Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters host cells by interacting with membrane-bound angiotensin-converting enzyme 2 (ACE2), a vital element in the renin–angiotensin system (RAS), which regulates blood pressure, fluid balance, and cardiovascular functions. We herein evaluate existing evidence for the molecular alterations within the RAS pathway (e.g., ACE2 and angiotensin II) during SARS-CoV-2 infection and subsequent Coronavirus Disease 2019 (COVID-19). This includes reports regarding potential effect of RAS blockade (e.g., ACE inhibitors and angiotensin II receptor blockers) on ACE2 expression and clinical outcomes in patients with co-morbidities commonly treated with these agents. The collective evidence suggests a dual role for ACE2 in COVID-19, depending on the stage of infection and the coexisting diseases in individual patients. This information is further discussed with respect to potential therapeutic strategies targeting RAS for COVID-19 treatment.
Keywords: SARS-CoV-2, COVID-19, Renin-angiotensin system (RAS), Angiotensin-converting enzyme 2 (ACE2), Angiotensin II (Ang II), RAS blockade
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) uses angiotensin-converting enzyme 2 (ACE2) as a receptor for cell entry and, therefore, might have an effect on the renin–angiotensin system (RAS) in the body [1], [2]. RAS imbalance has been implicated in the pathogenesis of hypertension, heart failure, and chronic kidney disorder, which are commonly treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). Notably, worse outcomes are reported in COVID-19 patients with co-morbidities commonly treated with these agents [3]. The involvement of ACE2 in SARS-CoV-2 infection, and the speculation regarding the harmful or beneficial effects of RAS blockade medications [4], [5] has fueled debate over the exact role of the RAS in COVID-19 pathophysiology and clinical outcomes.
SARS-CoV-2 viral entry is mainly mediated via the respiratory system, where ACE2 is abundantly expressed in nasal and oral mucosa and lung alveolar epithelium [6], [7]. ACE2 is a vital element in the RAS pathway, catalyzing the conversion of a peptide hormone angiotensin I (Ang I) to Ang 1–9 as well as angiotensin II (Ang II) into Ang 1–7, thereby counteracting the harmful effects of Ang II [8], [9], [10]. ACE2 is mostly bound to the cell membrane, mediating SARS-CoV-2 viral entry, while also existing in circulation as a soluble form. SARS-CoV-2 infection is thought to reduce ACE2 surface expression in target cells, leading to local or systemic RAS imbalance and tissue damage [11], [12]. The rationale of the concept stems from previous studies on SARS-CoV [13], [14]. Kuba et al. showed that SARS-CoV spike protein binding to ACE2 significantly reduced ACE2 cell surface expression in the HEK293 cell line and in the lungs of SARS-CoV-infected mice [13]. In a separate study, mouse pulmonary SARS-CoV infection resulted in ACE2-dependent myocardial infection and downregulation of ACE2 mRNA and protein expression in heart tissue [14]. Consistent with the observations in mouse models, immunohistochemistry of autopsied heart tissue samples from patients who died from SARS-CoV infection revealed the presence of SARS-CoV and marked reduction of ACE2 protein expression in heart tissue [14]. Given the sequence homology of the spike proteins in the two coronaviruses, SARS-CoV-2 might follow a similar mode of action to downregulate ACE2 expression, thereby depriving the lungs and other organs of an important protection mechanism against viral infection. However, the precise relationship between ACE2 protein levels, viral infectivity, and severity of SARS-CoV-2 infection is not fully understood.
COVID-19 is often worse in patients with pre-existing health conditions, including hypertension, cardiovascular disease, diabetes, and chronic kidney disorder [15]. These conditions are associated with an overactive RAS and are commonly treated with ACEIs or ARBs [16]. These agents are reported to upregulate ACE2 expression in animal models, raising concern about the safety of RAS blockade therapy in patients with COVID-19. There has been speculation that RAS blockade might increase susceptibility to SARS-CoV-2 infection and the likelihood of severe illness from COVID-19 [5]. However, recent observational studies suggested that ACEIs and ARBs would be helpful in treating COVID-19 [17], [18]. A possible explanation behind this paradox might lie in the stage of infection and the presence of co-morbidities (e.g., hypertension, diabetics, heart failure, or chronic kidney disorder) commonly treated with these agents. In this review, we provide a comprehensive analysis of current evidence for the spatiotemporal expression of ACE2, including membrane-bound ACE2 and the circulating soluble form, and its dynamic interplay with Ang II during SARS-CoV-2 infection. We also discuss the potential therapeutic targets within the RAS pathway for COVID-19 treatment.
SARS-CoV-2 host interaction
SARS-CoV-2 uses ACE2 as a functional receptor to infect human epithelial cells [19]. In particular, the receptor-binding domain (RBD) of the S1 subunit of the spike protein directly binds to the peptidase domain (PD) of ACE2 [20], [21], [22]. Following binding to ACE2, SAR-CoV-2 can enter host cells by two distinct mechanisms. First, the spike protein of the SARS-CoV-2 might be processed by transmembrane serine protease 2 (TMPRSS2) at the plasma membrane. The priming cleaves spike protein into the S1 and S2 subunits, revealing the S2 fusion peptide and facilitating membrane fusion with the host cell surface [1]. Alternatively, in cells where TMPRSS2 activity is insufficient, SARS-CoV-2 can enter the cell through endocytosis, in which endosomal proteases, such as cathepsin B and cathepsin L, facilitate S protein priming [23]. Through both mechanisms, receptor binding can result in internalization of the virus-containing complexes together with ACE2, resulting in potential loss of ACE2 receptor expression at the cell surface [13], [23] and subsequent release of genetic material, amplification, and exocytosis of virion particles, leading to viral spread in the body. Alternatively, SARS-CoV-2 viral entry might also be mediated through ACE2-independent pathways that involve CD209L/L-SIGN and CD209/DC-SIGN [24].
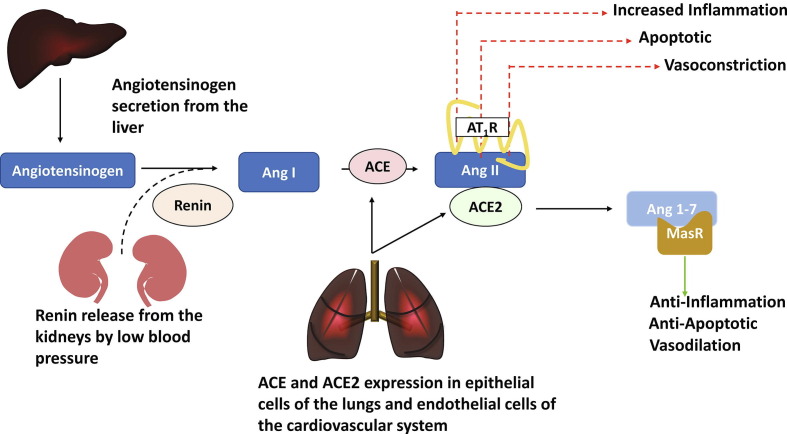
ACE2 is a vital element of the RAS, a crucial hormone cascade that regulates blood pressure, body fluid, and electrolyte balance [8], [9], [25]. The system operates through two complementary yet opposing biochemical pathways: the ACE2/Ang 1–7/MasR pathway versus the ACE/Ang II/AT1R pathway (Fig. 1 ). Ang II binds to the angiotensin II type 1 receptor (AT1R), which activates several signaling cascades to elicit potent vasoconstriction, fibrotic, and proinflammatory effects [26]. By contrast, the ACE2/Ang 1–7/MasR pathway antagonizes the harmful effects of Ang II by utilizing ACE2 to convert Ang II into Ang 1–7, triggering several signaling pathways to produce beneficial effects, including vasodilation, antiapoptosis, and anti-inflammation (Fig. 1) [27], [28], [29]. Under healthy conditions, the RAS is fine-tuned to keep the right levels of Ang II, thereby ensuring normal blood pressure, electrolyte balance, as well as cardiovascular and kidney functions. However, overexpression of Ang II can cause hypertension or fibrous tissue to build up in the heart, kidneys, and lungs, leading to multiorgan dysfunction. An increase in Ang II levels has been implicated in the pathogenesis of hypertension [30], heart failure [31], and renal dysfunction [32]. Notably, these conditions are frequent comorbidities in patients with COVID-19 who develop severe illness [33]. Observational studies have shown that plasma Ang II levels were significantly elevated in patients with severe COVID-19 compared with mild cases [34], [35], [36]. Increases in Ang II levels resulting from possible SARS-CoV-2-mediated loss of ACE2 function could drive severe lung injury, as observed in patients critically ill with COVID-19 [13], [34], [36]. However, the exact interplay between RAS components and COVID-19 severity is not yet fully understood.
Figure 1.
Circulating renin–angiotensin system (RAS). RAS begins with angiotensinogen secretion from the liver and renin release from kidneys, stimulated by low blood arterial pressure and reduced sodium chloride levels. Renin converts angiotensinogen to angiotensin I (Ang I). Angiotensin-converting enzyme (ACE), expressed in endothelial cells of multiple organs, including those within the cardiovascular system, acts on Ang I to produce the principal pressor hormone angiotensin II (Ang II). Ang II interacts with the Ang II type 1 receptor (AT1R) to regulate a variety of effector functions, including vascular contraction, endothelial function, protein synthesis, fibrosis, hypertrophy, and inflammation. Alternatively, Ang II can also be cleaved by ACE2 to form angiotensin 1–7. The physiological functions of angiotensin 1–7 are mediated through the Mas receptor. These are beneficial responses that antagonize the harmful effects of Ang II/AT1R.
ACE2 distribution and regulation
ACE2 is abundantly expressed in the nasal and oral mucosa and lung alveolar epithelium [6], which might explain why SARS-CoV-2 transmits via respiratory droplets and causes primary lung injury. ACE2 is also expressed in the heart, kidneys, liver, testes, intestine, brain, and other tissues [6], [37]. It is speculated that SARS-CoV-2 virus, following initial respiratory entry, travels through the bloodstream to infect other organs expressing ACE2. This is supported by reports that clinical samples of saliva, stools, and urine of patients with COVID-19 tested positive for the SARS-CoV-2 virus [38]. ACE2 expression is affected by a variety of physiological factors and pathological conditions. Clinical evidence shows that ACE2 expression is upregulated in hypertension [39], heart failure [40], and chronic kidney disorder [41] despite hyperactivation of the RAS and upregulated ACE/Ang II/AT1R signaling often observed in these diseases. Thus, innate physiological efforts to counterbalance these effects and reduce Ang II levels could explain the observed increases in endogenous ACE2 expression, suggesting an important feedback mechanism at play [42]. Indeed, ACE2 gene and protein levels were significantly increased in mice models following induction of severe acute pancreatitis (SAP) [43]. We suspect the elevated ACE2 levels in patients with underlying comorbidities are linked to severe outcomes in COVID-19 by enhancing virus uptake.
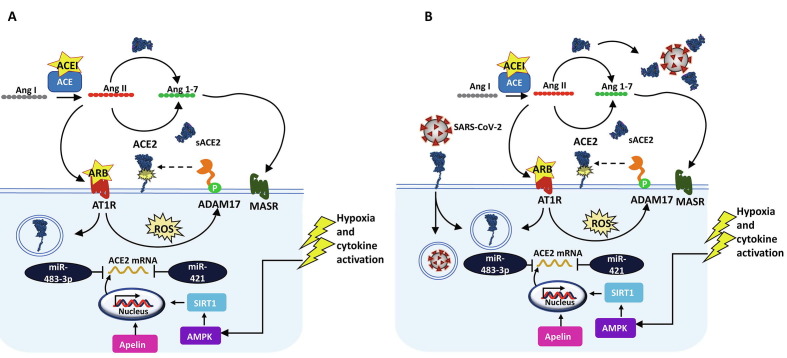
ACE2 expression is regulated in a spatiotemporal manner. At the transcriptional level, ACE2 mRNA expression is stimulated by sirtuin 1 (SIRT1) or apelin under energy stress conditions (e.g., hypoxia) (Fig. 2 ). Several post-transcriptional factors can suppress ACE2 expression, such as miRNA-421, by blocking translation of the mRNA transcript [44]. ACE2 is also subjected to post-translational regulation via Ang II feedback mechanisms. ACE2 expression can be downregulated through Ang II/AT1R-mediated internalization and degradation [45]. Conversely, elevated Ang II levels can promote activation of a disintegrin metalloproteinase 17 (ADAM17), resulting in proteolytic cleavage of ACE2 at its extracellular domain and subsequent release of a soluble form into the blood circulation [46]. The role of the soluble ACE2 is yet to be understood but might serve as a scavenger or decoy against SARS-CoV-2. To support this notion, a preclinical study showed that recombinant human soluble ACE2 (rhsACE2) retained a full binding affinity to SARS-CoV-2 spike protein [47]. Whereas membrane-bound ACE2 receptor mediates viral entry into host cells, circulating ACE2 might counteract SARS-CoV-2 viral spread in the body.
Figure 2.
Angiotensin-converting enzyme 2 (ACE2) regulation. (a) ACE2 expression is regulated transcriptionally via apelin signaling and sirtuin 1 (SIRT1) activation in response to energy stress [e.g., hypoxia or AMP kinase (AMPK) activation], transcription modulators, such as miR-421 and miR-483-3p, and post-translational mechanisms that involve feedback signaling of angiotensin II (Ang II). Ang II binding to Ang II type 1 receptor (AT1R) generates reactive oxygen species (ROS), stimulating a disintegrin metalloproteinase 17 (ADAM17), which cleaves cell-surface ACE2 and activates several signaling cascades, such as MAPK and ERK1/2. (b) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-mediated endocytosis might also result in downregulation of ACE2 surface expression [72], [73], [74], [75], [76].
RAS-blocking medication: harmful or helpful?
Clinical data have consistently shown that patients with hypertension or cardiovascular disease are more likely to develop severe illness from COVID-19 [48]. There was concern that RAS-blocking medications, such as ACEIs and ARBs, could worsen clinical outcomes of patients with COVID-19 because of their potential ability to upregulate ACE2 expression on the cell surface, leading to a greater number of potential sites for viral entry. In this context, ACEIs and ARBs have been reported to upregulate ACE2 mRNA and protein levels in several animal models of disease (e.g., myocardial infarction, hypertension, heart failure, cardiomyopathy, myocarditis, and atherosclerosis) as well as in healthy conditions [49], [50], [51]. However, little evidence is available about the effects of RAS blockade on ACE2 tissue expression in humans [52].
Observational studies identified no greater risk of severe COVID-19 in patients taking RAS-blocking agents compared with other antihypertensive treatments or no treatments [53], [54], [55]. A recent meta-analysis of 42,926 patients with COVID-19 concluded that the clinical use of ACEIs and ARBs was not associated with increased risk of COVID-19 death [56]. Yet, other retrospective analyses suggested that RAS-blocking drugs provide beneficial effects in patients with COVID-19, especially in hypertension cohorts [17], [18]. Notably, a 2-million patient cohort study revealed ACEIs and ARBs were associated with a significantly lower risk of hospitalization (hazard ratio (HR) 0.74 [95% confidence interval (CI) 0.65–0.83]) and intubation/death (HR 0.84 [95% CI 0.76–0.93]) in patients with hypertension [57]. Importantly, this study focused on patients with hypertension; patients with a history of diabetes, renal failure, chronic respiratory disease, or cardiovascular disease were excluded from the study to mitigate indication bias.
Observational studies can be complicated by confounding variables, such as age, sex, comorbidities, and concomitant medications. Therefore, a conclusive determination on the clinical effect of ACEIs and ARBs in COVID-19 requires well-controlled, randomized clinical trials. Several randomized clinical trials have evaluated the effect of discontinuing ACEI/ARB treatment in patients hospitalized with COVID-19. In the Brace Corona Trial, participants (N = 659) were randomized into temporary suspension of ACEI/ARB therapy (N = 334) versus continued use (N = 325) followed by assessment of clinical outcomes (e.g., number of the days alive and out of the hospital) [58], [59]. This study concluded that, among patients hospitalized with COVID-19 infection and receiving chronic ACEI/ARB medication, suspending ACEI/ARB provided no significant effect on clinical outcomes [59]. Similar findings were also recently reported for the REPLACE COVID trial (152-patient randomized open-label trials) in which there was no observable difference in COVID-19 severity between participants who continued ACEI/ARB therapy and those who discontinued treatment [60]. Another prospective randomized clinical trial (SWITCH-COVID) is investigating the clinical impact of switching RAS inhibitors in patients with COVID-19 with other antihypertensive medications that are not reported to affect ACE2 expression (NCT04493359). Overall, current clinical evidence suggests ACEIs and ARBs should not be discontinued in patients with COVID-19 with underlying diseases (e.g., hypertension or cardiovascular diseases).
Therapeutics targeting the RAS
Based on the engagement of ACE2 in SARS-CoV-2 infection and the consequential RAS imbalance with COVID-19 severity, there has been tremendous interest in exploring the therapeutic potential of targeting RAS components in patients with COVID-19. Theoretically, lowering the tissue expression of ACE2 could block SARS-CoV-2 viral entry into host cells, but the loss of ACE2 function would result in elevated levels of circulating Ang II and overactivation of RAS in patients with COVID-19. Therefore, therapeutic approaches are intended to inhibit viral entry while striking a balance in the RAS cascade. Among these, rhsACE2 is currently being investigated in clinical trials (NCT04335136). This class of molecules shares a unique dual mode of action that is thought to: (i) imitate physiological soluble ACE2, acting as a potential decoy and sequestering the virus in circulation; and (ii) replenish ACE2 activity to restore RAS balance [47], [61]. Preclinical studies showed that rhsACE2 was capable of binding and neutralizing SARS-CoV-2, reducing viral loads by a factor of 1,000–5,000 [47]. Several engineered forms of rhsACE2 are also under development, including mimetic peptides of the N-terminal human ACE2 [62], mutant rhsACE2 to increase affinity for viral spike protein [63], and ACE2-Fc fusion proteins to extend circulatory half-lives [64]. Other strategies include the infusion of synthetic Ang 1–7 peptide (NCT04375124 and NCT04332666) or Ang 1–7 analogs [65], and the repurposed use of ACEIs and ARBs. Administering exogenous Ang 1–7 could aid in maintaining the blood pressure of patients with severe COVID-19, reducing inflammation, while also potentially modulating ACE2 expression; although this still needs to be demonstrated clinically [66], [67]. Further information about the clinical development of therapeutics for COVID-19 treatment is available in recent reviews [68], [69], [70].
Concluding remarks
SARS-CoV-2 utilizes the ACE2 receptor to infect host cells, but the precise relationships between ACE2 levels, viral infectivity, and severity of the subsequent COVID-19 are not well understood. ACE2 is widely distributed in the body, which could explain the vulnerability to the novel coronavirus. ACE2 tissue expression is often upregulated in patients with cardiovascular diseases, potentially increasing infection risks, but it might be reduced upon SAR-CoV-2 infection. The loss of ACE2 expression, when a certain threshold is crossed, would disrupt local or systemic RAS homeostasis, leading to hyperinflammation and organ damage, as observed in patients with COVID-19. Additional studies are needed to assess the dynamic changes in serum ACE2, Ang II, and Ang 1–7 in patients with COVID-19 at baseline, during disease progression, and in response to therapeutic intervention. Such efforts could help identify human or viral factors that underpin whether patients with COVID-19 will develop severe symptoms.
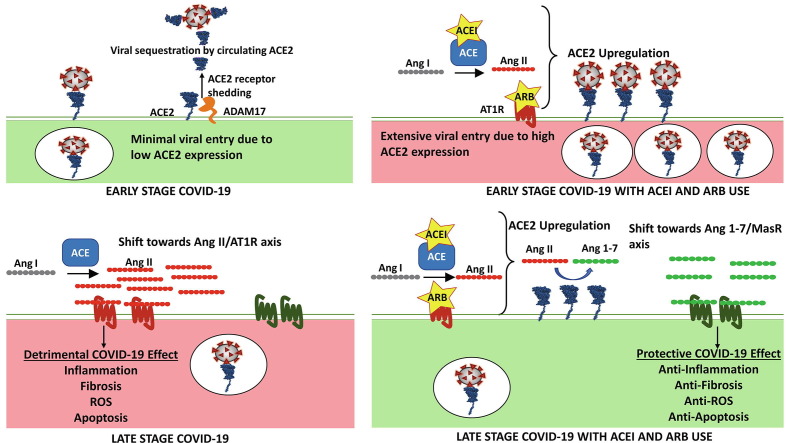
The unfolding story highlights the complex and dynamic interaction between SARS-CoV-2 and RAS components. With the limited data available, we attempt to propose a dual role for ACE2 in COVID-19 pathology [4], dependent on the stage of infection (Fig. 3 ). As a functional receptor for SARS-CoV-2, ACE2 is upregulated in several disease conditions or possibly in patients taking ACEIs and ARBs. The widespread expression of ACE2 could increase the risk of SARS-CoV-2 infection and subsequent COVID-19 severity. SARS-CoV-2 spike protein binding to ACE2 receptor reduces ACE2 cell surface expression through endocytosis or potentially through shedding mechanisms [71]. The loss of ACE2 would downregulate the ACE2/Ang 1–7/MasR pathway, and increased levels of Ang II would trigger harmful effects, such as inflammation, fibrosis, and apoptosis, as observed in patients with COVID-19. Therefore, therapeutic agents, such as ACEIs and ARBs and other RAS-targeting agents, might be helpful to mitigate the deleterious effects by restoring RAS balance in patients with COVID-19. The dynamic and spatiotemporal regulation of ACE2 expression emphasizes the need to understand the impact of RAS modulation during the course of SARS-CoV-2 infection and subsequent COVID-19 progression.
Figure 3.
Proposed dual role of renin–angiotensin system (RAS) in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and Coronavirus 2019 (COVID-19) progression. (a) The SARS-CoV-2 virus exploits the angiotensin-converting enzyme 2 (ACE2) receptor expressed on the cell-surface membrane as the initial host entry point. During the internalization process, the virus hijacks ACE2 through endocytosis and potentially activates receptor-shedding mechanisms. At the same time, soluble serum ACE2 may help scavenge SARS-CoV-2 in circulation. (b) SARS-CoV-2-driven downregulation of ACE2 leads to increased angiotensin II (Ang II) activity, which induces detrimental cellular conditions that can worsen severe COVID-19 outcome. (c) RAS inhibitors, such as angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), might upregulate ACE2 expression, leading to robust SARS-CoV-2 host entry during early stages of infection. (d) However, continued use of these drugs during late stages of COVID-19 can help mitigate and restore virus induced decreases in ACE2 expression. This will shift the angiotensin balance towards the Ang 1–7/MasR axis, providing a protective effect. Depending on the stage of infection, the ACEI/ARB output signal might switch between detrimental and protective during COVID-19 progression [4].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Steven Kozlowski, Gerald Feldman, Gowra Jagadeesh, and Frank Weichold for their critical review and comments on the manuscript. This work was funded by the U.S. Food and Drug Administration (FDA). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This article reflects the views of the authors and should not be construed to represent the views or policies of the FDA.
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiese O.J., Allwood B.W., Zemlin A.E. COVID-19 and the renin–angiotensin system (RAS): a spark that sets the forest alight? Med. Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020 doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommerstein R., Kochen M.M., Messerli F.H., Grani C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martines R.B., Ritter J.M., Matkovic E., Gary J., Bollweg B.C., Bullock H., et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg. Infect. Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 9.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 10.Sampaio W.O., Henrique de Castro C., Santos R.A., Schiffrin E.L., Touyz R.M. Angiotensin-1-7 counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 11.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandy K., Salunke A., Pathak S.K., Pandey A., Doctor C., Puj K., et al. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab. Syndr. 2020;14:1017–1025. doi: 10.1016/j.dsx.2020.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaman M.A., Oparil S., Calhoun D.A. Drugs targeting the renin-angiotensin-aldosterone system. Nat. Rev. Drug Discov. 2002;1:621–636. doi: 10.1038/nrd873. [DOI] [PubMed] [Google Scholar]
- 17.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baral R., White M., Vassiliou V.S. Effect of renin-angiotensin-aldosterone system inhibitors in patients with COVID-19: a systematic review and meta-analysis of 28,872 patients. Curr. Atheroscler. Rep. 2020;22:61. doi: 10.1007/s11883-020-00880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa L.B., Perez L.G., Palmeira V.A., Macedo E., Cordeiro T., Ribeiro V.T., Lanza K., et al. Insights on SARS-CoV-2 molecular interactions with the renin–angiotensin system. Front Cell. Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.559841. 559841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 21.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 23.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 uses clathrin-mediated endocytosis to gain access into cells. J. Biol. Chem. 2020;296 doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.R. Amraie, M.A. Napoleon, W. Yin, J. Berrigan, E. Suder, G. Zhao, et al., CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells, bioRxiv 2020; 2020.06.22.165803
- 25.Nishiyama A., Kobori H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin. Exp. Nephrol. 2018;22:1231–1239. doi: 10.1007/s10157-018-1567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunyady L., Catt K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 27.Grobe J.L., Mecca A.P., Lingis M., Shenoy V., Bolton T.A., Machado J.M., et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-1-7. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 28.Jiang F., Yang J., Zhang Y., Dong M., Wang S., Zhang Q., et al. Angiotensin-converting enzyme 2 and angiotensin 1–7: novel therapeutic targets. Nat. Rev. Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R.A. Santos, A.C. Simoes e Silva, C. Maric, D.M. Silva, R.P. Machado, I. de Buhr, et al., Angiotensin-1-7; is an endogenous ligand for the G protein-coupled receptor Mas, Proc. Natl. Acad. Sci. U. S. A. 2003; 100: 8258–63 [DOI] [PMC free article] [PubMed]
- 30.Navar L.G., Mitchell K.D., Harrison-Bernard L.M., Kobori H., Nishiyama A. Review: Intrarenal angiotensin II levels in normal and hypertensive states. J. Renin Angiotensin Aldosterone Syst. 2001;2(1_Suppl.):S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serneri G.G., Boddi M., Cecioni I., Vanni S., Coppo M., Papa M.L., et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ. Res. 2001;88:961–968. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 32.Cao W., Jin L., Zhou Z., Yang M., Wu C., Wu L., et al. Overexpression of intrarenal renin-angiotensin system in human acute tubular necrosis. Kidney Blood Press Res. 2016;41:746–756. doi: 10.1159/000450564. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.N. Liu, Y. Hong, R.-G. Chen, H.-M. Zhu , High rate of increased level of plasma angiotensin II and its gender difference in COVID-19: an analysis of 55 hospitalized patients with COVID-19 in a single hospital, WuHan, China, medRxiv 2020; 2020.2004.2027.20080432
- 36.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit. Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hikmet F., Méar L., Edvinsson Å., Micke P., Uhlén M., Lindskog C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong H.W., Kim S.M., Kim H.S., Kim Y.I., Kim J.H., Cho J.Y., et al. Viable SARS-CoV-2 in various specimens from COVID-19 patients. Clin. Microbiol. Infect. 2020;26:1520–1524. doi: 10.1016/j.cmi.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto B.G.G., Oliveira A.E.R., Singh Y., Jimenez L., Gonçalves A.N.A., Ogava R.L.T., et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020;222:556–563. doi: 10.1093/infdis/jiaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goulter A.B., Goddard M.J., Allen J.C., Clark K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizuiri S., Aoki T., Hemmi H., Arita M., Sakai K., Aikawa A. Urinary angiotensin-converting enzyme 2 in patients with CKD. Nephrology. 2011;16:567–572. doi: 10.1111/j.1440-1797.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 42.Bos J.M., Hebl V.B., Oberg A.L., Sun Z., Herman D.S., Teekakirikul P., et al. Marked up-regulation of ACE2 in hearts of patients with obstructive hypertrophic cardiomyopathy: implications for SARS-CoV-2-mediated COVID-19. Mayo Clin. Proc. 2020;95:1354–1368. doi: 10.1016/j.mayocp.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Wang J., Liu R., Qi H., Wen Y., Sun F., et al. Severe acute pancreatitis is associated with upregulation of the ACE2-angiotensin-1-7-Mas axis and promotes increased circulating angiotensin-1-7. Pancreatology. 2012;12:451–457. doi: 10.1016/j.pan.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Lambert D.W., Lambert L.A., Clarke N.E., Hooper N.M., Porter K.E., Turner A.J. Angiotensin-converting enzyme 2 is subject to post-transcriptional regulation by miR-421. Clin. Sci. 2014;127:243–249. doi: 10.1042/CS20130420. [DOI] [PubMed] [Google Scholar]
- 45.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I., et al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J. Biol. Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang X., Shi L., Wang Y., Xiao W., Duan G., Yang H., et al. The association of hypertension with the severity and mortality of COVID-19 patients: Evidence based on adjusted effect estimates. J. Infect. 2020;81:e44–e47. doi: 10.1016/j.jinf.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreutz R., Algharably E.A.E., Azizi M., Dobrowolski P., Guzik T., Januszewicz A., et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID–19. Cardiovasc. Res. 2020;16:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jessup J.A., Gallagher P.E., Averill D.B., Brosnihan K.B., Tallant E.A., Chappell M.C., et al. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am. J. Physiol. Heart and Circulatory Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 51.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 52.Vuille-dit-Bille R.N., Camargo S.M., Emmenegger L., Sasse T., Kummer E., Jando J., et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trifirò G., Massari M., Da Cas R., Menniti Ippolito F., Sultana J., Crisafulli S., et al. Renin-angiotensin-aldosterone system inhibitors and risk of death in patients hospitalised with COVID-19: a retrospective Italian cohort study of 43,000 patients. Drug Saf. 2020;43:1297–1308. doi: 10.1007/s40264-020-00994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semenzato L., Botton J., Drouin J., Baricault B., Vabre C., Cuenot F., et al. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R.D. Lopes, A.V.S. Macedo, P.G.M. de Barros E Silva, R.J. Moll-Bernardes, A. Feldman, G. D'Andréa Saba Arruda, et al., Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: Impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)--the BRACE CORONA Trial, Am. Heart J. 2020; 226, 49–59 [DOI] [PMC free article] [PubMed]
- 59.Lopes R.D., Macedo A.V.S., de Barros E., Silva P.G.M., Moll-Bernardes R.J., Dos Santos T.M., Mazza L., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen J.B., Hanff T.C., William P., Sweitzer N., Rosado-Santander N.R., Medina C., et al. Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial. Lancet Respir. Med. 2021;9:275–284. doi: 10.1016/S2213-2600(20)30558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karoyan P., Vieillard V., Gómez-Morales L., Odile E., Guihot A., Luyt C.E., et al. Human ACE2 peptide-mimics block SARS-CoV-2 pulmonary cells infection. Commun. Biol. 2020;4:192. doi: 10.1038/s42003-021-01736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan K.K., Dorosky D., Sharma P., Abbasi S.A., Dye J.M., Kranz D.M., et al. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369:1261–1265. doi: 10.1126/science.abc0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu P., Wysocki J., Souma T., Ye M., Ramirez V., Zhou B., et al. Novel ACE2-Fc chimeric fusion provides long-lasting hypertension control and organ protection in mouse models of systemic renin angiotensin system activation. Kidney Int. 2018;94:114–125. doi: 10.1016/j.kint.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 65.Sansoe G., Aragno M., Wong F. Covid-19 and liver cirrhosis: focus on the non-classical renin–angiotensin system and implications for therapy. Hepatology. 2021 doi: 10.1002/hep.31728. https://aasldpubs.onlinelibrary.wiley.com/journal/15273350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wösten-van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-1-7; or an angiotensin II receptor antagonist. J. Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 67.Imanpour H., Rezaee H., Nouri-Vaskeh M. Angiotensin 1–7: a novel strategy in COVID-19 treatment. Adv. Pharm. Bull. 2020;10:488–489. doi: 10.34172/apb.2020.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poduri R., Joshi G., Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell. Signal. 2020;74 doi: 10.1016/j.cellsig.2020.109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Twomey J.D., Luo S., Dean A.Q., Bozza W.P., Nalli A., Zhang B. COVID-19 update: the race to therapeutic development. Drug Resist. Updates. 2020;53 doi: 10.1016/j.drup.2020.100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cardone M., Yano M., Rosenberg A.S., Puig M. Lessons learned to date on COVID-19 hyperinflammatory syndrome: considerations for interventions to mitigate SARS-CoV-2 viral infection and detrimental hyperinflammation. Front. Immunol. 2020;11:1131. doi: 10.3389/fimmu.2020.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 73.Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin. Sci. 2014;126:507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 74.Kemp J.R., Unal H., Desnoyer R., Yue H., Bhatnagar A., Karnik S.S. Angiotensin II-regulated microRNA 483–3p directly targets multiple components of the renin-angiotensin system. J. Mol. Cell. Cardiol. 2014;75:25–39. doi: 10.1016/j.yjmcc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott A.J., O'Dea K.P., O'Callaghan D., Williams L., Dokpesi J.O., Tatton L., et al. Reactive oxygen species and p38 mitogen-activated protein kinase mediate tumor necrosis factor alpha-converting enzyme (TACE/ADAM-17) activation in primary human monocytes. J. Biol. Chem. 2011;286:35466–35476. doi: 10.1074/jbc.M111.277434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koka V., Huang X.R., Chung A.C.K., Wang W., Truong L.D., Lan H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]