Abstract
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in children is associated with better outcomes than in adults. The inflammatory response to COVID-19 infection in children remains poorly characterised.
Methods
We retrospectively analysed the medical records of 127 laboratory-confirmed COVID-19 patients aged 1 month to 16 years from Wuhan and Jingzhou of Hubei Province. Patients presented between January 25th and March 24th 2020. Information on clinical features, laboratory results, plasma cytokines/chemokines and lymphocyte subsets were analysed.
Findings
Children admitted to hospital with COVID-19 were more likely to be male (67.7%) and the median age was 7.3 [IQR 4.9] years. All but one patient with severe disease was aged under 2 and the majority (5/7) had significant co-morbidities. Despite 53% having viral pneumonia on computed tomography (CT) scanning only 2 patients had low lymphocyte counts and no differences were observed in the levels of plasma proinflammatory cytokines, including interleukin (IL)-2, IL-4, IL-6, tumour necrosis factor (TNF)-, and interferon (IFN)- between patients with mild, moderate or severe disease.
Interpretations
We observed that the immune responses of children to COVID-19 infection is significantly different from that seen in adults. Our evidence suggests that SARS-CoV-2 does not trigger a robust inflammatory response or ‘cytokine storm’ in children with COVID-19, and this may underlie the generally better outcomes seen in children with this disease.
Research in context.
Evidence before this study
We searched PubMed without language restriction for studies published until January 19, 2021, using the search terms “SARS-CoV-2″ or “novel coronavirus” or “COVID-19″ and “immune responses” or “innate immunity” or “cytokine” or “subset of lymphocyte” and “children” or “adolescent”. Previously published research describes that severe and fatal cases in children are relatively rare. However, the inflammatory responses to COVID-19 infection in children still needs further study.
Added value of this study
We analysed data from 127 laboratory-confirmed COVID-19 patients aged 1 month to 16 years in Hubei province to explore the immune responses to SARS-CoV-2 infection presenting to hospital with COVID-19. Cell numbers of CD3+, CD4+, CD8+ and natural killer T cells were within mostly normal limits even in more severe cases, and the levels of immunoglobulins, and proinflammatory cytokines, including interleukin (IL)−2, IL-4, IL-6, tumour necrosis factor (TNF)-α, and interferon (IFN)-γ were not generally elevated regardless of disease severity.
Implications of all the available evidence
The immune response to SARS-CoV-2 infection of children is significantly different from that seen in adults. The inflammatory responses seen even in children with viral pneumonia on CT are relatively mild and do not trigger the “cytokine storm” seen in some adults with COVID-19. This implies anti-cytokine therapies may not be effective in children with COVID-19.
Alt-text: Unlabelled box
1. Introduction
In December 2019, a novel coronavirus was identified in Wuhan China named by the World Health Organization (WHO) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 has 88% sequence homology with SARS-like coronavirus [1,2]. The WHO declared novel coronavirus disease 2019 (COVID-19) after infection by SARS-CoV-2 a public health emergency of international concern [3]. By 19 Jan 2021, there had been 96.01 million cases of COVID-19 and 2,049,352 deaths globally [4].
The immune system plays a crucial role in mediating the response to SARS-CoV-2 infection, with three major classes of pattern recognitions receptors (PRRs): toll-like receptors, RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs) [5,6]. These can all trigger downstream signalling effectors, such as Nuclear Factor-B and IRF3/7, which in turn stimulate anti-viral effector systems including those mediated through CD4+, CD8+, Natural Killer (NK) cells, and macrophages [7]. Increased release and transcription of proinflammatory cytokines, which may lead to a “cytokine storm”, result in elevated plasma levels of interleukin (IL)−2, IL-6, IL-7, IL-10, IL-1, tumour necrosis factor (TNF)-, and interferon (IFN)- in some severe or critically unwell subjects. In adults, cytokine responses are correlated with disease severity and poor prognosis in COVID-19 patients [8,9]. Moreover, pathological findings of COVID-19 biopsy samples have shown interstitial mononuclear inflammatory infiltrates in lung tissues [10]. What's more, complement has been reported as a target of COVID-19 [11], and complement activation has been found that the C3a generation and C3-fragment deposition from the lung biopsy samples of severe COVID-19 patients [12].
However, much less is known about the inflammatory responses in children with COVID-19. Children in general have much milder disease, although a rare subgroup has been identified with a Kawasaki like syndrome termed paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) [13,14].
In this study, we aimed to investigate the immune response of children with COVID-19, by measuring immunoglobin, complement, proinflammatory cytokines, and lymphocyte subsets, and in particular T-cell responses during the early and late stage of SARS-CoV-2 infection, and the difference between age groups, to provide insight into the role of the immune response in children with COVID-19.
2. Methods
2.1. Study population and clinical samples
We performed a retrospective cohort to study the immune response of 127 COVID-19 children subjects aged 1 month to 16 years, who were admitted into the Wuhan Children's Hospital and Jingzhou First People's Hospital during January 25th and March 24th, 2020. The diagnosis of COVID-19 was based on the guidelines issued under the New Coronavirus Pneumonia Prevention and Control Program (4th to 7th edition) published by the National Health Commission of China[15], all included cases were confirmed by real-time reverse transcriptase polymerase-chain-reaction (RT-PCR) using nasal swab specimens. Analyses were performed on the first blood sample collected after admission.
This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Medical Ethical Committees (2020-R120). Due to the urgent need to collect data on this emerging infectious disease, the requirement for written informed consent was waived.
2.2. COVID-19 clinical classification
Cases were classified into mild, moderate, severe and severe/critical cases following the guidelines issued by the National Health Commission of China (7th edition) [15].
2.2.1. Mild
Mild clinical symptoms without lung infiltration on chest X ray or chest computed tomography (CT) scan.
2.2.2. Moderate
Fever, cough and other symptoms present alongside lung infiltrations on chest X ray or chest CT scan.
2.2.3. Severe (children-specific definition)
The subject was classified as severe if at least one of the following diagnosis conditions was met:
-
a)
Shortness of breath as defined by age (<2 months, RR ≥ 60 per min; 2–12 months, RR ≥ 50 per min; 1–5 years, RR ≥ 40 per min;> 5 years, RR ≥ 30 per min).
-
b)
Oxygen saturations whilst breathing room air and at rest 93%;
-
c)
Respiratory distress, cyanosis, or intermittent apnoea;
-
d)
Drowsiness and convulsions;
-
e)
Refusal to eat or feeding difficulties, signs of dehydration.
2.2.4. Critical
One of the follow conditions has to be met:
-
a)
Respiratory failure requiring mechanical ventilation
-
b)
Cardiovascular Shock.
-
c)
Patients with other organ dysfunction needing intensive care unit treatment.
2.3. Cytokine assays
The levels of plasma cytokines or chemokines (IL-2, IL-4, IL-6, IL-10, TNF-, and IFN-) was quantified by enzyme linked immunosorbent assay (ELISA) kits. All assays were performed according to the manufacturer's instructions.
2.4. Flow cytometric analysis
Peripheral blood mononuclear cells (PBMCs) from 127 patients were obtained and processed in the Clinical Lab of Wuhan Children Hospital. The PBMCs were harvested by centrifugation and stained using a commercially available kit (Hangzhou Cellgene Biotech Co. Ltd). Cell numbers of total T, CD4+ T, CD8+ T, B cells and NK cells were analysed by flow cytometry on and LSR Fortessa Cell analyzer (BD Biosciences) and data processed using the FolwJo software.
2.5. Statistical analysis
Continuous variables were described using means and standard deviations (SD) or median (IQR). Comparisons across groups were performed with independent t-tests for normally distributed data or by Mann-Whitney U tests. Chi-square tests and Fisher's exact test were applied for categorical variables as appropriate. Univariate and multivariate logistic regression analysis was performed to explore the relationship between each variable and the risk of severity of COVID-19. All analyses were analysed using IBM SPSS statistics version 26.0.
2.6. Role of the funding source
This study was funded by National Natural Foundation of China (No. 81,970,653). The funder had no role in study design, data collection, analysis, and interpretation or writing of this research. Dr. Mei had access to the full database and took the decision to submit this manuscript for publication.
3. Results
3.1. Clinical characteristics
A total of 127 children with COVID-19 were admitted to hospital, 67.72% (86/127) were male, and the median age was 7.3 [IQR 4.9] years (Table 1). The subjects were classified into mild, moderate, and severe/critical groups following the guidelines of the National Health Commission of China. Fifty-seven children were diagnosed as mild type COVID-19, including one 1-month-old boy with an atrial septal defect. There were 63 moderate type COVID-19 subjects, including one 4-year-old boy with acute lymphoblastic leukaemia (ALL) and another 1-month-old boy with intracranial haemorrhage. Severe/critical type (n = 7) included 2 fatalities, one in a patient with intussusception and multiorgan failure, and one in a patient with an intracranial malignant tumour (2 fatalities case report, supplementary material). 3 of the other 5 severe/critical patients also had comorbidities, including at the time of initial presentation one with ALL on maintenance chemotherapy, two with atrial septal defects. The other two were otherwise healthy at initial presentation.
Table 1.
Demographic, baseline features of 127 children or adolescence with COVID-19. Values are numbers, mean±SD (standard deviation) or medians (interquartile ranges) unless stated otherwise.
| Total | Mild | Moderate | Severe and Critical | |
|---|---|---|---|---|
| No. of patients | 127 | 57 | 63 | 7 |
| Age, yr | 7.27 (4.85) | 8.49 (4.46) | 5.73 (4.85) | 1.64 (2.85) |
| Distribution, No. of patients | ||||
| 0–12m | 28 | 11 | 12 | 5 |
| 13–24m | 2 | 0 | 1 | 1 |
| >25 m, ≤5y | 14 | 8 | 6 | 0 |
| >5y, ≤10y | 45 | 19 | 25 | 1 |
| >10y | 38 | 19 | 19 | 0 |
| Male (%) | 67.72 | 71.93 | 66.67 | 71.43 |
| Weight, kg | 35.06 (23.9) | 36.76 (24.93) | 36.28 (22.85) | 11.60 (8.08) |
| Height, cm | 124.5 (36.49) | 128.25 (35.10) | 126.90 (34.78) | 78.80 (27.58) |
| Vital Signs, median [IQR] | ||||
| Temperature, °C | 36.5 [36.2–36.8] | 36.5 [36.2–36.7] | 36.5 [36.3–36.9] | 36.7 [36.2–37.0] |
| Pulse, per/min | 96 [88–110] | 96 [90–107] | 93 [88–101] | 145 [110–160] |
| Respiratory rate, per/min | 22 [20–26] | 23 [20–25] | 22 [20–25] | 26 [25–45] |
| Comorbidity | ||||
| Acute lymphoblastic leukaemia | 2 | 0 | 1 | 1 |
| Intussusception | 1 | 0 | 0 | 1 |
| Atrial septal defect | 3 | 1 | 0 | 2 |
| Intracranial malignant tumour | 1 | 0 | 0 | 1 |
| Intracranial haemorrhage | 1 | 0 | 1 | 0 |
| Co-infection | ||||
| Mycoplasma IgM (+) | 60 | 36 | 21 | 3 |
| CMV IgM (+) | 4 | 0 | 4 | 0 |
| CMV IgG (+) | 38 | 15 | 21 | 2 |
| EB IgM/VCA (+) | 3 | 1 | 2 | 0 |
| EB IgG/VCA (+) | 49 | 21 | 24 | 4 |
| Lenghth days of hospitalisation (d) | 15.41 (7.86) | 14.02 (5.81) | 15.24 (6.78) | 30.00 (19.58) |
| Fatal or MV | 2 | 0 | 0 | 2 |
COVID-19, coronavirus disease 2019; CMV, cytomegalovirus; EBV, Epstein-Barr virus. MV, mechanical ventilation.
All patients with severe/critical disease were less than 2 years old (1 month – 16 months) except one 8-year-old boy who developed myocardial damage. CT changes of pneumonia were present in 68/127 patients who underwent CT scans. Mild cases were defined according to the guideline issued by the National Health Commission of China and were without manifestations on CT scan. 2 out of 5 children with non-pneumonia manifestations were diagnosed as severe, one with atrial septal defects and one who died with an intracranial malignant tumour. Except for the two patients who died, all other patients (125) recovered to and were discharge from hospital, with an average length of stay of 15.4 days across the whole group. 47.24% (60/127) subjects tested positive of mycoplasma IgM, 4 subjects tested positive for cytomegalovirus (CMV), and 3 subjects tested positive for Epstein-Barr virus (EBV) IgM/VCA. Table 2 shows the results of blood tests and CT scan results for the full sample. Lymphopenia (lymphocyte count, <1.0 109 per litre) was only present in 2 patients (1.57%), including one 6-month-old boy (0.37 109/L) with intracranial malignant tumour who died and one 9-year-old boy (0.96 109/L).
Table 2.
Clinical characteristics of 127 children or adolescence with COVID-19. Values are mean±SD (standard deviation) or medians (interquartile ranges) unless stated otherwise.
| Total | Mild | Moderate | Severe/Critical | |
|---|---|---|---|---|
| White blood cells (4–10 109 cells per L) | 6.61 (2.09) | 6.61 (1.88) | 6.77 (2.17) | 5.33 (2.57) |
| Neutrophil (2–7 109 cells per L) | 2.94 (1.98) | 3.36 (2.58) | 2.72 (1.25) | 1.89 (1.80) |
| Lymphocytes (1–4 109 cells per L) | 3.07 (1.52) | 2.70 (0.96) | 3.35 (1.82) | 3.37 (1.81) |
| Eosinophil (0.05–0.5 109 cells per L) | 0.24 (0.38) | 0.15 (0.16) | 0.03 (0.03) | 0.13 (0.17) |
| Platelet (100–300 109 cells per L) | 298.90 (95.87) | 283.96 (74.42) | 296.15 (96.12) | 314.14 (28.47) |
| C-reactive protein (<0.75 mg/L) | 1.42 (1.97) | 2.09 (2.75) | 0.75 (0.01) | 0.75 (0.01) |
| Procalcitonin (≤0.05 ng/ml) | 0.07 (0.12) | 0.07 (0.15) | 0.06 (0.05) | 0.26 (0.24) |
| Fibrinogen (2–4 g/L) | 2.17 (0.64) | 2.23 (0.65) | 2.15 (0.65) | 2.07 (0.28) |
| D-dimer (0–0.55 mg/L) | 0.44 (1.12) | 0.24 (0.31) | 0.67 (1.63) | 0.15 (0.04) |
| Alanine aminotransferase (21–27 IU/L) | 29.74 (58.59) | 24.96 (10.99) | 47.29 (85.83) | 19.57 (22.90) |
| Aspartate transferase (15–46 IU/L) | 36.38 (61.71) | 15.07 (8.43) | 44.50 (81.46) | 27.71 (6.63) |
| Creatinine (58–110umol/L) | 37.38 (14.02) | 41.68 (13.50) | 33.30 (13.68) | 37.5 (7.64) |
| Blood urea nitrogen (3.2–7.1 mmol/L) | 4.20 (1.52) | 4.41 (1.28) | 3.98 (1.68) | 4.75 (0.72) |
| Calcium (2.1–2.55 mmol/L) | 2.47 (0.12) | 2.46 (0.11) | 2.48 (0.13) | 2.45 (0.14) |
| Infiltration of lung | ||||
| Pneumonia No. | 68 | 0 | 63 | 5 |
| non-pneumonia No. | 59 | 57 | 0 | 2 |
| No. of lobes affected, median[IQR] | 1 [1–2] | 0 | 2 [1–2] | 1 [1–3.5] |
Data are mean (standard deviation), unless otherwise indicate. COVID-19, coronavirus disease 2019.
3.2. T-cell immune responses to SARS-CoV-2
Both helper T (Th) cells (CD3+CD4+) and suppressor T cells (CD3+CD8+) counts in children with COVID-19 were within normal levels (Table 3). There were no significant differences in cell counts between the mild, moderate and severe/critical groups with COVID-19. The number of CD3+, CD3+CD4+, CD19+ B cells, and the ratio of CD4+/CD8+ were significantly lower for those aged 10 or above (Table 4).
Table 3.
T cell number and proinflammatory cytokines of 127 children or adolescence with COVID-19. Values are medians (interquartile ranges) unless stated otherwise.
| Total | Mild | Moderate | Severe and Critical | |
|---|---|---|---|---|
| CD3+T cell proportion (38.56–70.06%) | 68.09 [63.61–72.95] | 69.36 [63.72–73.28] | 67.33 [62.96–71.09] | 70.49 [66.87–72.35] |
| CD3+T cell number (805–4459cell/L) | 2073 [1675–2779] | 1955 [1561–2391] | 2231 [1840–3230] | 2280 [1717–2644] |
| CD3+CD4+T cell proportion (14.21%−36.99%) | 35.81 [30.78–39.19] | 35.12 [30.52–39.03] | 35.90 [32.11–44.00] | 35.89 [33.24–37.03] |
| CD3+CD4+T cell number (345–2350cell/L) | 1012 [669–1569] | 911 [669–1120] | 1103 [739–1942] | 1010 [839–1391] |
| CD3+CD8+T cell proportion (13.24%−38.53%) | 27.52 [21.84–31.49] | 29.27 [25.93–31.49] | 25.00 [19.48–28.89] | 28.32 [26.32–31.15] |
| CD3+CD8+T cell number (314–2080cell/L) | 743 [556–1022] | 743 [556–977] | 806 [556–1200] | 1022 [692–1220] |
| Natural killer proportion (7.92%−33.99%) | 9.97 [5.71–14.59] | 9.87 [5.66–13.32] | 10.81 [5.97–15.12] | 11.80 [6.79–14.62] |
| Natural killer cell number (210–1514cell/L) | 316 [187–470] | 261 [150–471] | 370 [236–470] | 225 [182–417] |
| Lymphocyte (25%−40%) | 18.15 [15.35–22.61] | 19.05 [15.60–20.91] | 17.43 [13.41–23.34] | 16.50 [14.43–19.14] |
| CD19+B cell number (240–1317 cell/L) | 557 [372–822] | 515 [374–661] | 654 [364–950] | 528 [450–649] |
| CD4+/CD8+T ratio (0.96–2.05) | 1.34 [1.09–1.78] | 1.33 [1.06–1.59] | 1.47 [1.14–1.95] | 1.21 [1.16–1.34] |
| CD4+CD25+T cell proportion (%) | 4.93 [3.64–5.70] | 5.13 [4.19–5.62] | 4.31 [3.36–6.18] | 5.15 [3.74–6.73] |
| Human IL-2 (0–11.4pg/ml) | 1.43 [1.21–1.67] | 1.32 [1.18–1.56] | 1.50 [1.34–1.70] | 1.54 [1.33–1.68] |
| Human IL-4 (0–12.9pg/ml) | 2.68 [2.07–3.33] | 2.52 [2.03–3.11] | 2.74 [2.23–3.34] | 2.83 [2.55–3.42] |
| Human IL-6 (0–20.9pg/ml) | 3.98 [3.04–5.66] | 3.79 [2.95–4.97] | 4.17 [3.19–7.34] | 3.17 [2.68–3.59] |
| Human IL-10 (0–5.9pg/ml) | 3.50 [3.05–4.61] | 3.26 [2.88–3.89] | 4.01 [3.32–5.66] | 3.26 [3.05–4.13] |
| Human TNF-α (0–5.5pg/ml) | 1.62 [1.26–2.23] | 1.52 [1.19–1.82] | 1.78 [1.31–2.36] | 1.76 [1.5–2.39] |
| Human IFN-γ (0–17.3pg/ml) | 2.87 [2.23–3.91] | 2.87 [2.12–4.05] | 2.94 [2.38–3.89] | 2.78 [1.85–3.01] |
Table 4.
Levels of T cell number and proinflammatory cytokines of 127 children or adolescence with COVID-19 in different age stages. Values are medians (interquartile ranges) unless stated otherwise.
| Total | ≤24m | >25 m, ≤5y | >5, <=10y | >10y | |
|---|---|---|---|---|---|
| CD3+T cell proportion (38.56–70.06%) | 68.09 [63.61–72.95] | 66.44 [59.76–75.26] | 65.66 [59.87–67.23] | 68.97 [64.06–72.35] | 69.98 [65.31–73.79] |
| CD3+T cell number (805–4459cell/L) | 2073 [1675–2779] | 3234 [2329–4403] | 2426 [2082–3617] | 1971 [1647–2287] | 1868 [1554–2267] |
| CD3+CD4+T cell proportion (14.21%−36.99%) | 35.81 [30.78–39.19] | 41.81 [35.95–46.25] | 30.79 [27.96–35.52] | 35.90 [30.63–39.18] | 32.10 [28.74–36.86] |
| CD3+CD4+T cell number (345–2350cell/L) | 1012 [669–1569] | 2033.5 [1757–2456] | 1205 [891–1403] | 970 [670–1123] | 740 [610–904] |
| CD3+CD8+T cell proportion (13.24%−38.53%) | 27.52 [21.84–31.49] | 23.46 [18.09–27.52] | 27.72 [22.54–30.91] | 27.07 [22.13–31.12] | 28.80 [26.10–31.92] |
| CD3+CD8+T cell number (314–2080cell/L) | 743 [556–1022] | 1100 [716–1281] | 1010 [788–1590] | 698 [481–983] | 707 [513–759] |
| Natural killer proportion (7.92%−33.99%) | 9.97 [5.71–14.59] | 5.905 [4.73–8.54] | 12.21 [4.44–18.91] | 11.04 [6.05–13.43] | 9.81 [6.20–16.52] |
| Natural killer cell number (210–1514cell/L) | 316 [187–470] | 289 [220–425] | 437 [308–696] | 316 [197–407] | 282 [145–476] |
| Lymphocyte (25%−40%) | 18.15 [15.35–22.61] | 21.37 [17.04–28.14] | 19.96 [17.53–27.79] | 17.69 [15.09–20.22] | 16.19 [14.09–20.69] |
| CD19+B cell number (240–1317 cell/L) | 557 [372–822] | 999 [725–1687] | 799 [534–1204] | 538 [358–651] | 408 [329–540] |
| CD4+/CD8+T ratio (0.96–2.05) | 1.34 [1.09–1.78] | 1.87 [1.44–2.68] | 1.34 [1.22–1.81] | 1.30 [1.10–1.61] | 1.14 [0.91–1.39] |
| CD4+CD25+T cell proportion (%) | 4.93 [3.64–5.70] | 4.86 [3.53–5.59] | 4.51 [3.68–5.02] | 4.65 [3.12–5.77] | 5.34 [4.38–6.75] |
| Human IL-2 (0–11.4pg/ml) | 1.43 [1.21–1.67] | 1.40 [1.27–1.69] | 1.52 [1.27–1.65] | 1.47 [1.25–1.95] | 1.36 [1.18–1.50] |
| Human IL-4 (0–12.9pg/ml) | 2.68 [2.07–3.33] | 2.72 [1.78–3.29] | 2.53 [2.36–2.94] | 2.58 [2.09–3.43] | 2.71 [2.16–3.24] |
| Human IL-6 (0–20.9pg/ml) | 3.98 [3.04–5.66] | 4.36 [3.51–7.33] | 3.43 [2.71–5.13] | 4.14 [3.14–6.63] | 3.70 [3.06–4.97] |
| Human IL-10 (0–5.9pg/ml) | 3.50 [3.05–4.61] | 4.53 [3.47–7.08] | 3.60 [3.01–4.35] | 3.71 [3.11–5.00] | 3.19 [2.79–3.49] |
| Human TNF-α (0–5.5pg/ml) | 1.62 [1.26–2.23] | 1.80 [1.43–2.40] | 1.65 [1.28–1.93] | 1.63 [1.27–2.68] | 1.50 [0.92–1.83] |
| Human IFN-γ (0–17.3pg/ml) | 2.87 [2.23–3.91] | 3.01 [2.57–3.80] | 3.14 [2.54–4.06] | 2.78 [2.11–4.78] | 2.81 [1.85–3.38] |
3.3. Plasma cytokine profiles and immunoglobulins
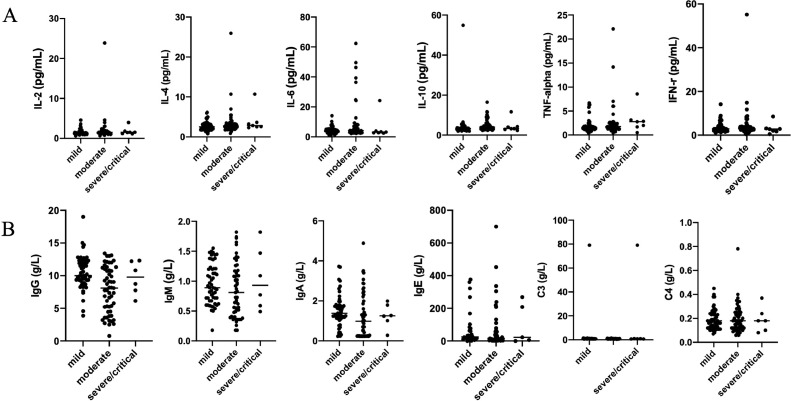
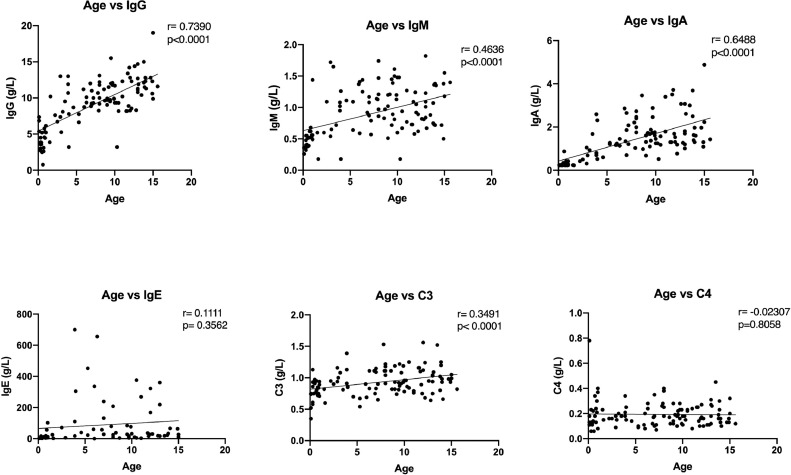
To investigate the potential role of proinflammatory cytokines/chemokines in SARS-CoV-2 infection, we measured the levels of IL-2, IL-4, IL-6, TNF-, and IFN- in plasma by ELISA. Interestingly levels were generally low and there were no strong associations with disease severity (Table 3). Similarly, Complement (C3 and C4) and immunoglobulins (IgG, IgA, IgM, and IgE) did not differ between mild, moderate and severe/critical cases (Fig. 1). Levels of immunoglobulins (IgG, IgA, IgM and C3) were significantly higher in older children, while IgE and C4 were not associated with age (Fig. 2). We observed weak associations between levels of cytokines and frequency of T cell subsets, but apart from a negative correlation between CD3+ counts and TNF- levels none of these survived corrections for multiple testing.
Fig. 1.
Levels of cytokines, immunoglobulins and complement in children with COVID-19 patients. A. Cytokines (IL-2, IL-4, IL-6, IL-10, TNF-, and IFN-) in patients in different groups; B. Immunoglobulins (IgG, IgA, IgM, and IgE) and complement (C3 and C4) in different groups.
Fig. 2.
Levels of immunoglobulins (IgG, IgA, IgM, IgE) and complement (C3, C4) relative to age in COVID-19 patients. IgG, IgA, IgM and C3 were significantly higher in older children, while IgE and C4 were not associated with age in COVID-19 patients.
3.4. Association of inflammatory markers with disease severity
To analyse potential markers of disease severity, we compared potential markers in mild cases, moderate and severe/critical groups. Individual analyses showed significant association between severity and age, CRP, IL6, IL10 and d-dimer levels (Table 5). However, in a multivariate logistic regression analysis showed there is no independent predictor of severe disease (Table 6). We also undertook a sensitivity analysis using 3 groups (mild, moderate and severe/critical groups) by separating the moderate and severe/critical subgroups. No significant differences were seen in the critical group compared with the other groups, although these data should be interpreted with caution due to the small number of subjects.
Table 5.
Individual analyses severity risk factors for patients with COVID-19.
| Age | CRP | IL6 | IL10 | D-dimer | |
|---|---|---|---|---|---|
| Mann-Whitney U | 1068.500 | 480.500 | 1293.500 | 951.000 | 682.500 |
| Wilcoxon W | 2149.500 | 2020.500 | 4533.500 | 4191.000 | 3028.500 |
| Z | −3.986 | −2.297 | −2.083 | −3.928 | −2.181 |
| Sig. | 0.000 | 0.022 | 0.037 | 0.000 | 0.029 |
CRP, C-reactive protein; IL, interleukin.
Table 6.
Multivariate logistic regression analysis of severity risk factors for patients with COVID-19.
| Variables | OR (95%CI) | P value |
|---|---|---|
| Age | −0.981 (−0.036–0.012) | 0.331 |
| CRP | 1.905 (−0.001–0.048) | 0.062 |
| IL6 | −1.448 (−0.37–0.006) | 0.154 |
| IL10 | 1.605 (−0.013–0.117) | 0.114 |
| D-dimer | 1.709 (−0.011–0.139) | 0.093 |
CRP, C-reactive protein; IL, interleukin.
4. Discussions
In this paper we present novel data on the immune responses seen in children hospitalised with COVID-19. A total 127 children with COVID-19 were admitted to hospital. The majority were male (67.7%), and the median age was 7.3 years. Patients with severe disease were younger (only 1 was over the age of 2) and generally had significant co-morbidity. The immune responses are significantly different from adults. Neither numbers of helper T cells and suppressor T cells were associated with severity of disease. Furthermore, our data show that SARS-CoV-2 does not trigger a severe inflammatory response or ‘cytokine storm’ in children, which potentially explains the much better outcomes seen in children infected with SARS-CoV-2. This was despite the presence of viral pneumonia on CT scanning in over half of the children studied. It is possible some of the cases with co-existing pathologies such as cancer may have had a degree of immunoparesis as a consequence of this underlying pathology. Interestingly, over half of the children in this cohort were Mycoplasma positive.
Lymphopenia is common in adults with COVID-19, but seems to be rare in children. In adults, lymphocyte counts also show negative correlations with the severity of disease and with the viral copy numbers from nasal or throat swab specimens [16,17]. The subsets of lymphocytes which are reduced in number in adult patients include CD4+ T cells, CD8+ T cells, B cells, NK cells, memory and regulatory T cells [16,18]. In adults, lymphocyte counts will increase during the convalescent stage of disease. In one recent study, enrichment of genes in apoptosis and P53 signalling pathways from bronchoalveolar lavage fluid (BALF) and peripheral blood mononuclear cell (PBMC) was observed suggesting, SARS-CoV-2 infection probably induces lymphocyte apoptosis [19]. Our data are in keeping with one previous small case series of 34 children in which only one case presented with lymphopenias [20]. Two preliminary reports have suggested that in contrast CD4+ T cell count and CD8+ T cell count may increase in children during COVID-19 [21,22]. Our study shows that for children with COVID-19 the numbers of CD4+ T cells, CD8+T cells, B cells, NK cells are not reduced, even in moderate or severe disease. In addition, the cell number of different lymphocyte subsets did not change between measures taken at admission and immediately before discharge.
Importantly, our data are also in keeping with a recent large epidemiology study characterising demographic features in children with COVID19 in China. This study reported that half of the patients with severe disease were less than one year of age [23]. Our study also shows that the severe/critical cases often have comorbidities which might be an important explanation for case severity amongst children with COVID-19.
Cytokines are important mediators of the inflammatory response to COVID-19 in adults and are associated with disease severity. A number of clinical trials are ongoing in adults assessing anti-cytokine approaches to improve outcomes in COVID19. However, little is known regarding the cytokine response in children with COVID19. We found that the levels of a range of proinflammatory cytokines including IL-2, IL-4, IL-6, TNF- and IFN- were no different between mild, moderate and severe/critical groups of children with COVID-19. These data strongly suggest that the better outcomes for children with COVID19, even in the presence of significant viral pneumonia on CT scanning, may be because children do not mount the profound inflammatory response to infection with SARS-CoV-2 seen in some adults and are thus less prone to multi-organ damage. In addition, it would seem unlikely that anti-cytokine approaches will be effective in patients in this age group. Recently, a subgroup of children infected with SARS-CoV-2 have been described who go on to develop a severe inflammatory condition which shows some similarities to Kawasaki syndrome, and which has been called Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS) [13]. These patients do show increased inflammatory markers including CRP and ferritin and some also have elevated markers of myocardial damage: it is conceivable the child in our study who died aged 7 had this condition. However, this appears to be a very rare complication.
This study has some limitations. First, this is a retrospective and observational study using pre-existing electronic medical record data. Second, there are few children with severe disease due to COVID-19 and so we may have missed small differences in cytokine levels or cellular responses between the groups with different disease severity. Finally, we did not have measures of viral load and hence cannot take this into account in our analyses. Nonetheless, we observed that the majority of children included in the study admitted to hospital with COVID19 have generally a less severe outcome than adults unless they have significant co-morbidities, and that this may in part be due to the failure of younger patients to mount a major cytokine response to the SARS-CoV-2.
Acknowledgments
Funding
This study was funded by National Natural Science Foundation of China (No. 81970653), and Key Program of Natural Science Foundation of Ningbo City (202003N4019).
Data sharing statement
The original contributions presented in the study are included in the article and supplementary material, further for information on data sharing please contact the corresponding author.
Contributors
GQ designed, analysed and interpreted the results, and wrote the Article. YZ, YX, WH collected data and analysed the results. IPH interpreted results and critically reviewed the manuscript. JY, HL, LD, and LR designed and analysed the results. MY and JM designed, interpreted the results, and coordinated the study.
Declaration of Interests
We declare no competing interests.
Acknowledgements
IPH is a National Institute for Health Research (NIHR) Senior Investigator.
Footnotes
Funding: National Natural Foundation of China (No. 81970653) and Key Program of Natural Science Foundation of Ningbo City (202003N4019).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.100831.
Contributor Information
Liemin Ruan, Email: lmruan@tom.com.
Maoqing Ye, Email: yemaoqing@fudan.edu.cn.
Jin Mei, Email: tibetcn@aliyun.com.
Appendix. Supplementary materials
References
- 1.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian G.Q., Yang N.B., Ding F. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-19) outbreak. Feb 18 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. 2020 (accessed Feb 18 2020).
- 4.Worldometer. COVID-19 coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/ (accessed Jan 19 2021).
- 5.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Jamilloux Y., Henry T., Belot A. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelemans T., Kikkert M. Viral Innate Immune Evasion and the Pathogenesis of Emerging RNA Virus Infections. Viruses. 2019;11(10):961. doi: 10.3390/v11100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10222):8–14. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risitano A.M., Mastellos D.C., Huber-Lang M. Complement as a target in COVID-19? Nature Rev Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao T., Hu M., Zhang X. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020 2020.03.29.20041962. [Google Scholar]
- 13.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viner R.M., Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1742. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Commission of the People's Republic of China. The notice of launching guideline on diagnosis and treatment of the novel coronavirus pneumonia (4th edition). Jan 27 2020. 2020. http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67.shtml (accessed Jan 27 2020).
- 16.Diao B., Wang C., Tan Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley B., Roufosse C.A., Osborn M., Naresh K.N. Convalescent donor SARS-COV-2-specific cytotoxic T lymphocyte infusion as a possible treatment option for COVID-19 patients with severe disease - has not received enough attention till date. Br J Haematol. 2020;189(6):1062–1063. doi: 10.1111/bjh.16780. [DOI] [PubMed] [Google Scholar]
- 18.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong Y., Liu Y., Cao L. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann P., Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D., Li H., Lu X.X. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen F., Liu Z.S., Zhang F.R. First case of severe childhood novel coronavirus pneumonia in China. Zhonghua er ke za zhi Chin J Pediatr. 2020;58(3):179–182. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Dong Y., Mo X., Hu Y. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.