Abstract
Amino acid metabolism plays an important role in controlling blood pressure by regulating the production of NO and ROS. The present study examined amino acid levels in the serum of Dahl SS rats and SS.13BN rats fed a low or high salt diet. We observed that 8 of 27 amino acids responded to a high salt diet in SS rats. Thus, we hypothesized that a defect in amino acids may contribute to the development of salt-induced hypertension. L-phenylalanine was used to treat SS rats with a low or high salt diet. The results demonstrated that L-phenylalanine supplementation significantly enhanced the serum nitrite levels and attenuated the high salt-induced hypertension in SS rats. Low levels of BH4 and nitrite and the impaired vascular response to acetylcholine were rescued by L-phenylalanine supplementation. Moreover, increased GTP cyclohydrolase (GCH1) mRNA, levels of BH4 and nitrite, and reduced superoxide production were observed in the kidneys of hypertensive SS rats with L-phenylalanine. The antihypertensive effects of L-phenylalanine might be mediated by enhancing BH4 biosynthesis and decreasing superoxide production from NO synthase, thereby protecting vascular and kidney function with reduced ROS and elevated NO levels. The present study demonstrated that L-phenylalanine supplementation restored vascular function, suggesting L-phenylalanine represented a potential target to attenuate high salt-sensitive hypertension through GCH1-BH4.
Introduction
High salt-induced hypertension and cardiovascular diseases are important risk factors for death worldwide [1, 2]. Although restricted salt intake is known to be beneficial, excessive salt intake still persists in some areas, especially in North China [3]. Dahl salt-sensitive (SS) rats are widely used for the study of hypertension and show the phenotype similar to human salt-sensitive hypertension [4].
Increasing evidence has indicated that high salt-induced hypertension is a metabolic syndrome [5–7], and this condition could be prevented and influenced by diets in humans and rats [8–10]. Compound protein diets were more protective against hypertension than purified AIN-76A rodent food in SS rats, implying that the amino acid composition of diets contributed to increased blood pressure [10]. However, high dietary protein (30% protein) exacerbated hypertension and renal damage in the kidneys of SS rats [11]. Additionally, a higher intake of branched chain amino acid (BCAA), particularly valine, was associated with a higher risk of incident hypertension [12]. Taken together, these results strongly suggest that the amino acid composition in the diet is closely related to control of blood pressure [13]. It was not clear how dietary amino acids affect blood pressure.
Our previous studies demonstrated that the metabolic features, especially amino acid metabolism, were significantly different between SS rats and SS.13BN rats [14]. In addition, the levels of amino acids were significantly altered in both the plasma and kidney of hypertensive SS rats [15, 16]. The same results were observed in Wistar rats fed high salt diets [17]. Moreover, supplementation with aspartate or L-arginine could increase the levels of NO and attenuate hypertension, implying that improving amino acid metabolism may lower blood pressure induced by high salt diets [18–20]. However, a comprehensive evaluation of amino acids in high salt diets has not been performed.
L-phenylalanine (L-phe), as the essential amino acid, is converted to tyrosine, and effects on blood pressure are potentially due to the changes in tyrosine levels by modulate the production of DOPA (3,4-dihydroxyPhe) and dopamine [21]. Besides, L-Phe can interfere with tetrahydrobiopterin (BH4) production, as a cofactor for aromatic amino acid hydroxylation, involved in the relaxation of the endothelium [22]. However, the crucial mechanistic link between L-phe, high salt induced hypertension and its potential impacts on vascular function and oxidation balance is lacking.
We hypothesize that amino acids metabolism may contribute to high salt induce hypertension and the aim of this study was to characterize the metabolic profiles of amino acids in SS rats. We evaluated whether these amino acids, especially L-phenylalanine, which is known to increase GCH activity and BH4 biosynthesis, would attenuate the increased blood pressure in high salt-induced Dahl SS rats.
Materials and methods
Animals and reagents
The diet used in the present study was obtained from Jiangsu Xietong (Nanjing, China). Dahl salt-sensitive (SS) rats and SS.13BN rats were purchased from Vital River (Beijing) and maintained with 12 h:12 h dark/light and free access to food and water. After adapting for 1 week, 7-week-old male rats were randomized into the low salt diet (0.4% NaCl, LS) and high salt diet (8% NaCl, HS) groups. Systolic blood pressure (SBP) was measured using tail-cuff methods with a computerized system (CODA-4, Kent, USA) as previously described [16]. All animal experiments were performed according to the guidelines of NIH and approved by the Animal Research Committee of Shaanxi Normal University (permit No.2017073).
For assessment of L-phenylalanine supplementation, all 7-week-old male SS rats were maintained on low salt diets and had free access to water. The L-Phe supplementation group was given free access to drinking water with 2.5% w/v L-Phe and low salt diets as described previously [23]. After 4 days of stable baseline blood pressure, the low salt diets were switched to a high salt diet for another 14 days (n = 7–9). At the end of the experiments, the rats were placed in metabolic cages and 24h urine samples were collected. All rats were fasted overnight and sacrificed. For chemical and amino acid analysis, serum and tissue samples were collected and stored at -80°C until analysis. All chemical reagents were obtained from Sigma. A double blind preclinical research was used to reduce the experimental bias.
Amino acid analysis
For free amino acids, 100 μl of separated serum was extracted with 200 μl of 8% sulfo-salicylic acid solution. After storage at -20°C for 30 min, the supernatants of the samples were collected after 10 min of centrifugation (13000 g at 4°C).
The concentrations of the amino acids were determined using an automated amino acid analyzer (S433-D System, Sykam, German). The analytical detector was set to measure a specific wavelength of 570 nm and 440 nm for proline and hydroxyproline. The buffer solutions, consisting of four different pH values (2.90, 4.20, 8.00), were purchased from Sykam (Germany). The flow rate of the amino acid analyzer was 0.6 ml/min, and the injection volume was 50 μl. The purchased standards of each individual amino acid were used for identification and quantification (external standard method), and the concentrations of individual free amino acid values are expressed in μM. During the running of the samples, a separate delivery pump for the ninhydrin reagent automatically mixes these two solutions, which are kept under nitrogen in the amino acid analyzer. The flow rate for the ninhydrin solution was 0.30 mL/min (130°C).
Aortic vascular isolation and reactivity measurement
For determination of the effects of L-Phe supplementation on vascular reactivity, aortic rings were isolated with the integrity maintained from all groups at 37°C. Then, vessels were precontracted with phenylephrine. The response of the aortic vessels to ACh was determined by measuring diameter of aortic vessels, as previously described [24].
Detection of BH4, nitrite, superoxide, urinary albumin, and NO synthase activity
Tissues were homogenized in 5 volumes of HEPES buffer (20 μM, pH 7.4) and extracted by centrifugation (10 min, 13000 g, 4°C). The supernatants were collected and measured by BCA protein assays (Thermo). The BH4 levels were measured from the aortic vasculature and kidney using a commercial ELISA kit at 480 nm. Quantification of total nitrite was performed as previously reported using nitrate reductase at 550 nm. Superoxide levels were estimated by a lucigenin chemiluminescence-based assay as previously described [25]. Tissue extracts were incubated with 5 μM lucigenin and 10 μM diphenyliodonium (DPI). These data were recorded in 5 consecutive 1-minute measurements and reported in relative light units per minute, normalized by the abundance of proteins. Urinary albumin and NO synthase activity were determined using kits purchased from the Nanjing Jiangcheng Bioengineering Institute (A028-2-1 and A014-2-2).
Aromatic amino acid and dopamine measurement
L-phenylalanine, tyrosine and dopamine in serum and the kidney were measured as previously described [26]. Briefly, the kidney tissues homogenate (1:10 w/v, PBS, pH = 7.4) or serum were de-proteinated with an equal volume of perchloric acid solution (0.59M) extracted by centrifugation (10 min, 13000 g, 4°C). The supernatant was then used to separate different compounds by HPLC (AntecDECADE II) and quantified using electrochemical detection at 210 nm. HPLC separation was performed using a C18 column (ALF-105 column, 50×1.0mm) and a mobile phase comprising Acetonitrile (2.5%), Octylamine (10 μL/L) and Perchloric acid (0.8 mL/L), pH 2, at a flow rate of 0.75 ml/min. Quantitation of dopamine, L-phenylalanine, and L-tyrosine was performed by comparison with external standards and normalized for total protein.
Quantitative RT-PCR
Total RNA was extracted from the kidney, and cDNA was synthesized using TaKaRa kits following the manufacturer’s protocol. The expression of GCH1 was analyzed by RT-PCR with SYBR Green PCR Master Mix. Rat GCH1 (forward: 5’-ATTTGTGGGAAGGGTCCA-3’, reverse: 5’-CAGATAACGCTGGCCTCA-3’) was obtained from BGI (Beijing, China). The PCR conditions were as follows: 35 cycles of 94°C for 10 s, 60°C for 20 s, and 82°C for 15 s of fluorescence collection. Expression was quantified by the comparative CT method and normalized to GAPDH with three repetitions.
Statistical analysis
All data were presented as the mean ± SEM. The normal distribution of amino acids contents were tested with SPSS 25.0 (IBM). A two-way analysis of variance was utilized to determine the differences in parameters between SS and SS.13BN rats on the different diets. Other data were analyzed by two-tailed t-tests. EC50 values were compared using Student’s t test. A p value <0.05 was considered statistically significant.
Results
Effects of high salt diets on amino acids in SS rats
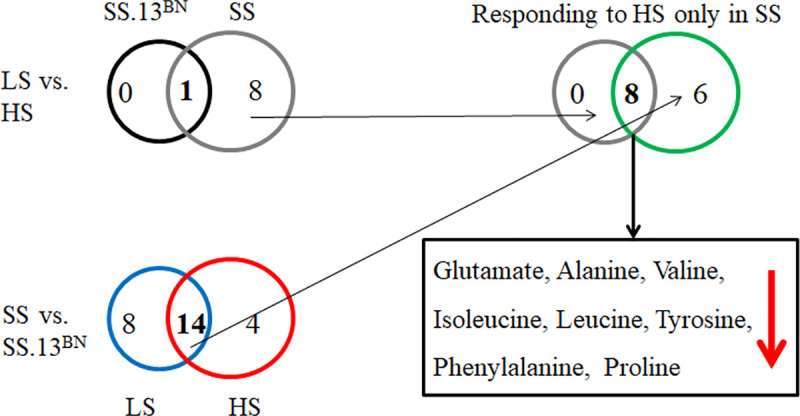
Dahl SS rats were significantly hypertensive after they were fed the 8.0% NaCl diet for 2 weeks, with an SBP of 158±7 mmHg compared with 110±5 mmHg on a 0.4% NaCl diet. While the level of blood pressure salt sensitivity was significantly lower in SS.13BN rats (109±4 in low salt and 121±5 mmHg in high salt diet) than SS rats. Compared with low salt diets, high salt diets caused significant reduction of tyrosine in both SS rats and SS.13BN rats (Table 1). Regardless of the diet, 14 of 27 amino acids were significantly different in SS rats and SS.13BN rats (Fig 1). Of the 14 amino acids differentially expressed between SS and SS.13BN rats both before and after high-salt exposure, a set of 8 amino acids responded to high salt only in SS rats: glutamate, alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, and proline, indicating that amino acids were associated with the development of high salt-induced hypertension. Considering the important role of L-phenylalanine in regulating vascular function and ROS levels, we added 2.5% w/v L-Phe for 2 weeks to further elucidate the effects of L-phenylalanine on the development of high salt-induced hypertension in SS rats.
Table 1. Data for amino acids in the serum of Dahl SS rats and SS.13BN rats fed a low salt or high salt diet (μM).
| SS.13BN-LS | SS.13BN-HS | SS-LS | SS-HS | |
|---|---|---|---|---|
| Phosphoserine | 70.7±8.6 | 67.1±10.5 | 63.0±9.6 | 61.9±9 |
| Taurine | 393.3±33.2 | 426.7±47.4 | 564.6±97.9c | 466.5±59.3 |
| Aspartic acid | 17.8±0.7 | 22.5±4.8 | 20.7±1.2c | 19.3±1.1 |
| Threonine | 302.6±44.3 | 300.4±25.2 | 403.7±46.0c | 328.4±138.2 |
| Serine | 336.5±45.8 | 389.8±35.5 | 499.2±57.8c | 482.6±42.2d |
| Asparagine | 69.1±13.1 | 64.6±6.2 | 97.3±11.8c | 84.8±11.3d |
| Glutamate | 229±18.3 | 206.0±37.4 | 386.1±46.0c | 283.3±49.6bd |
| Glycine | 546.3±76.5 | 627.8±76.0 | 796.9±101.5c | 817.5±73.3d |
| Alanine | 555.4±225.4 | 662.3±71.7 | 1076.9±89.8c | 849.8±75.2bd |
| Citrulline | 84.7±18.7 | 68.2±4.9 | 86.3±14.8 | 81.2±3.9d |
| Alpha aminobutyric acid | 17.8±1.2 | 15.8±2.6 | 27.1±10.2c | 20.1±1.7d |
| Valine | 163.1±28 | 180.8±22.7 | 267.8±42.3c | 214±17.8bd |
| Cystine | 25.9±0.3 | 25.1±1.1 | 38.7±11.7c | 28.2±1.9d |
| Methionine | 74.3±7.7 | 70.4±6.5 | 95.8±36.26 | 87.1±4.9d |
| Isoleucine | 112.0±13.9 | 123.9±10.3 | 168.9±22.3c | 142.1±12.2bd |
| Leucine | 203.5±29.6 | 236.1±27.01 | 330.5±38.5c | 278.4±28.7bd |
| Tyrosine | 127.1±12.4 | 111.8±8.4a | 159.5±15.2c | 137.9±11.1bd |
| Phenylalanine | 113.9±12.9 | 128.6±13.3 | 178.6±25.1c | 149.9±11.4bd |
| Histidine | 42.5±16 | 64.4±25.8 | 68.9±11.0c | 64.6±5.3 |
| 3-methylhistidine | 1.7±1 | 5.7±4.0 | 5.9±2.6c | 6.3±2.1 |
| 1-methyl histidine | 23.5±1.9 | 20.3±2.8 | 30.5±5.6c | 29.8±7.1d |
| Tryptophan | 107.2±8.2 | 121.85±16.6 | 137.2±11.8c | 119.6±11.1b |
| Ornithine | 240.8±27.6 | 276.74±51.8 | 361.3±30.5c | 325.3±24.8 |
| Lysine | 340.3±46.1 | 384.77±42.9 | 661.2±66.4c | 588.3±60.8d |
| Arginine | 74.2±60.7 | 38.14±38.9 | 39.9±45.99 | 37.7±17.9 |
| Hydroxyproline | 220.7±5.9 | 214.83±4.3 | 226.1±8.79 | 224.9±7.3d |
| Proline | 185.5±20.6 | 180.93±9.9 | 277.6±34.4c | 231.0±17.6bd |
a p<0.05 SS.13BN-LS vs SS.13BN-HS
b p< 0.05 SS-LS vs SS-HS
c p<0.05 SS.13BN-LS vs SS-LS
d p<0.05 SS.13BN-HS vs SS-HS. n = 6–8. Data are expressed as mean ± SD.
Fig 1. Numbers and overlaps of differentially (p<0.05) abundant amino acids in the serum of Dahl SS rats and SS.13BN rats fed a low salt or high salt diet.
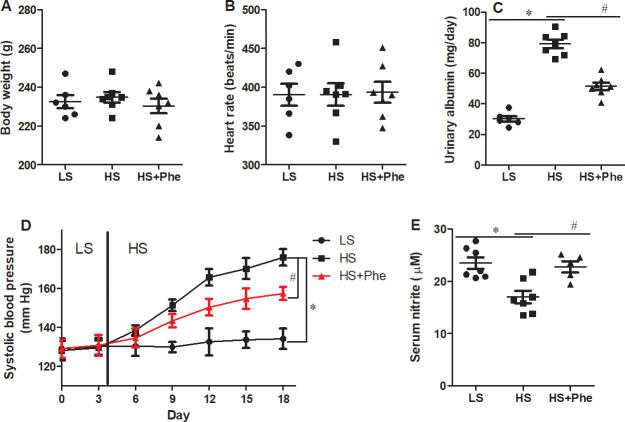
Effects of L-Phe on blood pressure
The body weight and heart rate were not different between the groups (Fig 2). Although baseline systolic blood pressure did not differ between the groups, L-Phe supplementation significantly attenuated high salt-induced hypertension in the SS rats (p<0.05). By the end of the experiments, systolic blood pressure of the L-Phe supplementation group was 18 mmHg lower than that of the vehicle group. Besides, the urinary albumin was significantly decreased in HS-Phe group than HS group. Significant increase of serum nitrite was also detected in HS-Phe group compared with the hypertensive HS group (Fig 2E).
Fig 2.
Effects of phenylalanine supplementation on body weight (A), heart rate (B), systolic pressure (C), serum nitrite (D), and urinary albumin (E) in Dahl SS rats fed a low salt or high salt diet. Each value represents the mean ± SEM, n = 6–8, * p<0.05 vs. LS group, # p<0.05 vs. HS group.
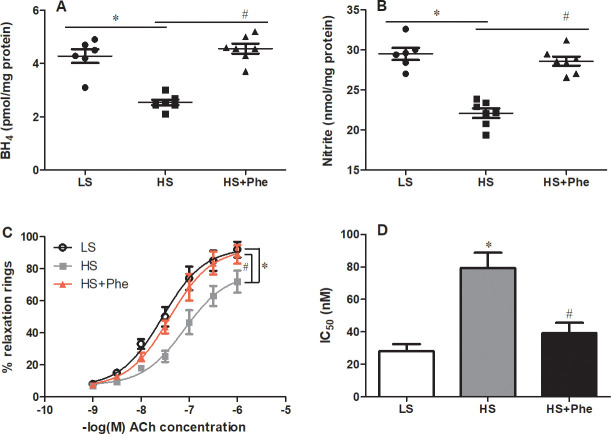
Effects of L-Phe on aortic vascular function
Further detailed studies in the vasculature revealed significantly lower BH4 levels in SS rats fed a high salt diet than in those fed a low salt diet; these levels were restored to low salt diet values following L-Phe treatment. Consistent with the effects on the BH4 levels, L-Phe supplementation significantly increased the nitrite levels in the vasculature of hypertensive SS rats (Fig 3A and 3B). Importantly, endothelial-dependent relaxation induced by ACh in L-Phe treatment group was significantly improved compared with that of the high salt diet control, as reflected by the leftward shift of the dose-response curves and the lower IC50 (28.16±7.24 nM in LS group, 79.46±16.38 nM in HS group, and 39.3±10.87 nM in HS-Phe group, Fig 3C and 3D).
Fig 3.
Effects of phenylalanine on BH4 (A), nitrite (B), vascular reactivity (C), and the corresponding IC50 value (D) in the aortas of Dahl SS rats fed a low salt or high salt diet. Each value represents the mean ± SEM, n = 6–8, * p<0.05 vs. LS group, # p<0.05 vs. HS group.
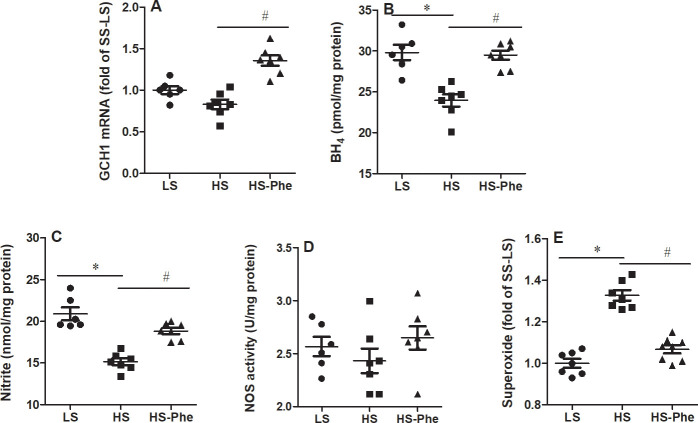
Effects of L-Phe on GTP cyclohydrolase, BH4, nitrite, and superoxide in the kidney
It should be noticed that the expression of GTP cyclohydrolase (GCH1) mRNA was no significant changes between LS group and HS group. Whereas, increased levels of GCH1 mRNA was observed in the L-Phe supplementation group compared with the high salt group (Fig 4A). Although levels of nitrite were restored to those on low salt diets, the activity of NO synthase showed no significant changes between the groups (Fig 4B and 4C). Moreover, L-Phe supplementation restored the lower BH4 and higher superoxide in the kidney of hypertensive SS rats than those on low salt diet (Fig 4D and 4E).
Fig 4.
Effects of phenylalanine on GCH1 mRNA (A), BH4 (B), nitrite (C), NOS activity (D), and ROS (E) in the kidneys of Dahl SS rats fed a low salt or high salt diet. Each value represents the mean ± SEM, n = 6–8, * p<0.05 vs. LS group, # p<0.05 vs. HS group.
Effects of L-Phe on tissue phenylalanine, tyrosine, and dopamine
L-Phe is catalyzed to tyrosine via the enzyme of phenylalanine hydroxylase in vivo. The decreased level of serum L-phenylalanine in the serum of hypertensive rats was reversed in the HS-Phe group (Table 2). Although levels of tyrosine were increased in HS-Phe group, there were no significant differences between HS and HS-Phe in the serum. However, the L-phe/tyrosine ratio was increased flowing with L-Phe supplement, implying that L-Phe was absorbed oral gavage. No significant rise of L-Phe/tyr was observed in both serum and the kidney (Table 2). There was trend of increased dopamine, but it did not reach statistical significance.
Table 2. Effects of L-phenylalanine supplementation on levels of L-phenylalanine, tyrosine, L-phe/try, and dopamine in both serum and the kidney of SS rats.
| Serum (μM) | Kidney (pmol/mg protein) | |||||
|---|---|---|---|---|---|---|
| LS | HS | HS+L-Phe | LS | HS | HS+L-Phe | |
| L-phenylalanine | 175.21±13.64 | 141.36±11.40* | 169.84±13.58# | 13.02±1.05 | 12.56±0.79 | 12.57±1.61 |
| Tyrosine | 163.41±10.36 | 135.72±12.74* | 156.53±11.71 | 7.35±0.75 | 7.79±0.84 | 7.76±0.64 |
| L-phe/tyr | 1.12±0.16 | 1.09±0.19 | 1.12±0.24 | 1.77±0.62 | 1.61±0.43 | 1.73±0.37 |
| Dopamine | 0.598±0.047 | 0.496±0.052 | 0.547±0.038 | 10.72±2.81 | 8.40±1.96 | 9.84±3.31 |
* p<0.05 vs. LS group
# p<0.05 vs. HS group. n = 6. Data are expressed as mean ± SD.
Discussion
Altered amino acids in Dahl SS rats
In the present study, we quantitatively analyzed the amino acid contents and showed that only 8 of 27 amino acids responded to high salt in SS rats. Among these amino acids, branched-chain amino acids (Val, Ile, Leu) are structural constituents of proteins that were significantly decreased in hypertensive rats. However, higher BCAA levels were associated with a higher risk of incident hypertension [27]. In the International Study of Macronutrients and Blood Pressure (INTERMAP study), higher intakes of glutamic acid and glycine were related to lower and higher blood pressure, respectively [28]. Although the precise mechanisms responsible for this antihypertensive effect of Pro and Tyr are still unknown, they may involve the inhibition of angiotensin-converting enzyme or production of vasodilators [29, 30]. Compared with those in SS.13BN rats, levels of glycine and methionine were increased in SS rats, which was consistent with previous reports in the plasma and kidney [16, 31]. We proposed that altered amino acids might be responsible, at least in part, for the development of high salt-induced hypertension in Dahl SS rats. Of course, more investigations need to be performed.
L-Phe altered NO and superoxide levels
Elevated blood pressure has been demonstrated in SS rats with decreased NO [32]. Although there was no significant difference in arginine and NO synthase activity between low salt and high salt diets, the NO levels were significantly decreased in hypertensive SS rats [33], which was due to decreased BH4 in the kidney and reduced NO production in the vasculature by uncoupling NOS. Confirming previous findings, we found that L-Phe supplementation reversed the inhibitory GCH1-BH4 complex in both the vasculature and kidneys, thus likely contributing to the increased NO production and decreased blood pressure [22, 23]. Furthermore, BH4, as an allosteric and essential cofactor for NO synthase, plays a critical role in the stabilization of the NO synthase dimer and increases the affinity of NO synthase for arginine [22, 34]. The decreased L-Phe in the hypertensive SS rats was associated with lower BH4, which led to increased sensitivity to high salt diets. Taken together, these data suggested that exogenous phenylalanine may increase BH4-dependent NO production, leading to decreased blood pressure in hypertensive SS rats.
The production of superoxide by NO synthase plays an important role in the development of hypertension [35]. BH4 has been reported to be a powerful oxidant of O2• [36]. The observed decreased superoxide in hypertensive SS rats may result in a shift away from the production of ROS by NOS to the production of NO by the increased BH4 with L-Phe supplementation [37]. The effects of L-Phe supplementation on oxidative stress need further study.
L-Phe improved endothelial function and reduced oxidative damage in the kidney
Endothelial function plays a key role in controlling blood pressure and the development of cardiovascular disease [38]. Our results confirmed that acetylcholine-induced relaxation in high salt diet group was significantly lower than that in SS rats fed low salt diets. However, L-Phe treatment improved vascular function in hypertensive SS rats. These results were consistent with previous studies on treatment with BH4 or L-Arg [39, 40]. Thus, we believe that L-Phe attenuates the development of salt-induced hypertension in SS rats by improving endothelial function.
The deficiency in GCH activity was likely contributed to the reduced BH4 levels [41]. Although there were no differences in the GCH1 mRNA level between the low and high salt groups, elevated mRNA of GCH1 was observed in HS-Phe group compared with HS group. This finding may be due to insufficient L-Phe contents in hypertensive rats. Correspondingly, this result was particularly relevant to the present study because L-Phe increased the mRNA expression of GCH1. Elevated NO levels and reduced ROS levels may promote sodium excretion and protect the kidney from ischemia [42]. The unchanged BH4 level in SS.13BN rats, which was reported previously [41], was consistent with phenylalanine in this strain when fed a high salt diet.
Obviously, phenylalanine hydroxylase also could convert L-phe to tyrosine, which was further catalyzing the conversion of tyrosine to dopamine and adrenaline. Although levels of dopamine were not altered followed with L-Phe treatment, increased blood pressure were attenuated in HS-Phe group indicating that dopamine may be not involved in salt induced hypertension [43]. Consistent with our previous results by GC-MS, no difference of L-phenylalanine and tyrosine were identified in the kidney of SS rats fed on 16wks high salt diets [16]. Previous studies confirmed that L-arginine could restore NO bioavailability through NOS-NO pathway, but L-arginine was not lack of efficacy but also high postinfarction mortality [44, 45], which suggested that L-Phe treatment may be more benefits to attenuate hypertension and to restore vascular function.
We conclude that there were several altered amino acids in hypertensive SS rats that could contribute to salt-induced hypertension. L-Phenylalanine supplementation can directly affect levels of BH4 and nitrite, thus attenuating salt-induced hypertension in SS rats through the improved vascular and kidney function.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the Youth Fund for Humanities and Social Sciences Research of the Ministry of Education (Grant No. 19YJC190023), the China Postdoctoral Science Foundation funding project (Grant No. 2019M663924XB), and the Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2018JQ1033, 2021JQ-309). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cappuccio FP, Campbell NR (2017) Population dietary salt reduction and the risk of cardiovascular disease: a commentary on recent evidence. The Journal of Clinical Hypertension 19: 4–5. 10.1111/jch.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco V, Oparil S (2006) Salt Sensitivity, a Determinant of Blood Pressure, Cardiovascular Disease and Survival. Journal of the American College of Nutrition 25: 247S–255S. 10.1080/07315724.2006.10719574 [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Guo X, Lu Z, Tang J, Li Y, et al. (2019) Cardiovascular Diseases Deaths Attributable to High Sodium Intake in Shandong Province, China. Journal of the American Heart Association 8: e010737. 10.1161/JAHA.118.010737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson DL, Dwinell MR, Greene AS, Kwitek AE, Roman RJ, et al. (2008) Chromosome substitution reveals the genetic basis of Dahl salt-sensitive hypertension and renal disease. American Journal of Physiology-Renal Physiology 295: F837. 10.1152/ajprenal.90341.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Gu D, Huang J, Rao DC, Jaquish CE, et al. (2009) Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. The Lancet 373: 829–835. 10.1016/S0140-6736(09)60144-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Hou E, Wang L, Wang Y, Yang L, et al. (2015) Reconstruction and analysis of correlation networks based on GC–MS metabolomics data for young hypertensive men. Analytica Chimica Acta 854: 95–105. 10.1016/j.aca.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Hou E, Wang Z, Sun N, He L, et al. (2014) Analysis of metabolites in plasma reveals distinct metabolic features between Dahl salt-sensitive rats and consomic SS.13(BN) rats. Biochem Biophys Res Commun 450: 863–869. 10.1016/j.bbrc.2014.06.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, et al. (2006) Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 47: 296–308. 10.1161/01.HYP.0000202568.01167.B6 [DOI] [PubMed] [Google Scholar]
- 9.Mattson DL, Meister CJ, Marcelle ML (2005) Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741. 10.1161/01.HYP.0000153318.74544.cc [DOI] [PubMed] [Google Scholar]
- 10.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, et al. (2004) Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiological Genomics 16: 194–203. 10.1152/physiolgenomics.00151.2003 [DOI] [PubMed] [Google Scholar]
- 11.De Miguel C, Lund H, Mattson DL (2011) High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274. 10.1161/HYPERTENSIONAHA.110.154302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirmiran P, Teymoori F, Asghari G, Azizi F (2019) Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Archives of Iranian Medicine (AIM) 22. [PubMed] [Google Scholar]
- 13.Jennings A, MacGregor A, Welch A, Chowienczyk P, Spector T, et al. (2015) Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. The Journal of nutrition 145: 2130–2138. 10.3945/jn.115.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin W, Liu Z, Zheng X, Chen M, Gao D, et al. (2018) High-salt diet affects amino acid metabolism in plasma and muscle of Dahl salt-sensitive rats. Amino Acids 50: 1407–1414. 10.1007/s00726-018-2615-6 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Sun Q, Sun N, Liang M, Tian Z (2017) Mitochondrial dysfunction and altered renal metabolism in Dahl salt-sensitive rats. Kidney and Blood Pressure Research 42: 587–597. 10.1159/000479846 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Liu X, Zhang C, Wang Z (2018) High salt diet induces metabolic alterations in multiple biological processes of Dahl salt-sensitive rats. The Journal of nutritional biochemistry 56: 133–141. 10.1016/j.jnutbio.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Zheng L, Luo R, Zhao X, Han Z, et al. (2015) A 1 H NMR-based metabonomic investigation of time-dependent metabolic trajectories in a high salt-induced hypertension rat model. RSC Advances 5: 281–290. [Google Scholar]
- 18.Fujii S, Zhang L, Igarashi J, Kosaka H (2003) L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension 42: 1014–1020. 10.1161/01.HYP.0000094557.36656.D0 [DOI] [PubMed] [Google Scholar]
- 19.Chen PY, Sanders PW (1991) L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. The Journal of clinical investigation 88: 1559–1567. 10.1172/JCI115467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou E, Sun N, Zhang F, Zhao C, Usa K, et al. (2017) Malate and aspartate increase L-arginine and nitric oxide and attenuate hypertension. Cell reports 19: 1631–1639. 10.1016/j.celrep.2017.04.071 [DOI] [PubMed] [Google Scholar]
- 21.Soares-da-Silva P, Pestana M, Vieira-Coelho MA, Fernandes MH, Albino-Teixeira A (1995) Assessment of renal dopaminergic system activity in the nitric oxide-deprived hypertensive rat model. British Journal of Pharmacology 114: 1403–1413. 10.1111/j.1476-5381.1995.tb13362.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell BM, Dorrance AM, Webb RC (2004) Phenylalanine improves dilation and blood pressure in GTP cyclohydrolase inhibition-induced hypertensive rats. Journal of cardiovascular pharmacology 43: 758–763. 10.1097/00005344-200406000-00004 [DOI] [PubMed] [Google Scholar]
- 23.Heikal L, Starr A, Hussein D, Prieto-Lloret J, Aaronson P, et al. (2018) l-Phenylalanine Restores Vascular Function in Spontaneously Hypertensive Rats Through Activation of the GCH1-GFRP Complex. JACC: Basic to Translational Science 3: 366–377. 10.1016/j.jacbts.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakanishi K, Vinten-Johansen J, Lefer DJ, Zhao Z, Fowler r W. C., et al. (1992) Intracoronary L-arginine during reperfusion improves endothelial function and reduces infarct size. American Journal of Physiology-Heart and Circulatory Physiology 263: H1650–H1658. 10.1152/ajpheart.1992.263.6.H1650 [DOI] [PubMed] [Google Scholar]
- 25.Münzel T, Afanas’ ev IB, Kleschyov AL, Harrison DG (2002) Detection of superoxide in vascular tissue. Arteriosclerosis, thrombosis, and vascular biology 22: 1761–1768. 10.1161/01.atv.0000034022.11764.ec [DOI] [PubMed] [Google Scholar]
- 26.Atherton ND, Green A (1988) HPLC measurement of phenylalanine in plasma. Clin Chem 34: 2241–2244. [PubMed] [Google Scholar]
- 27.Yamakado M, Nagao K, Imaizumi A, Tani M, Toda A, et al. (2015) Plasma free amino acid profiles predict four-year risk of developing diabetes, metabolic syndrome, dyslipidemia, and hypertension in Japanese population. Scientific reports 5: 11918. 10.1038/srep11918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan Q, Stamler J, Griep LMO, Daviglus ML, Van Horn L, et al. (2015) An update on nutrients and blood pressure. Journal of atherosclerosis and thrombosis: 30000. 10.5551/jat.30000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cicero AF, Aubin F, Azais-Braesco V, Borghi C (2013) Do the lactotripeptides isoleucine-proline-proline and valine-proline-proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am J Hypertens 26: 442–449. 10.1093/ajh/hps044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deijen J, Wientjes C, Vullinghs H, Cloin P, Langefeld J (1999) Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain research bulletin 48: 203–209. 10.1016/s0361-9230(98)00163-4 [DOI] [PubMed] [Google Scholar]
- 31.Tanada Y, Okuda J, Kato T, Minamino-Muta E, Murata I, et al. (2017) The metabolic profile of a rat model of chronic kidney disease. PeerJ 5: e3352. 10.7717/peerj.3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowley AW Jr. xs(2008) Renal medullary oxidative stress, pressure-natriuresis, and hypertension. Hypertension 52: 777–786. 10.1161/HYPERTENSIONAHA.107.092858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szentiványi M Jr, Zou A-P, Mattson DL, Soares P, Moreno C, et al. (2002) Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 283: R266–R272. 10.1152/ajpregu.00461.2001 [DOI] [PubMed] [Google Scholar]
- 34.Yoneyama T, Hatakeyama K (2001) Ligand binding to the inhibitory and stimulatory GTP cyclohydrolase I/GTP cyclohydrolase I feedback regulatory protein complexes. Protein Science 10: 871–878. 10.1110/ps.38501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klahr S (2001) The role of nitric oxide in hypertension and renal disease progression. Nephrology Dialysis Transplantation 16: 60–62. 10.1093/ndt/16.suppl_1.60 [DOI] [PubMed] [Google Scholar]
- 36.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, et al. (2001) Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1288. 10.1161/01.cir.103.9.1282 [DOI] [PubMed] [Google Scholar]
- 37.Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS (2008) Ratio of 5, 6, 7, 8-tetrahydrobiopterin to 7, 8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. American Journal of Physiology-Heart and Circulatory Physiology 294: H1530–H1540. 10.1152/ajpheart.00823.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyoshi A, Suzuki H, Fujiwara M, Masai M, Iwasaki T (1997) Impairment of endothelial function in salt-sensitive hypertension in humans. American journal of hypertension 10: 1083–1090. 10.1016/s0895-7061(97)00226-4 [DOI] [PubMed] [Google Scholar]
- 39.Hong H-J, Hsiao G, Cheng T-H, Yen M-H (2001) Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38: 1044–1048. 10.1161/hy1101.095331 [DOI] [PubMed] [Google Scholar]
- 40.Artigues C, Richard V, Roussel C, Lallemand F, Henry J-P, et al. (2000) Increased endothelium-monocyte interactions in salt-sensitive hypertension: effect of L-arginine. Journal of cardiovascular pharmacology 35: 468–473. 10.1097/00005344-200003000-00018 [DOI] [PubMed] [Google Scholar]
- 41.Taylor NE, Maier KG, Roman RJ, Cowley AW Jr., (2006) NO synthase uncoupling in the kidney of Dahl S rats: role of dihydrobiopterin. Hypertension 48: 1066–1071. 10.1161/01.HYP.0000248751.11383.7c [DOI] [PubMed] [Google Scholar]
- 42.Vassileva I, Mountain C, Pollock DM (2003) Functional role of ETB receptors in the renal medulla. Hypertension 41: 1359–1363. 10.1161/01.HYP.0000070958.39174.7E [DOI] [PubMed] [Google Scholar]
- 43.Pinho MJ, Serrão MP, Soares-da-Silva P (2007) High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. American Journal of Physiology-Renal Physiology 292: F1452–F1463. 10.1152/ajprenal.00465.2006 [DOI] [PubMed] [Google Scholar]
- 44.Rochette L, Lorin J, Zeller M, Guilland J-C, Lorgis L, et al. (2013) Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacology & Therapeutics 140: 239–257. 10.1016/j.pharmthera.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 45.Sun T, Zhou WB, Luo XP, Tang YL, Shi HM (2010) Oral L-arginine supplementation in acute myocardial infarction therapy: a meta-analysis of randomized controlled trials. Clinical Cardiology 32: 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.