Abstract
Background:
Psoriasis is associated with increased cardiovascular risk that is not captured by traditional proinflammatory biomarkers.
Objective:
To investigate the relationship between Psoriasis Area and Severity Index, circulating proinflammatory biomarkers, and vascular health in psoriasis.
Methods:
In patients with psoriasis and in age and sex-matched controls, 273 proteins were analyzed with the Proseek Multiplex Cardiovascular disease reagents kit and Inflammatory reagents kit (Olink Bioscience), whereas vascular endothelial inflammation and health were measured via direct transcriptomic analysis of brachial vein endothelial cells.
Results:
In psoriasis, chemokine ligand 20 (CCL20), interleukin (IL) 6, and IL-17A were the top 3 circulating proinflammatory cytokines. Vascular endothelial inflammation correlated with CCL20 (r = 0.55; P<.001) and less so with IL-6 (r = 0.36; P = .04) and IL-17A (r = 0.29; P = .12). After adjustment for potential confounders, the association between CCL20 and vascular endothelial inflammation remained significant (β = 1.71; P = .02). In nested models, CCL20 added value (χ2 = 79.22; P <.001) to a model already incorporating the Psoriasis Area and Severity Index, Framingham risk, high-sensitivity C-reactive protein, Il-17A, and IL-6 (χ2 = 48.18; P<.001) in predicting vascular endothelial inflammation.
Limitations:
Our study was observational and did not allow for causal inference in the relationship between CCL20 and cardiovascular risk.
Conclusion:
We demonstrate that CCL20 expression has a strong association with vascular endothelial inflammation, reflects systemic inflammation, and may serve as a potential biomarker of impaired vascular health in psoriasis.
Keywords: atherosclerosis, biomarkers, cardiovascular disease, endothelial cell, inflammation, psoriasis, vascular health
INTRODUCTION
Psoriasis is a chronic inflammatory skin disease associated with atherosclerosis and a 50% higher risk of cardiovascular disease compared with that for matched controls.1 Cardiovascular risk calculators incorporating traditional cardiovascular risk factors underestimate risk in psoriasis, and both the joint American Academy of Dermatology –National Psoriasis Foundation and American College of Cardiology/American Heart Association guidelines now suggest integrating psoriasis into clinical decision making when determining the risk of a cardiovascular event.2–4 These recommendations, although a critical first step toward risk-reduction strategies in psoriasis, may not fully reflect the underlying influence of systemic inflammatory profiles on the risk of cardiovascular disease. Further tools and biomarkers are therefore required to refine cardiovascular risk strategies in the psoriatic population.
Impaired endothelial vascular health is the first step in the development of cardiovascular disease.5 The endothelium maintains vascular tone and stiffness, as well as regulates leukocyte trafficking and leukocyte and platelet vascular adhesion.6 Activated endothelium expresses inflammatory cytokines and adhesion molecules that attract leukocytes and contribute to atherosclerotic plaque progression.7 Direct investigation of the vascular endothelial profile has shown that the endothelium in psoriasis is inflamed and activated and lends insight into the pathophysiology of early atherosclerosis and cardiovascular disease development.8
Identifying circulating biomarkers of impaired vascular health in psoriasis has the potential to improve cardiovascular risk stratification and may be informative regarding biological pathways contributing to disease. Therefore, the purpose of this investigation was to use high-throughput targeted proteomic panels to investigate the relationship between psoriasis skin activity, inflammatory biomarkers, and vascular health as a first step toward improved cardiovascular risk-stratification tools in psoriasis.
METHODS
Subject recruitment
Patients with a diagnosis of psoriasis by a board-certified dermatologist, as well as age- and sex-matched controls, were recruited from New York University Langone Health between September 2017 and April 2019 as part of an ongoing study investigating vascular endothelial health in psoriasis. Exclusion criteria, among others, included a prior diagnosis of clinical cardiovascular disease, active aspirin or statin usage, a recent (<1 month) change in biologic, oral, or light therapy (for psoriasis), and a diagnosis of other autoimmune diseases aside from psoriasis, psoriatic arthritis, or both. Controls were recruited through community advertising targeted to allow age and sex matching to psoriasis patients. The detailed research methodology has been previously reported and approved by New York University School of Medicine Institutional Review Board.8
Vascular endothelial profiling
Participants (psoriasis and control) arrived fasting (>4 hours) and underwent clinical and anthropometric assessments as previously published.9 A 20-gauge angiocatheter was inserted into the brachial vein in the forearm and 3 J-shaped vascular guide-wires were sequentially advanced into the brachial vein. The cells obtained from the J wires were washed in dissociation buffer, spun down, and resuspended in isolation buffer. The solution was then incubated with biotinylated mouse antihuman monoclonal antibody directed against CD146 (1:200; Millipore Sigma) and endothelial cells were isolated with streptavidin magnetic FlowComp Dynabeads (1:100). Endothelial cells underwent messenger RNA extraction with an RNAqueous micro-RNA isolation kit (Invitrogen). Messenger RNA was converted to complementary DNA (Quantabio) and amplified via PerfeCTa PreAmp SuperMix (Quantabio). TaqMan (Life Technologies) primers were used on an Applied Biosystems 7500 Fast Real-Time PCR System. As previously described,7 we created a composite value of the mean brachial vein endothelial transcriptome, which represents the messenger RNA expression of known endothelial inflammatory activation biomarkers, including ICAM1, VCAM1, CXCL1, CXCL10, CX3XL1, CCL3, COX-2, interleukin (IL) 8, and IL-1β. In prior studies, these biomarkers of endothelial activation and damage were shown to be upregulated in psoriasis patients, to be linearly associated with the Psoriasis Area and Severity Index (PASI), and to improve with standard cardiovascular risk-reduction therapies such as aspirin.10 To ensure reproducibility across analysis, results are represented as normalized to human acidic ribosomal protein for each sample and gene.11 Outliers including mean brachial vein endothelial transcriptome greater than 2 standard deviations from the mean were excluded from the analysis.
Targeted proteomic panels
As previously described and reported, aliquots of stored serum samples were analyzed with the Proseek Multiplex Cardiovascular disease reagents kit and Inflammatory reagents kit (Olink Bioscience) assessing a total of 273 unique proinflammatory proteins.12 Briefly, oligonucleotide-labeled antibody probes with proximity extension assay technology bind to their designed target. These antibody pairs attach to their designed target and create a new DNA amplicon. The amplicons were quantified with a Fluidigm BioMark HD real-time polymerase chain reaction platform. Data are reported as normalized protein expression, a unit of measurement based on a log2 scale.
Statistical analysis
Continuous data are presented as mean ± standard deviation for normal data or median (interquartile range) for nonnormally distributed data, as appropriate. Categoric data are presented as total number (percentage). Normally distributed continuous variables were assessed through a Student t test, whereas nonnormally distributed continuous variables were assessed through Wilcoxon’s rank sum test. Categoric variables were assessed through χ2 analysis. Pearson correlation was used to determine the relationship between protein levels, PASI, and vascular endothelial transcript expression. Linear regression and mixed-methods linear regression to incorporate PASI into multivariate-adjusted and nested models were used as noted. Regression analysis data are reported as a β coefficient (95% confidence interval) and P value. P < .05 was considered statistically significant. All analyses were performed in Stata (version 14, StataCorp).
RESULTS
Participant characteristics
The demographics and clinical characteristics of 23 psoriasis participants were compared with that of 15 controls and are listed in Table I. Subjects were well matched for age, sex, and body mass index. Both psoriasis and control groups were at low 10-year risk of a cardiovascular event, with no difference between groups (Framingham risk score 2.3 [interquartile range 1.4–4.2] vs 1.7 [interquartile range 1.0–3.0]; P = .21). The average PASI score was 4.8 (interquartile range 2.7–10.8), indicating moderate disease activity, with approximately 50% of subjects receiving biologic therapy. As expected, protein levels of IL-17A were higher in the psoriasis cohort (Table I).
Table I.
Characteristics of patient with psoriasis and matched healthy controls
| Variable | Psoriasis (N = 23) | Healthy controls (N = 15) | P value |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age, y | 42.0 ± 14 | 40.6 ± 14 | .71 |
| Male sex | 11 (48) | 8 (53) | .74 |
| Hyperlipidemia | 1 (4) | 0 | .41 |
| Type 2 diabetes mellitus | 0 | 0 | >.99 |
| Body mass index, kg/m2 | 27 (24–30) | 25 (23–30) | .58 |
| Hypertension | 0 | 0 | >.99 |
| Current smoker | 0 | 0 | >.99 |
| Lipid treatment | 0 | 0 | >.99 |
| Clinical and laboratory values | |||
| Total cholesterol, mg/dL | 173 ± 26 | 156 ± 26 | .21 |
| HDL cholesterol, mg/dL | 49 ± 14 | 51 ± 12 | .63 |
| LDL cholesterol, mg/dL | 108 (75–112) | 84 (68–115) | .44 |
| Triglycerides, mg/dL | 85 (59–113) | 65 (56–103) | .24 |
| Framingham risk score, median (IQR), % | 2.3 (1.4–4.2) | 1.7 (1.0–3.0) | .21 |
| hs-CRP, median (IQR), mg/L | 1.8 (0.5–4.1) | 1.4 (0.5–1.5) | .41 |
| Psoriasis severity | |||
| Psoriasis Area and Severity Index score, median (IQR) | 4.8 (2.7–10.8) | N/A | N/A |
| Systemic or biologic treatment | 11 (49) | N/A | N/A |
| Psoriasis duration, median (IQR), y | 18.5 (10–22.5) | N/A | N/A |
| Proinflammatory cytokines | |||
| CCL20, NPX | 6.3 ± 1.4 | 5.2 ± 0.7 | .009 |
| IL-17A, NPX | 2.5 ± 2.1 | 1.2 ± 1.0 | .03 |
| IL-6, NPX | 4.9 ± 1.6 | 4.2 ± 0.7 | .08 |
Data are presented as mean ± standard deviation or No. (%) unless otherwise stated. Significant at P < .05.
CCL20, Chemokine ligand 20; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; IQR, interquartile range; LDL, low-density lipoprotein; N/A, not applicable; NPX, normalized expression units on a log2 scale.
Proinflammatory protein profiling
To identify upregulated proteins, the proinflammatory protein expression profile was determined in serum from a subset of psoriasis patients with at least moderate psoriasis skin activity (≥3% body surface area of psoriasis). Consistent with the main cohort, subjects with psoriasis (n = 19) compared with controls (n = 15) were similar in age, sex, body mass index, Framingham risk score, and high-sensitivity C-reactive protein (Supplemental Table I available via Mendeley at https://data.mendeley.com/datasets/vnk9syjf8t/1). Out of 273 proteins, we identified 15 upregulated and 2 downregulated circulating proteins in blood (Supplemental Table II) between psoriasis and control (nominal P<.05). The top 3 upregulated proinflammatory cytokines were IL-17A (2.25-fold; P = .02), IL-6 (1.25-fold; P = .03), and chemokine ligand 20 (CCL20) (1.22-fold; P = .01) (Supplemental Table II). Only IL-17A levels, but not those of CCL20 and IL-6, differed between individuals receiving and not receiving biologic therapy (Supplemental Table III). No differences in the association between psoriasis duration (in years) and IL-17A (r = 0.18; P = .51), IL-6 (r = −0.03; P = .92), and CCL20 (r = 0.5; P = .85) were noted.
Relationship between circulating biomarkers, PASI, and vascular endothelial inflammation
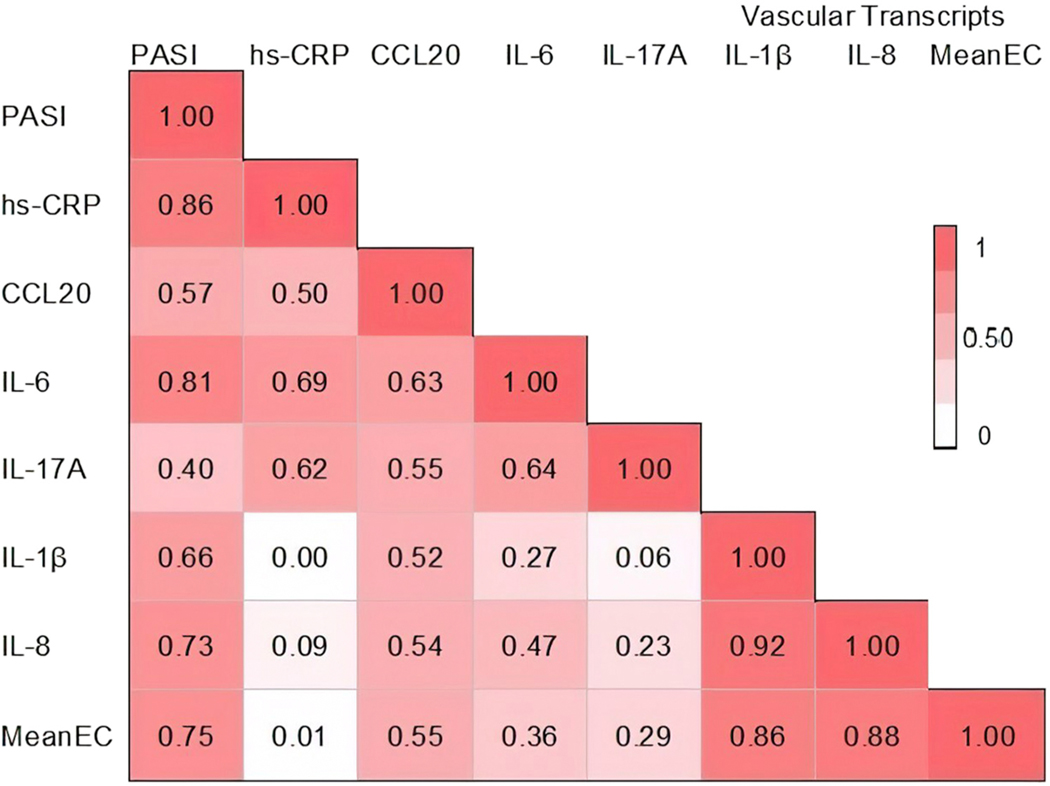
We previously investigated endothelial cell inflammatory transcripts as a surrogate for vascular endothelial inflammation and found them to be increased in psoriasis patients compared with controls and positively correlated with psoriasis skin severity.7 To identify which circulating biomarkers associate with vascular inflammation, we evaluated the correlation between psoriasis skin disease activity, circulating biomarkers, and vascular endothelial inflammation (Fig 1). As expected, PASI correlated with inflammatory biomarker high-sensitivity C-reactive protein (r = 0.86; P < .001), CCL20 (r = 0.57; P = .01), and IL-6 (r = 0.81; P <.001) but less so with IL-17A (r = 0.40; P = .08) (Fig 1). Among these circulating proteins, we noted a high correlation between mean brachial vein endothelial transcriptome and CCL20 (r = 0.55; P<.001) (Fig 1) and less of one with IL-6 (r = 0.36; P = .04) and IL-17A (r = 0.29; P = .12) (Fig 2).
Fig 1.
Correlation matrix between Psoriasis Area and Severity Index score, vascular endothelial inflammation, and common biomarkers of systemic inflammation in psoriasis. The mean brachial vein endothelial transcriptome expression of known vascular endothelial inflammatory activation biomarkers includes ICAM1, VCAM1, CXCL1, CXCL10, CX3XL1, CCL3, COX-2, interleukin 8, and interleukin 1-β. Color and corresponding correlation coefficient (r) indicate relative strength of association. CCL20, Chemokine ligand 20; hsCRP, high-sensitivity C-reactive protein; IL, interleukin; MeanEC, mean brachial vein endothelial transcriptome expression; PASI, Psoriasis Area and Severity Index.
Fig 2.
Linear regression models between Psoriasis Area and Severity Index score, chemokine ligand 20, and mean vascular endothelial inflammation in psoriasis. Regression plot and correlation coefficient between (A) Psoriasis Area and Severity Index score, (B) chemokine ligand 20, and mean vascular endothelial. P <.05 for significance. CCL20, Chemokine ligand 20; NPX, normalized expression units on a log2 scale; PASI, Psoriasis Area and Severity Index.
Assessment of CCL20 and endothelial inflammation
Vascular inflammation and endothelial dysfunction is a key step in the development of atherosclerosis.13 Because CCL20 displayed the highest correlation with mean brachial vein endothelial transcriptome, we evaluated the strength of the association between them in multiple models. CCL20 remained significantly associated with mean brachial vein endothelial transcriptome after adjustment for Framingham risk (β = 1.75; P < .01) and, after further adjustment for individuals receiving biologic therapy, IL-17A, IL-6, and high-sensitivity C-reactive protein (β = 1.71; P = .02) (Table II).
Table II.
Linear regression between vascular endothelial inflammation and circulating proinflammatory biomarkers
| Mean vascular endothelial inflammation | β coefficient | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| CCL20 | 1.74 | 0.72 to 2.77 | .002 |
| Model 2 | |||
| CCL20 | 1.75 | 0.65 to 2.85 | .003 |
| Framingham risk | −1.38 | − 19.1 to 17.2 | .88 |
| Model 3 | |||
| CCL20 | 1.71 | 0.23 to 3.20 | .02 |
| Framingham risk | 0.27 | −22.12 to 22.76 | .98 |
| Biologic therapy | 1.57 | −2.59 to 5.7 | .44 |
| IL-17A | −0.23 | −1.56 to 1.10 | .72 |
| IL-6 | 0.53 | −1.59 to 2.65 | .61 |
| hs-CRP | −0.22 | −0.87 to 0.4 | .49 |
Significant at P < .05.
CCL20, Chemokine ligand 20; CI, confidence interval; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin.
Finally, mixed-effects multivariable linear regression with the incorporation of stepwise nested models (with the corresponding χ2) was used to assess the incremental value and effect size in the association between traditional measures of cardiovascular risk, inflammation in psoriasis, and the outcome of vascular endothelial inflammation (mean brachial vein endothelial transcriptome). The addition of high-sensitivity C-reactive protein (χ2 = 1.18; P = .56), IL17A, and IL-6 (χ2 = 2.65; P < .62) did not improve the predictive value to a model already incorporating Framingham risk (Supplemental Table III). Adding PASI to the model was predictive of mean brachial vein endothelial transcriptome (χ2 = 48.18; P < .001). Finally, the addition of CCL20 provided the highest incremental value to the model (χ2 = 79.22; P < .001), highlighting the potential of CCL20 beyond psoriasis skin severity and other inflammatory biomarkers to predict vascular endothelial inflammation in psoriasis.
DISCUSSION
In this well-phenotyped cohort of psoriasis patients with moderate skin severity compared with that of controls, we used a targeted proteomic approach of 273 proteins to show that CCL20, IL-17A, and IL-6 were the 3 highest differentially expressed cytokines in psoriasis. CCL20 showed a strong association with psoriasis skin severity and displayed the highest point estimate in its correlation with vascular endothelial inflammation. After adjustment for other known drivers of vascular inflammation and cardiovascular risk, CCL20 added predictive value in the assessment of vascular endothelial inflammation in psoriasis.
Psoriasis is a complex, chronic, immunomediated disease typically affecting skin and nails; however, emerging evidence suggests a distinct association between chronic inflammation and cardiometabolic conditions, including cardiovascular disease.14 The risk of cardiovascular disease is at least 50% higher in subjects with psoriasis than matched controls, whereas those with a history of moderate to severe plaque psoriasis have up to a 3-fold higher risk of cardiovascular disease1 and a 40% higher risk of death from cardiovascular disease.15 Even individuals with mild psoriasis have a 30% increased risk of a myocardial infarction.15 The odds of other vascular diagnoses are also elevated, including a 70% higher risk of cerebrovascular disease and almost 2-fold risk of peripheral vascular disease compared with that of matched controls.16 Current hypotheses to explain elevated cardiovascular risk include cumulative exposure to altered systemic inflammatory profiles along with insulin resistance, obesity, hyperlipidemia, hypertension, and platelet activation leading to endothelial dysfunction; premature atherosclerosis; and, ultimately, cardiovascular events.10,17 To further expand on these prior epidemiologic and mechanistic studies, we show that CCL20 is one of the most significantly upregulated proinflammatory cytokines in psoriasis, which also associates with vascular endothelial inflammation.
CCL20 is a chemokine that selectively attracts dendritic cells, effector/memory T lymphocytes, and naive B cells.18 In psoriasis, IL-17A, IL-22, and tumor necrosis factor α induce keratinocyte CCL20 expression, which enhances T-cell infiltration.19 Within the vasculature, CCL20 induces lymphocyte migration and triggers pathways similar to those activated by low-density lipoprotein cholesterol.20 Vascular smooth muscle cells express CCL20, with expression higher in human atherosclerotic lesions.20 Additionally, in animal models, genetic deletion of CCL20 receptors in Apoe−/− atherosclerosis-prone mice decreases endothelial inflammation and atherogenesis.21 These studies suggest a mechanistic pathogenic mediator and highlight potential utility of CCL20 as a biomarker of vascular endothelial inflammation, which may ultimately serve as a therapeutic target to reduce cardiovascular risk in psoriasis.
In psoriasis, there remains a challenge in appropriately quantifying and stratifying patients at elevated risk of a cardiovascular event. Various imaging tools have been proposed to evaluate vascular inflammation (and cardiovascular risk) in psoriasis, including [18F]-fluorodeoxyglucose positron emission tomography–computed tomography,22 yet this remains economically ineffective and labor intensive, and exposes the patient to prolonged radiation. Glycoproteins such as glycoprotein acetylation, a nuclear magnetic resonance signal of the inflammasome response, have also been suggested as biomarkers of systemic and vascular inflammation in psoriasis23–25; however the prospective utility remains to be defined.26
Given the need to identify vascular inflammatory risk in psoriasis, this study shows that circulating CCL20 is a top upregulated cytokine and displays a high correlation with ex vivo assessment of vascular endothelial inflammation compared with established markers such as high-sensitivity C-reactive protein, IL-17A, and IL-6.27 The addition of CCL20 to nested models incorporating traditional cardiovascular disease risk factors, PASI, and other circulating biomarkers revealed incremental value in predicting vascular endothelial inflammation. These findings suggest that CCL20 deserves further study as a circulating biomarker to capture the summative risk related to multiple inflammatory pathways in psoriasis. Studies are needed to evaluate the association between circulating serum CCL20, coronary atherosclerosis burden, and hard cardiovascular outcomes such as myocardial infarction, stroke, and cardiovascular-related death.
LIMITATIONS
Our study has limitations. The cross-sectional design did not allow causal inference and, as an observational study, does not permit studying the potential influence on future cardiovascular disease risk. Additionally, our cohort may underrepresent endothelial inflammation because the median PASI score was 5, which correlates with mild to moderate skin disease; however, this score was similar to those in other studies showing that even individuals with mild psoriasis have an independent risk of cardiovascular disease up to 30% above that of matched controls.1,15 Last, half of the patients recruited in this study were receiving immunomodulating therapies, which may have affected the absolute level of cytokines, including IL-17A, IL-6, and CCL20. However, when data were stratified by biologic therapy, we did not observe a statistically significant difference between groups in CCL20 levels.
CONCLUSION
In conclusion, using direct, ex vivo analyses, we demonstrated that CCL20 is significantly correlated with PASI score and vascular endothelial inflammation. Our data suggest that CCL20 reflects vascular inflammation better than well-established markers of inflammation in psoriasis, including IL-6, IL-17A, and high-sensitivity C-reactive protein. We propose that CCL20 may serve as a biomarker of systemic inflammation and vascular health in this population and allow cardiovascular risk stratification; however, further investigation is warranted to confirm these findings.
Supplementary Material
CAPSULE SUMMARY.
Our data add to the increasing knowledge regarding the role of systemic inflammation in psoriasis and increased risk of cardiovascular disease.
Our results warrant further investigation regarding novel biomarkers, such as chemokine ligand 20, that capture cardiovascular risk to allow better risk stratification and disease mitigation in psoriasis.
Acknowledgments
Funding sources: Supported by a National Institutes of Health (NIH, Bethesda, MD) training grant (T32HL098129), NIH Clinical and Translational Science Awards at New York University Awards (New York, NY) (UL1TR001445, KL2TR001446, and TL1TR001447), American Heart Association Career Development Grant (Dallas, TX) (18CDA34080540), and Dermatology Foundation Research Grant to Dr Garshick; and an American Heart Association Career Development Grant (Dallas, TX) (18CDA34110203AHA) and American Society of Hematology grant (18-A0–00-1001884) to Dr Barrett. Dr Scher was supported, in part, by NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR074500, the Riley Family Foundation, the Beatriz Snyder Foundation, the Rheumatology Research Foundation, and a National Psoriasis Foundation Grant. Dr Berger was supported, in part, by NIH grants R01HL139909, R01HL114978, and R35HL144993.
IRB approval status: This study was approved by the NYU IRBU.
Abbreviations used:
- CCL20
chemokine ligand 20
- IL
interleukin
- PASI
Psoriasis Area and Severity Index
Footnotes
Conflicts of interest
None disclosed.
REFERENCES
- 1.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. [DOI] [PubMed] [Google Scholar]
- 2.Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775.e1–775.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073–1113. [DOI] [PubMed] [Google Scholar]
- 4.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary. Circulation. 2019;140:e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desideri G, Ferri C. Endothelial activation. Sliding door to atherosclerosis. Curr Pharm Des. 2005;11:2163–2175. [DOI] [PubMed] [Google Scholar]
- 6.Michiels C. Endothelial cell functions. J Cell Physiol. 2003;196: 430–443. [DOI] [PubMed] [Google Scholar]
- 7.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007; 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 8.Garshick MS, Barrett T, Wechter T, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39:787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jelic S, Lederer DJ, Adams T, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garshick MS, Tawil M, Barrett TJ, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Tomalin L, Lee J, et al. Reduction of inflammatory and cardiovascular proteins in the blood of patients with psoriasis: differential responses between tofacitinib and etanercept after 4 weeks of treatment. J Invest Dermatol. 2018;138:273–281. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013; 2:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700–703. [DOI] [PubMed] [Google Scholar]
- 17.Aksentijevich M, Lateef SS, Anzenberg P, Dey AK, Mehta NN. Chronic inflammation, cardiometabolic diseases and effects of treatment: psoriasis as a human model. Trends Cardiovasc Med. 2020;30:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schutyser E,Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. [DOI] [PubMed] [Google Scholar]
- 19.Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulateCCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvayrac O, Rodriguez-Calvo R, Alonso J, et al. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol. 2011;31:2733–2741. [DOI] [PubMed] [Google Scholar]
- 21.Wan W, Lim JK, Lionakis MS, et al. Genetic deletion of chemokine receptor Ccr6 decreases atherogenesis in ApoE-deficient mice. Circ Res. 2011;109:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey AK, Joshi AA, Chaturvedi A, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2:1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 2017;15: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ormseth MJ, Chung CP, Oeser AM, et al. Utility of a novel inflammatory marker, GlycA, for assessment of rheumatoid arthritis disease activity and coronary atherosclerosis. Arthritis Res Ther. 2015;17:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baran A, Mysliwiec H, Szterling-Jaworowska M, Kiluk P, Swiderska M, Flisiak I. Serum YKL-40 as a potential biomarker of inflammation in psoriasis. J Dermatolog Treat. 2018;29:19–23. [DOI] [PubMed] [Google Scholar]
- 26.Joshi AA, Lerman JB, Aberra TM, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res. 2016;119:1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.