Abstract
Much remains unknown about the transformation of proprioceptive afferent input from the periphery to the cortex. Until recently, the only recordings from neurons in the cuneate nucleus (CN) were from anesthetized animals. We are beginning to learn more about how the sense of proprioception is transformed as it propagates centrally. Recent recordings from microelectrode arrays chronically implanted in CN have revealed that CN neurons with muscle-like properties have a greater sensitivity to active reaching movements than to passive limb displacement, and we find that these neurons have receptive fields that resemble single muscles. In this review, we focus on the varied uses of proprioceptive input and the possible role of CN in processing this information.
Keywords: proprioceptive system, muscle receptor, sensorimotor control, movement, dorsal column, reaching
Introduction:
Proprioception is generated by a variety of receptors that encode mechanical strain and deformation caused by the movement of all parts of the body, including the trunk, head and limbs. Chief among these receptors are muscle spindles that encode muscle length and the speed of length change (Proske & Gandevia, 2012). Golgi tendon organs that respond to active muscle force, joint receptors responding to loads and extreme positions, and skin receptors activated by movement-related stretch of the skin (J. Houk & Simon, 1967). This diverse set of receptors supplies information throughout the cerebral cortex and cerebellum and underlies all aspects of proprioception, from simple spinal reflexes to complex supraspinal reflexes as well as the planning and execution of voluntary movements. Information from these same receptors is also necessary for the conscious perception of the position and motion of our limbs, a perception that remains largely in the background (Proske & Gandevia, 2012) causing it to be referred to colloquially as the “hidden” sixth sense.
A significant portion of afferents from these receptors project directly or indirectly to a caudal brainstem region referred to as the dorsal column nuclei (DCN) complex (Loutit, Vickery, & Potas, 2021; Mountcastle, 2011). This complex of nuclei is in an ideal position to regulate these inputs. Early work examining their structure and function was primarily conducted on cat models, almost always while under sedation (Andersen, Eccles, Oshima, & Schmidt, 1964; Andersen, Eccles, Schmidt, & Yokota, 1964; Cooke, Larson, Oscarsson, & Sjölund, 1971; Rosén & Sjölund, 1973), with notable exceptions (Ghez & Pisa, 1972).
We have recently begun to record in awake monkeys from the cuneate nucleus (CN) (Suresh et al., 2017), the portion of the DCN that carries signals from the arms to the thalamus (Rosén & Sjölund, 1973). Such recordings now allow us to make observations that were previously impossible under sedation. For example, our results show that the sensitivity of many CN neurons differs for actively generated reaches and passive limb displacements of the arm. Those neurons that appear to receive input from muscle spindles are typically more sensitive during active movement. Furthermore, we found that the tuning of CN neurons for movements in different directions is quite similar to what we would expect from receptors of a single muscle, matching the results of previous studies using single muscle stretches (Hummelsheim & Wiesendanger, 1985; Rosén & Sjölund, 1973) but contrasting with a study using electrical stimulation of peripheral nerves (Witham & Baker, 2011). In this review, we will attempt to reconcile the apparent inconsistencies in the previous literature, focusing on two major areas: proprioceptive gain modulation and convergence of afferent input in DCN. In doing so, we hope to provide a perspective from which to examine previous DCN research and to design new studies to illuminate how proprioceptive information is processed as it moves from the periphery to the brain.
Proprioceptive gain modulation in the cuneate nucleus:
Sensory gating, or the attenuation of afferent input, is a feature of many sensory systems (Azim & Seki, 2019). During saccadic eye movements, visual information is attenuated to avoid blurred images caused by the movement of the eye (Binda & Morrone, 2018; Bremmer, Kubischik, Hoffmann, & Krekelberg, 2009; Crevecoeur & Kording, 2017; Holt, 1903). Similarly, tactile sensations arising during active touch are significantly weaker than the same stimuli presented passively (Cohen & Starr, 1987; Schmidt, Schady, & Torebjörk, 1990). These observations have led to the hypothesis that the nervous system turns down the gain on sensory receptors when the information they are transmitting is likely to be noisy (Ghez & Pisa, 1972). As the somatosensory gateway to the brain, neurons in the DCN complex are a logical site of proprioceptive gating.
Consistent with the sensory gating hypothesis, CN receives descending signals from the somatosensory and motor cortices (Andersen, Eccles, Schmidt, et al., 1964; Leiras, Velo, Martín-Cora, & Canedo, 2010; Loutit et al., 2021). Their effect on afferent transmission has been the subject of experiments conducted mostly in anesthetized cats. Stimulation of these cortical areas leads to both excitatory and inhibitory effects, though early studies focused primarily on the inhibitory ones (Aguilar, Rivadulla, Soto, & Canedo, 2003; Andersen, Eccles, Oshima, et al., 1964; Andersen, Eccles, Schmidt, et al., 1964). Much like the effect of cortical stimulation, the afferent volley from stimulating the second of two peripheral nerves in close succession is markedly attenuated, suggesting that inhibitory circuitry within CN also contributes to the attenuation of afferent signals (Andersen, Eccles, Oshima, et al., 1964).
The potential functional role of this afferent attenuation was studied more directly by recording medial lemniscus field potentials evoked by stimulation of the tactile superficial radial nerve in cats (Ghez & Pisa, 1972). The resulting afferent volleys, which would have been generated by axons supplying RFs throughout the forearm and paw were attenuated during stepping. Without finer spatial resolution, it would have been difficult to see combined enhancement and attenuation of the effects, if it were there. As a means to determine the mechanism giving rise to the attenuation they applied Wall’s technique (Wall, 1958), which measures the amplitude of the antidromic potential in the peripheral nerve in response to CN stimulation. This amplitude is correlated with the extent of depolarization in the presynaptic terminal, called primary afferent depolarization (PAD), itself an indirect measure of presynaptic inhibition. In these experiments, PAD increased in a velocity-dependent manner throughout a step, suggesting that presynaptic effects on the inputs to CN mediate at least some of the sensory gating of tactile signals.
The problem of the blurring of retinal images during rapid eye movements was recognized already in the 11th century by the Persian scholar Alhazen (Saliba & Sabra, 1992). Over 100 years ago, Holt proposed that vision is simply suppressed during saccades (Holt, 1903), but we now know that a more selective filtering of visual input occurs (Binda & Morrone, 2018). The sensation of a shirtsleeve sliding over the skin during reaching may be analogous to blurred vision during a saccade, contributing noise that the somatosensory system might appropriately attenuate. However, uniformly gating all somatosensory signals during movement, including muscle length changes or unexpected object contact, could cause blindness to critical sources of feedback. In CN, perhaps as in the visual system, there is evidence of gain modulation that is more complex than simple gating (Leiras et al., 2010; Palmeri, Bellomo, Giuffrida, & Sapienza, 1999). The experiments described above that yielded predominantly inhibitory effects in CN (Andersen, Eccles, Oshima, et al., 1964) relied on broadly distributed cortical stimulation. In other experiments that matched the receptive field of the stimulated cortical area to that of the CN neuron, the effect was typically excitatory. As the receptive fields became more dissimilar, the effect of stimulation was more likely to be inhibitory (Palmeri et al., 1999), leading to a “spotlighting” effect.
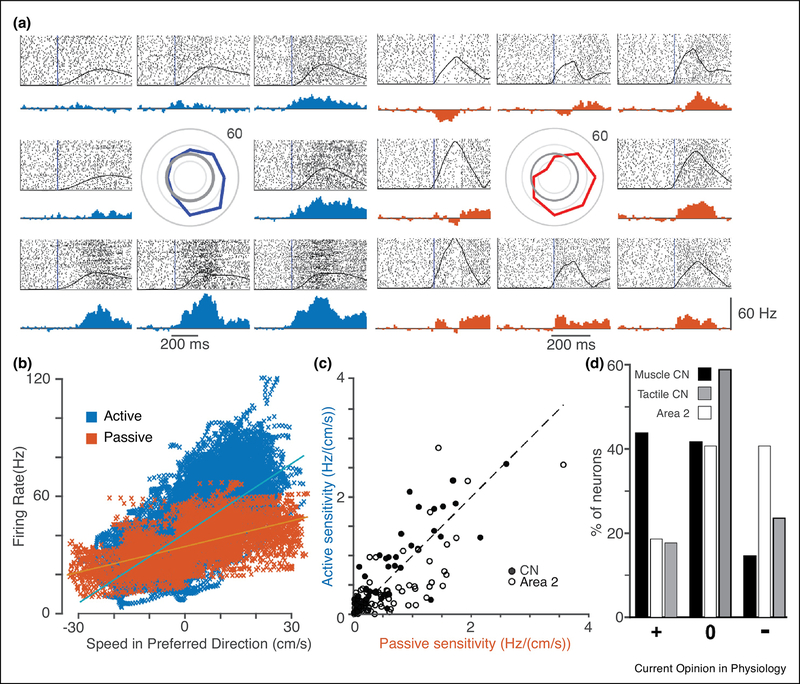
While these results were for CN neurons with cutaneous receptive fields, the idea of more flexible gain modulation might well apply broadly across the somatosensory system. We investigated this question with extracellular recordings from implanted electrode arrays that allowed us to record single CN neurons from awake, behaving monkeys. We compared the movement sensitivity of CN neurons during reaching to that of passive limb perturbations. Fig 1A shows the response of one example neuron that appeared to receive input from the anterior deltoid. We fit sinusoidal tuning curves to the responses and found the neuron’s preferred direction (PD) using simple linear models (Georgopoulos, Kalaska, Caminiti, & Massey, 1982).
Figure 1:
CN neurons with muscle-like inputs tend to respond more strongly to reaching movements than to passive arm perturbations. A) Responses recorded from a single CN neuron that appeared to receive input from muscle spindles in the anterior deltoid. The monkey grasped the handle of a planar manipulandum and made “center-out” movements in eight directions (left group of eight responses). We applied force perturbations in the same eight directions when the hand was at rest in the center-hold position prior to 50% of the movements (right group of responses). Raster plots (above) and trial-averaged firing rate histograms (below) are shown for each movement direction, positioned relative to the center of each group of plots. In the center of the plots is the tuning curve of the neuron. Overlaid on the raster plots are trial averaged hand speed traces for each direction. B) Scatter plot relating the firing rate of the example neuron in A to the hand speed in the PD. Each data point represents a single 10 ms time bin, color coded by condition. Blue and orange lines represent the best linear model fit from hand velocity to firing rate. C) Summary of the active and passive sensitivity of spindle-receiving CN neurons (filled circles, three monkeys,48 neurons) and somatosensory cortical area 2 (open circles, two monkeys, 86 neurons) neurons. D) Percentage of neuros with’ sensitivity that was significantly enhanced (+), was unchanged (0), or was attenuated (−) in the active case compared to the passive case.
In addition to deriving the PD of each neuron, these linear models allowed us to infer the sensitivity of each neuron’s firing rate to the speed of movement. We compared these inferred sensitivities between the active and passive conditions. The slope of the fitted lines in Fig 1B represents the sensitivity for both active (blue) and passive (orange) limb movements. In this example, the sensitivity of the active movements was larger (1.3 Hz/(cm/s)) than that of the of the passive condition (0.8 Hz/(cm/s)). Across all muscle-like CN neurons (those that had receptive fields that resembled muscles with no tactile response), the active sensitivity tended to be greater than the passive sensitivity (Fig 1C, filled circles). To make statistical comparisons between active and passive sensitivity, we used bootstrapping to estimate the confidence interval of the sensitivity difference for each neuron (Efron & Tibshirani, 1986). We then counted those neurons with significantly enhanced or attenuated sensitivity. Across three monkeys, the sensitivity of muscle-like CN neurons was more than twice as likely to be enhanced during active reaching than attenuated (Fig 1D, black bars). There was no such bias in CN neurons with tactile receptive fields (Fig 1D, gray bars). We also performed this analysis for neurons recorded under the same conditions from area 2, a mixed cutaneous and proprioceptive area of cerebral cortex. We found that unlike CN, area 2 neuron sensitivities were somewhat more likely to be attenuated during active movement than enhanced (open symbols and bars, Fig 1 C, D), in contrast with an earlier study whose methods didn’t take into account differences in kinematics and found no significant difference across conditions (London & Miller, 2013). This may reflect additional attenuation that occurs after signals pass through CN, for which there is some evidence (Chapman, Jiang, & Lamarre, 1988; Dale & Cullen, 2017). The functional role of this added inhibition is not clear.
Gain modulation in CN could arise from multiple sources including descending modulatory input to CN, altered gamma drive to muscle spindles, and altered transmission of the afferent input through spinal interneurons. Muscle spindles receive descending gamma drive that directly modulates their sensitivity (Prochazka, Hulliger, Zangger, & Appenteng, 1985). During locomotion, gamma drive is modulated substantially, particularly so during less stereotypic gait (Bennett, De Serres, & Stein, 1996; Ellaway, Taylor, & Durbaba, 2015). Although gamma drive has the potential to explain the context-dependence that we observe in CN, its modulation during reaching has not been well studied and extrapolating to reaching from quadrupedal locomotion in cats is problematic (Jones, Wessberg, & Vallbo, 2001). Experiments using methods insensitive to gamma drive, such as measurement of PAD, have found similar enhancement in proprioceptive spinal interneurons (Confais, Kim, Tomatsu, Takei, & Seki, 2017), evidence that reach-related enhancement is likely not wholly due to alterations in gamma drive. Experiments designed to further identify the site or sites of proprioceptive gain modulation would make an important contribution to our understanding of this system.
Functionally, gain modulation serves at least two purposes. First, it can enhance or attenuate the intensity of the conscious experience of a sensation, as demonstrated in previous psychophysical studies (Juravle, Binsted, & Spence, 2017; Schmidt et al., 1990). Perhaps more importantly, sensory gain must be optimized for motor control. For example, the gain of the stretch reflex is reduced in muscles that would otherwise oppose the generation of fast movements (Adams & Hicks, 2005). Throughout the gait cycle of normal walking, the stretch reflex is maximal during stance and completely suppress in the transition from stance to swing (Sinkjær, Andersen, & Larsen, 1996). Recently, groups have begun to investigate the consequences of disrupting these gain-modulating pathways, leading to profound motor deficits, including oscillatory movements that are consistent with an underdamped feedback control system (Fink et al., 2014). Gain modulation at every level of the somatosensory neuraxis (including fusimotor drive to the spindles) likely underlies the flexibility of multiple hierarchical feedback control loops (Kurtzer, Pruszynski, & Scott, 2008; Nashed, Crevecoeur, & Scott, 2014; Pruszynski et al., 2011; Scott, 2004, 2016; Scott, Cluff, Lowrey, & Takei, 2015; Weiler, Gribble, & Pruszynski, 2019).
Convergence properties in CN and area 2
In addition to selective modulation of gain, sensory afferent pathways may also combine inputs across space and differing modalities. The evidence for such convergence in CN is mixed. In one study, 87% of CN neurons responded to electrical stimulation of more than one peripheral nerve, even across modalities (Witham & Baker, 2011). Other experiments, in which individual muscles were stretched, found very little convergence (Hummelsheim & Wiesendanger, 1985; Rosén & Sjölund, 1973).
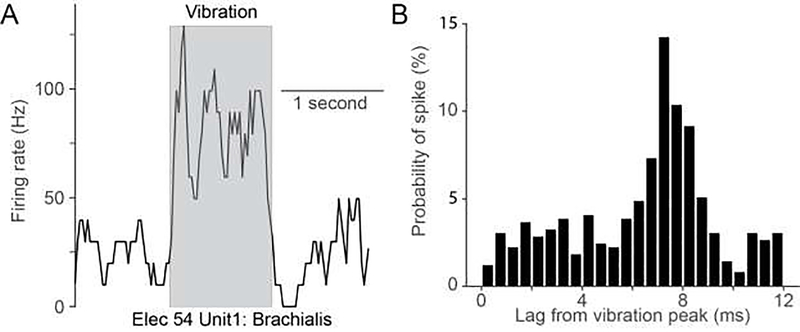
We estimated the extent of convergence in CN with two complementary methods: mapping receptive fields using vibratory stimuli and examining the spatial tuning of single CN neurons during passive arm movements. A good fraction (~50%) of neurons in CN that appeared to have muscle-like receptive fields from manual testing responded robustly to ~100 Hz muscle vibration, a stimulus that strongly activates muscle spindles (Fig 2A). Fig 2B shows a neuron with a phase-locked response to vibration with a lag of ~8 ms from the peak voltage driving the vibrator. Despite these strong responses from individual muscles, it was quite rare that a given CN neuron could be driven by vibration of more than one muscle. Attempts to evoke similar responses in area 2 were uniformly unsuccessful. We speculated that the inability to drive area 2 neurons may be due to their receiving convergent input not only from multiple muscles but also cutaneous afferents, thereby diluting the effect of the spindle input from a single vibrated muscle. It would be informative to repeat this experiment in thalamus and somatosensory cortical area 3a, as both regions have neurons which receive exclusively muscle inputs.
Figure 2:
Neurons in CN are strongly activated by 100 Hz sinusoidal vibration. A) Example CN response to vibration of brachialis. During 100 Hz vibration (grey box), firing rates increased to ~100 Hz, and returned to baseline immediately after stimulation ended. B) Time-dependent probability of the occurrence of the first spike after peak indentation suggests that this example CN neuron was phase-locked to the vibration.
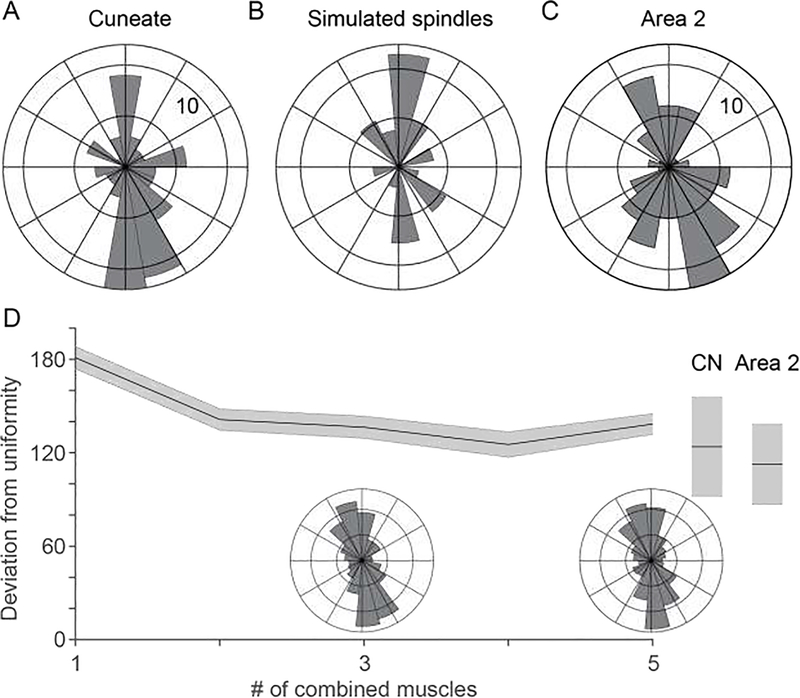
We found a striking nonuniformity in the distribution of CN PDs (Fig. 3A) and asked whether it might also be evidence of limited convergence. To this end, we used DeepLabCut, a markerless motion tracking system (A. Mathis et al., 2018), an OpenSim musculoskeletal model (Chan & Moran, 2006; Delp et al., 2007), and a simple model of the spindle response to muscle length change (a one-half power law mapping muscle lengthening to firing rate (J. C. Houk, Rymer, & Crago, 1981)) to simulate the activity of muscle spindles throughout the 18 major muscles of the arm during the passive limb movements. These simulated muscle spindle PDs were also highly nonuniform, falling primarily along the axis towards and away from the body, qualitatively like that of CN (Fig 3B). We reasoned that convergence of multiple muscles would cause a significantly more uniform distribution.
Figure 3:
Both CN and area 2 appear to inherit strongly bimodal distributions of preferred direction from the biomechanics of the arm during passive limb displacement. A) Distribution of CN preferred directions during passive arm movements. B) PD distribution from a population of simulated proximal arm muscle spindles. C) Distribution of PDs for area 2 neurons for passive arm movements. D) Convergence of simulated muscle spindle afferents from multiple muscles slightly decreases mean absolute deviation from uniformity. Inset polar histograms show the PD distribution for simulated spindles from different numbers of muscles. Deviation from nonuniformity for actual CN and area 2 distributions plotted at the extreme of the plot. Shaded areas indicate one standard deviation of the mean across bootstrap iterations.
However, when we analyzed area 2 similarly, we found those PD distributions to be only slightly more uniform than CN, but not statistically so (Fig 3C). This was unexpected, given out intuiton about convergence and an earlier report of a PD distribution in area 2 that was by eye, more nearly uniform (Prud’homme & Kalaska, 1994). Accordingly, we simulated the PD distributions neurons receiving convergent excitatory and inhibitory inputs from the spindles of different numbers of muscles, examining the changes in distribution with increasing convergence. While this slightly increased the distribution uniformity, the change was considerably less than we anticipated (Fig 3D) indicating that this tool is too crude to address the question of convergence with any precision.
Proprioceptive neuroscience is in need of better tools to precisely measure and control the relevant movement-related variables. Unlike vision or touch, which offer the means to activate receptors with nearly arbitrary spatial and temporal patterns (Killebrew et al., 2007; Korenberg & Naka, 1988), the mechanics of the muscles of the limb cause virtually unavoidable correlations during natural movements (Mollazadeh, Aggarwal, Thakor, & Schieber, 2014; Santello, Flanders, & Soechting, 1998). Opto- and chemogenetic methods are promising, potentially allowing for fine-grained experimental circuit dissection and control of afferent signals during behavior (M. W. Mathis, Mathis, & Uchida, 2017; Sauerbrei et al., 2018; Smith, Bucci, Luikart, & Mahler, 2016; Tashima et al., 2018), including targeted activation of muscle spindle afferents (Kubota et al., 2019).
Proprioceptive streams and their relevance to motor control
For a “hidden” sense, proprioception plays several vital roles. Proprioceptive inputs to the anterior and posterior parietal cortices, as well as to the secondary somatosensory cortex in the superior bank of the Sylvian fissure, contribute individually to a variety of disparate functions, including movement planning, online movement correction, as well as the conscious perception of limb state (Pavlides, Miyashita, & Asanuma, 1993; Rushworth, Nixon, & Passingham, 1997; Wolpert, Goodbody, & Husain, 1998). The ideal location of the dorsal column nuclei to combine and modulate these inputs for the diverse function they subserve (Loutit et al., 2021) is the final topic of this review.
Area 2 is the earliest cortical area with a large proportion of neurons having combined cutaneous and muscle inputs. For this reason, some consider it not to belong with areas 3a, 3b, and 1 as part of S1. The confluence of these inputs within hand area 2 is thought to be important for stereognosis, for which a knowledge of hand conformation combined with object contact points is critical (Gardner, Babu, Ghosh, Sherwood, & Chen, 2007; Rincon-Gonzalez, Warren, Meller, & Helms Tillery, 2011; Yau, Kim, Thakur, & Bensmaia, 2016). The role of arm area 2 is less obvious, but its conjunction of tactile and proprioceptive information may be important in localizing the limb relative to nearby objects in the environment. Its strong connections to area 5 in the posterior parietal cortex reinforce this possibility (Padberg, Cooke, Cerkevich, Kaas, & Krubitzer, 2018).
The posterior parietal cortex (PPC), including area 5, is considered “multimodal association cortex”, neither strictly sensory nor motor, and related to multiple interoceptive and exteroceptive sensory modalities. Interestingly, area 2 neurons retain a prominent force component (Prud’homme & Kalaska, 1994) which is eliminated in area 5, perhaps to accommodate the multimodal convergence with vision in area 7 (Hamel-Pâquet, Sergio, & Kalaska, 2006). In humans, a stroke causing a lesion in the right PPC can cause a profound neglect of the left side of the body. More precise ablations in area 5 of monkeys impair reaching in darkness but not in light, while area 7 lesions have the opposite effect: reaching in the light is impaired, but not in darkness (Rushworth et al., 1997). A human patient with a lesion in area 5 “loses” her arm when it leaves her view for more than a few seconds, but it returns when it becomes visible again (Wolpert et al., 1998). This apparent role of PPC in updating limb position is closely related to its contribution to movement planning and may also involve the secondary somatosensory cortex.
The descending connections to CN from the sensorimotor cortex suggest that CN has a key role in flexibly modulating the gain of somatosensory input, although this would not rule out other lower-level mechanisms. Such gain modulation could serve to focus attention on a class of receptors or a portion of the limb under different behavioral contexts, and may also be necessary to generate complex, context-dependent reflex activity. Experiments to monitor the inputs to CN, for example in the dorsal root ganglia, under similar behavioral conditions, will be an important next step in understanding this processing.
Our own results, including sensory mappings of proprioceptive CN neurons and recording of their activity during behavior, point to a CN that receives potent connections from only a small number of afferent inputs. This finding is not altogether surprising—recent evidence shows that only a small number of synapses (4–8 for cutaneous receptors) dominate the firing of CN neurons despite far larger numbers of synapses observed anatomically on these neurons (Bengtsson, Brasselet, Johansson, Arleo, & Jörntell, 2013). This anatomical rather than physiological observation may also underlie the discrepancy with the much broader convergence estimates based on electrical stimulation that might synchronously recruit more of these afferents (Witham & Baker, 2011). The purpose of this apparently broad, yet weak convergence from the periphery is still an open question. One possible answer is that it may enable greater plasticity in sensory processing. Much like the analogous pruning process in the cerebral cortex, CN has many inputs that are lost late in development as descending corticobulbar fibers invade the dorsal column nuclei (Fisher & Clowry, 2009). Furthermore, recent studies have shown that the change in cortical representation observed after loss of peripheral input (Jain, Qi, Collins, & Kaas, 2008) has its origin in dorsal column remapping (Kambi et al., 2014). These observations suggest that both in development and in recovery from injury, CN may optimize the strength of its diverse peripheral inputs for the proprioceptive functions carried out by more central brain structures.
Summary
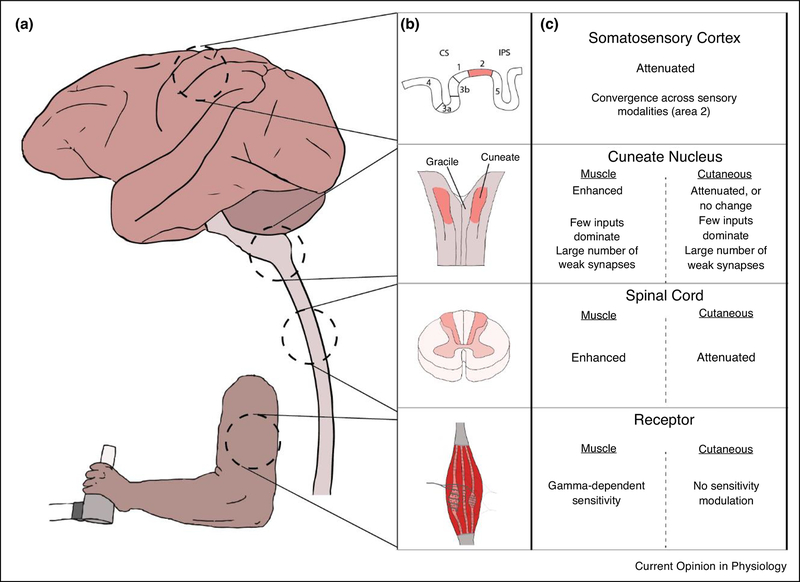
Figure 4 presents a high-level summary of the convergence and sensitivity properties of somatic sensation presented in this review. In the periphery, the sensitivity of muscle spindles, the main receptor considered in this review, is modulated in a complex, behavior-dependent manner by descending gamma drive. Golgi tendon organs, which signal force, and cutaneous receptors lack this descending control. Additional mechanisms within the spinal cord allow the sensitivity to all somatosensory modalities to be modulated. As a general rule, muscle afferent input is primarily enhanced during active movement, while cutaneous input is attenuated. Gain modulation in the spinal cord is critically important for spinal motor circuitry to produce controlled movements. Within the main cuneate nucleus, this modality-dependent movement sensitivity is largely maintained. At this first site for convergence between afferents in the brain, there appears to be quite low behaviorally-relevant convergence between muscle inputs during typical movements, although there is some evidence that multiple cutaneous submodalities (i.e., rapidly adapting and slowly adapting receptors) may converge on single CN neurons (Suresh et al., 2017). There is also evidence of a larger number of latent synapses during development and recruited in response to injury that may only contribute meaningfully to firing when they are activated with an unusually high intensity, such as by electrical stimulation of the peripheral nerve. Finally, neurons within area 2 of the somatosensory cortex are the first neurons in the cortical somatosensory pathway for which there is clear evidence of broad convergence across muscle and tactile modalities. Its anatomical position between the single-modality primary somatosensory areas and the even more broadly convergent receptive fields of posterior parietal cortex suggest an early role in the development of an internal body map for planning and controlling movement. How the more uniform (relative to CN) attenuation of input during movement might relate to such a functional role remains unclear.
Figure 4:
Overview of convergence and sensitivity properties along the somatosensory neuraxis A) Diagram of somatosensory areas discussed in this review. From top to bottom, in dashed circles: somatosensory cortex (adapted from a Scalable Brain Atlas of the macaque brain (Bakker, Tiesinga, & Kötter, 2015), dorsal column nuclei, spinal cord, and muscle receptors. Those areas most relevant to this review are expanded in B) and highlighted in light red. C) Summaries of the convergence and sensitivity in each of these areas. For brevity, we condensed a complex literature to the primary direction of sensitivity modulation (“attenuated” or “enhanced”), together with a similarly high-level overview of the convergence at each region. See text and references for more nuanced detail.
Acknowledgments
This work was supported by grants from the NINDS to Miller (R01 NS095162, R01 NS095251) and Versteeg (F31 NS092356).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, & Hicks AL (2005). Spasticity after spinal cord injury. Spinal Cord, 43, 577–586. 10.1038/sj.sc.3101757 [DOI] [PubMed] [Google Scholar]
- Aguilar J, Rivadulla C, Soto C, & Canedo A (2003). New Corticocuneate Cellular Mechanisms Underlying the Modulation of Cutaneous Ascending Transmission in Anesthetized Cats. Journal of Neurophysiology, 89(6), 3328–3339. 10.1152/jn.01085.2002 [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Oshima T, & Schmidt RF (1964). Mechanisms of synaptic transmission in the Cuneate Nucleus. Journal of Neurophysiology, 27(6), 1096–1116. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14223972 [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, & Yokota T (1964). Identification of relay cells and interneurons in the Cuneate Nucleus. Journal of Neurophysiology, 27(6), 1080–1095. 10.1152/jn.1964.27.6.1080 [DOI] [PubMed] [Google Scholar]
- Azim E, & Seki K (2019). Gain control in the sensorimotor system This review comes from a themed issue on Motor control systems. Current Opinion in Physiology, 8, 177–187. 10.1016/j.cophys.2019.03.005This review explores sensory gain modulation across many sensory systems, from the lamprey olfactory bulb to monkey muscle spindles. It presents a broad overview of the different roles of gain modulation, as well as deficits associated with its loss. The authors review both novel and previously published experiments in which sensory gain changes enhance or attenuate incoming afferent signals, perhaps to reduce sensory noise and to finetune inputs for use by the motor systems. In one experiment, experimenters demonstrated that the sensitivity to muscle and cutaneous input of cervical spinal neurons differs qualitatively. As in our results from CN, the sensitivity of neurons receiving muscle inputs was enhanced during active movement, while the sensitivity of neurons receiving cutaneous input was attenuated.
- Bakker R, Tiesinga P, & Kötter R (2015). The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics, 13(3), 353–366. 10.1007/s12021-014-9258-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Brasselet R, Johansson RS, Arleo A, & Jörntell H (2013). Integration of Sensory Quanta in Cuneate Nucleus Neurons In Vivo. PLoS ONE, 8(2). 10.1371/journal.pone.0056630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, De Serres SJ, & Stein RB (1996). Regulation of soleus muscle spindle sensitivity in decerebrate and spinal cats during postural and locomotor activities. Journal of Physiology, 495(3), 835–850. 10.1113/jphysiol.1996.sp021636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda P, & Morrone MC (2018). Vision During Saccadic Eye Movements. Annual Review of Vision Science, 4(1), 193–213. 10.1146/annurev-vision-091517-034317 [DOI] [PubMed] [Google Scholar]
- Bremmer F, Kubischik M, Hoffmann KP, & Krekelberg B (2009). Neural dynamics of saccadic suppression. Journal of Neuroscience, 29(40), 12374–12383. 10.1523/JNEUROSCI.2908-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SS, & Moran DW (2006). Computational model of a primate arm: from hand position to joint angles, joint torques and muscle forces. J. Neural Eng, 3, 327–337. 10.1088/1741-2560/3/4/010 [DOI] [PubMed] [Google Scholar]
- Chapman CE, Jiang W, & Lamarre Y (1988). Modulation of lemniscal input during conditioned arm movements in the monkey. Experimental Brain Research, 72(2), 316–334. 10.1007/BF00250254 [DOI] [PubMed] [Google Scholar]
- Cohen LG, & Starr A (1987). LOCALIZATION, TIMING AND SPECIFICITY OF GATING OF SOMATOSENSORY EVOKED POTENTIALS DURING ACTIVE MOVEMENT IN MAN (Vol. 110). Retrieved from https://academic.oup.com/brain/article-abstract/110/2/451/467280 [DOI] [PubMed] [Google Scholar]
- Confais J, Kim G, Tomatsu S, Takei T, & Seki K (2017). Nerve-specific input modulation to spinal neurons during a motor task in the monkey. Journal of Neuroscience, 37(10), 2612–2626. 10.1523/JNEUROSCI.2561-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JD, Larson B, Oscarsson O, & Sjölund B (1971). Origin and termination of cuneocerebellar tract. Experimental Brain Research, 13(4), 339–358. 10.1007/BF00234336 [DOI] [PubMed] [Google Scholar]
- Crevecoeur F, & Kording KP (2017). Saccadic suppression as a perceptual consequence of efficient sensorimotor estimation. ELife, 6. 10.7554/eLife.25073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A, & Cullen KE (2017). The Ventral Posterior Lateral Thalamus Preferentially Encodes Externally Applied Versus Active Movement: Implications for Self-Motion Perception. Cerebral Cortex, 29(1), 305–318. 10.1093/cercor/bhx325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, … Thelen DG (2007). OpenSim: Open-Source Software to Create and Analyze Dynamic Simulations of Movement. IEEE Transactions on Biomedical Engineering, 54(11), 1940–1950. 10.1109/TBME.2007.901024 [DOI] [PubMed] [Google Scholar]
- Efron B, & Tibshirani R (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science, 1(1), 54–75. 10.1214/ss/1177013815 [DOI] [Google Scholar]
- Ellaway PH, Taylor A, & Durbaba R (2015, August 1). Muscle spindle and fusimotor activity in locomotion. Journal of Anatomy. Blackwell Publishing Ltd. 10.1111/joa.12299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AJP, Croce KR, Huang ZJ, Abbott LF, Jessell TM, & Azim E (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature, 509(7498), 43–48. 10.1038/nature13276These authors examined the critical role of presynaptic inhibition in the spinal cord in the generation of motor behavior in mice. One experiment selectively targeted a class of spinal interneurons that synapse onto presynaptic terminals connecting proprioceptive afferents to motor neurons. Activation of these neurons produced primary afferent depolarization (PAD), the hallmark of presynaptic inhibition. A second experiment selectively ablated this class of interneurons, producing motor oscillations that disrupted reaching. The oscillations would be modeled with an underdamped sensory-motor reflex loop. These experiments suggest that presynaptic inhibition in the SC is critical to prevent undamped feedback during movement.
- Fisher T, & Clowry GJ (2009). Elimination of muscle afferent boutons from the cuneate nucleus of the rat medulla during development. Neuroscience, 161(3), 787–793. 10.1016/j.neuroscience.2009.04.009This paper examines the developmental pruning of proprioceptive inputs to CN in the rat. Using a transganglionic labelling toxin, experimenters measured the density of synapses in the CN at different timepoints in development. They found a dramatic, 40% reduction in the number of synaptic boutons from muscle receptors in CN from postnatal days 7 to 42. The simultaneous invasion of corticocuneate fibers during this developmental window suggests that descending drive may play a part in organizing the ascending inputs, possibly providing signaling needed to generate the cortical somatotopic map.
- Gardner EP, Babu KS, Ghosh S, Sherwood A, & Chen J (2007). Neurophysiology of Prehension. III. Representation of Object Features in Posterior Parietal Cortex of the Macaque Monkey. Journal of Neurophysiology, 98(6), 3708–3730. 10.1152/jn.00609.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, & Massey JT (1982). On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 2(11), 1527–1537. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7143039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, & Pisa M (1972). Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Research, 40(1), 145–155. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4338259 [DOI] [PubMed] [Google Scholar]
- Hamel-Pâquet C, Sergio LE, & Kalaska JF (2006). Parietal area 5 activity does not reflect the differential time-course of motor output kinetics during arm-reaching and isometric-force tasks. Journal of Neurophysiology, 95(6), 3353–3370. 10.1152/jn.00789.2005 [DOI] [PubMed] [Google Scholar]
- Holt EB (1903). Eye-movement and central anaesthesia. The Psychological Review: Monograph Supplements, 4(1), 1–45. Retrieved from https://psycnet.apa.org/record/1926-02629-001 [Google Scholar]
- Houk JC, Rymer WZ, & Crago PE (1981). Dependence of dynamic response of spindle receptors on muscle length and velocity. Journal of Neurophysiology, 46(1), 143–166. 10.1152/jn.1981.46.1.143 [DOI] [PubMed] [Google Scholar]
- Houk J, & Simon W (1967). Responses of Golgi tendon organs to forces applied to muscle tendon. Journal of Neurophysiology, 30(6), 1466–1481. 10.1152/jn.1967.30.6.1466 [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, & Wiesendanger M (1985). Neuronal responses of medullary relay cells to controlled stretches of forearm muscles in the monkey. Neuroscience, 16(4), 989–996. 10.1016/0306-4522(85)90111-3 [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger R, Wiesendanger M, & Bianchetti M (1985). The projection of low-threshold muscle afferents of the forelimb to the main and external cuneate nuclei of the monkey. Neuroscience, 16(4), 979–987. 10.1016/0306-4522(85)90110-1 [DOI] [PubMed] [Google Scholar]
- Jain N, Qi HX, Collins CE, & Kaas JH (2008). Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. Journal of Neuroscience, 28(43), 11042–11060. 10.1523/JNEUROSCI.2334-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, & Vallbo ÅB (2001). Directional tuning of human forearm muscle afferents during voluntary wrist movements. Journal of Physiology, 536(2), 635–647. 10.1111/j.1469-7793.2001.0635c.xd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juravle G, Binsted G, & Spence C (2017). Tactile suppression in goal-directed movement. Psychonomic Bulletin and Review, 24(4), 1060–1076. 10.3758/s13423-016-1203-6 [DOI] [PubMed] [Google Scholar]
- Kambi N, Halder P, Rajan R, Arora V, Chand P, Arora M, & Jain N (2014). Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nature Communications, 5(1), 1–10. 10.1038/ncomms4602When the dorsal columns are damaged, cortical receptive field boundaries shift, causing the deafferented region to be invaded by inputs from surrounding receptive fields. This has been interpreted as a consequence of cortical plasticity, but this paper challenges that interpretation. The researchers exposed the trigeminal nucleus and the face area of cortex in monkeys, months after cutting the dorsal roots innervating the hand. Lidocaine injected into the trigeminal nucleus blocked the remapped hand inputs, which remained intact following lidocaine injection in the face somatosensory cortex. These results suggest that remapping at the level of DCN is responsible for the altered cortical representation, not cortical plasticity per se.
- Killebrew JH, Bensmaïa SJ, Dammann JF, Denchev P, Hsiao SS, Craig JC, & Johnson KO (2007). A dense array stimulator to generate arbitrary spatio-temporal tactile stimuli. Journal of Neuroscience Methods, 161(1), 62–74. 10.1016/j.jneumeth.2006.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenberg MJ, & Naka KI (1988). White-noise analysis in visual neuroscience. Visual Neuroscience, 1(3), 287–296. 10.1017/S0952523800001942 [DOI] [PubMed] [Google Scholar]
- Kubota S, Sidikejiang W, Kudo M, Inoue K, Umeda T, Takada M, & Seki K (2019). Optogenetic recruitment of spinal reflex pathways from large-diameter primary afferents in non-transgenic rats transduced with AAV9/Channelrhodopsin 2. The Journal of Physiology, 597(19), 5025–5040. 10.1113/JP278292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, & Scott SH (2008). Long-Latency Reflexes of the Human Arm Reflect an Internal Model of Limb Dynamics. Current Biology, 18(6), 449–453. 10.1016/J.CUB.2008.02.053 [DOI] [PubMed] [Google Scholar]
- Leiras R, Velo P, Martín-Cora F, & Canedo A (2010). Processing Afferent Proprioceptive Information at the Main Cuneate Nucleus of Anesthetized Cats. Journal of Neuroscience, 30(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- London BM, & Miller LE (2013). Responses of somatosensory area 2 neurons to actively and passively generated limb movements. Journal of Neurophysiology, 109(6), 1505–1513. 10.1152/jn.00372.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutit AJ, Vickery RM, & Potas JR (2021). Functional organization and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub. Journal of Comparative Neurology, 529(1), 187–220. 10.1002/cne.24942 [DOI] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, & Bethge M (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nature Neuroscience, 21(9), 1281–1289. 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- Mathis MW, Mathis A, & Uchida N (2017). Somatosensory Cortex Plays an Essential Role in Forelimb Motor Adaptation in Mice. Neuron, 93(6), 1493–1503.e6. 10.1016/j.neuron.2017.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollazadeh M, Aggarwal V, Thakor NV, & Schieber MH (2014). Principal components of hand kinematics and neurophysiological signals in motor cortex during reach to grasp movements. Journal of Neurophysiology, 112(8), 1857–1870. 10.1152/jn.00481.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB (2011). Central Nervous Mechanisms in Mechanoreceptive Sensibility. In Comprehensive Physiology (pp. 789–878). Hoboken, NJ, USA: John Wiley & Sons, Inc. 10.1002/cphy.cp010318 [DOI] [Google Scholar]
- Nashed JY, Crevecoeur F, & Scott SH (2014). Rapid online selection between multiple motor plans. Journal of Neuroscience, 34(5), 1769–1780. 10.1523/JNEUROSCI.3063-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Cooke DF, Cerkevich CM, Kaas JH, & Krubitzer L (2018). Cortical connections of area 2 and posterior parietal area 5 in macaque monkeys. Journal of Comparative Neurology. 10.1002/cne.24453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeri A, Bellomo M, Giuffrida R, & Sapienza S (1999). Motor cortex modulation of exteroceptive information at bulbar and thalamic lemniscal relays in the cat. Neuroscience, 88(1), 135–150. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10051195 [DOI] [PubMed] [Google Scholar]
- Pavlides C, Miyashita E, & Asanuma H (1993). Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. Journal of Neurophysiology, 70(2), 733–741. 10.1152/jn.1993.70.2.733 [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, & Appenteng K (1985). “Fusimotor set”: new evidence for α-independent control of γ-motoneurones during movement in the awake cat. Brain Research, 339(1), 136–140. 10.1016/0006-8993(85)90632-8 [DOI] [PubMed] [Google Scholar]
- Proske U, & Gandevia SC (2012). The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiological Reviews, 92(4), 1651–1697. 10.1152/physrev.00048.2011 [DOI] [PubMed] [Google Scholar]
- Prud’homme MJ, & Kalaska JF (1994). Proprioceptive activity in primate primary somatosensory cortex during active arm reaching movements. Journal of Neurophysiology, 72(5), 2280–2301. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7884459 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, & Scott SH (2011). Primary motor cortex underlies multi-joint integration for fast feedback control. Nature, 478(7369), 387–390. 10.1038/nature10436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Gonzalez L, Warren JP, Meller DM, & Helms Tillery S (2011). Haptic interaction of touch and proprioception: Implications for neuroprosthetics. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 19(5), 490–500. 10.1109/TNSRE.2011.2166808 [DOI] [PubMed] [Google Scholar]
- Rosén I, & Sjölund B (1973). Organization of group I activated cells in the main and external cuneate nuclei of the cat: identification of muscle receptors. Experimental Brain Research, 16(3), 221–237. 10.1007/bf00233327 [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, & Passingham RE (1997). Parietal cortex and movement. I. Movement selection and reaching. Experimental Brain Research, 117(2), 292–310. 10.1007/s002210050224 [DOI] [PubMed] [Google Scholar]
- Saliba G, & Sabra AI (1992). The Optics of Ibn Al-Haytham: Books I-III, on Direct Vision. Journal of the American Oriental Society, 112(3), 528. 10.2307/603118 [DOI] [Google Scholar]
- Santello M, Flanders M, & Soechting JF (1998). Postural hand synergies for tool use. Journal of Neuroscience, 18(23), 10105–10115. 10.1523/jneurosci.18-23-10105.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbrei B, Guo JZ, Mischiati M, Guo W, Kabra M, Verma N, … Hantman A (2018, February 15). Motor cortex is an input-driven dynamical system controlling dexterous movement. BioRxiv. bioRxiv 10.1101/266320 [DOI] [Google Scholar]
- Schmidt RF, Schady WJL, & Torebjörk HE (1990). Gating of tactile input from the hand - I. Effects of finger movement. Experimental Brain Research, 79(1), 97–102. 10.1007/BF00228877 [DOI] [PubMed] [Google Scholar]
- Scott SH (2004). Optimal feedback control and the neural basis of volitional motor control. Nature Reviews Neuroscience. Nature Publishing Group. 10.1038/nrn1427 [DOI] [PubMed] [Google Scholar]
- Scott SH (2016). A Functional Taxonomy of Bottom-Up Sensory Feedback Processing for Motor Actions. Trends in Neurosciences, 39(8), 512–526. 10.1016/j.tins.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Scott SH, Cluff T, Lowrey CR, & Takei T (2015). Feedback control during voluntary motor actions. Current Opinion in Neurobiology, 33, 85–94. 10.1016/j.conb.2015.03.006 [DOI] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, & Larsen B (1996). Soleus stretch reflex modulation during gait in humans. Journal of Neurophysiology, 76(2), 1112–1120. 10.1152/jn.1996.76.2.1112 [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, & Mahler SV (2016, April 1). DREADDs: Use and application in behavioral neuroscience. Behavioral Neuroscience. American Psychological Association Inc. 10.1037/bne0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh AK, Winberry JE, Versteeg C, Chowdhury R, Tomlinson T, Rosenow JM, … Bensmaia SJ (2017). Methodological considerations for a chronic neural interface with the cuneate nucleus of macaques. Journal of Neurophysiology, 118(6), 3271–3281. 10.1152/jn.00436.2017This paper describes the methods used to implant multi-electrode arrays chronically in the dorsal brainstem of monkeys, allowing the first-ever recordings of single CN neurons during behavior. Fields in the medial lemniscus were recorded previously from cats during stepping (Ghez & Pisa, 1972). The surgical procedures that led, eventually, to a high success rate and yield of neuron are described. In this paper the authors describe a topography of CN, including a detailed somatotopic mapping that guided electrode array design specifications, and the first single neuron proprioceptive signals recorded in CN of an awake behaving animal.
- Tashima R, Koga K, Sekine M, Kanehisa K, Kohro Y, Tominaga K, … Tsuda M (2018). Optogenetic activation of non-nociceptive aβ fibers induces neuropathic pain-like sensory and emotional behaviors after nerve injury in rats. ENeuro, 5(1). 10.1523/ENEURO.0450-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD (1958). Excitability changes in afferent fibre terminations and their relation to slow potentials. The Journal of Physiology, 142(1), i3–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16992026 [PMC free article] [PubMed] [Google Scholar]
- Weiler J, Gribble PL, & Pruszynski JA (2019). Spinal stretch reflexes support efficient hand control. Nature Neuroscience, 22(4), 529–533. 10.1038/s41593-019-0336-0 [DOI] [PubMed] [Google Scholar]
- Witham CL, & Baker SN (2011). Modulation and transmission of peripheral inputs in monkey cuneate and external cuneate nuclei. Journal of Neurophysiology, 106(5), 2764–2775. 10.1152/jn.00449.2011In this paper, the experimenters studied the convergence properties of CN neurons in the anaesthetized monkey. They recorded extracellularly from both CN and external cuneate nucleus neurons while stimulating various peripheral nerves. A large percentage of neurons responded to the electrical stimulation of more than a single peripheral nerve, evidence of wide convergence across both receptor modality and location. These effects were found for both primarily proprioceptive and primarily cutaneous-responsive CN neurons, as well as neurons in the cerebellar-projecting external cuneate. These results present an interesting contrast to our own results and earlier results (Hummelsheim, Wiesendanger, Wiesendanger, & Bianchetti, 1985), which yielded much lower estimates of convergence.
- Wolpert DM, Goodbody SJ, & Husain M (1998). Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience, 1(6), 529–533. 10.1038/2245 [DOI] [PubMed] [Google Scholar]
- Yau JM, Kim SS, Thakur PH, & Bensmaia SJ (2016, February 1). Feeling form: The neural basis of haptic shape perception. Journal of Neurophysiology. American Physiological Society. 10.1152/jn.00598.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]