Abstract
INTRODUCTION:
Current guidelines recommend intravenous (IV) proton pump inhibitor (PPI) therapy in peptic ulcer bleeding (PUB). We aimed to compare the efficacy of oral and IV administration of PPIs in PUB.
METHODS:
We performed a systematic search in 4 databases for randomized controlled trials, which compared the outcomes of oral PPI therapy with IV PPI therapy for PUB. The primary outcomes were 30-day recurrent bleeding and 30-day mortality. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for dichotomous outcomes, while weighted mean differences (WMDs) with CI were calculated for continuous outcomes in meta-analysis. The protocol was registered a priori onto PROSPERO (CRD42020155852).
RESULTS:
A total of 14 randomized controlled trials reported 1,951 peptic ulcer patients, 977 and 974 of which were in the control and intervention groups, respectively. There were no statistically significant differences between oral and IV administration regarding 30-day rebleeding rate (OR = 0.96, CI: 0.65–1.44); 30-day mortality (OR = 0.70, CI: 0.35–1.40); length of hospital stay (WMD = −0.25, CI: −0.93 to –0.42); transfusion requirements (WMD = −0.09, CI: −0.07 to 0.24); need for surgery (OR = 0.91, CI: 0.40–2.07); further endoscopic therapy (OR = 1.04, CI: 0.56–1.93); and need for re-endoscopy (OR = 0.81, CI: 0.52–1.28). Heterogeneity was negligible in all analysis, except for the analysis on the length of hospitalization (I2 = 82.3%, P = 0.001).
DISCUSSION:
Recent evidence suggests that the oral administration of PPI is not inferior to the IV PPI treatment in PUB after endoscopic management, but further studies are warranted.
INTRODUCTION
A common source of upper gastrointestinal bleeding is peptic ulcer bleeding (PUB), with a prevalence of 30% (1), and it has a high mortality, estimated between 3% and 14% (2). PUB management is based on 2 methods: urgent endoscopy and, if necessary, endoscopic hemostasis and conservative treatment after that.
Current protocols recommend 3 days of treatment with intravenous (IV) proton pump inhibitor (PPI), but in cases where patients tolerate it, oral treatment may also be considered. The European Society of Gastrointestinal Endoscopy guideline from 2015 cautiously intimates that if the patient's condition permits, high-dose oral PPI may be an alternative in those able to tolerate oral medications (3). The American College of Gastroenterology guideline from 2012 advocates that only patients with low-risk ulcers can receive oral PPI therapy instead of IV one (4). The American Society of Gastrointestinal Endoscopy guideline from 2012 does recommend IV PPI and does not mention oral administration as a possible treatment option (5).
Since the publication of the above guidelines, 2 meta-analyses investigated the differences in outcomes between the oral and IV administration of PPIs (6,7).
The meta-analysis from 2016 included 7 randomized controlled trials (RCTs) with 859 patients, and the other one from 2017 included 9 RCTs with 1,036 patients. These meta-analyses concluded that oral PPI is an equally safe treatment option after the initial endoscopic management. However, both analyses had several limitations.
A third meta-analysis by Sachar et al. (8) from 2014 focused on the continuous and intermittent IV administration of PPIs and found that intermittent PPI therapy is comparable with the current guideline recommended regime in patients with endoscopically treated high-risk bleeding ulcers.
IV administration of PPI is more complicated compared with the oral route. Long-term IV cannulation may result in thrombophlebitis and can serve as a gateway for other infections. The management of IV cannula needs specially trained nursing staff, while the continuous infusion reduces the mobility and the comfort of the patients. IV medication carries significantly more iatrogenic risks than their oral equivalents. Finally, the same dose of IV PPI can cost many times more than oral (9).
Given the above-detailed issues with IV administration and the advantages of oral administration, we wanted to compare the efficacy of the 2 administration routes in RCTs and analyze whether future RCTs are needed.
METHODS
Protocol and registration
We reported the meta-analysis and systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (10). Our work was performed following the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (11). The protocol was registered in PROSPERO under registration number CRD42020155852 (12).
Systematic search
Our PICO items were as follows: We looked for studies on patients admitted with bleeding peptic ulcers after endoscopic assessment (P) that compare 2 PPI treatment regimes: oral (I) or IV (C). The primary outcomes were 30-day rebleeding and 30-day mortality. Secondary outcomes were overall mortality, total rebleeding during follow-up, blood transfusion requirement, length of hospital stay, need for surgery, re-endoscopy, and further endoscopic treatment (O).
The following search key was used on October 26, 2020, in MEDLINE (through PubMed), Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Scopus: (“oral” or “per os” or “po” or “p.o.”) and (“intravenous” or “bolus” or “parenteral” or “iv” or “i.v.”) and (“proton pump inhibitor” or “PPI” or “proton-pump inhibitor”) and (random*). No filters or language restrictions were applied.
Eligibility criteria
All RCTs that compared oral with IV PPI administration in PUB, regardless of Forrest classification, with any reported clinical outcome (mortality, rebleeding, re-endoscopy, further endoscopic treatment, blood transfusion, length of hospital stay, and surgery) were included in our analysis. Full-text articles and conference abstracts were eligible. There was no language restriction imposed.
Screening and selection
Articles yielded by the initial search were imported into a reference management program (EndNote X7; Clarivate Analytics, Philadelphia, PA). The same software was used to remove duplicates by searching for articles with overlapping publication years, authors, or titles. Two independent reviewers (E.C. and H.S.) screened the records by title, abstract, and full text against the eligibility criteria. Disagreements were resolved by consensus and the involvement of the corresponding author (B.E.).
Data extraction
From the eligible studies, 2 review authors (E.C. and H.S.) independently extracted relevant data. Disagreements were resolved by consensus and the involvement of the corresponding author (B.E.).
Data were extracted and manually introduced into a purpose-designed Excel sheet (Office 365; Microsoft, Redmond, WA). Data were collected on the year of publication, study and publication type, geographical location, number of cases and controls, and basic demographics (age and sex). Data were also collected on the Forrest classification of the PUB, details of endoscopic procedure before PPI treatment, PPI therapy before endoscopic diagnosis and treatment, type of the oral PPI therapy after the intervention/comparator PPI, the oral and IV treatment regimes (doses, timing, and other specifics of the drugs). Most importantly, data on rebleeding, transfusion requirements (data provided in mL and L were converted in internationally used 300-mL packed red blood cell units), length of hospital stay, mortality, need for surgery, re-endoscopy, and further endoscopic treatment were recorded as well.
Risk-of-bias assessment and quality of evidence
The risk of bias was assessed using the RoB 2: A revised Cochrane risk-of-bias tool for randomized trials (13) by 2 independent reviewers (E.C. and S.K.). Disagreements were resolved by consensus and the involvement of the corresponding author (B.E.).
To rate the quality and evidence of the results, we used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) method by 2 independent reviewers (E.C. and S.K.) (14).
Statistical methods
Odds ratios (ORs) were calculated for dichotomous outcomes and weighted mean differences (WMDs) for continuous variables, both with 95% confidence intervals (CIs). The random-effects model was applied at all analyses with the DerSimonian-Laird estimation (15). Statistical heterogeneity was assessed using Cochrane Q and the I2 statistics. According to the Cochrane Handbook, heterogeneity could be interpreted as moderate between 30% and 60%, as substantial between 50% and 90%, and as considerable above 75% (16).
To analyze precision and determine whether additional RCTs are needed or might influence our results, we tried to conduct Trial Sequential Analysis (TSA). It is a tool for quantifying data's statistical reliability in the cumulative meta-analysis, adjusting significance levels for sparse data. TSA was performed with TSA program version 0.9 beta (available from www.ctu.dk/tsa).
To assess the probability that future studies in a similar setting would have the same result, we calculated the prediction interval (PI) for our meta-analysis' primary outcomes (16).
Sensitivity analyses (the leave-one-out method) were also performed. Publication bias was assessed by the visual inspection of the funnel plots and by the Egger test, where a significant test result (P < 0.1) indicates the presence of bias (17). Statistical analyses were performed with Stata 16 (StataCorp).
RESULTS
Study selection
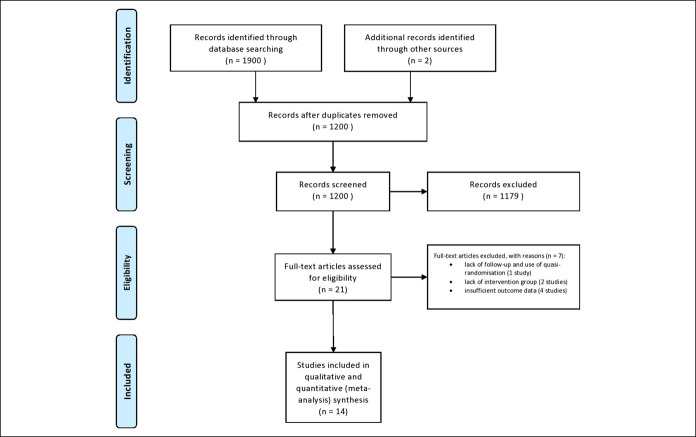
Our search yielded a total of 1,900 articles, 184 in MEDLINE (through PubMed), 1,053 in Embase, 348 in the Cochrane Central Register of Controlled Trials (CENTRAL), and 315 in Scopus. Two articles were identified by cross-referencing. A total of 21 potentially eligible articles were identified, from which 7 were excluded with reasons (18–24). Details of the search and selection are in Figure 1.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
Description of the studies included
Our research analysed 14 RCTs with a pooled study population of 1,951 PUB patients, with 977 controls and 974 in the intervention group (9,25–37). The number of patients in individual studies ranged from 25 to 323. Eleven of the included studies were from Asia (9,26,28–32,34–37), 1 study was from Europe (27), and 2 from North America (25,33). Thirteen of the 14 studies included cases with Forrest Ia, Ib, IIa, and IIb. By contrast, a single study included cases where endoscopic treatment was not necessary according to current guidelines (Forrest IIc and III) (37). The main characteristics of the included studies are shown in Table 1.
Table 1.
Main characteristics of the included studies
| Author (yr) | Location | Per os PPI | Mean age (yr) | Male % of total | Forrest classification | Incidence of 30-d rebleeding (% of total) | 30-d mortality (% of total) | ||
| IV PPI | IA/IB | IIA/IIB/IIC | III | ||||||
| Bajaj et al. (25) (2007) | Wisconsin, USA | 12 | 63 | 14% | 3 | 5 | 4 | 2/25 (8) | 0 (0) |
| 13 | 4 | 4 | 5 | ||||||
| Chen et al. (26) (2015) | Taiwan, Taipeia | 156 | No data | No data | 156b | 0 | 18/323 (5.5) | 3/323 (0.9) | |
| 167 | 167 | 0 | |||||||
| Focareta et al. (27) (2004) | Rotondo, Italy | 45 | No data | No data | 30 | 57c | 0 | No data | No data |
| 42 | 0 | ||||||||
| Jang et al. (28) (2006) | Seoul, Korea | 19 | 59.3 | 76.3% | 6 | 13 | 0 | 3/38 (7.8) | 1/38 (2.6) |
| 19 | 4 | 15 | 0 | ||||||
| Javid et al. (29) (2009) | Kashmir, India | 45 | 35.6 | No data | 19 | 26 | 0 | No data | No data |
| 45 | 18 | 27 | 0 | ||||||
| Kim et al. (30) (2012) | Korea | 54 | No data | 80.1% | 21 | 33 | 0 | 4/106 (3.7) | No data |
| 52 | 18 | 34 | 0 | ||||||
| Mostaghni et al. (31) (2011) | Shiraz, Iran | 44 | 59.4 | 74.1% | 14 | 30 | 0 | 9/85 (10.5) | 2/85 (2.3) |
| 41 | 12 | 29 | 0 | ||||||
| Sung et al. (32) (2014) | Hong Kong, China | 126 | 64 | 74% | 55 | 71 | 0 | 17/244 (6.9) | No data |
| 118 | 51 | 67 | 0 | ||||||
| Theyventhiran et al. (33) (2013) | United States | 52 | No data | No data | 108c | 0 | 0 | No data | 0/108 |
| 56 | 0 | 0 | |||||||
| Tsai et al. (34) (2009) | Taipei, Taiwan | 78 | 68.7 | 72.4% | 33 | 44 | 0 | 25/156 (16)d | 3/156 (1.9) |
| 78 | 31 | 47 | 0 | ||||||
| Valizadeh Toosi et al. (35) (2018) | Sari, Iran | 90 | No data | 63% | 90b | 0 | 7/178 (3.9) | 4/178 (2.2) | |
| 88 | 88 | 0 | |||||||
| Yen et al. (36) (2012) | Taipei, Taiwan | 50 | 63.9 | 71% | 22 | 28 | 0 | No data | 0/100 (0) |
| 50 | 21 | 29 | 0 | ||||||
| Yilmaz et al. (37) (2006) | Diyarbakir, Turkey | 99 | 52.7 | 68.7% | 0 | 37 | 62 | 12/211 (5.6) | 5/211 (2.4) |
| 112 | 0 | 30 | 82 | ||||||
| Karim et al. (9) (2020) | Pakistan | 104 | 56.3 | 60.5% | 40 | 65 | 0 | 11/200 (5.5) | 14/200 (7) |
| 96 | 35 | 80 | 0 | ||||||
IV, intravenous; PPI, proton pump inhibitor.
Chen et al. (2015) was a multicenter, while Kim et al. (2012) was a 2-center study; all the other randomized controlled trials included were single-center.
In the Chen et al. (2015) and Valizadeh et al. (2018) studies, there were no details with regards the specific Forrest classification of the bleeding ulcers, and only a wider interval was given.
In the Focareta et al. (2004) and Theyventhiran et al. (2013) studies, it was not specified nor the number of patients who received the IV treatment, neither the po treatment.
In Tsai et al. (2009) study, the explanation for the higher rebleeding rate is the use of epinephrine injection as single and primary method of hemostasis.
Eleven studies used the currently recommended IV treatment regime (8 mg PPI/hr) (9,25,28–33,35,37), while 3 used IV bolus (32,34,37) and 1 reduced dosage of PPI (3.375 mg/hr) (26). Different PPIs were used as comparators, including pantoprazole (9,25,28,35), omeprazole (29,31,37), rabeprazole (30,34), lansoprazole (26,36), and esomeprazole (27,32,33). Further details of the management are shown in Table 1 (Supplementary Digital Content 1, http://links.lww.com/CTG/A571).
Primary outcomes
Recurrent bleeding
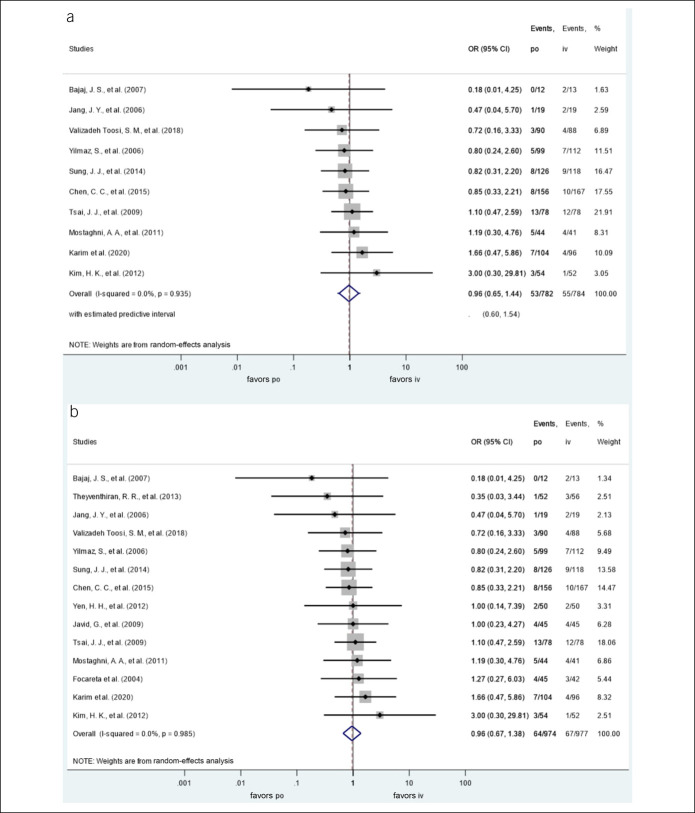
The oral administration was not associated with the risk of 30-day recurrent bleeding, OR = 0.96, CI 95% (0.65–1.44), P = 0.857; PI: 0.60–1.54. There was no heterogeneity among the included studies (I2 = 0%). There were 53 episodes of recurrent bleedings in 782 enrolled subjects in the oral group and 55 in 784 in the IV group (Figure 2a).
Figure 2.
(a) ORs for 30-day recurrent bleeding with oral proton pump inhibitors (vs IV proton pump inhibitors). (b) ORs for total recurrent bleeding with oral proton pump inhibitors (vs IV proton pump inhibitors). CI, confidence interval; IV, intravenous; OR, odds ratio; po, per os.
We performed a subgroup analysis, and it did not demonstrate a difference in the risk of 30-day rebleeding between the bolus and continuous PPI administration. The OR of rebleeding in the case of continuous administration was 0.93 (CI: 0.59–1.46) and 1.10 (CI: 0.47–2.59) in the case of administration in a bolus both compared against per os treatment.
The oral administration was not associated with the risk of total recurrent bleeding either, OR = 0.96, CI 95% (0.67–1.38), P = 0.812. There was no heterogeneity among the included studies (I2 = 0%). There were 64 episodes of recurrent bleeding in 974 enrolled subjects in the oral group and 67 in 977 in the IV group (Figure 2b).
In the case of the 3-day recurrent bleeding, the results were similar, with no significant differences noted: OR = 1.07, CI 95% (0.63–1.80), P = 0.799; I2 = 0%. There were 33 episodes of recurrent bleeding in 553 enrolled subjects in the oral group and 31 in 551 in the IV group (see Figure 1, Supplementary Digital Content 3, http://links.lww.com/CTG/A573).
Mortality
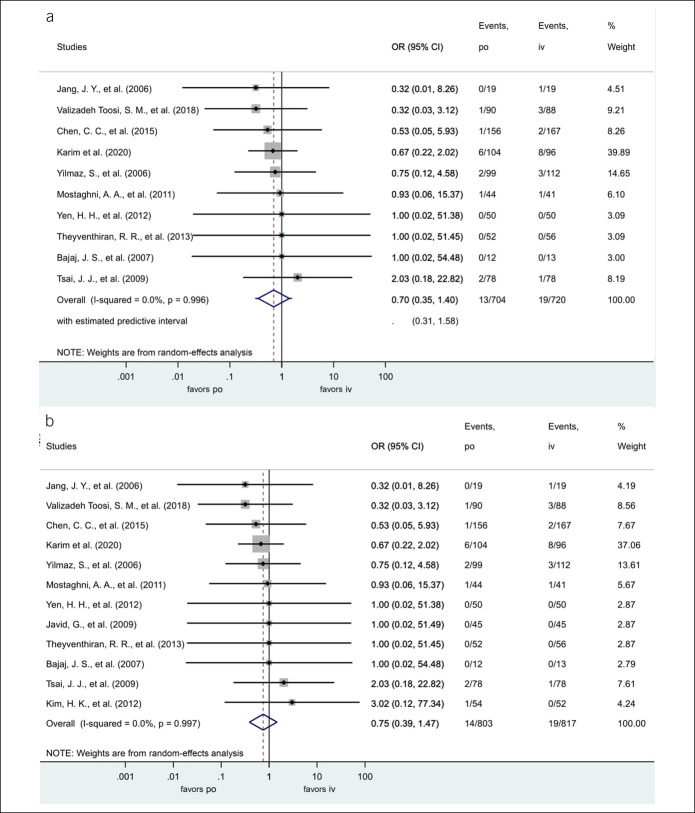
Regarding the other primary outcome, the oral administration was not associated with the risk of 30-day mortality, OR = 0.70, CI 95% (0.35–1.40), P = 0.314, PI: 0.31–1.58. There was no heterogeneity among the included studies (I2 = 0%). There were 13 deaths in 704 enrolled subjects in the oral group and 19 in 720 in the IV group (Figure 3a).
Figure 3.
(a) ORs for 30-day mortality with oral proton pump inhibitors (vs IV proton pump inhibitors). (b) ORs for total mortality with oral proton pump inhibitors (vs IV proton pump inhibitors). CI, confidence interval; IV, intravenous; OR, odds ratio; po, per os.
The total mortality figures were similar to that of the 30-day mortality. The oral administration was not associated with the risk of death, OR: 0.75, CI 95% (0.39–1.47), P = 0.405. There was no heterogeneity among the included studies (I2 = 0%). There were 14 deaths in 803 enrolled subjects in the oral and 19 in 817 in the IV group (Figure 3b).
Secondary outcomes
Concerning the secondary outcomes, the oral administration was not associated with the risk of re-endoscopy (OR = 0.81, 95% CI: 0.52–1.28, P = 0.371, I2 = 0%), need for further endoscopic therapy (OR = 1.04, 95% CI: 0.56–1.93, P = 0.894; I2 = 0%), and need for surgery (OR = 0.91, 95% CI: 0.40–2.07, P = 0.829; I2 = 0%) (see Figures 2–4, Supplementary Digital Content 4, Supplementary Digital Content 5, Supplementary Digital Content 6, http://links.lww.com/CTG/A574, http://links.lww.com/CTG/A575, http://links.lww.com/CTG/A576).
The oral administration was not associated with increased risk in terms of blood transfusion requirements (WMD = −0.09, 95% CI: −0.07 to 0.24, P = 0.270; I2 = 16.3%) or length of hospitalization (WMD = −0.25, 95% CI: −0.93 to 0.42; I2 = 82.3%, P = 0.001) (see Figures 5, 6, Supplementary Digital Content 7, Supplementary Digital Content 8, http://links.lww.com/CTG/A577, http://links.lww.com/CTG/A578).
Additional analysis
Considerable heterogeneity was observed only in the case of the hospitalization length (I2 = 82.3%, P < 0.001). Therefore, we performed a sensitivity analysis that showed that the study by Yen et al. (36) was the outlier. The association did not change by removing this study, but the heterogeneity disappeared I2 = 20.5%, P = 0.273. Moreover, sensitivity analyses (the leave-one-out method) did not identify influential studies (see Figures 17–26, Supplementary Digital Content 19, Supplementary Digital Content 20, Supplementary Digital Content 21, Supplementary Digital Content 22, Supplementary Digital Content 23, Supplementary Digital Content 24, Supplementary Digital Content 25, Supplementary Digital Content 26, Supplementary Digital Content 27, and Supplementary Digital Content 28, http://links.lww.com/CTG/A589, http://links.lww.com/CTG/A590, http://links.lww.com/CTG/A591, http://links.lww.com/CTG/A592, http://links.lww.com/CTG/A593, http://links.lww.com/CTG/A594, http://links.lww.com/CTG/A595, http://links.lww.com/CTG/A596, http://links.lww.com/CTG/A597, and http://links.lww.com/CTG/A598).
To find out whether additional RCTs are needed or might influence our results, we tried to conduct a TSA. However, the software refused to draw the figure as the accrued information size was substantially below the required information size.
To assess the probability that future studies in a similar setting would have the same result, we calculated the estimated predictive intervals in the case of 30-day rebleeding and 30-day mortality. The analyses could not unequivocally conclude whether further studies would have negative or positive results, PI: 0.6–1.54 and PI: 0.31–1.58, respectively.
Risk of bias and quality of evidence assessment
Visual assessment of the Funnel plots and Egger tests did not suggest publication bias (see Figures 7–16, Supplementary Digital Content 9, Supplementary Digital Content 10, Supplementary Digital Content 11, Supplementary Digital Content 12, Supplementary Digital Content 13, Supplementary Digital Content 14, Supplementary Digital Content 15, Supplementary Digital Content 16, Supplementary Digital Content 17, and Supplementary Digital Content 18, http://links.lww.com/CTG/A579, http://links.lww.com/CTG/A580, http://links.lww.com/CTG/A581, http://links.lww.com/CTG/A582, http://links.lww.com/CTG/A583, http://links.lww.com/CTG/A584, http://links.lww.com/CTG/A585, http://links.lww.com/CTG/A586, http://links.lww.com/CTG/A587, and http://links.lww.com/CTG/A588).
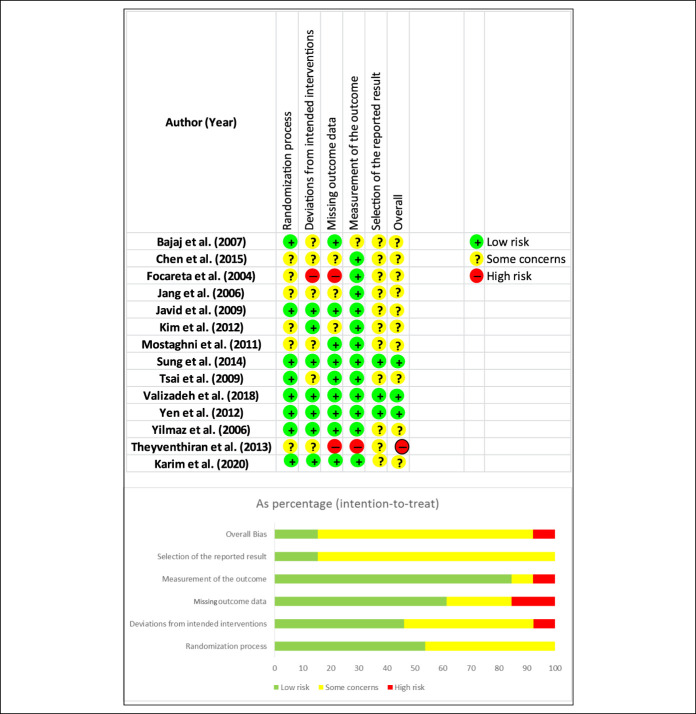
The risk of bias, according to the RoB 2: A revised Cochrane risk-of-bias tool for randomized trials (13), identified some concerns. Of the 14 studies, only 3 carried a low risk of bias (9,32,35,36). Because of the lack of detailed information in the individual studies, some concerns were present in most domains, especially for the reporting of results. There was a low risk of bias across the studies concerning the outcome measurement. Details of the risk assessment are shown in Figure 4.
Figure 4.
Top: Results of the Cochrane Risk of Bias assessment tool 2 for randomized controlled trials. Bottom: Risk of bias assessment for domains. Points evaluated: randomized process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall risk of bias.
DISCUSSION
Findings from this meta-analysis, involving nearly 2,000 patients in 14 RCTs, imply that oral administration of PPIs is noninferior compared with IV administration in PUB. No statistically significant difference was found regarding the risks of rebleeding, mortality, repeat interventions (endoscopy, endoscopic treatment, or surgery), nor in need for transfusion or length of hospitalization between oral and IV PPIs.
Thirty-day recurrent bleeding occurred in about 7% of all participants in both IV and oral administration, and the 3-day rebleeding rate was around 6%. Our results suggest that recurrent bleeding is an early complication of PUB, and the risk of rebleeding was not increased, regardless of the interval. Death occurred very rarely across the RCTs; 1.8% and 2.6% of participants died in the oral and IV groups, respectively. As PUB patients do not die from bleeding but decompensation of their comorbidities, we think that the most important primary outcome of PPI treatment of PUB is rebleeding.
Given that mortality and rebleeding rates (around 2% and 7%, respectively) are low in patients with PUB, a large number of patients would have been required to show the difference (if there is any), so that type II error may distort the conclusions of the individual studies. As shown by the wide estimated predictive intervals of 30-day rebleeding and mortality (which included 1 in all cases), our meta-analysis is likely suffering from type II error as well, so that further, sufficiently powered studies are needed. However, the analyses of recent data unequivocally suggest clinical noninferiority of PPI's oral administration compared with IV in all outcomes examined.
In our meta-analysis, the only significant statistical heterogeneity among the studies included affected the analysis on the length of hospitalization and could be explained with a single RCT. The heterogeneity was no longer detectable on exclusion of the study of Yen et al. (36). In this study, participants were discharged after a mean length of hospitalization of 3.9 and 1.8 days (P < 0.01) in the IV and oral groups, respectively. None of the other studies demonstrated a difference in the length of hospital stay. The 1.8 days of mean length of hospital stay was an extreme outlying result. In all the other RCTs, the mean length of hospitalization was longer than 3 days in the IV and oral groups.
Not only the oral vs IV but also the IV continuous vs bolus administration of PPI is of interest. The previous evidence in a meta-analysis of Sachar et al., involving 13 RCTs with more than 1,700 participants, showed no difference in three-, seven-, and thirty-day rebleeding risk bolus and continuous IV administration of PPIs were compared in patients with high-risk bleeding ulcers. There was no statistically significant heterogeneity detected in their analyses (8). Our study could meta-analyze 2 subgroups, 1 with bolus and 1 with continuous IV PPI administration. The meta-analytical calculations showed no clinically significant difference in either of the 2 subgroups compared with oral administration. Seventy-two hours of IV PPI administration may not be necessary even in patients with high-risk ulcer stigmata requiring endoscopic therapy, and there is likely no added benefit of intermittent IV PPI compared with oral PPI based on the findings. Therefore, we can state that oral, IV bolus and continuous IV administration of PPIs all seem equally effective in PUB treatment. These are powerful arguments against the common use of 72 hours of IV PPIs in a continuous infusion.
Potential explanations for the clinical noninferiority of the different routes of administration of PPIs in PUB
The equal clinical efficacy shown in our meta-analysis is most likely explained by the pharmacokinetic and pharmacodynamic properties of PPIs. We know from the study of Shin et al. that omeprazole reaches its peak serum levels within an hour in the case of oral administration in a rat model. The serum half-life of omeprazole and the other PPIs is about 1 hour. Still, the acid-activated PPIs covalently bind to the gastric H+, K+-ATPase and can maintain their beneficial acid-suppressing effect, even when they are barely detectable in the serum. PPIs differ in their time to maximal plasma concentration, but they vary between 1 and 5 hours (38).
The study of Javid et al. (29) gave valuable insights on the acid-reducing potential of omeprazole, pantoprazole, and rabeprazole: All could equally reduce pH regardless of the administration route (IV or per os).
Strengths
To date, this is the most comprehensive meta-analysis in the topic with a rigorous and up-to-date methodology. There was no significant statistical heterogeneity in the analysis, meaning that clinical differences (different types, dosages, bolus, or continuous IV PPI administration) do not substantially distort the results of the analysis.
Limitations
Regarding the limitations, the most critical one was the lack of sample size estimation in 9 studies (9,25–29,31,33,35,37). Only 2 of the studies (31,33) specified pre-emptive PPI use before endoscopy, and 1 of the studies included participants who did not need endoscopic therapy (37). The majority of the included RCTs focused on the high-risk PUB patients (proportion of 92%, CI: 90–93); although approximately 42%–77% of patients have Forrest III, low-risk ulcers in studies on general PUB populations (39,40). We detected a significant difference among the studies in the approach of the endoscopic treatment of the bleeding ulcer. Also, the development of endoscopic hemostasis in recent decades is a vital aspect of PUB treatment and might have caused a degree of chronological bias. However, neither earlier (before 2010) nor later individual RCTs (after 2010) confirmed the oral PPI's inferiority against IV. There was no need for additional meta-analytical calculation as the outcomes were not statistically different between the 2 arms in any studies in the 2 subgroups. There were also major differences in both the oral and IV treatment regimes because 4 studies (26,27,34,36) used bolus IV PPI administration rather than the currently recommended continuous administration. Differences were also noted regarding the active substances as well as dosages and timing (see Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A571). Out of the 14 included studies, 3 were available only as conference abstracts with limited data. Another critical issue might be the heterogeneity among the populations. Eight studies enrolled men predominantly (28,30–32,34–37), and 1 study enrolled very few men (25). Also, major differences were observed concerning the studied populations' age. Interestingly, none of the apparent clinically heterogeneous aspects translated into statistical heterogeneity. Finally, the included studies were not free of biases, as discussed in the results and shown in Figure 4.
Grade of evidence
All the limitations were downgrading items when we assessed the evidence levels for all outcomes following the GRADE approach (see Table 2, Supplementary Digital Content 2, http://links.lww.com/CTG/A572). Based on the results and the careful assessment of the evidence level, the certainty levels were very low for each outcome.
We can conclude that IV PPI—either as a continuous infusion or intermittent dosing compared with oral PPI, as an adjunct to endoscopic therapy receiving in most patients in the meta-analysis—does not appear to provide clinical benefit.
CONFLICTS OF INTEREST
Guarantor of the article: Bálint Erőss, MD, PhD.
Specific author contributions: B.E. and E.C. conceptualized and designed the study in cooperation with H.S., Z.S., and N.V.; B.E., E.C. in cooperation with Z.S. and S.K. constructed the search query and performed the search process; E.C. and H.S. screened the articles for eligibility and performed the data extraction; E.C., B.E., E.H., and D.P. conducted the quality assessment; E.C. and B.E. wrote the article; L.H. performed the statistical analysis; Z.S., S.K., P.H., and D.P. provided valuable feedback after critically reviewing the first drafts of the manuscript. All authors reviewed and approved the final manuscript for publication.
Financial support: The project titled “GINOP-2.3.2-15-2016-00048—STAY ALIVE” is co-financed by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020. Human Resources Development Operational Programme Grant, Grant Number: EFOP‐3.6.2‐16‐2017‐00006—LIVE LONGER is cofinanced by the European Union (European Regional Development Fund) within the framework of Programme Széchenyi 2020.
Potential competing interests: None to report.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A571, http://links.lww.com/CTG/A572, http://links.lww.com/CTG/A573, http://links.lww.com/CTG/A574, http://links.lww.com/CTG/A575, http://links.lww.com/CTG/A576, http://links.lww.com/CTG/A577, http://links.lww.com/CTG/A578, http://links.lww.com/CTG/A579, http://links.lww.com/CTG/A580, http://links.lww.com/CTG/A581, http://links.lww.com/CTG/A582, http://links.lww.com/CTG/A583, http://links.lww.com/CTG/A584, http://links.lww.com/CTG/A585, http://links.lww.com/CTG/A586, http://links.lww.com/CTG/A587, http://links.lww.com/CTG/A588, http://links.lww.com/CTG/A589, http://links.lww.com/CTG/A590, http://links.lww.com/CTG/A591, http://links.lww.com/CTG/A592, http://links.lww.com/CTG/A593, http://links.lww.com/CTG/A594, http://links.lww.com/CTG/A595, http://links.lww.com/CTG/A596, http://links.lww.com/CTG/A597, http://links.lww.com/CTG/A598
Contributor Information
Endre Csiki, Email: endre_csiki@yahoo.com.
Hanna Szabó, Email: szabohannaum@gmail.com.
Lilla Hanák, Email: hanaklilla92@gmail.com.
Zsolt Szakács, Email: szaki92@gmail.com.
Szabolcs Kiss, Email: kissszabolcs1995@gmail.com.
Nóra Vörhendi, Email: vorinocci@gmail.com.
Dániel Pécsi, Email: daniel.pecsi1991@gmail.com.
Eszter Hegyi, Email: eszter.hegyi@aok.pte.hu.
Péter Hegyi, Email: hegyi2009@gmail.com.
REFERENCES
- 1.Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: Patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011;60(10):1327–35. [DOI] [PubMed] [Google Scholar]
- 2.van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 2008;22(2):209–24. [DOI] [PubMed] [Google Scholar]
- 3.Gralnek IM, Dumonceau JM, Kuipers EJ, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47(10):a1–46. [DOI] [PubMed] [Google Scholar]
- 4.Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol 2012;107(3):345–60; quiz 61. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JH, Fisher DA, Ben-Menachem T, et al. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc 2012;75(6):1132–8. [DOI] [PubMed] [Google Scholar]
- 6.Jian Z, Li H, Race NS, et al. Is the era of intravenous proton pump inhibitors coming to an end in patients with bleeding peptic ulcers? Meta-analysis of the published literature. Br J Clin Pharmacol 2016;82(3):880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tringali A, Manta R, Sica M, et al. Comparing intravenous and oral proton pump inhibitor therapy for bleeding peptic ulcers following endoscopic management: A systematic review and meta-analysis. Br J Clin Pharmacol 2017;83(8):1619–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sachar H, Vaidya K, Laine L. Intermittent vs continuous proton pump inhibitor therapy for high-risk bleeding ulcers: A systematic review and meta-analysis. JAMA Intern Med 2014;174(11):1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karim R, Hameed R, Ali K, et al. Comparison of oral versus intravenous proton pump inhibitors in preventing re-bleeding from peptic ulcer after successful endoscopic therapy. Cureus 2020;12(1):e6741. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151(4):264–9, w64. [DOI] [PubMed] [Google Scholar]
- 11.Chandler J, Hopewell S. Cochrane methods—Twenty years experience in developing systematic review methods. Syst Rev 2013;2:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth A, Clarke M, Dooley G, et al. PROSPERO at one year: An evaluation of its utility. Syst Rev 2013;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015;350:h870. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 16.IntHout J, Ioannidis JPA, Rovers MM, et al. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkun AN, Adam V, Sung JJ, et al. Cost effectiveness of high-dose intravenous esomeprazole for peptic ulcer bleeding. PharmacoEconomics 2010;28(3):217–30. [DOI] [PubMed] [Google Scholar]
- 19.Barkun AN, Herba K, Adam V, et al. The cost-effectiveness of high-dose oral proton pump inhibition after endoscopy in the acute treatment of peptic ulcer bleeding. Aliment Pharmacol Ther 2004;20(2):195–202. [DOI] [PubMed] [Google Scholar]
- 20.Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology 2008;134(7):1836–41. [DOI] [PubMed] [Google Scholar]
- 21.Liang CM, Lee JH, Kuo YH, et al. Intravenous non-high-dose pantoprazole is equally effective as high-dose pantoprazole in preventing rebleeding among low risk patients with a bleeding peptic ulcer after initial endoscopic hemostasis. BMC Gastroenterol 2012;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phulpoto JA, Bhatti ZA, Shaikh A. Comparison of oral and intravenous proton pump inhibitor in patients with high risk bleeding peptic ulcers. Rawal Med J 2013;38(1):7–10. [Google Scholar]
- 23.Schaffalitzky de Muckadell OB, Havelund T, Harling H, et al. Effect of omeprazole on the outcome of endoscopically treated bleeding peptic ulcers. Randomized double-blind placebo-controlled multicentre study. Scand J Gastroenterol 1997;32(4):320–7. [DOI] [PubMed] [Google Scholar]
- 24.Shavakhi A, Ataei S, Ataei M, et al. The comparison of oral omeprazole and intravenous pantoprazole effects in high risk upper gastrointestinal bleeding patients. J Isfahan Med Sch 2008;26(90):242–8. [Google Scholar]
- 25.Bajaj JS, Dua KS, Hanson K, et al. Prospective, randomized trial comparing effect of oral versus intravenous pantoprazole on rebleeding after nonvariceal upper gastrointestinal bleeding: A pilot study. Dig Dis Sci 2007;52(9):2190–4. [DOI] [PubMed] [Google Scholar]
- 26.Chen CC, Fang YJ, Lee JY, et al. Oral versus intravenous proton pump inhibitors in preventing peptic ulcer rebleeding after endoscopic hemostasis. J Gastroenterol Hepatol (Aust) 2015;30:5. [Google Scholar]
- 27.Focareta R, Ciarleglio A, Piai G, et al. Proton-pump inhibitor (PPI) and acute peptic ulcer bleeding: Effectiveness of oral esomeprazole vs intravenous omeprazole in reducing the risk of recurrent bleeding. X Congresso Nazionale delle Malattie Digestive, Turin, 27–31 March 2004.
- 28.Jang JY, Joo KR, Hwangbo Y, et al. A comparison of the effect of high-dose oral and intravenous proton pump inhibitor on the prevention of rebleeding after endoscopic treatment of bleeding peptic ulcers. Korean J Gastrointest Endosc 2006;33(1):6–11. [Google Scholar]
- 29.Javid G, Zargar SA, U-Saif R, et al. Comparison of p.o. or i.v. proton pump inhibitors on 72-h intragastric pH in bleeding peptic ulcer. J Gastroenterol Hepatol 2009;24(7):1236–43. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Kim JS, Kim TH, et al. Effect of high-dose oral rabeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. Gastroenterol Res Pract 2012;2012:317125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostaghni AA, Hashemi ST, Heydari ST. Comparison of oral and intravenous proton pump inhibitor on patients with high risk bleeding peptic ulcers: A prospective, randomized, controlled clinical trial. Iran Red Crescent Med J 2011;13(7):458–63. [PMC free article] [PubMed] [Google Scholar]
- 32.Sung JJ, Suen BY, Wu JC, et al. Effects of intravenous and oral esomeprazole in the prevention of recurrent bleeding from peptic ulcers after endoscopic therapy. Am J Gastroenterol 2014;109(7):1005–10. [DOI] [PubMed] [Google Scholar]
- 33.Theyventhiran RR, Menon J, Singh M, et al. A comparison between intravenous infusion and oral proton pump inhibitors (PPI) following pre-emptive intravenous infusion of a PPI in acute peptic ulcer bleed (PUB) post endoscopic hemostasis. Gastroenterology 2013;144(5 Suppl 1):S80–1. [Google Scholar]
- 34.Tsai JJ, Hsu YC, Perng CL, et al. Oral or intravenous proton pump inhibitor in patients with peptic ulcer bleeding after successful endoscopic epinephrine injection. Br J Clin Pharmacol 2009;67(3):326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valizadeh Toosi SM, Elahi Vahed AR, Maleki I, et al. Comparison of oral versus intravenous proton pump inhibitors in preventing re-bleeding from peptic ulcer after successful endoscopic therapy. Middle east J Dig Dis 2018;10(4):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen HH, Yang CW, Su WW, et al. Oral versus intravenous proton pump inhibitors in preventing re-bleeding for patients with peptic ulcer bleeding after successful endoscopic therapy. BMC Gastroenterol 2012;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz S, Bayan K, Tüzün Y, et al. A head to head comparison of oral vs intravenous omeprazole for patients with bleeding peptic ulcers with a clean base, flat spots and adherent clots. World J Gastroenterol 2006;12(48):7837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 2013;19(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med 1994;331(11):717–27. [DOI] [PubMed] [Google Scholar]
- 40.Groenen MJM, Kuipers EJ, Hansen BE, et al. Incidence of duodenal ulcers and gastric ulcers in a Western population: Back to where it started. Can J Gastroenterol 2009;23(9):604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.