Background
As of March 25, 2021, more than 124 million people globally have been diagnosed with a coronavirus disease 2019 (COVID-19) infection and almost 2.7 million have died from COVID-19.1 This international pandemic was met with the rapid development and Food and Drug Administration approval under Emergency Use Authorization (EUA) of 2 effective COVID-19 mRNA vaccines (Pfizer-BioNTech and Moderna) in December 2020. Unfortunately, within days, severe allergic reactions to the COVID-19 mRNA vaccines occurred, creating a potential barrier to large-scale vaccination efforts. To address this unmet need amidst great uncertainty, in December 2020, we published initial algorithms to help the allergist guide safe vaccination in individuals with allergy histories.2
Since then, more than 46 million individuals have been fully vaccinated and more than 2 million additional Americans receive their vaccinations daily.3 The Centers for Disease Control and Prevention (CDC), using Vaccine Adverse Event Reporting System and V-safe voluntary reporting data, described the rate of anaphylaxis after receipt of the mRNA COVID-19 vaccines as 4.5 cases per million doses administered, with 89% occurring within the 15- to 30-minute observation period.4, 5, 6 This is comparable to anaphylaxis rates with other vaccines including the inactivated influenza vaccine (1.4 per million), pneumococcal polysaccharide vaccine (2.5 per million), and the live attenuated herpes zoster vaccine (9.6 per million).4 However, prospective cohort data from more than 60,000 Mass General Brigham (MGB) employees found a higher incidence rate of anaphylaxis to the mRNA COVID-19 vaccines at 2.47 per 10,000 vaccinations.7 The marked difference in observed incidence rates likely relates to incomplete CDC capture of cases, although the MGB cohort may have a higher rate than some US populations because of demographic or geographic effects.
Janssen COVID-19 Vaccine
On February 27, 2021, the Food and Drug Administration issued EUA approval for a third COVID-19 vaccine from Janssen (Johnson and Johnson) in individuals 18 years or older. This is an adenovirus type 26 vectored vaccine encoding a stabilized variant of the severe acute respiratory syndrome coronavirus 2 spike protein, showing high efficacy with 100% protection from death or hospitalization (similar to the mRNA COVID-19 vaccines available under EUA in the United Sates) following the currently recommended single dose. The pivotal phase III trial data reported urticaria in 5 vaccinated individuals and 1 individual who received placebo in the 7 days following vaccination. One hypersensitivity reaction deemed likely related to vaccine, not classified as anaphylaxis, was reported in 1 vaccinated individual, with urticaria beginning 2 days following vaccination and angioedema of the lips without respiratory distress beginning 4 days following vaccination.8 In addition, 1 case of anaphylaxis among 110,000 in an ongoing open-label study in South Africa has been reported following the Janssen COVID-19 vaccine administered in clinical studies.9
Updated CDC Vaccination Guidance
Despite increasing knowledge, the mechanism of allergic reactions to any of the COVID-19 vaccines remains unclear but the excipients within these vaccines remain a possibility. Polyethylene glycol (PEG) is the common excipient in both mRNA COVID-19 vaccines, whereas polysorbate 80 is the excipient in the Janssen COVID-19 vaccine. PEG and polysorbate are structurally related, and skin testing has shown cross-reactive hypersensitivity in rare cases when evaluation to both excipients has been pursued. Polysorbate 80 as an excipient cause of anaphylaxis is extremely rare, with just 1 case report of vaccine anaphylaxis thought to be related to polysorbate 80 in the literature.10
At the time of publication, updated CDC guidance11 states (1) if you are allergic to PEG, you should not receive an mRNA COVID-19 vaccine and instead speak to your physician about receiving the Janssen COVID-19 vaccine; (2) if you are allergic to polysorbate 80, you should not receive the Janssen COVID-19 vaccine and instead speak to your physician about receiving the mRNA COVID-19 vaccines; (3) a history of polysorbate allergy is a precaution rather than a contraindication to mRNA vaccination12; and (4) vaccination of these individuals (ie, those with PEG or polysorbate allergy histories) should be undertaken only under the supervision of a health care provider experienced in the management of severe allergic reactions.12 Therefore, the CDC suggests that individuals with a contraindication to the mRNA COVID-19 vaccines (due to a history of possible PEG allergy) may be considered for the Janssen COVID-19 vaccine and similarly, individuals with a contraindication to the Janssen COVID-19 vaccine (due to a history of possible polysorbate allergy) may be considered for the mRNA COVID-19 vaccines. The CDC also provides guidance around use of Janssen COVID-19 vaccine if the recipient develops a severe allergic reaction to dose 1 of an mRNA COVID vaccine, allowing for Janssen vaccination provided a delay between mRNA and Janssen vaccination of at least 28 days.11 There are currently no efficacy data on this “mix and match” approach, and we do not know the long-term durability of protection from any of the current COVID-19 vaccines. Additionally, on April 13, 2021, the CDC placed the Janssen vaccine on “pause” while investigating adverse events of thrombocytopenia and central venous thrombosis.
Prevaccine Risk Stratification: Outcomes
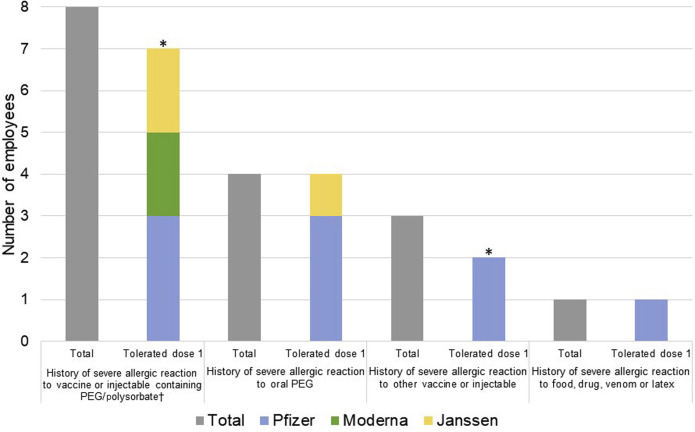
Since vaccination efforts were initiated at MGB, 472 employees with high risk allergy histories had allergist guidance prior to vaccination and we recommended 15 minute (n = 209, 44%) and 30 minute (n = 241, 51%) observations after mRNA vaccination. Of 22 (5%) referred for pre-mRNA vaccination excipient skin testing, 16 completed skin testing to date (Figure 1 ). Our previous protocols were used.2 Referral reasons included a history of a severe allergic reaction to a vaccine or injectable with PEG/polysorbate (n = 8), oral PEG (n = 4), other vaccine or injectable (n = 3), and food, drug, venom, or latex (n = 1). Only 1 employee, with a history of oral PEG allergy, was skin test positive to methylprednisolone acetate (4 mg/mL intradermal, 10 × 30 mm), which contains PEG as an excipient. This employee subsequently tolerated the Janssen COVID-19 vaccine. Among the skin test negative individuals (n = 15) who received the first dose (n = 13), no allergic reactions were observed (9 Pfizer-BioNTech, 2 Moderna, 2 Janssen). At the time of publication, 2 employees with negative skin testing await COVID-19 vaccination.
Figure 1.
Prevaccine high risk patients and dose 1 vaccination outcome (n = 16). Among the 16 individuals skin tested after risk stratification, only 1 individual was skin test positive (oral PEG severe allergic reaction, tolerated Janssen vaccine). Thirteen of the 15 skin test negative individuals tolerated the initial dose of COVID-19 vaccine. ∗Two skin test negative individuals are awaiting dose 1 of the COVID-19 vaccine: 1 employee with a history of severe allergic reaction to vaccine or injectable containing PEG/polysorbate and 1 employee with a history of severe allergic reaction to vaccine or injectable. †One employee experienced pruritus on lower back immediately after Pfizer dose 1 was given 10 mg cetirizine with complete resolution of symptoms in 30 minutes. Tolerated Pfizer dose 2 without any allergic symptoms.
Prevaccine Risk Stratification: Algorithm
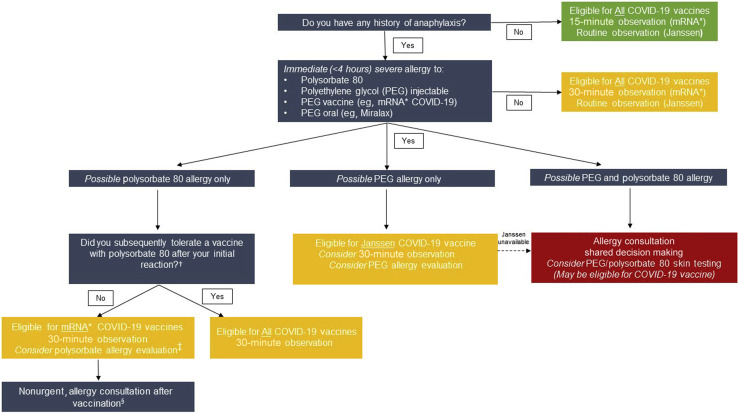
With additional clinical data and approval of the third COVID-19 vaccine in the United States, we propose a modified approache to the evaluation of patients with reported allergy histories that remain consistent with CDC guidance (Figure 2 ). Although these algorithm provides guidance, until COVID-19 vaccine supply increases, the primary role of the allergist is to enable patients to safely receive the first vaccine available to them. This may require allergist evaluation for PEG and/or polysorbate allergy depending on vaccine availability.
Figure 2.
Risk stratification pathways for COVID-19 vaccination in patients with possible PEG or polysorbate allergy. The primary role of the allergist is to enable patients to safely receive the first vaccine available to them. This may require allergist evaluation for PEG and/or polysorbate allergy depending on vaccine availability. Individuals with any history of anaphylaxis, per CDC guidance, would be monitored for 30 minutes after mRNA COVID-19 vaccination. Individuals without a PEG or polysorbate allergy are eligible to receive all COVID-19 vaccines and observation time would depend on which vaccine was being given and whether there was a previous history of anaphylaxis. Individuals with a polysorbate 80 only allergy would be further assessed by asking “Did you tolerate a polysorbate 80 vaccine after your initial reaction” to a polysorbate 80 injectable or vaccine. Individuals with PEG-only allergy are eligible to receive Janssen COVID-19 vaccine without allergy evaluation. ∗mRNA COVID-19 vaccines from Pfizer-BioNTech and Moderna. †See Table I. ‡Consider allergy evaluation of polysorbate allergy history if patient preference is for the Janssen vaccine and it is available. §Polysorbate allergy evaluation may be useful in guiding the future use of injectables and vaccines with polysorbate.
Similar to our initial algorithm,2 individuals with any history of anaphylaxis should continue to be monitored for 30 minutes after receiving an mRNA COVID-19 vaccine. Following current CDC guidance,11 , 12 individuals who self-report a PEG-allergy only can be considered for Janssen COVID-19 vaccine if available, whereas individuals who self-report a polysorbate-only allergy can be considered for mRNA COVID-19 vaccines after shared decision making with their physician. For COVID-19 vaccine-naive individuals, clarification of polysorbate allergy can be easily assessed by asking about tolerance of other common vaccines with polysorbate 80 (Table I ). In patients with a history of PEG anaphylaxis, cross-reactivity to polysorbate 80 and other PEG derivatives may be a significant problem13 and more data are needed to assess whether these individuals will tolerate the low concentrations of polysorbate 80 present in the Janssen vaccine (and other severe acute respiratory syndrome coronavirus 2 vaccines in development). Pending the CDC's ongoing evaluation of the Janssen vaccine, our algorithms may require additional modifications.
Table I.
Select vaccines containing polysorbate excipients
| Polysorbate | Vaccine name | Vaccine type | Total mg per dose |
|---|---|---|---|
| Polysorbate 20 | Havrix (adult) | HepA | 0.050 |
| Polysorbate 20 | Flublok | Influenza | 0.028 |
| Polysorbate 20 | Flublok Quad | Influenza | 0.028 |
| Polysorbate 20 | Havrix (child) | HepA | 0.025 |
| Polysorbate 20 | Sanofi∗ | SARS-CoV-2† | Unknown |
| Polysorbate 20 | Twinrix | HepA + HepB | Not specified |
| Polysorbate 80 | Flucelvax Quad | Influenza | ≤1.50 |
| Polysorbate 80 | Fluad | Influenza | 1.18 |
| Polysorbate 80 | Flulaval Quad | Influenza | ≤0.887 |
| Polysorbate 80 | Fluarix Quad | Influenza | ≤0.55 |
| Polysorbate 80 | Jansen COVID-19 | SARS-CoV-2 | 0.16 |
| Polysorbate 80 | Boostrix | Tdap | ≤0.10 |
| Polysorbate 80 | Infanrix | DTaP | ≤0.10 |
| Polysorbate 80 | Kinrix | DTaP + IPV | ≤0.10 |
| Polysorbate 80 | Pediarix | DTaP + HepB + IPV | ≤0.10 |
| Polysorbate 80 | Prevnar 13 | Pneumococcal 13-valent | ≤0.10 |
| Polysorbate 80 | Shingrix | Zoster | 0.080 |
| Polysorbate 80 | Gardasil | HPV | 0.050 |
| Polysorbate 80 | Gardasil 9 | HPV | 0.050 |
| Polysorbate 80 | Heplisav-B | HepB | 0.050 |
| Polysorbate 80 | Vaxelis | Dtap-IPV-Hib-HepB | ≤0.030 |
| Polysorbate 80 | Trumenba | Meningococcal Group B | 0.018 |
| Polysorbate 80 Polysorbate 80 |
AstraZeneca Sanofi∗ |
SARS-CoV-2† SARS-CoV-2†AS03 adjuvant |
≤0.007 mg 4.86 mg |
| Polysorbate 80 | JE-Vax | Japanese encephalitis | ≤0.0074 |
| Polysorbate 80 | Pentacel | DTaP + IPV + Hib | 0.0050 |
| Polysorbate 80 | Quadracel | DTaP + IPV | 0.0050 |
| Polysorbate 80 | RotaTeq | Rotavirus | Not specified |
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
The SARS-CoV-2 Sanofi vaccine contains polysorbate 20 (unknown mg/dose) with polysorbate 80 in the AS03 adjuvant (4.86 mg/dose).
Not approved by the Food and Drug Administration.
Conclusions
Severe allergic reactions to COVID-19 vaccines remain exceedingly rare, and the mechanism of these reactions requires further investigations. All vaccine sites should continue to observe higher risk individuals following CDC guidelines and have staff trained in recognizing and managing anaphylaxis. The vast majority of individuals with high risk allergy histories will not need excipient skin testing prior to vaccination. As our experience and knowledge with COVID-19 variants vaccines increases, we must continue to remain flexible with our approach. Our updated prevaccine risk stratification algorithm can be used in conjunction with the previously published skin testing guidance.2 In the meantime, the potential life-saving benefit of vaccination makes it essential that allergists continue to carefully evaluate and advise all patients with allergy histories and prevent denying access to the vaccine unnecessarily.
Acknowledgments
We thank many colleagues in the Mass General Brigham health system for the design and implementation of the COVID-19 vaccination program, including Paul D. Biddinger, MD, Upeka Samarakoon, MS, PhD, MPH, Rajesh Patel, MD, MPH, Caroline L. Sokol, MD, PhD, Micelle E. Conroy, MD, Leeann Ouimet, MBA, Allen Judd, BA, Lily Li, MD, Tanya M. Laidlaw, MD, David I. Hong, MD, Anna M. Feldweg, MD, Nahal Beik, PharmD, BCPS, Christian M. Mancini, BS, Aimee Foster, MS, FNP-BC, and Kenisha Lewis.
Footnotes
No funding was received for this work.
Conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University & Medicine. https://coronavirus.jhu.edu/map.html Available from:
- 2.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations Available from:
- 4.Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T., et al. First month of COVID-19 vaccine safety monitoring— United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC COVID-19 Response Team; Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal K.G., Robinson L.B., Camargo C.A., Shenoy E.S., Banerji A., Landman A.B.∗, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen Pharmaceutical Companies Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Janssen Covid-19 vaccine to prevent coronavirus disease 2019 (Covid-19) https://www.janssenlabels.com/emergency-use-authorization/Janssen+COVID-19+Vaccine-HCP-fact-sheet.pdf Available from:
- 9.Lovelace B., Jr. J&J says two trial participants had severe allergic reactions after getting Covid vaccine. https://www.cnbc.com/2021/02/26/jj-says-two-people-had-severe-allergic-reactions-after-getting-covid-vaccine.html Available from:
- 10.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. bcr0220125797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Information about COVID-19 vaccines for people with allergies. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html Available from:
- 12.Centers for Disease Control and Prevention Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States United States Department of Health and Human Services. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#Contraindications Available from:
- 13.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]