysis with surgical pathology in Crohn's disease. Am J Gastroenterol 2007;102(11):2541–50. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Ko HM, Torres J, et al. Luminally polarized mural and vascular remodeling in ileal strictures of Crohn's disease. Hum Pathol 2018;79:42–9. [DOI] [PubMed] [Google Scholar]
- 25.Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015;110(3):432–40. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Mao R, Huang S, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology 2018;2018:171221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberhuber G, Stangl PC, Vogelsang H, et al. Significant association of strictures and internal fistula formation in Crohn's disease. Virchows Arch 2000;437(3):293–7. [DOI] [PubMed] [Google Scholar]
- 28.Burke JP, Mulsow JJ, O'Keane C, et al. Fibrogenesis in Crohn's disease. Am J Gastroenterol 2007;102(2):439–48. [DOI] [PubMed] [Google Scholar]
- 29.Coffey JC, O'Leary DP, Kiernan MG, et al. The mesentery in Crohn's disease: Friend or foe? Curr Opin Gastroenterol 2016;32(4):267–73. [DOI] [PubMed] [Google Scholar]
- 30.Borley NR, Mortensen NJ, Jewell DP, et al. The relationship between inflammatory and serosal connective tissue changes in ileal Crohn's disease: Evidence for a possible causative link. J Pathol 2000;190(2):196–202. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan AL, Warren BF, Gear MW, et al. Fat-wrapping in Crohn's disease: Pathological basis and relevance to surgical practice. Br J Surg 1992;79(9):955–8. [DOI] [PubMed] [Google Scholar]
- 32.Mao R, Kurada S, Gordon IO, et al. The mesenteric fat and intestinal muscle interface: Creeping fat influencing stricture formation in Crohn's disease. Inflamm Bowel Dis 2019;25(3):421–6. [DOI] [PubMed] [Google Scholar]
- 33.Pennington L, Hamilton SR, Bayless TM, et al. Surgical management of Crohn's disease. Influence of disease at margin of resection. Ann Surg 1980;192(3):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fazio VW, Marchetti F, Church M, et al. Effect of resection margins on the recurrence of Crohn's disease in the small bowel. A randomized controlled trial. Ann Surg 1996;224(4):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frolkis AD, Lipton DS, Fiest KM, et al. Cumulative incidence of second intestinal resection in Crohn's disease: A systematic review and meta-analysis of population-based studies. Am J Gastroenterol 2014;109(11):1739–48. [DOI] [PubMed] [Google Scholar]
- 36.Dias CC, Rodrigues PP, Fernandes S, et al. The risk of disabling, surgery and reoperation in Crohn's disease - A decision tree-based approach to prognosis. PLoS One 2017;12(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias CC, Portela F, Fernandes S, et al. The timing of early therapeutic strategies has a significant impact on Crohn's disease prognosis. Dig Liver Dis 2018;50(5):462–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.