Graphical abstract
Keywords: Side effect, Adverse event, SARS-CoV-2, COVID-19, mRNA vaccine, Acceptance, Pfizer BioNtech, BNT162b2
Abstract
Introduction
Concerns are prevailing about the safety and side effects of the BNT162b2 mRNA vaccine for coronavirus disease 2019 (COVID-19).
Methods
A randomized, cross-sectional study was performed to investigate the side effects of the BNT162b2 vaccine using an independent online questionnaire gathering responses from healthcare workers (HCWs) with detailed review of organ systems.
Results
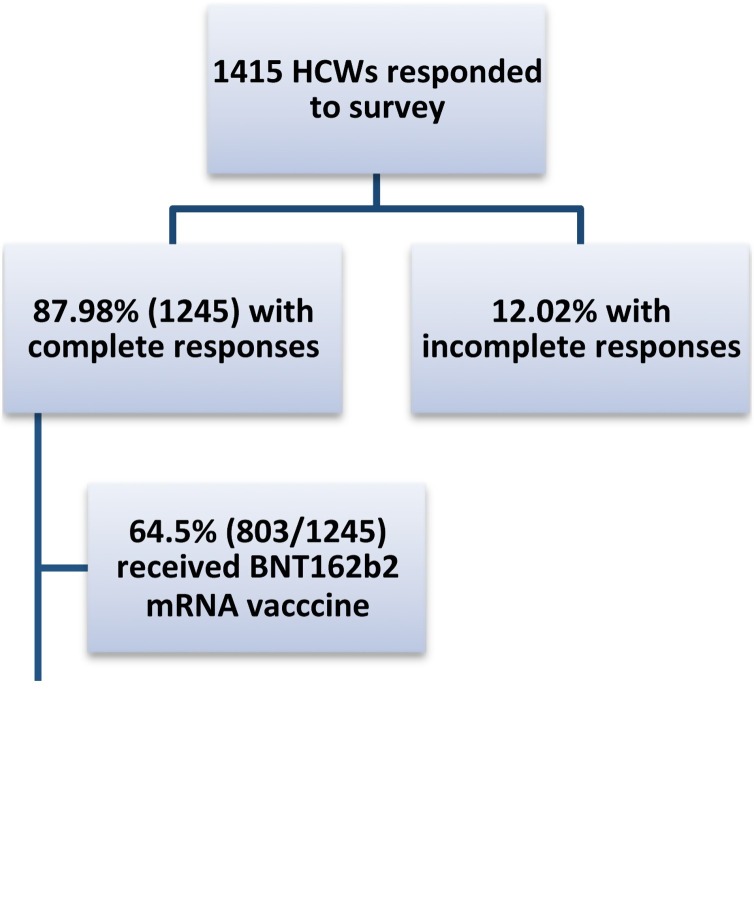
Of all HCWs, 87.98% (1245/1415) completed the survey. Of them, 64.5% (803/1245) received the BNT162b2 mRNA vaccine and reported at least one or more symptoms (classified based on organ systems and occurrence rate) post vaccination. Of these, 640/803 (79.7%) were able to continue activities of daily living (ADL), 103/803 (12.83%) had trouble temporarily to perform ADL, 99/803 (12.33%) took time off work temporarily, 20/803 (2.49%) required help from an outpatient provider, 5/803 (0.62%) required help from an emergency department and 2/803 (0.25%) required hospitalization. Despite this, 97.61% intended to have the second dose and 92.9% had already received it.
Conclusions
Commonly reported symptoms (occurrence in descending order) were soreness, fatigue, myalgia, headache, chills, fever, joint pain, nausea, muscle spasm, sweating, dizziness, flushing, feelings of relief, brain fogging, anorexia, localized swelling, decreased sleep quality, itching, tingling, diarrhoea, nasal stuffiness and palpitations. Despite this, remarkable acceptance for the second dose of the BNT162b2 vaccine was found among HCWs.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused a global pandemic in a short period of time, imposing challenges on medical services, researchers, epidemiologists and policy makers about the nature of the virus; and posing challenges for a successful vaccine outcome (Umakanthan et al., 2020). SARS-CoV-2 is transmitted via respiratory droplets from face-to-face contact or contaminated surfaces. Although the role of aerosol spread in humans remains unclear, this mode of transmission is still a major concern (Ganyani et al., 2020). The symptoms of coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2 infection, include fever, generalized weakness/fatigue, myalgia, dry cough, shortness of breath, nausea, vomiting, diarrhoea, headache, anosmia, ageusia and rhinorrhoea (Mao et al., 2020a, Mao et al., 2020b). Common complications of COVID-19 include pneumonia; acute respiratory distress syndrome; acute liver failure; cardiac abnormalities with troponin elevation; acute heart failure dysrhythmias; myocarditis; prothrombotic coagulopathy resulting in venous and arterial thromboembolic events; acute renal failure; neurologic manifestations, including impaired consciousness and acute cerebrovascular disease; and shock (Chen et al., 2020, Levi et al., 2020, Long et al., 2020, Mao et al., 2020b, Middeldorp et al., 2020, Rodriguez-Morales et al., 2020). As of 22 February 2021, there had been 500,000 deaths related to COVID-19 in the USA (CDC, 2021a).
The US Food and Drug Administration has authorized two mRNA vaccines to prevent COVID-19: the BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) on 11 December 2020, and the mRNA-1273 COVID-19 vaccine (Moderna) on 18 December 2020. On 12 December 2020, the Advisory Committee on Immunization Practices (ACIP) issued an interim recommendation for the use of a two-dose regimen of the BNT162b2 mRNA vaccine (which was shown to have 95% efficacy in protection against COVID-19) in persons aged ≥16 years (Oliver et al., 2020, Polack et al., 2020). The recommendation for a two-dose regimen of the mRNA-1273 vaccine (which was shown to have 94.1% efficacy in preventing COVID-19, including severe disease) was issued by ACIP on 19 December 2020 (Baden et al., 2021). The first phase of the vaccination programme was primarily focused on healthcare workers (HCWs) and long-term care facility (LTCF) residents. The BNT162b2 vaccine consists of two doses (30 μg, 0.3 mL each), administered intramuscularly, 3 weeks apart (CDC, 2020). A large population-based study found that allergic reactions to vaccines generally occur at a rate of 1.31 (95% confidence interval 0.90–1.84) cases per million vaccine doses, with no fatalities reported (McNeil et al., 2016). Current reports from the Centers for Disease Control and Prevention (CDC) suggest that anaphylactic reactions to the BNT162b2 mRNA vaccine may occur more frequently compared with other vaccines (CDC COVID-19 Response Team, 2021b).
Overall, the COVID-19 mRNA-based vaccination programme has generated many concerns, questions and continuing arguments concerning the safety issues of both the new mRNA vaccines among HCWs and the general population in the USA. A recent study on self-reported side effects with the mRNA-1273 vaccine among HCWs showed a broad spectrum of symptomatology, with most symptoms being non‐life threatening, and high acceptance of the vaccine among HCWs (Kadali et al., 2021). However, there are limited data and literature on the side effects of the BNT162b2 mRNA vaccine that focus specifically on a detailed review of organ systems, and the rate of occurrence of symptoms after receipt of this vaccine. The objective of this sub-study was to analyse the safety and detailed side-effect profile of the BNT162b2 vaccine using a self-reported online survey questionnaire among HCWs. A random population of HCWs was selected to investigate the side effects of these vaccines using responses from the survey questionnaire [consisting of a more detailed review of organ systems in comparison to the data collected by CDC through the Vaccine Adverse Event Reporting System (VAERS)] (Gee et al., 2021).
Methods
Design and sample selection
After obtaining institutional review board approval, a cross-sectional study was conducted by circulating an independent online survey questionnaire through an internet-based survey platform (Survey Monkey), which gathered anonymous responses from HCWs from verified healthcare communities representing various parts of the country during the early phase of COVID-19 vaccination. No personal identifications were obtained. The Survey Monkey web link was distributed to coordinators of healthcare institutions and verified communities of HCWs via social media (e.g. Facebook). Informed consent was obtained at the beginning of the survey. Participants who voluntarily agreed and consented to proceed and who chose to receive one of the two mRNA-based COVID-19 vaccines were automatically allowed to move forward to answer subsequent questions about the side effects and other variables. Those who chose ‘None of them’ were diverted to a disqualified page. The study obtained feedback in anonymous mode regarding the side-effect and benefit profile during the post-vaccination period.
Duration of study
The Survey Monkey web link was left open and kept active to collect responses for approximately 6 weeks. The responses were collected between 24 January 2021 and 10 March 2021.
Responses were obtained from 1415 HCWs (Figure 1 ) who reported receiving one or two doses of either the BNT162b2 mRNA vaccine or the mRNA-1273 vaccine. Of 1415 responses, 1245 were complete responses. Only the complete responses related to the BNT162b2 mRNA vaccine were included in the final analysis of this sub-study (Figure 1).
Figure 1.
Classification of survey responses from healthcare workers (HCWs).
Results
This study primarily focused on the BNT162b2 mRNA vaccine. Of the 1245 vaccine recipients who completed the survey, 803 (64.5%) received the BNT162b2 mRNA vaccine and 35.5% (442) received the mRNA-1273 vaccine (Figure 1). Among the responses from recipients of the BNT162b2 vaccine, 7.1% were aged 18–30 years, 38.85% were aged 31–40 years, 29.51% were aged 41–50 years, 16.69% were aged 51–60 years, 7.2% were aged 61–70 years, 0.37% were aged 71–80 years, and 0.25% were aged 81–90 years. The average age of the participants was 43 years. Of the responses, 86.55% were from females and the remainder were from males. The majority of participants had a higher level of education, with either a doctoral or professional medical degree or a Master’s degree (Table 1 ).
Table 1.
Demographic data of study participants reporting receipt of BNT162b2 mRNA vaccine.
| Percentage responded (%) | Number responded (n) | |
|---|---|---|
| Age group (years) | ||
| 18–30 | 7.1% | 57 |
| 31–40 | 38.85% | 312 |
| 41–50 | 29.51% | 237 |
| 51–60 | 16.69% | 134 |
| 61–70 | 7.22% | 58 |
| 71–80 | 0.37% | 3 |
| 81–90 | 0.25% | 2 |
| Gender | ||
| Female | 86.55% | 695 |
| Male | 13.45% | 108 |
| Level of education (answered by 627 HCWs) | ||
| Doctorate or professional medical degree | 58.69% | 368 |
| Master’s degree | 22.97% | 144 |
| Bachelor’s degree | 9.41% | 59 |
| Associate degree | 5.74% | 36 |
| High school graduate (working in healthcare setting) | 3.19% | 20 |
HCWs, healthcare workers.
In total, 92.9% of the HCWs who received the BNT162b2 mRNA vaccine had received both doses and 7.1% had only received the first dose at the time of this study. Those who had received the BNT162b2 vaccine reported various symptoms based on their complete review of organ systems: localized symptom/s, 719/803 (89.54%); generalized symptom/s, 610/803 (75.97%); musculoskeletal symptom/s, 428/803 (53.3%); gastrointestinal symptom/s, 172/803 (21.42%); psychological/psychiatric symptom/s, 133/803 (16.56%); neurological symptom/s, 102/803 (12.7%); head/ear/eyes/nose/throat symptom/s, 97/803 (12.08%); endocrine symptom/s, 83/803 (10.34%); cardiovascular symptom/s, 48/803 (5.98%); respiratory symptom/s, 21/803 (2.61%); urinary symptom/s, 10/803 (1.24%); and allergic symptom/s (other than localized/generalized rash), 10/803 (1.24%) (Table 2 ).
Table 2.
Event rate classified based on review of organ systems and descending order of occurrence.
| Symptoms after the first and/or second dose of the BNT162b2 mRNA vaccine | Percentage reported | Number of HCWs reporting symptom (n) |
|---|---|---|
| Generalized symptom/s | ||
| Generalized weakness/fatigue | 58.89% | 391 |
| Headache | 45.48% | 302 |
| Chills | 36.60% | 243 |
| Fever | 21.99% | 146 |
| Sweating | 9.64% | 64 |
| Dizziness | 9.04% | 60 |
| Flushing | 8.13% | 54 |
| Localized symptom/s | ||
| Sore arm/pain | 88.04% | 707 |
| Localized swelling at the injection site | 5.48% | 44 |
| Itching | 5.35% | 43 |
| Lymphadenopathy (axillary or regional) | 3.36% | 27 |
| Rasha | 2.49% | 20 |
| Residual skin discoloration | 1.25% | 10 |
| Bleeding | 0.37% | 3 |
| Loss of hair locally | 0.12% | 1 |
| Musculoskeletal symptom/s | ||
| Muscle pain/myalgia | 45.70% | 367 |
| Arthritis/joint pains | 16.56% | 133 |
| Muscle stiffness/spasm | 9.59% | 77 |
| Gastrointestinal symptom/s | ||
| Nausea | 15.94% | 128 |
| Decreased appetitea | 5.73% | 46 |
| Diarrhoea | 4.61% | 37 |
| Abdominal pain | 3.11% | 25 |
| Vomiting | 1.49% | 12 |
| Heartburn | 1.12% | 9 |
| Constipation | 0.37% | 3 |
| Food intolerance | 0.25% | 2 |
| Psychological and/or psychiatric symptom/s | ||
| Feelings of joy/relief/gratitude | 6.35% | 51 |
| Decreased sleep quality | 5.35% | 43 |
| Anxiety | 2.49% | 20 |
| Increase in sleep | 2.12% | 17 |
| Psychological stress | 0.75% | 6 |
| Decrease in memory | 0.75% | 6 |
| Depression | 0.37% | 3 |
| Manic/hypermanic mood changes | 0.37% | 3 |
| Behavioural changes | 0.12% | 1 |
| Neurological symptom/s | ||
| Brain fogging or reduced mental clarity/attention/concentration | 5.85% | 47 |
| Tingling | 4.86% | 39 |
| Numbness | 2.86% | 23 |
| Vertigo like symptoms | 2.49% | 20 |
| Paralysis/extremity weakness | 0.62% | 5 |
| Incoordination | 0.50% | 4 |
| Reactivation of shingles | 0.25% | 2 |
| Loss of consciousness/fainting | 0.25% | 2 |
| Seizures | 0.12% | 1 |
| Facial weakness | 0.12% | 1 |
| Head/ear/eyes/nose/throat symptom/s | ||
| Nasal stuffiness | 4.61% | 37 |
| Sore throat | 2.99% | 24 |
| Runny nose | 2.24% | 18 |
| Ringing sensation in ears | 1.99% | 16 |
| Ear pain | 1.12% | 9 |
| Eye pain | 0.87% | 7 |
| Blurring of vision | 0.50% | 4 |
| Changes in hearing | 0.37% | 3 |
| Hoarseness | 0.37% | 3 |
| Flashing lights | 0.25% | 2 |
| Ear discharge | 0.12% | 1 |
| Bleeding gums | 0.12% | 1 |
| Endocrine symptom/s | ||
| Decreased appetiteb | 5.73% | 46 |
| Heat/cold intolerance | 3.24% | 26 |
| Increased thirst | 1.12% | 9 |
| Increased appetite | 0.87% | 7 |
| Increased urine production | 0.25% | 2 |
| Cardiovascular symptom/s | ||
| Palpitations | 4.36% | 35 |
| Chest pain | 1.12% | 9 |
| Blood pressure changes | 0.87% | 7 |
| Syncope | 0.12% | 1 |
| Respiratory symptom/s | ||
| Shortness of breath | 1.99% | 16 |
| Cough | 0.87% | 7 |
| Wheezing | 0.25% | 2 |
| Urinary symptom/s | ||
| Urgent need to urinate | 0.75% | 6 |
| Frequent urination at night | 0.37% | 3 |
| Difficulty in urination | 0.12% | 1 |
| Pain or burning on urination | 0.12% | 1 |
| Allergic symptom/s | ||
| Rasha | 2.49% | 20 |
| Hives | 0.62% | 5 |
| Swelling in mouth/ throat | 0.37% | 3 |
| Atopic eczema | 0.25% | 2 |
| Swelling of lips or tongue | 0.12% | 1 |
| Effect on activity or need for medical attention | ||
| Trouble to perform regular daily living activities temporarily | 12.83% | 103 |
| Required transient time off from work | 12.33% | 99 |
| Required to seek help from outpatient provider | 2.49% | 20 |
| Required to seek help from emergency department provider | 0.62% | 5 |
| Required to hospitalize and subsequent inpatient care | 0.25% | 2 |
HCWs, healthcare workers.
Included in localized and allergic symptom classification.
Included in gastrointestinal and endocrine symptom classification.
Generalized symptoms
The main generalized symptoms that were reported were generalized weakness or fatigue (58.9%, 473/803), headache (44.83%, 360/803), chills (35.99%, 289/803), fever (22.04%, 177/803), sweating (9.22%, 74/803), dizziness (8.34%, 67/803) and flushing (7.1%, 57/803).
Localized symptoms
Approximately 88.04% (707/803) of HCWs reported a sore arm or pain at the injection site as their primary localized side effect, followed by localized swelling at the injection site (5.48%, 44/803), itching (5.35%, 43/803), lymphadenopathy (axillary or regional) (3.36%, 27/803), rash (2.49%, 20/803), residual skin discoloration (1.25%, 10/803) and bleeding (0.37%, 3/803).
Musculoskeletal symptoms
Myalgia (muscle pain) (45.7%, 367/803), arthritis or joint pain (16.56%, 133), and muscle stiffness/spasm (9.59%, 77/803) were reported by the recipients. Of note, paradoxically, two HCWs (Participants #38 and 915) with a prior history of chronic psoriasis reported improvement in their psoriatic symptoms.
Gastrointestinal symptoms
Nausea (15.94%, 128/803), diarrhoea (4.61%, 37/803), decreased appetite (5.73%, 46/803), abdominal pain (3.11%, 25/803), vomiting (1.49%, 12/803), heartburn (1.12%, 9/803) and constipation (0.37%, 3/803) were reported by the recipients.
Psychological or psychiatric symptoms
Decreased sleep quality (5.35%, 43/803), feelings of joy/relief/gratitude (6.35%, 51/803), anxiety (2.49%, 20/803), increased sleep (2.12%, 17/803), psychological stress (0.75%, 6/803), decrease in memory (0.75%, 6/803), manic/hypermanic mood changes (0.37%, 3/803), depression (0.37%, 3/803) and behavioural changes (0.12%, 1/803) were reported by the recipients.
Neurological symptoms
Brain fogging or reduced mental clarity/attention/concentration (5.85%, 47/803), tingling of the extremity at the injection site (4.86%, 39/803), numbness (2.86%, 23/803), vertigo-like symptoms (2.49%, 20/803), paralysis/extremity weakness (0.62%, 5/803), lack of coordination (0.5%, 4/803), loss of consciousness/fainting (0.25%, 2/803), facial weakness (0.12%, 1/803) and seizures (0.12%, 1/803) were reported by the recipients. Of note, two participants reported reactivation of herpes or shingle-like lesions after receiving the vaccine.
Head/eyes/ears/nose/mouth/throat symptoms
Nasal stuffiness (4.61%, 37/803), sore throat (2.99%, 24/803), runny nose (2.24%, 18/803), ringing sensation in the ears (1.99%, 16/803), ear pain (0.87%, 7/803), blurred vision (0.5%, 4/803), eye pain (0.87%, 7/803), changes in hearing (0.37%, 3/803), flashing lights (0.25%, 2/803), hoarseness (0.37%, 3/803), ear discharge (0.12%, 1/803) and bleeding gums (0.12%, 1/803) were reported by the recipients.
Endocrine symptoms
Decreased appetite (5.73%, 46/803), heat or cold intolerance (3.24%, 26/803), increased thirst (1.2%, 9/803), increased appetite (0.87%, 7/803) and increased urine production (0.25%, 2/803) were reported by the recipients.
Cardiovascular symptoms
Palpitations/racing heart (4.36%, 35/803), chest pain (1.12%, 9/803), and blood pressure changes and syncope (0.87%, 7/803) were reported by the recipients.
Respiratory symptoms
Shortness of breath (1.99%, 16/803), coughing (0.87%, 7/803) and wheezing (0.25%, 2/803) were reported by the recipients.
Allergic/skin symptoms (except for rash)
Hives (0.62%, 5/803), swelling of the lips (0.12%, 1/803), swelling in the mouth/throat (0.37%, 3/803) and atopic eczema (0.25%, 2/803) were reported by the recipients.
Urinary symptoms
Urgent urination (0.75%, 6/803), increased frequency of urination (0.37%, 3/803), difficulty in urination (0.12%, 1/803) and dysuria (0.12%, 1/803) were reported by the recipients.
The extent of the impact of these symptoms on the vaccine recipients was evaluated. Overall, 640/803 (79.7%) had no issues and were able to continue their daily routine activities, 103/803 (12.83%) had trouble to perform regular daily activities temporarily, and 99/803 (12.33%) required time off work temporarily. Only 20/803 (2.49%) needed help from an outpatient provider, 5/803 (0.62%) needed help from an emergency department provider, and 2/803 (0.25%) needed hospitalization and subsequent inpatient care (Table 2).
Infection rate among vaccinated group
In total, 10/803 HCWs (1.25%) reported that they had tested positive for COVID-19 during the period between the first and second doses of the BNT162b2 vaccine, and no HCWs reported that they had tested positive for COVID-19 after the second dose of the BNT162b2 vaccine.
Benefits of vaccines other than for COVID-19
In total 2.14% (12/803) HCWs reported improvement or resolution of chronic symptoms after receiving the BNT162b2 vaccine. Specifically, two HCWs with a prior history of chronic psoriatic arthritis reported improvement in their psoriatic joint pain.
Discussion
This study aimed to analyse the safety and detailed side-effect profile of the BNT162b2 vaccine among HCWs in the USA. Based on the above results, vaccine recipients can primarily expect the following symptoms during the early phase of the post-vaccination period: localized soreness; generalized weakness; myalgia; headache; chills; fever; joint pain; and nausea. Muscle stiffness or spasm, sweating, dizziness, flushing, feelings of joy/relief/gratitude, brain fogging or reduced mental clarity/attention/concentration, decreased appetite, localized swelling at the injection site, decreased sleep quality, itching, tingling, diarrhoea, nasal stuffiness, palpitations and/or high heart rate were reported as other predominant symptoms. Of note, 97.61% of the BNT162b2 vaccine recipients intended to receive a second dose of the vaccine irrespective of side effects after the first dose, conflicting schedules and personal apprehension. Moreover, 92.62% of the BNT162b2 vaccine recipients had already received their second dose by the time they responded to the survey.
At this point, the aetiology of side effects or reactions to the BNT162b2 vaccine remains unclear. The CDC estimates suggest that anaphylaxis (trouble with breathing, swelling of the face and throat, rash, low blood pressure) occurs soon after vaccination in 11 cases per million doses among recipients of the BNT162b2 vaccine (CDC COVID-19 Response Team, 2021b). In total, up to 13 January 2021, there had been 16 deaths involving non-LTCF residents and 46 cases of anaphylaxis following administration of the BNT162b2 vaccine according to the VAERS report (Gee et al., 2021). Furthermore, a report from Norway of 23 deaths among elderly recipients of the BNT162b2 vaccine raised understandable safety concerns; however, further investigations are in progress to determine whether these deaths were related to the vaccine, or whether they represent an expected number of deaths among frail individuals who may already have had a limited life expectancy (Torjesen, 2021). Thirteen of the deaths in the Norwegian report have been investigated to date, and common adverse reactions to the mRNA vaccines, such as fever, nausea and diarrhoea, may have contributed to the fatal outcomes in some of the frail patients (Torjesen, 2021). Investigations are in progress, but the underlying conditions (e.g. heart disease, cancer, stroke, probable pulmonary embolism and frail health) were considered to be the cause of death in frail patients (Gee et al., 2021). However, in the present study, there were no reports of anaphylaxis or death due to the BNT162b2 vaccine.
As such, in accordance with the CDC guidelines, it is recommended that all vaccine recipients should be observed for at least 15 min after receiving the vaccine, with adrenaline available at the vaccination site in case it is needed. It is the inactive ingredients or excipients (including egg protein, gelatin, formaldehyde, thimerosal and neomycin), rather than the active ingredients, that cause the allergic reactions. CDC recommends that individuals with a history of anaphylaxis to polyethylene glycol (PEG), PEG derivatives or polysorbate should avoid both mRNA COVID-19 vaccines (CDC, 2020).Excipients (which are used in the vaccine to stimulate a stronger immune response, prevent bacterial contamination, and stabilize the potency of the vaccine during transportation and storage) are the major contributors to specific IgE-mediated and immediate reactions associated with vaccines (Banerji et al., 2020).
In this study, 10 HCWs (1.25%) reported that they had tested positive for COVID-19 during the period between the first and second doses of the BNT162b2 vaccine. There are concerns about whether COVID-19 can be caused by the vaccine; however, this is unlikely because these mRNA vaccines did not use live SARS-CoV-2 virus in their development. If COVID-19 is observed soon after vaccination, it is improbable that it is caused by the vaccine; instead, the infection may have been caused by failure of the vaccine (studies on this are in progress), pre-existing infection before vaccine administration or infection at the time of vaccination.
There is a need to monitor further reports on the side effects of the vaccines as the vaccination programme continues. Approximately 6.35% of vaccine recipients reported feelings of joy/relief/gratitude post vaccination, and less than 2.5% of participants stated that they have decided not to have the second dose. These can be taken as positive signs. At best, most of the vaccine recipients (HCWs) took the challenge to end the deadly pandemic, irrespective of side effects experienced by them. Nevertheless, before receiving an mRNA vaccine that acts against this deadly virus, which has caused millions of deaths worldwide within 1 year, vaccine recipients need to balance the risks of possible non-life threatening adverse events with the potential benefit.
This study has several limitations. As this was an independent study investigating detailed self-reported symptoms through anonymous responses, the receipt of vaccine doses by study participants and their reported symptoms were not verified or confirmed, or recorded or documented officially by the study investigators. Most of the symptoms reported above occurred in the early post-vaccination phase of the vaccine. The latent effects of this vaccine were not studied or included in this study. No specific data on the initial timing of the onset of symptoms after vaccine administration or the duration of symptoms were obtained in this study. Some respondents may have incorrectly blamed the vaccine for several systemic side effects that they developed soon after vaccination, although symptoms of their pre-existing chronic medical problems may have contributed to these side effects, or they could be an unfortunate coincidence from new underlying medical problems that were not related to the vaccine. Chronic medical problems, such as heart attacks, blood disorders, cancer, stroke and other rare illnesses, occurred before the pandemic and will continue to occur. Acute and chronic health issues may be triggered after vaccine administration, as shown in this study, where two HCWs reported reactivation of herpes or shingle-like infections. The vaccine could be found to be responsible if a thorough investigation is performed and if certain health problems occur at a higher-than-normal rate. If not, it is more likely to be an unfortunate coincidence that these effects occurred after administration of the vaccine. Other examples, including rare cases of Bell’s palsy and other neurological diseases, have been reported since administration of the COVID-19 vaccine; however, to date there is no clear suggestion that the vaccine played any role. Similarly, it was reported that a physician in Florida developed a fatal blood disorder after receiving a COVID-19 vaccine, which raised concerns that it was triggered by the vaccine, although this condition did not occur among the tens of thousands of subjects in the clinical trials (Robert, 2021).
Conclusions
This detailed review of organ systems found that localized soreness, generalized weakness, myalgia, headache, chills, fever, joint pain and nausea were the most commonly reported symptoms, followed by muscle stiffness or spasm, sweating, dizziness, flushing, feelings of joy/relief/gratitude, brain fogging or reduced mental clarity/attention/concentration, decreased appetite, localized swelling at the injection site, decreased sleep quality, itching, tingling, diarrhoea, nasal stuffiness, palpitations and/or high heart rate. The majority (97.61%) of the vaccine recipients intended to receive a second dose of the vaccine, irrespective of side effects after the first dose, conflicting schedules and personal apprehension. This indicates a remarkable acceptance rate for this vaccine, which can be considered as a positive sign. At best, most of the vaccine recipients (HCWs) took the challenge to end the deadly pandemic, irrespective of side effects experienced by them. If COVID-19 occurs after vaccination (1.25% of vaccine recipients in this study reported that they had tested positive for COVID-19 in the period between the first and second doses), this is unlikely to be caused by the vaccine; instead, the infection may be caused by failure of the vaccine (studies on this are in progress), pre-existing infection before vaccine administration, or infection at the time of vaccination. The design and methods used in this study may provide direction to assess the safety and detailed side-effect profile of mRNA-based COVID-19 vaccines among pregnant women, lactating women and breastfeeding infants to add to the existing literature related to these specific populations.
Authors’ contributions
All authors contributed significantly to this work.
Conceptualization, proposal writing and supervised data collection: Renuka Ananth Kalyan Kadali and Ravali Janagama.
Data analysis: Renuka Ananth Kalyan Kadali, Ravali Janagama, and Sharanya Peruru.
Data management: Renuka Ananth Kalyan Kadali, Ravali Janagama, and Sharanya Peruru. Manuscript writing: Renuka Ananth Kalyan Kadali, Ravali Janagama.
Critical reviewing and editing of the manuscript: Renuka Ananth Kalyan Kadali, Ravali Janagama, and Srikrishna Malayala.
All authors have read and approved the manuscript.
Ethical approval
Exempt approval for this survey study was obtained from the Institutional Review Board at Cape Fear Valley Health System, 1638 Owen Drive, Fayetteville, NC 28304.
Funding
None.
Conflict of interest
None declared.
Acknowledgements
The authors wish to thank Dr Folarin for his timely COVID-19 updates and preparedness meetings at Harnett Health System, Dr Ramu G. Sudhagoni for his suggestions about data analysis approaches, and Sailaja Kadali (from Richard Montgomery High School, MD, USA) for assistance with data calculations and her excellent secretarial assistance.
References
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji A., Wickner P.G., Saff R., Stone C.A., Jr, Robinson L.B., Long A.A. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9(4):1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2020. COVID-19 vaccination: clinical considerations. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html [Accessed 8 March 2021] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta, GA: 2021. COVID-19 death data and resources. Available at: https://www.cdc.gov/nchs/covid19/mortality-overview.htm [Accessed 8 March 2021] [Google Scholar]
- CDC COVID-19 Response Team, Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine – United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.T., Shao S.C., Hsu C.K., Wu I.W., Hung M.J., Chen Y.C. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24:346. doi: 10.1186/s13054-020-03009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganyani T., Kremer C., Chen D., Torneri A., Faes C., Wallinga J. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.17.2000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J., Marquez P., Su J., Calvert G.M., Liu R., Myers T. First month of COVID-19 vaccine safety monitoring – United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadali R.A.K., Janagama R., Peruru S., Gajula V., Madathala R.R., Chennaiahgari N. Adverse effects of COVID-19 mRNA-1273 vaccine: a randomized, cross-sectional study on healthcare workers with detailed self-reported symptoms. J Med Virol. 2021:1–10. doi: 10.1002/jmv.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–40. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao R., Qiu Y., He J.S., Tan J.Y., Li X.H., Liang J. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert H.S. Harvard Health Blog; 2021. COVID-19 vaccines: safety, side effects and coincidence. Available at: https://www.health.harvard.edu/blog/covid-19-vaccines-safety-side-effects-and-coincidence-2021020821906 [Accessed 11 March 2021] [Google Scholar]
- Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torjesen I. Covid-19: Norway investigates 23 deaths in frail elderly patients after vaccination. BMJ. 2021;372:n149. doi: 10.1136/bmj.n149. [DOI] [PubMed] [Google Scholar]
- Umakanthan S., Sahu P., Ranade A.V., Bukelo M.M., Rao J.S., Abrahao-Machado L.F. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19) Postgrad Med J. 2020;96:753–758. doi: 10.1136/postgradmedj-2020-138234. [DOI] [PMC free article] [PubMed] [Google Scholar]