Abstract
Currently, the world has been devastated by an unprecedented pandemic in this century. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the agent of coronavirus disease 2019 (COVID-19), has been causing disorders, dysfunction and morphophysiological alterations in multiple organs as the disease evolves. There is a great scientific community effort to obtain a therapy capable of reaching the multiple affected organs in order to contribute for tissue repair and regeneration. In this regard, mesenchymal stem cells (MSCs) have emerged as potential candidates concerning the promotion of beneficial actions at different stages of COVID-19. MSCs are promising due to the observed therapeutic effects in respiratory preclinical models, as well as in cardiac, vascular, renal and nervous system models. Their immunomodulatory properties and secretion of paracrine mediators, such as cytokines, chemokines, growth factors and extracellular vesicles allow for long range tissue modulation and, particularly, blood-brain barrier crossing. This review focuses on SARS-CoV-2 impact to lungs, kidneys, heart, vasculature and central nervous system while discussing promising MSC's therapeutic mechanisms in each tissue. In addition, MSC's therapeutic effects in high-risk groups for COVID-19, such as obese, diabetic and hypertensive patients are also explored.
Keywords: SARS-CoV-2, MSC, Cytokine storm, Immunomodulation, Cell therapy
Graphical abstract

1. Introduction
The outbreak of a new coronavirus has changed the global routine. In late December 2019, most of the staff at Huanan Seafood Wholesale Market, Wuhan, China, presented clinical symptoms resembling viral pneumonia [[1], [2], [3]]. The disease rapidly spread among other Chinese provinces and then to other countries. The pathogen causing the novel infectious disease was identified as a coronavirus, initially called 2019 novel Coronavirus (2019-nCoV) and then renamed Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses [4]. The new disease was designated as coronavirus disease 2019 (COVID-19) and classified as a pandemic on March 11, 2020, by the World Health Organization [5].
Since treatment is still lacking, researchers around the world have been seeking drugs, vaccines, and other novel therapies. Stem cell therapy has emerged as a potential treatment for the dysfunctions in multiple organs affected by COVID-19. Mesenchymal stem cells (MSCs) are the most suitable stem cell type due to their immunomodulatory properties, as well as general safety regarding MSC interventions [6]. Their major therapeutic factor is the secretion of paracrine mediators such as cytokines, chemokines, growth factors, and extracellular vesicles, which promote tissue regeneration and repair [[7], [8], [9]].
This article discusses how MSCs therapy could help in the treatment of multiple organs affected by COVID-19.
1.1. The virus
SARS-CoV-2 belongs to the Coronaviridae family and is classified as a β-CoV (Betacoronavirus genus) [4]. It is the seventh coronavirus capable of infecting humans. However, unlike most of the other coronaviruses, it can cause severe respiratory syndrome, similar to SARS-CoV and MERS-CoV, which caused epidemics in the last two decades [3].
The new coronavirus has a tropism for cells that present human angiotensin-converting enzyme 2 (ACE2), a protein involved in the renin-angiotensin system (RAS) regulation. This receptor is widely expressed in many organs, including the lungs, heart, kidneys and intestine [[10], [11], [12], [13]]. Once the viral RNA is inside the target cell, replication and translation processes begin, assembling new virions that will be released via the secretory pathway [14].
SARS-CoV-2 is transmitted through exposure to droplets or aerosol exhaled by infected individuals or by direct contact with contaminated fomites [15,16]. SARS-CoV-2 enters the human airway and infects type-II pneumocytes and alveolar macrophages as soon as it reaches the alveoli [17,18]. Generally, in viral infections, host cells are expected to identify viral elements through pattern recognition receptors that activate nuclear factor NF-kB and interferon (IFN) regulatory factors, inducing the synthesis of type-I antiviral interferons and chemoattractants. In contrast to common respiratory viruses, the new coronavirus evades antiviral effects from type-I interferons, as these proteins are barely produced [19]. Chemokines first attract innate immune cells such as monocytes, dendritic cells, and natural killer (NK) cells, which are responsible for subsequently recruiting lymphocytes, the agents of the adaptive immune system. An effective response from lymphocytes is crucial for a favorable outcome in which CD4+ T cells help B cells to produce neutralizing antibodies against the viral S protein, and help CD8+ T cells to exterminate infected cells [12,17].
Otherwise, a dysfunctional immune response may lead to a hyperinflammatory state, which in turn can progress to a phenomenon known as cytokine storm. In this scenario, the patient's immune system overreacts unloading numerous cytokines such as interleukin-2 (IL-2), IL-6, IP-10 (CXCL10), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) into the bloodstream, affecting multiple organs. The cytokine storm is considered the main pathologic mechanism of this viral disease and is strongly associated with critical cases (Fig. 1 ) [[20], [21], [22]].
Fig. 1.
SARS-CoV-2 structure and infection.
Infection and associated inflammatory triggering by SARS-CoV-2. SARS-CoV-2 virion representation displaying its spike (S) glycoprotein, membrane protein and envelope protein composing the external envelope, while the viral RNA is located internally, surrounded by nucleocapsid protein. During alveolar epithelium infection, SARS-CoV-2′ S protein binds to ACE2 receptors and is modified by serine protease TMPRSS2, present on the cell's surface. Then, SARS-CoV-2 infects the cell, triggering cellular damage that progresses to pyroptosis. Cell death releases multiple proinflammatory cytokines, such as IL-2, IL-6, TNF-α and MCP-1, that in dysregulated immune responses may cause persistent tissue inflammation.
ACE2 - angiotensin-converting enzyme 2; IFN-γ - interferon-gamma; IL - interleukin; IP-10 - CXCL10; TNF-α - tumor necrosis factor-alpha; MCP-1 - monocyte chemoattractant protein-1; MIP-1α - macrophage inflammatory protein.
COVID-19 is a complex disease that displays a wide spectrum of clinical presentations, ranging from asymptomatic infection to death-threatening cases [23]. The most common clinical symptoms are mild-to-moderate and comprise fever, dry cough, myalgia, fatigue, and dyspnea. Other manifestations include loss of smell and taste and gastrointestinal disorders [21,[24], [25], [26]]. Advanced age, obesity, diabetes, and cardiovascular diseases (CVD) are some of the risk factors that aggravate COVID-19 [3,24,27,28]. Complications associated with severe cases include acute respiratory distress syndrome (ARDS), multiple organ failure, and disseminated intravascular coagulation [29].
1.2. The cell
MSCs are promising tools in cell therapy due to their multipotent differentiation, immunoregulation and tissue regeneration effects [7,8]. Easy to obtain, MSCs are found in most vascularized tissues such as bone marrow, adipose tissues, umbilical cord, placenta and even menstrual blood, but can also be generated from embryonic stem cells [[30], [31], [32], [33]]. These cells are considered safe, even in allogeneic environment, avoiding immune responses due to their low expression of MHC-I and MHC-II [8,34].
MSCs migrate towards damaged tissue areas, curbing the injury and preserving the tissue. Their therapeutic effects are mainly through paracrine signaling of growth and survival factors, such as vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), fibroblast growth factor (FGF), and insulin-like growth factor-1 (IGF-1), which promote angiogenesis, cell survival and cell proliferation [35,36].
Furthermore, MSCs can directly inhibit the release of proinflammatory cytokines, such as TNF-α and IFN-γ by immunosuppressive responses in the innate and adaptive immune systems [[36], [37], [38]]. For example, MSCs can interact with Th2 lymphocytes, attenuating NK cell responses and inducing changes in M1 macrophages to M2 anti-inflammatory macrophages. MSC's IL-10 production can suppress Th17 lymphocyte expansion through JAK/STAT/SOC3, while TNF-α production is reduced. Through the release of TGF-β, MSCs are one of the key regulators of Treg lymphocyte populations [39].
Extracellular vesicles (EVs) are another MSCs mechanism of action that involves carrying biological messengers into injured sites. EVs comprise microvesicles and exosomes, which contain transcription factors, growth factors, cytokines, mRNAs, and microRNAs (miRNAs), and are responsible for the angiogenic, anti-inflammatory, and anti-apoptotic effects [[40], [41], [42], [43]]. EVs are nanovesicles measuring 30–150 nm that originate from direct budding of the cell membrane or via endosomal secretion. They are composed of cytosolic contents and a lipid bilayer similar to their mother cell [9,44], and therefore, are able to mimic cell interactions between the source and the target [41,45].
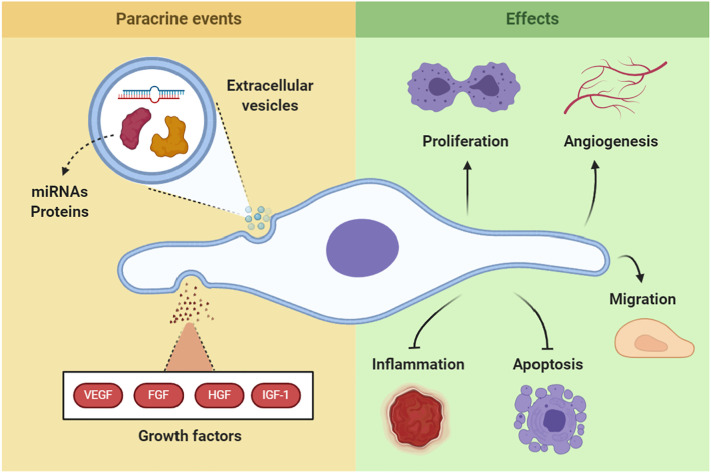
Therefore, MSCs and MSC-EVs are considered potential instruments in therapy due to acting simultaneously in crucial mechanisms of tissue damage. Namely through tissue remodeling, by modulating inflammatory cells and signals, enhancing tissue survival, and favoring angiogenesis, while presenting low risk of immunogenicity and tumorigenicity [41]. Thus, MSCs are suitable candidates for cell therapy in COVID-19 (Fig. 2 ).
Fig. 2.
MSC's therapeutic mechanisms.
MSC's main therapeutic mechanisms. MSC paracrine signaling either through soluble growth factors or through EV carrying multiple molecules, promotes angiogenesis and induces resident tissue cells to survive and proliferate, while inhibiting inflammation progression and cell apoptosis. Direct viral damage to the tissue and dysregulated immune responses are key pathophysiologic mechanisms in multiple organ dysfunctions in COVID-19. Therefore, MSC's ability to induce tissue remodeling may be beneficial to patients with COVID-19, improving recovery and restraining long-term dysfunction.
VEGF – vascular endothelial growth factor; FGF – fibroblast growth factor; HGF – hepatocyte growth factor; IGF-1 – insulin-like growth factor-1.
2. MSCs-based therapies for multiple organs affected by COVID-19
At first, health care professionals and researchers thought that COVID-19 was simply a respiratory disease. However, many recent findings demonstrate that this infection is more intricate than expected, affecting a myriad of organs, culminating in multiple organ dysfunction [[46], [47], [48]]. SARS-CoV-2 tropism is not limited to the upper and lower respiratory tract. Renal, cardiovascular, nervous, and gastrointestinal systems are also targets given their high expression of ACE2 [[49], [50], [51], [52]]. Single-cell RNA-sequence analysis has identified enriched ACE2 expression in lung type II pneumocytes, enterocytes, stomach epithelial cells, kidney proximal tubules, podocytes, myocardium cells, arterial smooth muscle cells, and pancreatic beta-cells [53,54]. These data indicate that direct viral tissue damage in addition to a dysregulated immune response, is an important pathophysiologic mechanisms of multiple organ dysfunction in COVID-19.
Hereafter, we report on how the SARS-CoV-2 virus damages the lungs, heart, vasculature, kidneys and nervous system; how MSCs act and which mechanisms they adopt on certain injuries, proving their therapeutic potential; how comorbidities such as obesity, diabetes, and hypertension can complicate the infection.
2.1. Lungs
The lungs are the most affected organs by SARS-CoV-2, developing intra-alveolar fibrin with hyaline membrane, including proliferative intra-alveolar and interstitial fibroblasts, bronchopneumonia and necrotizing bronchiolitis in some cases [55,56] The interaction among the virus, pneumocytes, and alveolar macrophages establishes a cascade of inflammatory events, producing cytokines, chemokines, and stimulating inflammatory cells infiltration [57,58].
Many studies reported that lungs of patients with COVID-19 presented diffuse alveolar damage with inflammatory cell infiltration in the alveolar cavity, including macrophages, monocytes, CD4+ T lymphocytes, eosinophils, and neutrophils, along with alveolar wall thickening. In addition, alveolar septum edema exhibited CD68+ macrophages, CD20+ B cells, CD8+ T cells, type II pneumocyte hyperplasia, and interstitial fibrosis, besides inclusions of SARS-CoV-2 in type II pneumocytes [[57], [58], [59], [60], [61], [62], [63]]. Moreover, thrombotic microvascular lesions were observed, apparently mediated by an intense activation and deposition of complement system proteins within the pulmonary septum microvasculature, such as C5b-9, C4d, and MASP2 [58,64]. Some severe cases of infected patients showed lymphocytopenia, depletion of CD4+ and CD8+ T cells, and neutrophilia, besides massive macrophage and neutrophil infiltrates into the lung tissue [12,60]. Patients with severe disease also presented a panel of increased proinflammatory cytokines and chemokines such as IL-6, IL-8, TNF-α, MCP1, and RANTES, as compared to patients with non-severe disease. Inflammatory cell infiltration exacerbates inflammation and exudation, which promotes alveoli dysfunction and a vicious cycle of continuous cytokine release, leading to cytokine storm. These findings demonstrate that pyroptosis and apoptosis contribute to lung injury, with pyroptosis predominating [12]. Therefore, the therapeutic alternative of MSC use might assist in the improvement of severe lung injury caused by direct infection and immunopathological lesions.
MSC's therapeutic potential has received considerable attention in preclinical settings, with marked therapeutic effects in multiple respiratory diseases, including ARDS, as observed in patients with COVID-19 [65]. MSCs have demonstrated many possible mechanisms for improving ARDS resolution through their anti-inflammatory and anti-apoptotic effects on host cells, reducing lung alveolar epithelium permeability, increasing alveolar fluid clearance, and enhancing host mononuclear cell phagocytic activity [66]. Therefore, MSCs are currently under investigation as a potential therapy for COVID-19.
One of the critical mechanisms implicated in COVID-19 pathology is alveolar edema and infiltration, mainly due to loss of epithelial selective permeability. Therefore, it is crucial to recover alveolar epithelial integrity with concomitant edema resolution [65]. The ability of MSCs to restore alveolar fluid clearance has been noted in ex vivo perfused human lungs. Intrabronchial administration of either MSCs or MSC-conditioned media (MSC-CM) in lipopolysaccharide (LPS)-induced ARDS has been shown to reduce lung edema and normalize alveolar fluid clearance [67]. Mouse models also demonstrated diminished apoptosis in airway epithelial cells [68,69]. Moreover, in vitro, MSCs or MSC-CM prevented epithelial barrier dysfunction, selective permeability loss, and consequent disruption of fluid transport by restoring epithelial sodium transporters and tight junctions [70,71]. Another mechanism MSCs can improve epithelial integrity and regulation is by transferring healthy mitochondria to epithelial cells, reducing oxidative damage and apoptosis, thus increasing survival in mice [72,73]. Immunomodulation can also be regulated by direct mitochondrial transfer, either to T cells, stimulating Treg phenotype that restricts inflammatory responses, or to macrophages, promoting enhanced phagocytosis [74,75].
The therapeutic potential of MSCs was assessed in other respiratory viruses. Immediate MSC therapy significantly attenuated H9N2 avian influenza virus-induced acute lung injury and inflammation in mice. They were capable of increasing the survival rate, decreasing lung edema and histological injury, improving gas exchange, and reducing alveolar proinflammatory chemokines and cytokines, such as granulocyte macrophage colony-stimulating factor (GM-CSF), monokine induced by gamma interferon (MIG), IL-1α, IFN-γ, IL-6, and TNF-α [76]. In an in vivo pig model of swine H1N1 influenza, MSC-EVs reduced virus shedding and virus-induced production of proinflammatory cytokines, alleviating histopathological lesions in the lungs. When analyzed in vitro, MSC vesicles inhibited H1N1 influenza virus replication and virus-induced apoptosis in lung epithelial cells, mainly through RNA transfer [77]. Similar results were found in an H5N1-infected mouse model treated with MSCs [78]. MSCs have been hypothesized to neutralize free virus particles through the production of antibiotic proteins such as Cathelicidin antimicrobial peptide LL-37 (LL-37), which binds to virus and lung cell binding sites while also stimulating immunosuppression [79,80].
However, MSC effects in virus-induced lung injury appear to be viral strain-specific. H1N1-infected mice administered MSCs, although showing modestly reduced viral load and lower influenza-induced thrombocytosis, did not display any improvements in survival, histopathology, inflammatory profile resolution or prevention [65,81,82]. As the virus induced ARDS model is not yet fully established, with variable viral concentrations in inoculation dose, frequency and timing, MSC's therapeutic effect is still under question in viral scenarios.
Many cytokines and chemokines are involved in MSC's therapeutic capacity in respiratory diseases. Prostaglandin E2 (PGE2) produced by MSCs can drive macrophages from M1 to M2, an anti-inflammatory phenotype, increasing their production of anti-inflammatory cytokine IL-10 [20]. Another key potent anti-inflammatory protein secreted by MSCs is TNF stimulated gene-6 (TSG-6), which can contribute to decreasing inflammatory cytokine and cell counts in bronchoalveolar lavage fluid, while also contributing to fibrosis resolution [83]. Knockdown of TSG-6 expression in MSCs nullified most of these anti-inflammatory effects [84]. TSG-6 is involved in a well-described mechanism by acting on resident macrophages, decreasing TLR2/NFk-B signaling, and thereby decreasing the secretion of proinflammatory mediators [85,86]. Herpesvirus entry mediator (HVEM) and B- and T-lymphocyte attenuator (BTLA) signaling also seems to be involved in MSC response to ARDS, particularly its immunomodulatory effects. Transplanted lung MSCs expressing HVEM showed improved regulation of inflammatory responses in innate and adaptive immune cells in LPS-induced ARDS model [87].
Moreover, MSC therapy can be beneficial even in indirect COVID-19 injuries. Preclinical studies using ventilator-induced lung injury models in rats showed that treatment with MSCs or MSC-CM decreased bronchoalveolar liquid concentrations of cytokines and inflammatory cells [88,89]. MSCs can also prevent secondary bacterial infections in patients with COVID-19 through their anti-microbial capacity, showing reduced bacterial levels in the alveoli, blood, and spleen in mice as well as in ex vivo human lungs [67,90].
Although few studies have investigated MSC therapy in viral-induced ARDS, the collective literature suggests a moderate beneficial effect of MSCs in general ARDS. Therefore, as COVID-19 patients receive mainly support therapy, MSCs are promising therapeutic candidates. Further investigations are necessary, especially regarding MSC timing and frequency of administration, hopefully in the strongest evidence scenario: randomized double-blind clinical trials.
2.2. Blood vessels
Many clinical conditions, including general pneumonia, increase the risk of venous thromboembolism (VTE); hence, anticoagulants are routinely used as prophylaxis. [[91], [92], [93]]. However, COVID-19 seems to cause more frequent thrombotic disorders or endothelial damage than infections by other respiratory viruses such as SARS-CoV, MERS, and influenza [94]. Preliminary reports suggest that disseminated thrombotic events such as disseminated intravascular coagulation (DIC) occur in patients affected by COVID-19 [[95], [96], [97]]. Two studies from Netherlands and China with severely ill COVID-19 patients demonstrated that, respectively, 31% of 184 had thrombosis with most events being VTE, and that 25% of 81 developed VTE [98,99]. Out of the 1026 patients hospitalized with COVID-19, 40% were considered to be at a high risk of VTE, based on the Padua Prediction Score [100].
A retrospective multicenter study investigating 183 patients found higher levels of D-dimer and fibrin degradation products (~3.5- and ~1.9-fold, respectively) as well as increased prothrombin time (~14%) in COVID-related deaths. Approximately 71.4% of non-survivors vs 0.6% of survivors met the criteria for DIC [96]. Elevations in prothrombin time, D-dimer, and IL-6 levels are associated with a procoagulant profile and poor prognosis in patients with COVID-19 [101]. Furthermore, based on a meta-analysis of 400 severely ill patients, platelet count was inversely correlated with COVID-19 severity [102].
One aspect that still raises questions in COVID-19 is the dissociation between relatively well-preserved lung mechanics and the severity of hypoxemia. Patients with COVID-19 maintain high respiratory system compliance, indicating a well-preserved elastic lung tissue, but display a high shunt fraction, indicating poor gas exchange (i.e., low oxygenated blood leaving the lungs). In physiological settings, endothelial self-regulation due to low oxygen levels takes place to activate vasoconstriction in the alveoli-endothelium which is not properly exchanging gas. However, COVID-19 may dysregulate this mechanism, leading to hyperperfusion of inefficient alveoli causing lower pO2, even though the ventilation capacity is only moderately reduced [103,104].
As SARS-CoV-2 is capable of infecting ACE2-expressing cells, direct endothelial cell infection was detected in multiple organs in post-mortem patients with COVID-19, revealing viral endothelial inclusions and a high frequency of congested small lung vessels [105]. Therefore, the direct endothelial lesion pathway may have a central role in the onset of COVID-19 coagulopathy and thrombotic disorders. As risk factors for COVID-19 and thrombotic diseases, such as age [106], obesity [107], diabetes [107], and hypertension [108,109] overlap, vascular damage may be a critical enhancer of disease progression. The World Health Organization (WHO) has already recognized COVID-19-related thrombosis, recommending daily prophylactic low molecular weight heparin or twice daily subcutaneous unfractionated heparin use in severe cases even after hospital discharge [110,111].
Increased research efforts are being made to investigate possible therapeutic strategies aimed to reduce blood clot formation, vascular damage and reverse the inflammatory and procoagulant systemic state caused by COVID-19 [112,113]. MSC's proangiogenic signaling, enhancing endothelial cell survival, and its supportive role in vascular remodeling, make them great therapeutic candidates in endothelial disorders [[114], [115], [116]].
MSCs are potent stabilizers of the vascular endothelium, decreasing endothelial permeability and protecting against inflammatory disruption of barrier function. In ex vivo perfused human lungs, MSCs reduced extravascular lung water, improved lung endothelial barrier permeability and restored alveolar fluid clearance in LPS models [83]. Keratinocyte growth factor (KGF) was essential for the beneficial effect of MSCs on alveolar epithelial fluid transport, in part by restoring amiloride-dependent sodium transport [117]. MSCs could protect the endothelial barrier function and regulate inflammation as they secrete VEGF and HGF, contributing to endothelial barrier stabilization in pulmonary capillaries, endothelial cell apoptosis inhibition, endothelial VE-cadherin recovery, and proinflammatory factors reduction [66,116].
Epithelium-derived microparticles from pulmonary damage are a potential source of procoagulant mediators in the alveolar space in patients with ARDS [118]. There is also a positive feedback between coagulation and inflammation, optimizing the response to injury and resulting in a wide range of diseases related to excessive inflammation and/or thrombosis [119]. Regarding the uncontrolled coagulation and thrombotic events due to COVID-19, reports on transfused MSC indicate that they express tissue-factor, a procoagulant, potentially leading to thrombosis and complement activation. There is considerable concern regarding the use of MSCs in clinical trials investigating thrombotic pathologies, as most protocols include transfusion of MSCs to the blood circulation [119]. Since the injection of cells and appropriated solution might elicit coagulatory responses, to the best of our knowledge no clinical trials have been conducted using MSC in thrombogenic settings. However, this behavior is still in question, as MSC could decrease thrombi size in acute pulmonary thromboembolism mice [120,121].
Thus, we can speculate that MSC therapy could modulate inflammation in the endothelial cell microenvironment and regulate endothelial permeability during COVID-19, preventing dysfunction. Synergistically, continuing the WHO-recommended treatment with anticoagulants, MSCs could directly inhibit endothelium apoptosis, acting as a potent regulator of vascular remodeling and recuperating blood flow control. In fact, the simultaneous use of both strategies seems to inhibit procoagulant events related to MSC transfusion. [122].
2.3. Heart
Several studies have shown that CVD predisposes patients to severe COVID-19 conditions, increasing the probability of mortality [24,95,97,99]. However, the effects of SARS-CoV-2 specifically in the heart are not well known. COVID-19 patients without previous history of CVD displayed cardiovascular manifestations, such as increased protein levels of cardiac troponin, myoglobin, creatine kinase and NT-proBNP, in addition to clinical symptoms, in particular chest pressure, suggesting myocarditis involvement [96,98,[100], [101], [102], [103]]. Even small increases in troponin I levels (0.03–0.09 ng/mL) were significantly associated with death [123]. Myocardial injury occurs in ~25% of hospitalized patients and is associated with a greater need for mechanical ventilator support and higher hospital mortality [124].
One proposed cardiac pathophysiological mechanism is through direct myocardial injury, as SARS-CoV-2 seems to have the capacity to infect cardiomyocytes, pericytes and fibroblasts via the ACE2 pathway [125]. Furthermore, systemic cytokine-mediated injury, inflammatory cells infiltration, cardiac hypoxemia, coronary plaque rupture with acute myocardial infarction and adrenergic stress are other mechanisms implicated in COVID-19 cardiac pathology [124]. Post-mortem endomyocardial biopsy from COVID-19 positive patients found hypertrophied cardiomyocytes along with inflammatory infiltrates, focal edema, interstitial hyperplasia, fibrosis, degeneration, necrosis and signs of lymphocytic myocarditis [126].
Evidence suggesting that SARS-CoV-2 can cause myocardial lesions are corroborated by the structural similarity of the virus to SARS-CoV-1 (virus responsible for the 2002–2004 SARS epidemic) [103,127]. Both were found to be dependent on ACE-2 receptors for targeting and infecting human cells, and also to cause excessive cytokine responses, hypoxia and impaired left ventricular performance [64,94,96,105,106].
Previous studies indicated that SARS-CoV-1 infection, promotes ACE-2 dysregulation, arresting the cardioprotective effects of angiotensin 1–7 and leading to the synthesis of TNF-α [128,129]. Moreover, the activation of Smad signaling pathway triggers TGF-β signaling, which develops myocardium interstitial fibrosis [128,130].
To the best of our knowledge, no published study described the action of MSCs specifically in the heart of patients with COVID-19. Therefore, our review discusses the role and effectiveness of MSCs and MSC-EVs therapy based on similar cardiovascular manifestations.
Multiple studies reported the use of MSCs as beneficial for myocardial restoration [115,116,[131], [132], [133]]. In a model of Coxsackievirus B3-induced myocarditis in mice, MSCs exhibited a cardioprotective role by inhibiting the expression of NOD2, ASC, caspase-1, IL-1β, IL-18 and NLRP3 inflamassome in the left ventricle, which recovered myocardial contractility and fibrosis. They also reduced apoptosis, oxidative stress, intracellular viral particles production and TNF-α mRNA expression, while activated cardiac mononuclear cells and the IFN-γ protective pathway [114,134].
MSCs have also demonstrated beneficial effects in models of acute myocardial infarction. MSCs were able to reduce infarct size, preserve systolic and diastolic cardiac performance and activate resident cardiac stem cells, promoting its proliferation, migration and angiogenic capacity, which ultimately led to reduced fibrosis [66,83]. These results validate the cardioprotective, proangiogenic, and prosurvival effects of MSCs in the heart via immune system and tissue repair modulation [83,117,135,136].
Clinical trials involving the therapeutic use of MSCs in cardiac pathologies are still in very early phases. General safety has been demonstrated using mixed methodologies and MSCs source [137]. The main challenges encompass MSC's low tissue retention and survival after transplantation. However, initial results suggest that MSCs improved left ventricular function, questionnaire-assessed symptoms and quality of life, although more robust investigation is necessary [137].
Taken together, these data demonstrate the promising therapeutic potential of MSCs for various cardiovascular complications. Based on SARS-CoV-2 pathophysiology in the heart and the similarity observed in CVD, mainly in myocarditis, MSC therapy could constitute a potential treatment for COVID-19 related heart alterations.
2.4. Kidneys
SARS-CoV-2 has also tropism for the kidneys, with preference for renal proximal tubule epithelial cells and glomerular cells. These cell types are enriched with ACE2, TMPRSS2, and cathepsin L (CTSL), a facilitator of SARS-CoV-2 infection [49,53,138,139]. Therefore, this set of proteins forms a key point that allows viral entry, replication, and immune response establishment, thereby promoting acute kidney injury [49,140,141] Studies have shown viral particles in the peritubular space, renal tubular epithelial cytoplasm, podocytes, glomerular capillaries, and a lower proportion in distal tubule epithelium, where ACE2 is known to be expressed [50,105]. These findings show that SARS-CoV-2 can directly infect renal epithelia, promoting acute renal injury in patients with COVID-19 [50,142]. Recent research described major renal morphological changes in COVID-19 postmortem individuals, such as acute proximal tubular injury with loss of brush border, vacuolar degeneration, tubular lumen dilation, necrotic regions, and epithelium detachment with a bare tubular basement membrane [50,143] Tubular atrophy, mild interstitial fibrosis, Bowman's capsule rupture with global collapse of glomerular capillaries, podocytes vacuolization, and glomerular basement membrane retraction were also observed [50,143,144]. In addition, patients with COVID-19 showed renal dysfunction leading to increased levels of creatinine, urea, uric acid, proteinuria, hematuria, and inflammatory markers [12,50,[143], [144], [145]].
Although SARS-CoV-2 viral particles can promote renal injury by direct infection, some studies reported that the virus was not present in podocytes and tubules. It is suggested that the host inflammatory response to coronavirus is exacerbated, causing cytokines such as interferons to damage cells [143,144]. A study showed lymphocyte infiltration, high levels of complement system proteins, and macrophage infiltration in tubulointerstitial regions, in which proinflammatory cytokines derived from macrophages led to tubular injury, enhancing SARS-COV-2 cytotoxic effect [146]. Virus-induced inflammatory mediators cause indirect effects such as hypoxia, shock and rhabdomyolysis, damaging kidney tissues [145]. Besides, high levels of these inflammatory substances are responsible for rapid multiorgan failure and death [12].
MSCs are being extensively studied for their therapeutic potential to treat renal injury [147]. MSCs were able to reduce tubular cell apoptosis, inflammatory infiltrate, and interstitial fibrosis in rats with unilateral ureteral obstruction (UUO). Different mechanisms are controlled by the paracrine effect of MSCs in remodeling fibrosis. They inhibit transdifferentiation of activated myofibroblasts through TGFβ/Smad2/3 signaling pathway, reduce mesangial cell proliferation through PI3K/Akt and MAPK signaling and induce high expression levels of MMP2 and MMP9 [12] MSCs are able to reduce TGF-β, which has a key role in fibrogenesis because it stimulates ECM protein synthesis, fibroblast proliferation, and stress fiber synthesis, while decreases matrix degradation and promotes epithelial-mesenchymal transition of tubular cells [148,149]. MSC and MSC-CM improved tubulointerstitial fibrosis, decreased TGF-β expression, prevented degeneration of ZO-1 (a tight junction protein) in tubular epithelial cells, reduced mesangial expansion and suppressed excessive tubule dilatation in mice with diabetic nephropathy [150]. In addition, Li et al. reported that umbilical cord-derived MSCs promoted urine microalbumin improvement, glomerular hypertrophy repair, glomerular basement membrane thickening, fibrotic alteration, and podocyte process effacement, also in kidneys of mice with diabetic nephropathy [12] These data show that MSCs have great potential to improve tubulointerstitial fibrosis by different mechanisms in the kidneys of patients with COVID-19.
MSC also have the ability to promote anti-inflammatory outcomes at long distances through their direct and indirect paracrine effects. In mice with ischemic or reperfusion acute kidney injury (AKI), intravenously infused MSCs are incorporated into the lungs and secrete soluble factors into bloodstream that improve kidney injury and ischemic renal repair by suppressing inflammatory reactions [151]. Recent evidence has shown that a combination of exosomes with soluble factors secreted by MSCs contribute to anti-inflammatory response, repair, and regeneration of renal tissues. The miRNAs transported by MSC exosomes are involved in tubular epithelium protection and regeneration [150]. In a mice model of aristolochic acid-induced nephropathy, MSC EVs reduced creatinine and urea levels, tubular necrosis, and interstitial fibrosis, while also reduced profibrotic genes expression (e.g., αSMA, Col 1a1, and TGF-β) and cellular activity of fibroblasts, pericytes, and myofibroblasts. In addition, they reduced inflammatory cells, promoted renal cell proliferation, and reduced apoptosis [149]. MSC-EV's miRNAs were transferred to host cells, altering their genetic expression, inhibiting inflammatory and profibrotic pathways [149]. miRNAs are also involved in tubular epithelium protection and regeneration [150]. These data show that MSC EVs have antifibrotic and anti-inflammatory properties, attenuating the innate immune response and favoring renal regeneration.
The renoprotective feature of MSCs is attributed to their unique ability to modulate the inflammatory immune response. MSCs promoted accelerated M2-polarized macrophage infiltration, trophic factors secretion, tubular epithelium proliferation, renal function improvement and IL-6 and TNFα reduction in mice with rhabdomyolysis-induced AKI [152]. Other studies also reported that MSCs promoted an increased expression of IL-10 and IL-4 mRNA, decreased expression of IL-1β and TNF-α mRNA, and decreased inflammatory cells infiltration, reduced a proinflammatory (Th1) environment, and increased anti-inflammatory effects (Th2) [147,153,154].
MSC transplantation into the renal subcapsular region of rats with podocyte injury induced by puromycin promoted blood pressure reduction and decreased proteinuria, albuminuria, and serum creatinine. Renal regenerative and reparative effects of MSCs are also related to restoration of Wilms' tumor suppressor 1 (WT1) and VEGF expression and improvement of podocytes' functional and structural integrity, including proteins such as nephrin and podocin (slit diaphragm), synaptopodin, and podocalyxin (cytoskeletal proteins) [154]. Marrow mononuclear cell fraction, that includes MSC and other progenitor cells, can also promote renal tissue restoration [155].
Clinical trials with MSC administration in patients with AKI or sepsis-associated AKI, chronic kidney disease or diabetic nephropathy has been limited to a small number of early phase clinical trials. Overall, MSC demonstrated good safety and tolerability, however only modest alterations in plasma cytokine profiles were observed, without a clear signal of improved outcome for MSC-treated patients [[156], [157], [158]].
Beyond MSC's paracrine effect, mitochondrial transfer also plays a role in kidney injury regeneration and repair. Bone marrow-derived MSCs transferred mitochondria to proximal tubular epithelial cells of mice with diabetic nephropathy in vivo [150]. These embedded mitochondria suppressed apoptosis, regulated mitochondrial factors such as Bcl-2, Bax, and PGC-1α, inhibited reactive oxygen species (ROS) production, recovered transporter expression such as megalin and SGLT2, and repaired tubular structures [159]. Therefore, it was shown that MSCs have several mechanisms that promote renal regeneration and repair, thus unraveling the very promising therapeutic potential for patients affected by COVID-19.
2.5. Central nervous system
Several patients with COVID-19 have reported neurological events such as headache, dizziness, hypogeusia, and hyposmia [12,160]. In a systematic review of 554 patients who tested positive for COVID-19, the average prevalence of headaches was approximately 8% and dizziness was 7%–9.4% [63,[161], [162], [163]]. There are also many reports of cases that manifested more severe neurological symptoms, such as acute cerebrovascular diseases (acute ischemic stroke, cerebral venous sinus thrombosis, cerebral hemorrhage and subarachnoid hemorrhage), meningitis or encephalitis, acute hemorrhagic necrotizing encephalopathy, acute Guillain-Barré syndrome, hypopsia, and neuralgia [160,164,165]. In a large retrospective cohort study comparing 1916 COVID-19 patients and 1486 influenza patients adjusted for age, sex, and race, the likelihood of stroke was almost 8-fold higher for COVID-19 (odds ratio 7.6) [166].
The mechanisms underlying neural manifestations in COVID-19 are probably multifactorial: viral, immune, hypoxia, and hypercoagulability. Direct damage to the brain caused by SARS-CoV-2 is still under debate. Some authors have suggested direct brain infection as endothelial cells, arterial smooth muscle cells, glial cells, and neurons express ACE2 receptors in the brain [54,167]. In a preprint, SARS-CoV-2 was capable of infecting human brain organoids and the brain of mice overexpressing human ACE2 in vivo [168]. In another preprint, all 6 patients autopsies displaying affected brain tissues exhibited foci of SARS-CoV-2 infection and replication, particularly in astrocytes. In vitro, astrocyte infection changed energy metabolism, altered key proteins and metabolites ultimately reducing neuronal viability [169]. On the other hand, although autopsies showed SARS-CoV-2 could be detected in the brains of 21 (53%) of 40 examined patients, they were not associated with the severity of neuropathological changes, which were mainly attributed to mild neuroinflammatory or hypoxic changes [170,171]. To the best of our knowledge, no conclusive proof of direct neural infection has been demonstrated in live humans, as most studies did not find SARS-CoV-2 RNA in cerebrospinal fluid (CSF) or brain samples [[172], [173], [174], [175], [176], [177], [178]].
Cerebrovascular alterations such as large-vessel ischemic strokes and cerebral venous thrombosis are consequences of the procoagulant vascular state induced by COVID-19 [179]. Meanwhile, inflammatory cytokine storm provoked by SARS-CoV-2 infection may damage the blood-brain or blood-CSF barriers, oligodendrocytes and neurons [3]. The most common histopathologic findings in autopsies were astrogliosis, ischaemic lesions, neuronal degeneration and activated microglia in the brainstem [126,171].
Several studies have demonstrated positive results of MSC therapy in other neurological diseases, as they have high regenerative capacity and immunoregulatory function. In neurological complications (neurodegenerative diseases or neural inflammation), the main obstacle to treatment is the blood-brain barrier, which many drugs cannot cross to reach the target organ [45,180]. However, MSC EVs can overcome this barrier and solve the problem. Thus, from work already published, and where it is possible to parallel neurological manifestations to those caused by SARS-CoV-2, the performance and effectiveness of MSC and MSC-EVs are discussed.
Inflammatory conditions in the central nervous system (CNS) are involved in the pathogenesis of acute and chronic neurodegenerative diseases, such as brain and spinal cord trauma, and stroke [181]. In inflammatory responses, microglia are activated, leading to neurotoxic and proinflammatory factors release, including cytokines, ROS, NO, and eicosanoids that can damage neurons and glial cells [181,182]. There is also the activation and recruitment of neutrophils and monocyte-derived macrophages into the bloodstream, further inducing neurodegeneration [183]. Sun et al. demonstrated that therapy with umbilical cord-derived MSC-EVs helped to recover spinal cord injury by reducing levels of IFN-γ, IL-6, TNF-α, and MIP-1α in mice [182]. Another study using MPTP-induced mouse model, which kills dopamine-producing neurons, reported that bone marrow-derived MSCs decreased proinflammatory cytokines, suppressed microglia activation, recovered blood-brain barrier integrity and prevented neuronal death by releasing TGF-β1. As a result, MSCs promote neuroprotective effect against cytotoxicity [184].
MSC and MSC-EVs also release neurotrophic factors, rescuing neuromotor activity in spinal cord injury [185,186]. A study that evaluated MSC-EV therapy following stroke in mice found that the treatment helped to improve peripheral immune responses, alleviating the quality and quantity of immunosuppression [187]. MSCs were observed to promote neurovascular recovery and plasticity in other studies that performed the same analysis in rats [187,188]. Recently, using a stroke model Xin et al. reported that MSC-EVs carry miR-133b, which is suggested to be the main component involved in improving cerebral recovery in stroke. MSCs could increase miR-133b levels in ischemic brain tissues, promoting an increase in neuron growth [187,188]. Thus, MSCs have important neuromodulatory capacity that can be used therapeutically.
In clinical trials utilizing MSC for spinal cord injury, stroke or traumatic brain injury side effects related to the procedure noted, although none of them were followed with sequelae. Transplantation of MSCs resulted in neurologic improvement in the majority of clinical trials, with superior performance in motor function, sensory level and daily living activities [189]. MSCs have great potential to improve patient symptoms and quality of life, whereas traditional medicine offers no efficient treatment.
The research presented here demonstrates the promising therapeutic potential of MSC and MSC-EVs in the treatment of various neurological complications. Although far from ideal, those models illustrate the potential benefits MSC may have on the neurological pathology of COVID-19, in different time points of the disease progression.
2.6. Risk groups for COVID-19: obesity, diabetes and hypertension
The main groups at risk of developing severe COVID-19 are elderly and obese individuals, who often have related comorbidities such as hypertension and type 2 diabetes (T2D) [[190], [191], [192]]. The Centers for Disease Control and Prevention (CDC) reported that the majority of patients hospitalized with COVID-19 in march 2020 were elderly (≥65 years) and among them, most of those who were obese experienced complications [[193], [194], [195]]. Simonnet et al. [196] showed a high prevalence of mechanical ventilation support as Body Mass Index (BMI) increased, being greatest in patients with obesity (BMI > 35), independent of age, diabetes, and hypertension [196]. Overweight or severely obese patients with COVID-19 had elevated metabolic damage linked to pneumonia aggravation, with higher prevalence of mechanical ventilation, and death [190,194].
The enhanced pathophysiology observed in individuals with obesity and COVID-19 is not clear, but probably is multi-factorial, ranging from complement system hyperactivation, chronic inflammation and comorbidities progression [191,197]. There is a consensus that obesity negatively affects the immune response. In particular, inflammatory responses are triggered and develop to a constant low-grade systemic inflammation directly impacting the whole body [197]. This complex cellular event involves hyperplasia and hypertrophy of white adipose tissue cells and a macrophage profile switch to a proinflammatory state, that triggers the release of proinflammatory cytokines, such as TNF-α and IL-6. IL-6 is positively correlated to COVID-19 aggressiveness and is one of the main markers of lung injury [198,199]. Furthermore, weight excess is largely known to be positively associated with multiple respiratory diseases, such as asthma, obstructive sleep apnea syndrome, acute lung injury, and ARDS, even after adjusting for other risk factors [191].
Over time, this low-grade inflammation contributes to the development of insulin resistance and progression to T2D [198,[200], [201], [202]]. Individuals with T2D also have impaired immune system responses with altered activities of macrophages, NK cells, and neutrophils. In this scenario, lymphocytes induce production of proinflammatory cytokines due to impaired Treg function [203]. In a cohort study, patients with COVID-19 that had well-controlled levels of glycemia showed controlled COVID-19 symptoms, while T2D patients showed higher levels of neutrophils and IL-6 cytokines [204]. Higher levels of proinflammatory cytokines, D-dimmers, and a lower rate of survival were also associated with patients that had T2D and COVID-19 in another study [205]. Hyperglycaemia, regardless of diabetes status, was independently correlated to increased disease severity and mortality in 11,312 hospital patients with COVID [206].
The proinflammatory state seen in obesity can also contribute to the development of hypertension [207,208]. In multiple studies, hypertension was associated with poor COVID-19 outcome, including ARDS, need for intensive care unit, disease progression and mortality [24,[209], [210], [211]].
These data highlight the urgent need to develop therapies designed for potential high-risk COVID-19 patients [212]. The chronic pro-inflammatory state and impaired immunity typically found in patients with obesity, T2D and hypertension make it even more complex to design strategies aiming to treat COVID-19 based on controlling inflammation and preventing cytokine storm. In this regard MSC therapy may be a strategy to counteract COVID-19 especially in high-risk patients.
MSC therapy has positive metabolic and trophic effects in obesity models. In a preclinical systematic review, MSCs demonstrated strong positive effect in obese mice, improving glucose metabolism homeostasis, lipid profiles, non-alcoholic fatty liver disease and systemic inflammation [213]. Obese mice that received MSCs showed reduced liver fat deposition, IL-6 expression, blood glucose levels and increased glucose tolerance [214]. MSCs and MSC-CM have also presented a recovery effect on obesity-related cardiac alterations, reducing fibrosis, improving arrhythmias and exercise capacity [215].
In the T2D context, MSC transplantation has shown improvement in reversing peripheral insulin resistance and relieving β-cell destruction through paracrine activity [[216], [217], [218], [219]]. Moreover, in hypertension disease, MSC's effects can reduce arterial pressure and induce a decrease in cardiac hypertrophy while also stimulating vascular recovery through secretion of VEGF, IGF, and HGF [220,221]. MSC could also improve systolic blood pressure in renovascular and salt-sensitive hypertension in mice [222,223]. Therefore, although safety must still be thoroughly validated, MSC-based therapy might be appropriate to patients with COVID-19 who have obesity and co-morbidities, as MSC has broad potential therapeutic benefits.
2.7. Challenges
Currently, there are 308 clinical trials registered to assess the therapeutic potential of MSCs for clinical use in patients affected with COVID-19 [224]. Most of these clinical trials use bone marrow or umbilical cord as sources of MSCs, which are intravenously administered. These studies are in phases I and II, evaluating safety and efficacy, mainly in patients with exacerbated inflammatory responses. As the therapy is innovative, there is a need for caution when administering cells into patients. In preclinical trials, MSCs are quite efficient, but advanced clinical trials have fallen short of expectations. Clinical trials involving the use of MSC in patients with COVID-19 although presented partial improvement of outcome, have received critics regarding loose definitions and limited data availability [225,226]. Some aspects such as dose standardization, frequency of MSC doses and treatment timing, as defining the optimal stage of COVID-19 at which the cells should be injected, still need to be clarified. Also, the possibility of thromboembolism must always be taken into account [119]. It is important to recognize that in future investigations, patients need to be followed-up, not only in the short term, but also in the long term to detect undesirable adverse effects. Besides, long-haulers, long-COVID or post-acute COVID patients, in which variable symptoms can linger for months, might benefit from MSCs treatment, but clearer definition of post-COVID sequelae as well as solid evidence for MSC's effect in this context is still lacking [227].
In this review article, we summarized the reported evidence of MSCs potential benefits in multiple organs and systems affected by COVID-19 (Table 1 ). Collectively, MSCs 1) provide an anti-inflammatory environment via different mechanisms, decreasing cytokine, chemokine, and inflammatory cell infiltration; 2) improve pathological resolution, promoting tissue remodeling and reducing fibrosis; and 3) restore organ function, recovering the expression of proteins involved in cell structures in the lungs, kidneys, cardiovascular system, nervous system, and also in hypertensive, obesity, and diabetes models. Thus, MSCs are a very promising treatment strategy for patients with COVID-19, although some challenges will need to be addressed during translational research.
Table 1.
COVID-19 multi organ pathology and MSC's potential therapeutic mechanisms.
| Organs | SARS-CoV-2 pathogenesis | MSC therapeutic potential |
|---|---|---|
| Lungs | Cytokine storm | Immunomodulation → paracrine signaling (PGE2, TSG-6, HVEM-BTLA) |
| Diffuse alveolar injury | ↓ viral load - LL37 protein | |
| Hyaline membrane formation | ↓ epithelial cells apoptosis | |
| Exudate and cell infiltration inside alveoli | ↑ alveolar fluid clearance and angiogenesis | |
| Possible secondary infection | ↓ histological injury | |
| Progressive loss of function | ↓ pulmonary fibrosis (TGF-β1) | |
| Pulmonary fibrosis | Alveolar epithelial integrity recovery | |
| Heart | ↑cardiac troponin ↑myoglobin ↑creatine kinase ↑NT-proBNP | |
| ACE-2 dysregulation ↓angiotensin 1–7 ↑TNFα | ↑ myocardial contractility | |
| Myocarditis/microvascular injury/microthrombicardiomyopathy | ↓ apoptosis ↓oxidative stress | |
| Hypoxia | ↓TNF-α mRNA | |
| Arrhythmias | Activate resident cardiac stem cells | |
| Acute cardiac injury with cardiomyopathy, ventricular arrhythmias, and hemodynamic instability | Preserve cardiac function | |
| Myocardium fibrosis | ↓ infarct size ↓ fibrosis | |
| Blood vessels | Endothelial cell infection | |
| Hyperperfusion of inefficient | ||
| alveoli | ↑KGF ↑VEGF ↑HGF | |
| Procoagulant profile: | ↓ endothelial cell apoptosis | |
| ↑D-dimer | ↓ lung endothelial barrier permeability | |
| ↑fibrin degradation ↑IL-6 | ↑ angiogenesis | |
| ↑ thrombotic disorders | Preserves endothelial barrier function | |
| ↑COVID severity = ↓platelet | ||
| Kidneys | Directly infect renal epithelia | ↓ tubular cell apoptosis |
| Tubular injury | ↓ inflammatory infiltrate | |
| Necrotic lesions | ↓ TGF-β tissue remodeling ↓ fibrosis | |
| Epithelium detachment | ↑ podocytes function and integrity | |
| Bowman's capsule rupture | Nephroprotective effects | |
| Renal dysfunction | Regeneration of renal tubular cells through MSC-derived Evs | |
| Interstitial fibrosis | ||
| Central nervous system | Direct damage (?) | ↓IFN-γ ↓IL-6 ↓TNFα ↓MIP-1α |
| Glial cell hyperplasia | ↓ microglia activation | |
| Acute ischemic stroke/cerebral venous sinus thrombosis/cerebral hemorrhage | ↑ blood-brain barrier integrity ↑ angiogenesis | |
| Ischaemic lesions | ↓ neurocytotoxicity | |
| Neuron degeneration | ↑ miR-133b | |
| ↑ neurovascular recovery and plasticity |
PGE2 - prostaglandin E2; TSG-6 -TNF-stimulated gene-6; HVEM - Herpesvirus entry mediator; BTLA - B- and T-lymphocyte attenuator; LL-37 - cathelicidin antimicrobial peptides LL-37; TGF-β - transforming growth factor-beta; IFN-γ - interferon-gamma; IL-6 - interleukin 6; TNF-α - tumor necrosis factor-alpha; MIP-1α - macrophage inflammatory protein; KGF - keratinocyte growth factor; VEGF - vascular endothelial growth factor; HGF - hepatocyte growth factor.
Conflicts of interest
The authors declare no potential conflicts of interest.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, the “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, and the “Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)”.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
G.P.C. and A.A.T. were responsible for Conceptualization; G.P.C., A.A.A., A.L.F., M.A.R., G.P.O. and Y.M.A.A. for Data Curation and Writing the Original Draft; M.A.R. handled Data Visualization; G.P.C., K.R.S., E.A.C.C., A.C.S., S.N.C., L.C. and A.A.T. were responsible for Review & Editing; E.A.C.C., A.C.S., S.N.C., L.C. and A.A.T. were responsible for Supervision and Funding Acquisition.
Glossary
- ACE2 - Angiotensin-converting enzyme 2
- AKI - Acute kidney injury
- ARDS - Acute respiratory distress syndrome
- BMI - Body mass index
- CDC - Centers for Disease Control and Prevention
- CNS - Central nervous system
- CSF - cerebrospinal fluid
- CTSL - Cathepsin L
- CVD - Cardiovascular diseases
- DIC - Disseminated intravascular coagulation
- EV - Extracellular vesicles
- FGF - Fibroblast growth factor
- GM-CSF - Granulocyte macrophage colony-stimulating factor
- hACE2 mice - Transgenic mice expressing human ACE2
- HGF - Hepatocyte growth factor
- IFN - Interferon
- IGF - Insulin growth factor-1
- IL-2 - Interleukin-2
- KGF - Keratinocyte growth factor
- MCP-1 - Monocyte chemoattractant protein-1
- MIG - Monokine induced by interferon-gamma
- MIP-1α - Macrophage inflammatory protein
- MiRNA - microRNA
- MSC - Mesenchymal stem cells
- NK - Natural killer cells
- PDGF-DD - Platelet-derived growth factor-DD
- PGE2 - Prostaglandin E2
- RAS - Renin-angiotensin system
- RBD - Receptor-binding domain
- ROS - reactive oxygen species
- SARS-CoV-2 - Severe acute respiratory syndrome-coronavirus 2
- TGF-β - Transforming Growth Factor-beta
- TNF-α - Tumor necrosis factor-alpha
- TSG-6 - TNF-stimulated gene-6
- UUO - Unilateral ureteral obstruction
- VEGF - Vascular endothelial growth factor
- VTE - Venous thromboembolism
- WT1 - Wilms' tumor suppressor 1
- HVEM - Herpesvirus entry mediator
- BTLA - B- and T-lymphocyte attenuator
- LL-37 - Cathelicidin antimicrobial peptides LL-37
- T2D - Type 2 Diabetes
- S protein - SARS-CoV-2 Spike protein
- MSC-CM - Conditioned-media
References
- 1.Chang T.-H., Wu J.-L., Chang L.-Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J. Formos. Med. Assoc. 2020;119(5):982–989. doi: 10.1016/j.jfma.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. (n.d.). Coronavirus Disease (COVID-19) Pandemic. 2020.
- 3.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W.…Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Viruses, C. S. G. of the I. C. on T. of The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . 2020. Situation Report-51 SITUATION IN NUMBERS Total and New Cases in Last 24 Hours. [Google Scholar]
- 6.Ullah I., Subbarao R.B., Rho G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 2015;35(2):e00191. doi: 10.1042/BSR20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochon B., Kozubska M., Surygała G., Witkowska A., Kuźniewicz R., Grzeszczak W., Wystrychowski G. Mesenchymal stem cells—potential applications in kidney diseases. Int. J. Mol. Sci. 2019;20(10):2462. doi: 10.3390/ijms20102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crigna A.T., Daniele C., Gamez C., Balbuena S.M., Pastene D.O., Nardozi D., Brenna C., Yard B., Gretz N., Bieback K. Frontiers in Medicine. vol. 5. Frontiers Media S.A; 2018. Stem/stromal cells for treatment of kidney injuries with focus on preclinical models; p. 179. Issue JUN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji K., Kitamura S., Wada J. Secretomes from mesenchymal stem cells against acute kidney injury: possible heterogeneity. Stem Cells Int. 2018;2018(8693137) doi: 10.1155/2018/8693137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai Y., Kuba K., Ohto-Nakanishi T., Penninger J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J. 2010;74(3):405–410. doi: 10.1253/circj.CJ-10-0045. Circ J. [DOI] [PubMed] [Google Scholar]
- 11.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6(3):271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z., Wu M., Yao J., Guo J., Liao X., Song S., Li J., Duan G., Zhou Y., Wu X., Zhou Z., Wang T., Hu M., Chen X., Fu Y., Lei C., Dong H., Xu C., Hu Y.…Yan J. Caution on kidney dysfunctions of COVID-19 patients. SSRN Electron. J. 2020 doi: 10.1101/2020.02.08.20021212. 2020.02.08.20021212. [DOI] [Google Scholar]
- 13.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73(1):529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- 15.Meselson M. Droplets and aerosols in the transmission of SARS-CoV-2. N. Engl. J. Med. 2020;382(21):2063. doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., Crown K.K., Brett-Major D.M., Schnaubelt E.R., Broadhurst M.J., Lawler J.V., Reid S.P., Lowe J.J. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., O’Mahony L., Gao Y., Nadeau K., Akdis C.A. Allergy: European Journal of Allergy and Clinical Immunology. vol. 75. Blackwell Publishing Ltd; 2020. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19; pp. 1564–1581. Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., Zhang X., Cai J.-P., Zhou J., Yuan S., Kok K.-H., To K.K.-W., Chan I.H.-Y., Zhang A.J., Sit K.-Y.…Yuen K.-Y. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Inf. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020;71(6):1400–1409. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F., Caricchio R., Mahmud S., Hazen M.M., Halyabar O., Hoyt K.J., Han J., Grom A.A., Gattorno M., Ravelli A., De Benedetti F., Behrens E.M., Cron R.Q., Nigrovic P.A. Arthritis and Rheumatology. vol. 72. John Wiley and Sons Inc.; 2020. On the alert for cytokine storm: immunopathology in COVID-19; pp. 1059–1063. Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M.…Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X., Cao Y. Yuan, Lu X. Xia, Zhang J. Jin, Du H., Yan Y. Qin, Akdis C.A., Gao Y. Dong. Eleven faces of coronavirus disease 2019. Allergy Eur. J. Allergy Clin. Immunol. 2020;75(7):1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J.…Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y., Min P., Lee S., Kim S.-W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Korean Med. Sci. 2020;35(18) doi: 10.3346/jkms.2020.35.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J. Jin, Dong X., Cao Y. Yuan, Yuan Y. Dong, Yang Y. Bin, Yan Y. Qin, Akdis C.A., Gao Y. Dong. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur. J. Allergy Clini. Immunol. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 27.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020;92(6):568–576. doi: 10.1002/jmv.25748. John Wiley and Sons Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamba C., Singh S.P., Choudhury S. Can mesenchymal stem cell therapy be the interim management of COVID-19? Drug Discov. Ther. 2020 doi: 10.5582/ddt.2020.03032. advpub. [DOI] [PubMed] [Google Scholar]
- 31.Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L.…P??ault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.da Silva Meirelles L., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 33.Ding D.C., Shyu W.C., Lin S.Z. Cell Transplantation. vol. 20. SAGE PublicationsSage CA; Los Angeles, CA: 2011. Mesenchymal stem cells; pp. 5–14. Issue 1. [DOI] [PubMed] [Google Scholar]
- 34.Migliorini F., Rath B., Colarossi G., Driessen A., Tingart M., Niewiera M., Eschweiler J. Improved outcomes after mesenchymal stem cells injections for knee osteoarthritis: results at 12-months follow-up: a systematic review of the literature. Arch. Orthop. Trauma Surg. 2020;140(7):853–868. doi: 10.1007/s00402-019-03267-8. [DOI] [PubMed] [Google Scholar]
- 35.Kasten P., Beverungen M., Lorenz H., Wieland J., Fehr M., Geiger F. Comparison of platelet-rich plasma and VEGF-transfected mesenchymal stem cells on vascularization and bone formation in a critical-size bone defect. Cells Tissues Organs. 2012;196(6):523–533. doi: 10.1159/000337490. [DOI] [PubMed] [Google Scholar]
- 36.Tögel F., Zhang P., Hu Z., Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 2009;13(8b):2109–2114. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen. Med. 2019;4(1) doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y., Wang Y., Li Q., Liu K., Hou J., Shao C., Wang Y. Nature Reviews Nephrology. vol. 14. Nature Publishing Group; 2018. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases; pp. 493–507. Issue 8. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W., Xu J. Cell Proliferation. vol. 53. Blackwell Publishing Ltd; 2020. Immune modulation by mesenchymal stem cells. Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng C.Y., Hwang C. Il, Corney D.C., Flesken-Nikitin A., Jiang L., Öner G.M., Munroe R.J., Schimenti J.C., Hermeking H., Nikitin A.Y. MiR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment. Cell Rep. 2014;6(6):1000–1007. doi: 10.1016/j.celrep.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keshtkar S., Azarpira N., Ghahremani M.H. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1) doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindoso R.S., Collino F., Bruno S., Araujo D.S., Sant’Anna J.F., Tetta C., Provero P., Quesenberry P.J., Vieyra A., Einicker-Lamas M., Camussi G. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23(15):1809–1819. doi: 10.1089/scd.2013.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy M.B., Moncivais K., Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013;45(11):e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchiya A., Takeuchi S., Iwasawa T., Kumagai M., Sato T., Motegi S., Ishii Y., Koseki Y., Tomiyoshi K., Natsui K., Takeda N., Yoshida Y., Yamazaki F., Kojima Y., Watanabe Y., Kimura N., Tominaga K., Kamimura H., Takamura M., Terai S. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm. Regen. 2020;40(1) doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biol T.J., Bulut Ö., Gürsel İ. Mesenchymal stem cell derived extracellular vesicles: promising immunomodulators against autoimmune, autoinflammatory disorders and SARS-CoV-2 infection. Turk. J. Biol. 2020;44(SI-1):273–282. doi: 10.3906/biy-2002-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A.…Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 2020;14(9):865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaim S., Chong J.H., Sankaranarayanan V., Harky A. COVID-19 and multiorgan response. Curr. Probl. Cardiol. 2020;45(8) doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., Braun F., Lu S., Pfefferle S., Schröder A.S., Edler C., Gross O., Glatzel M., Wichmann D., Wiech T.…Huber T.B. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/nejmc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., Yi F., Yang H.C., Fogo A.B., Nie X., Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., Baldanti F., Paolucci S., Pelenghi S., Iotti G.A., Mojoli F., Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526(1):135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sekulic M., Harper H., Nezami B.G., Shen D.L., Sekulic S.P., Koeth A.T., Harding C.V., Gilmore H., Sadri N. Molecular detection of SARS-CoV-2 infection in FFPE samples and histopathologic findings in fatal SARS-CoV-2 cases. Am. J. Clin. Pathol. 2020;154(2):190–200. doi: 10.1093/ajcp/aqaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasquez-Bonilla W.O., Orozco R., Argueta V., Sierra M., Zambrano L.I., Muñoz-Lara F., López-Molina D.S., Arteaga-Livias K., Grimes Z., Bryce C., Paniz-Mondolfi A., Rodríguez-Morales A.J. A review of the main histopathological findings in coronavirus disease 2019. Hum. Pathol. 2020;105:74–83. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pernazza A., Mancini M., Rullo E., Bassi M., De Giacomo T., Rocca C. Della, d’Amati G. Early histologic findings of pulmonary SARS-CoV-2 infection detected in a surgical specimen. Virchows Arch. 2020;477(5):743–748. doi: 10.1007/s00428-020-02829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaefer I.-M., Padera R.F., Solomon I.H., Kanjilal S., Hammer M.M., Hornick J.L., Sholl L.M. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod. Pathol. 2020;33(11):2104–2114. doi: 10.1038/s41379-020-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S.…Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 60.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J. Thorac. Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao X.-H., He Z.-C., Li T.-Y., Zhang H.-R., Wang Y., Mou H., Guo Q., Yu S.-C., Ding Y., Liu X., Ping Y.-F., Bian X.-W. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020;30(6):541–543. doi: 10.1038/s41422-020-0318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao X.H., Li T.Y., He Z.C., Ping Y.F., Liu H.W., Yu S.C., Mou H.M., Wang L.H., Zhang H.R., Fu W.J., Luo T., Liu F., Guo Q.N., Chen C., Xiao H.L., Guo H.T., Lin S., Xiang D.F., Shi Y.…Bian X.W. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi = Chin. J. Pathol. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., Zhang S., Cao T., Yang C., Li M., Guo G., Chen X., Chen Y., Lei M., Liu H., Zhao J., Peng P., Wang C.-Y., Du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laffey J.G., Matthay M.A. Cell-based therapy for acute respiratory distress syndrome: biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017;196(3):266–273. doi: 10.1164/rccm.201701-0107CP. American Thoracic Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walter J., Ware L.B., Matthay M.A. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014;2(12):1016–1026. doi: 10.1016/S2213-2600(14)70217-6. Lancet Publishing Group. [DOI] [PubMed] [Google Scholar]
- 67.Lee J.W., Krasnodembskaya A., McKenna D.H., Song Y., Abbott J., Matthay M.A. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am. J. Respir. Crit. Care Med. 2013;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J., Rosenzwajg M., Matthay M.A., Lee J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015;192(3):324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shen Q., Chen B., Xiao Z., Zhao L., Xu X., Wan X., Jin M., Dai J., Dai H. Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol. Med. Rep. 2015;11(4):2831–2837. doi: 10.3892/mmr.2014.3092. [DOI] [PubMed] [Google Scholar]
- 70.Fang X., Neyrinck A.P., Matthay M.A., Lee J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010;285(34):26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goolaerts A., Pellan-Randrianarison N., Larghero J., Vanneaux V., Uzunhan Y., Gille T., Dard N., Planès C., Matthay M.A., Clerici C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am. J. Phys. Lung Cell. Mol. Phys. 2014;306(11):L975–L985. doi: 10.1152/ajplung.00242.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B.K., Jha K.A., Barhanpurkar A.P., Wani M.R., Roy S.S., Mabalirajan U., Ghosh B., Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33(9) doi: 10.1002/embj.201386030. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Court A.C., Le-Gatt A., Luz-Crawford P., Parra E., Aliaga-Tobar V., Bátiz L.F., Contreras R.A., Ortúzar M.I., Kurte M., Elizondo-Vega R., Maracaja-Coutinho V., Pino-Lagos K., Figueroa F.E., Khoury M. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2) doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackson M.V., Morrison T.J., Doherty D.F., McAuley D.F., Matthay M.A., Kissenpfennig A., O’Kane C.M., Krasnodembskaya A.D. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34(8):2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Xu J., Shi W., Chen C., Shao Y., Zhu L., Lu W., Han X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther. 2016;7(1) doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9(1) doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan M.C.W., Kuok D.I.T., Leung C.Y.H., Hui K.P.Y., Valkenburg S.A., Lau E.H.Y., Nicholls J.M., Fang X., Guan Y., Lee J.W., Chan R.W.Y., Webster R.G., Matthay M.A., Peiris J.S.M. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. 2016;113(13):3621 LP–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gordon Y.J., Huang L.C., Romanowski E.G., Yates K.A., Proske R.J., McDermott A.M. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr. Eye Res. 2005;30(5):385–394. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oliveira-Bravo M., Sangiorgi B.B., Schiavinato J.L. dos S., Carvalho J.L., Covas D.T., Panepucci R.A., Neves F. de A.R., Franco O.L., Pereira R.W., Saldanha-Araujo F. LL-37 boosts immunosuppressive function of placenta-derived mesenchymal stromal cells. Stem Cell Res Ther. 2016;7(1) doi: 10.1186/s13287-016-0448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Darwish I., Banner D., Mubareka S., Kim H., Besla R., Kelvin D.J., Kain K.C., Liles W.C. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gotts J.E., Abbott J., Matthay M.A. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am. J. Phys. Lung Cell. Mol. Phys. 2014;307(5):L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danchuk S., Ylostalo J.H., Hossain F., Sorge R., Ramsey A., Bonvillain R.W., Lasky J.A., Bunnell B.A., Welsh D.A., Prockop D.J., Sullivan D.E. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res Ther. 2011;2(3):27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Magaña-Guerrero F.S., Domínguez-López A., Martínez-Aboytes P., Buentello-Volante B., Garfias Y. Human amniotic membrane mesenchymal stem cells inhibit neutrophil extracellular traps through TSG-6. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-10962-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prockop D.J. Stem Cells. vol. 31. John Wiley & Sons, Ltd; 2013. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation; pp. 2042–2046. Issue 10. [DOI] [PubMed] [Google Scholar]
- 87.Cheng T., Feng Y., Chen X., Zhou J., Song Y. Lung-resident mesenchymal stem cells regulated the inflammatory responses in innate and adaptive immune cells through HVEM-BTLA pathway during ARDS. Exp. Cell Res. 2020;395(1) doi: 10.1016/j.yexcr.2020.112155. [DOI] [PubMed] [Google Scholar]
- 88.Curley G.F., Ansari B., Hayes M., Devaney J., Masterson C., Ryan A., Barry F., O’Brien T., Toole D.O., Laffey J.G. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118(4):924–932. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 89.Curley G.F., Hayes M., Ansari B., Shaw G., Ryan A., Barry F., O’brien T., O’toole D., Laffey J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 90.Krasnodembskaya A., Samarani G., Song Y., Zhuo H., Su X., Lee J.W., Gupta N., Petrini M., Matthay M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302(10):1003–1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Julien P., Julien G., Morgan C., Erika P., Thibault D., Fanny L., Emmanuelle J., Antoine R., Julien L., Sophie S., Nicolas C., Arthur D., Ahmed E.K., Raphaël F., Patrick G., Marion H., Emmanuelle J., Mercé J., Geoffrey L.…Antoine H. Pulmonary embolism in patients with COVID-19. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 92.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.-J., Harjola V.-P., Huisman M.V., Humbert M., Jennings C.S., Jiménez D., Kucher N., Lang I.M., Lankeit M., Lorusso R., Mazzolai L., Meneveau N., Ní Áinle F., Prandoni P., Pruszczyk P.…Zamorano J.L. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of. Eur. Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 93.Schünemann H.J., Cushman M., Burnett A.E., Kahn S.R., Beyer-Westendorf J., Spencer F.A., Rezende S.M., Zakai N.A., Bauer K.A., Dentali F., Lansing J., Balduzzi S., Darzi A., Morgano G.P., Neumann I., Nieuwlaat R., Yepes-Nuñez J.J., Zhang Y., Wiercioch W. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C. Der, Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J.…Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. Elsevier USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fan B.E., Chong V.C.L., Chan S.S.W., Lim G.H., Lim K.G.E., Tan G.B., Mucheli S.S., Kuperan P., Ong K.H. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 2020;95(6):E131–E134. doi: 10.1002/ajh.25774. [DOI] [PubMed] [Google Scholar]
- 96.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiol. Cardiothoracic Imaging. 2020;2(2) doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R., Tang C., Zhang N., Zhong N., Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. (CCLM) 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 102.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin. Chim. Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gattinoni L., Coppola S., Cressoni M., Busana M., Rossi S., Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. American Thoracic Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Santamarina M.G., Boisier D., Contreras R., Baque M., Volpacchio M., Beddings I. COVID-19: a hypothesis regarding the ventilation-perfusion mismatch. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-03125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. Lancet Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007;44(2):62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stein P.D., Beemath A., Olson R.E. Obesity as a risk factor in venous thromboembolism. Am. J. Med. 2005;118(9):978–980. doi: 10.1016/j.amjmed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 108.Huang L., Li J., Jiang Y. Association between hypertension and deep vein thrombosis after orthopedic surgery: a meta-analysis. Eur. J. Med. Res. 2016;21(1) doi: 10.1186/s40001-016-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lip G.Y.H. Hypertension and the prothrombotic state. J. Hum. Hypertens. 2000;14(10):687–690. doi: 10.1038/sj.jhh.1001051. [DOI] [PubMed] [Google Scholar]
- 110.Spyropoulos A.C., Lipardi C., Xu J., Peluso C., Spiro T.E., De Sanctis Y., Barnathan E.S., Raskob G.E. Modified IMPROVE VTE risk score and elevated D-dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;04(01):e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.WHO . 2020. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (2019-nCoV) Infection Is Suspected Interim Guidance 28 January 2020. [Google Scholar]
- 112.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Whyte C.S., Morrow G.B., Mitchell J.L., Chowdary P., Mutch N.J. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J. Thromb. Haemost. 2020;18(7):1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Au P., Tam J., Fukumura D., Jain R.K. Bone marrow–derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melero-Martin J.M., De Obaldia M.E., Kang S.Y., Khan Z.A., Yuan L., Oettgen P., Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ. Res. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Németh K., Leelahavanichkul A., Yuen P.S.T., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., Hu X., Jelinek I., Star R.A., Mezey É. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li J.-W., W. X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Stem Cells Dev. 2015;19:2368–2378. [PubMed] [Google Scholar]
- 118.Bastarache J.A., Wang L., Wang Z., Albertine K.H., Matthay M.A., Ware L.B. Intra-alveolar tissue factor pathway inhibitor is not sufficient to block tissue factor procoagulant activity. Am. J. Phys. Lung Cell. Mol. Phys. 2008;294(5):L874–L881. doi: 10.1152/ajplung.00372.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Coppin L., Sokal E., Stéphenne X. Thrombogenic risk induced by intravascular mesenchymal stem cell therapy: current status and future perspectives. Cells. 2019;8(10) doi: 10.3390/cells8101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng X., Li J., Yu X., Tan R., Zhu L., Wang J., Wang R., Gu G., Liu Q., Ren L., Wang C., Hu Q. Therapeutic effectiveness of bone marrow-derived mesenchymal stem cell administration against acute pulmonary thromboembolism in a mouse model. Thromb. Res. 2015;135(5):990–999. doi: 10.1016/j.thromres.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 121.Wang B., Wu S., Wang T., Ma Z., Liu K. Bone marrow-derived mesenchymal stem cells-mediated protection against organ dysfunction in disseminated intravascular coagulation is associated with peripheral immune responses. J. Cell. Biochem. 2017;118(10):3184–3192. doi: 10.1002/jcb.25964. [DOI] [PubMed] [Google Scholar]
- 122.Liao L., Shi B., Chang H., Su X., Zhang L., Bi C., Shuai Y., Du X., Deng Z., Jin Y. Heparin improves BMSC cell therapy: anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics. 2017;7(1):106–116. doi: 10.7150/thno.16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., Zhao S., Somani S., Van Vleck T., Vaid A., Chaudhry F., De Freitas J.K., Fayad Z.A., Pinney S.P., Levin M., Charney A., Bagiella E., Narula J., Glicksberg B.S.…Fuster V. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giustino G., Pinney S.P., Lala A., Reddy V.Y., Johnston-Cox H.A., Mechanick J.I., Halperin J.L., Fuster V. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC focus seminar. J. Am. Coll. Cardiol. 2020;76(17):2011–2023. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hendren N.S., Drazner M.H., Bozkurt B., Cooper L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Deshmukh V., Motwani R., Kumar A., Kumari C., Raza K. Histopathological observations in COVID-19: a systematic review. J. Clin. Pathol. 2020 doi: 10.1136/jclinpath-2020-206995. jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 127.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5(1) doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D.…Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Esai Selvan M., Spindler M.P., Tan J., van der Heide V., Gregory J.K.…Laserson U. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. Cell Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20(6):343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abraham A., Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020;9(1):28–38. doi: 10.1002/sctm.19-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rossi E., Goyard C., Cras A., Dizier B., Bacha N., Lokajczyk A., Guerin C.L., Gendron N., Planquette B., Mignon V., Bernabeu C., Sanchez O., Smadja D.M. Co-injection of mesenchymal stem cells with endothelial progenitor cells accelerates muscle recovery in hind limb ischemia through an endoglin-dependent mechanism. Thromb. Haemost. 2017;117(10):1908–1918. doi: 10.1160/TH17-01-0007. [DOI] [PubMed] [Google Scholar]
- 133.Smadja D.M., d’Audigier C., Guerin C.L., Mauge L., Dizier B., Silvestre J.-S.S., Cortivo L.D., Gaussem P., Emmerich J. Angiogenic potential of BM MSCs derived from patients with critical leg ischemia. Bone Marrow Transplant. 2012;47(7):997–1000. doi: 10.1038/bmt.2011.196. [DOI] [PubMed] [Google Scholar]
- 134.Miteva K., Pappritz K., Sosnowski M., El-Shafeey M., Müller I., Dong F., Savvatis K., Ringe J., Tschöpe C., Van Linthout S. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-20686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pati S., Gerber M.H., Menge T.D., Wataha K.A., Zhao Y., Baumgartner J.A., Zhao J., Letourneau P.A., Huby M.P., Baer L.A., Salsbury J.R., Kozar R.A., Wade C.E., Walker P.A., Dash P.K., Cox C.S., Doursout M.-F., Holcomb J.B. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6(9) doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pati S., Khakoo A.Y., Zhao J., Jimenez F., Gerber M.H., Harting M., Redell J.B., Grill R., Matsuo Y., Guha S., Cox C.S., Reitz M.S., Holcomb J.B., Dash P.K. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells Dev. 2011;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo Y., Yu Y., Hu S., Chen Y., Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11(5) doi: 10.1038/s41419-020-2542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allison S.J. SARS-CoV-2 infection of kidney organoids prevented with soluble human ACE2. Nat. Rev. Nephrol. 2020;16(6):316. doi: 10.1038/s41581-020-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Menon R., Otto E., Sealfon R., Nair V., Wong A., Theesfeld C., Chen X., Wang Y., Boppanna A., Luo J., Yang Y., Kasson P., Schaub J., Berthier C., Eddy S., Lienczewski C., Godfrey B., Dagenais S., Sohaney R.…Kretzler M. SARS-CoV-2 receptor networks in diabetic and COVID-19 associated kidney disease. MedRxiv Preprint Server Health Sci. 2020 doi: 10.1101/2020.05.09.20096511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan X., Xu D., Zhang H., Zhou W., Wang L., Cui X. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Farkash E.A., Wilson A.M., Jentzen J.M. Ultrastructural evidence for direct renal infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020 doi: 10.1681/ASN.2020040432. ASN.2020040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int. Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peleg Y., Kudose S., D’Agati V., Siddall E., Ahmad S., Nickolas T., Kisselev S., Gharavi A., Canetta P. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int. Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Diao B., Wang C., Wang R., Feng Z., Tan Y., Wang H., Wang C., Liu L., Liu Y., Liu Y., Wang G., Yuan Z., Ren L., Wu Y., Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. MedRxiv. 2020 doi: 10.1101/2020.03.04.20031120. 2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lira R., Oliveira M., Martins M., Silva C., Carvalho S., Stumbo A.C., Cortez E., Verdoorn K., Einicker-Lamas M., Thole A., de Carvalho L. Transplantation of bone marrow-derived MSCs improves renal function and Na++K+-ATPase activity in rats with renovascular hypertension. Cell Tissue Res. 2017;369(2):287–301. doi: 10.1007/s00441-017-2602-3. [DOI] [PubMed] [Google Scholar]
- 148.Gregorini M., Corradetti V., Rocca C., Pattonieri E.F., Valsania T., Milanesi S., Serpieri N., Bedino G., Esposito P., Libetta C., Avanzini M.A., Mantelli M., Ingo D., Peressini S., Albertini R., Dal Canton A., Rampino T. Mesenchymal stromal cells prevent renal fibrosis in a rat model of unilateral ureteral obstruction by suppressing the renin-angiotensin system via HuR. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0148542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kholia S., Herrera Sanchez M.B., Cedrino M., Papadimitriou E., Tapparo M., Deregibus M.C., Bruno S., Antico F., Brizzi M.F., Quesenberry P.J., Camussi G. Mesenchymal stem cell derived extracellular vesicles ameliorate kidney injury in aristolochic acid nephropathy. Front. Cell Develop. Biol. 2020;8:188. doi: 10.3389/fcell.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nagaishi K., Mizue Y., Chikenji T., Otani M., Nakano M., Konari N., Fujimiya M. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci. Rep. 2016;6(1) doi: 10.1038/srep34842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Jie, Zhang L., Wang N., Ding R., Cui S., Zhu F., Xie Y., Sun X., Wu D., Hong Q., Li Q., Shi S., Liu X., Chen X. Mesenchymal stem cells attenuate ischemic acute kidney injury by inducing regulatory T cells through splenocyte interactions. Kidney Int. 2013;84(3):521–531. doi: 10.1038/ki.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geng Y., Zhang L., Fu B., Zhang J., Hong Q., Hu J., Li D., Luo C., Cui S., Zhu F., Chen X. Mesenchymal stem cells ameliorate rhabdomyolysis-induced acute kidney injury via the activation of M2 macrophages. Stem Cell Res Ther. 2014;5(3):80. doi: 10.1186/scrt469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliveira-Sales E.B., Maquigussa E., Semedo P., Pereira L.G., Ferreira V.M., Câmara N.O., Bergamaschi C.T., Campos R.R., Boim M.A. Mesenchymal stem cells (MSC) prevented the progression of renovascular hypertension, improved renal function and architecture. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ornellas F.M., Ramalho R.J., Fanelli C., Garnica M.R., Malheiros D.M.A.C., Martini S.V., Morales M.M., Noronha I.L. Mesenchymal stromal cells induce podocyte protection in the puromycin injury model. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-55284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oliveira M., Lira R., Freire T., Luna C., Martins M., Almeida A., Carvalho S., Cortez E., Stumbo A.C., Thole A., Carvalho L. Bone marrow mononuclear cell transplantation rescues the glomerular filtration barrier and epithelial cellular junctions in a renovascular hypertension model. Exp. Physiol. 2019;104(5):740–754. doi: 10.1113/EP087330. [DOI] [PubMed] [Google Scholar]
- 156.Fazekas B., Griffin M.D. Mesenchymal stromal cell-based therapies for acute kidney injury: progress in the last decade. Kidney Int. 2020;97(6):1130–1140. doi: 10.1016/j.kint.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 157.Packham D.K., Fraser I.R., Kerr P.G., Segal K.R. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo-controlled, dose escalation study. EBioMedicine. 2016;12:263–269. doi: 10.1016/j.ebiom.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zilberman-Itskovich S., Efrati S. Mesenchymal stromal cell uses for acute kidney injury—current available data and future perspectives: a mini-review. Front. Immunol. 2020;11:1369. doi: 10.3389/fimmu.2020.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Konari N., Nagaishi K., Kikuchi S., Fujimiya M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-40163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., Paniz-Mondolfi A., Lagos-Grisales G.J., Ramírez-Vallejo E., Suárez J.A., Zambrano L.I., Villamil-Gómez W.E., Balbin-Ramon G.J., Rabaan A.A., Harapan H.…Sah R. Travel Medicine and Infectious Disease. vol. 34. Elsevier USA; 2020. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis; p. 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., Gervasoni C., Ridolfo A.L., Rizzardini G., Antinori S., Galli M. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M., Sakata H., Kondo K., Myose N., Nakao A., Takeda M., Haro H., Inoue O., Suzuki-Inoue K., Kubokawa K., Ogihara S., Sasaki T., Kinouchi H.…Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., Lantos J., Schenck E.J., Goyal P., Bruce S.S., Kahan J., Lansdale K.N., LeMoss N.M., Murthy S.B., Stieg P.E., Fink M.E., Iadecola C., Segal A.Z., Cusick M.…Navi B.B. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1366–1372. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baig A.M., Khaleeq A., Ali U., Syeda H. ACS Chemical Neuroscience. vol. 11. American Chemical Society; 2020. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms; pp. 995–998. Issue 7. [DOI] [PubMed] [Google Scholar]
- 168.Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., Lu P., Weizman O.-E., Liu F., Dai Y., Szigeti-Buck K., Yasumoto Y., Wang G., Castaldi C., Heltke J., Ng E., Wheeler J., Alfajaro M.M., Levavasseur E.…Iwasaki A. Neuroinvasion of SARS-CoV-2 in human and mouse brain. BioRxiv. 2020 doi: 10.1101/2020.06.25.169946. 2020.06.25.169946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Crunfli F., Carregari V.C., Veras F.P., Vendramini P.H., Reis-de-oliveira G., Silva-costa L.C., Saia-cereda V.M., Codo A.C., Parise P.L., Toledo-teixeira D.A., Souza G.F. De, Muraro S.P., Melo M.S., Almeida G.M., Mayara E., Firmino S., Paiva I.M., Manuella B., Silva S.…Davanzo G.G. SARS-CoV-2 Is Found in the Human Brain, Where It Infects Astrocytes and, to a Lesser Extent, Neurons. 2020. https://www.researchsquare.com/article/rs-104944/v1
- 170.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., Mushumba H., Fitzek A., Allweiss L., Dandri M., Dottermusch M., Heinemann A., Pfefferle S., Schwabenland M., Sumner Magruder D., Bonn S., Prinz M., Gerloff C., Püschel K.…Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet. Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., Adams G., Hornick J.L., Padera R.F.J., Sabeti P. Neuropathological features of Covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bellon M., Schweblin C., Lambeng N., Cherpillod P., Vazquez J., Lalive P.H., Schibler M., Deffert C. Cerebrospinal fluid features in SARS-CoV-2 RT-PCR positive patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Espíndola O. de M., Siqueira M., Soares C.N., Lima M.A.S.D. de, Leite A.C.C.B., Araujo A.Q.C., Brandão C.O., Silva M.T.T. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;96:567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Neumann B., Schmidbauer M.L., Dimitriadis K., Otto S., Knier B., Niesen W.-D., Hosp J.A., Günther A., Lindemann S., Nagy G., Steinberg T., Linker R.A., Hemmer B., Bösel J., groups, P. and the I. study Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2020;418(117090) doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., De Leacy R.A., Shigematsu T., Ladner T.R., Yaeger K.A., Skliut M., Weinberger J., Dangayach N.S., Bederson J.B., Tuhrim S., Fifi J.T. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020;382(20):e60. doi: 10.1056/nejmc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saiegh A. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke cerebrovascular disease. J. Neurol. Neurosurg. Psychiatry. 2020;0:1–3. doi: 10.1136/jnnp-2020-323522. [DOI] [PubMed] [Google Scholar]
- 178.Yin R., Feng W., Wang T., Chen G., Wu T., Chen D., Lv T., Xiang D. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J. Med. Virol. 2020;92(10):1782–1784. doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Román G.C., Spencer P.S., Reis J., Buguet A., Faris M.E.A., Katrak S.M., Láinez M., Medina M.T., Meshram C., Mizusawa H., Öztürk S., Wasay M. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J. Neurol. Sci. 2020;414 doi: 10.1016/j.jns.2020.116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gorabi A.M., Kiaie N., Barreto G.E., Read M.I., Tafti H.A., Sahebkar A. The therapeutic potential of mesenchymal stem cell–derived exosomes in treatment of neurodegenerative diseases. Mol. Neurobiol. 2019;56(12):8157–8167. doi: 10.1007/s12035-019-01663-0. [DOI] [PubMed] [Google Scholar]
- 181.Wyss-Coray T., Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35(3):419–432. doi: 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 182.Sun G., Li G., Li D., Huang W., Zhang R., Zhang H., Duan Y., Wang B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C. 2018;89:194–204. doi: 10.1016/j.msec.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 183.Lima R.R., Costa A.M.R., Souza R.D. de, Gomes-Leal W. Revista Paraense de Medicina. vol. 21. Scielo; 2007. Inflamação em doenças neurodegenerativas; pp. 29–34. [Google Scholar]
- 184.Chao Y.X., He B.P., Tay S.S.W. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J. Neuroimmunol. 2009;216(1–2):39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 185.Nosrat I.V., Widenfalk J., Olson L., Nosrat C.A. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev. Biol. 2001;238(1):120–132. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 186.Sakai K., Yamamoto A., Matsubara K., Nakamura S., Naruse M., Yamagata M., Sakamoto K., Tauchi R., Wakao N., Imagama S., Hibi H., Kadomatsu K., Ishiguro N., Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012;122(1):80–90. doi: 10.1172/JCI59251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xin H., Li Y., Cui Y., Yang J.J., Zhang Z.G., Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Y., Chopp M., Meng Y., Katakowski M., Xin H., Mahmood A., Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J. Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Badyra B., Sułkowski M., Milczarek O., Majka M. Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl. Med. 2020;9(10):1174–1189. doi: 10.1002/sctm.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. Nature Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and <scp>SARS-CoV</scp> −2: a population to safeguard. Diabetes Metab. Res. Rev. 2020;36(7) doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 192.Williamson E., Walker A., Bhaskaran K., Bacon S., Bates C., Morton C., Curtis H., Mehrkar A., Evans D., Inglesby P., Cockburn J., Mcdonald H., MacKenna B., Tomlinson L., Douglas I., Rentsch C., Mathur R., Wong A., Grieve R.…Goldacre B. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092999. 2020.05.06.20092999. [DOI] [Google Scholar]
- 193.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J.…Fry A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. American Physiological Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Petrilli C., Jones S., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K., Cerfolio R., Francois F., Horwitz L. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in new York City. BMJ. 2020 doi: 10.1101/2020.04.08.20057794. 2020.04.08.20057794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., Caizzo R., Caplan M., Cousin N., Duburcq T., Durand A., El kalioubie A., Favory R., Garcia B., Girardie P.…Verkindt H. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Butler M.J., Barrientos R.M. Brain, Behavior, and Immunity. vol. 87. Academic Press Inc; 2020. The impact of nutrition on COVID-19 susceptibility and long-term consequences; pp. 53–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chiappetta S., Sharma A.M., Bottino V., Stier C. COVID-19 and the role of chronic inflammation in patients with obesity. Int. J. Obes. 2020;44(8):1790–1792. doi: 10.1038/s41366-020-0597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dal Moro F., Vendramin I., Livi U. Medical Hypotheses. vol. 143. Churchill Livingstone; 2020. The war against the SARS-CoV2 infection: is it better to fight or mitigate it? p. 110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mraz M., Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014;222(3):113–127. doi: 10.1530/JOE-14-0283. BioScientifica Ltd. [DOI] [PubMed] [Google Scholar]
- 201.Oliver E., McGillicuddy F., Phillips C., Toomey S., Roche H.M. Postgraduate symposium: the role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. Proc. Nutr. Soc. 2010;69(2):232–243. doi: 10.1017/S0029665110000042. [DOI] [PubMed] [Google Scholar]
- 202.Thole A.A., Rodrigues-Cunha A.C.S., Carvalho S.N., Garcia-Souza E.P., Cortez E., Stumbo A.C., Carvalho L., Moura A.S. Progenitor cells and TNF-alpha involvement during morphological changes in pancreatic islets of obese mice. Tissue Cell. 2012;44(4):238–248. doi: 10.1016/j.tice.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 203.Erener S. Molecular Metabolism. vol. 39. Elsevier GmbH; 2020. Diabetes, infection risk and COVID-19; p. 101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., Li H., Zhang P., Song X., Chen X., Xiang M., Zhang C., Bai L., Xiang D., Chen M.M.…Li H. Association of Blood Glucose Control and Outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sardu C., D’Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Paolisso G., Marfella R. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Carrasco-Sánchez F.J., López-Carmona M.D., Martínez-Marcos F.J., Pérez-Belmonte L.M., Hidalgo-Jiménez A., Buonaiuto V., Suárez Fernández C., Freire Castro S.J., Luordo D., Pesqueira Fontan P.M., Blázquez Encinar J.C., Magallanes Gamboa J.O., de la Peña Fernández A., Torres Peña J.D., Fernández Solà J., Napal Lecumberri J.J., Amorós Martínez F., Guisado Espartero M.E., Jorge Ripper C.…Gómez Huelgas R. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 registry. Ann. Med. 2021;53(1):103–116. doi: 10.1080/07853890.2020.1836566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Drummond G.R., Vinh A., Guzik T.J., Sobey C.G. Nature Reviews Immunology. vol. 19. Nature Publishing Group; 2019. Immune mechanisms of hypertension; pp. 517–532. Issue 8. [DOI] [PubMed] [Google Scholar]
- 208.Vinciguerra M., Romiti S., Greco E. 2020. Atherosclerosis as Pathogenetic Substrate for Sars-Cov2 “‘Cytokine Storm.’”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Iaccarino G., Grassi G., Borghi C., Ferri C., Salvetti M., Volpe Massimo M. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian society of hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 210.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. JRAAS J. Renin-Angiotensin-Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rodilla E., Saura A., Jiménez I., Mendizábal A., Pineda-Cantero A., Lorenzo-Hernández E., Fidalgo-Montero M. del P., López-Cuervo J.F., Gil-Sánchez R., Rabadán-Pejenaute E., Abella-Vázquez L., Giner-Galvañ V., Solís-Marquínez M.N., Boixeda R., Peña-Fernández A. de la, Carrasco-Sánchez F.J., González-Moraleja J., Torres-Peña J.D., Guisado-Espartero M.E.…Gómez-Huelgas R. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J. Clin. Med. 2020;9(10):3136. doi: 10.3390/jcm9103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dietz W., Santos-Burgoa C. Obesity. vol. 28. Blackwell Publishing Inc; 2020. Obesity and its implications for COVID-19 mortality; p. 1005. Issue 6. [DOI] [PubMed] [Google Scholar]
- 213.Saleh F., Itani Leila, Calugi S., Grave R.D., El Ghoch M. Adipose-derived mesenchymal stem cells in the treatment of obesity: a systematic review of longitudinal studies on preclinical evidence. Curr. Stem Cell Res. Ther. 2018;13(6) doi: 10.2174/1574888X13666180515160008. [DOI] [PubMed] [Google Scholar]
- 214.Cao M., Pan Q., Dong H., Yuan X., Li Y., Sun Z., Dong X., Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther. 2015;6(1) doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Daltro P.S., Barreto B.C., Silva P.G., Neto P.C., Sousa Filho P.H.F., Santana Neta D., Carvalho G.B., Silva D.N., Paredes B.D., de Alcantara A.C., Freitas L.A.R., Couto R.D., Santos R.R., Souza B.S.F., Soares M.B.P., Macambira S.G. Therapy with mesenchymal stromal cells or conditioned medium reverse cardiac alterations in a high-fat diet–induced obesity model. Cytotherapy. 2017;19(10):1176–1188. doi: 10.1016/j.jcyt.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 216.Hao H., Liu J., Shen J., Zhao Y., Liu H., Hou Q., Tong C., Ti D., Dong L., Cheng Y., Mu Y., Liu J., Fu X., Han W. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats. Biochem. Biophys. Res. Commun. 2013;436(3):418–423. doi: 10.1016/j.bbrc.2013.05.117. [DOI] [PubMed] [Google Scholar]
- 217.Si Y., Zhao Y., Hao J., Liu J., Guo Y., Mu Y., Shen J., Cheng Y., Fu X., Han W. Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes. 2012;61(6):1616–1625. doi: 10.2337/db11-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Solis M.A., Moreno Velásquez I., Correa R., Huang L.L.H. Diabetology and Metabolic Syndrome. vol. 11. BioMed Central Ltd; 2019. Stem cells as a potential therapy for diabetes mellitus: a call-to-action in Latin America; p. 20. Issue 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun Y., Shi H., Yin S., Ji C., Zhang X., Zhang B., Wu P., Shi Y., Mao F., Yan Y., Xu W., Qian H. Human mesenchymal stem cell derived exosomes alleviate type 2 diabetes mellitus by reversing peripheral insulin resistance and relieving β-cell destruction. ACS Nano. 2018;12(8):7613–7628. doi: 10.1021/acsnano.7b07643. [DOI] [PubMed] [Google Scholar]
- 220.De Oliveira L.F., Almeida T.R., Ribeiro Machado M.P., Cuba M.B., Alves A.C., Da Silva M.V., Rodrigues Júnior V., Dias Da Silva V.J. Priming mesenchymal stem cells with endothelial growth medium boosts stem cell therapy for systemic arterial hypertension. Stem Cells Int. 2015;2015 doi: 10.1155/2015/685383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhu Z., Fang Z., Hu X., Zhou S. MicroRNAs e células-tronco mesenquimais: Esperança para a hipertensão pulmonar. Braz. J. Cardiovasc. Surg. 2015;30(3):380–385. doi: 10.5935/1678-9741.20150033. Sociedade Brasileira de Cirurgia Cardiovascular. [DOI] [Google Scholar]
- 222.Abumoawad A., Saad A., Ferguson C.M., Eirin A., Herrmann S.M., Hickson L.J., Goksu B.B., Bendel E., Misra S., Glockner J., Dietz A.B., Lerman L.O., Textor S.C. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020;97(4):793–804. doi: 10.1016/j.kint.2019.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hu Junping, Zhu Q., Xia M., Guo T.L., Wang Z., Li P.-L., Han W.-Q., Yi F., Li N. Transplantation of mesenchymal stem cells into the renal medulla attenuated salt-sensitive hypertension in Dahl S rat. J. Mol. Med. 2014;92(11):1139–1145. doi: 10.1007/s00109-014-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Trials C. 2020. Search of: MSC | COVID19 - List Results - ClinicalTrials.gov. [Google Scholar]
- 225.Khoury M., Rocco P.R.M., Phinney D.G., Krampera M., Martin I., Viswanathan S., Nolta J.A., LeBlanc K., Galipeau J., Weiss D.J. Cell-based therapies for coronavirus disease 2019: proper clinical investigations are essential. Cytotherapy. 2020;22(11):602–605. doi: 10.1016/j.jcyt.2020.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T., Huang L., Zhao P., Yuan X., Fan X., Zhang J.-Y., Song J., Zhang D., Jiao Y., Liu L.…Wang F.-S. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct. Targeted Ther. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Rajan S., Khunti K., Alwan N., Steves C., Greenhalgh T., Macdermott N., Sagan A., Mckee M. In the wake of the pandemic preparing for long COVID. 2021. http://www.euro.who.int/en/about-us/partners/ [PubMed]